Hypersensitivity of Intrinsically Photosensitive Retinal Ganglion Cells in Migraine Induces Cortical Spreading Depression
Abstract
:1. Introduction
2. Results
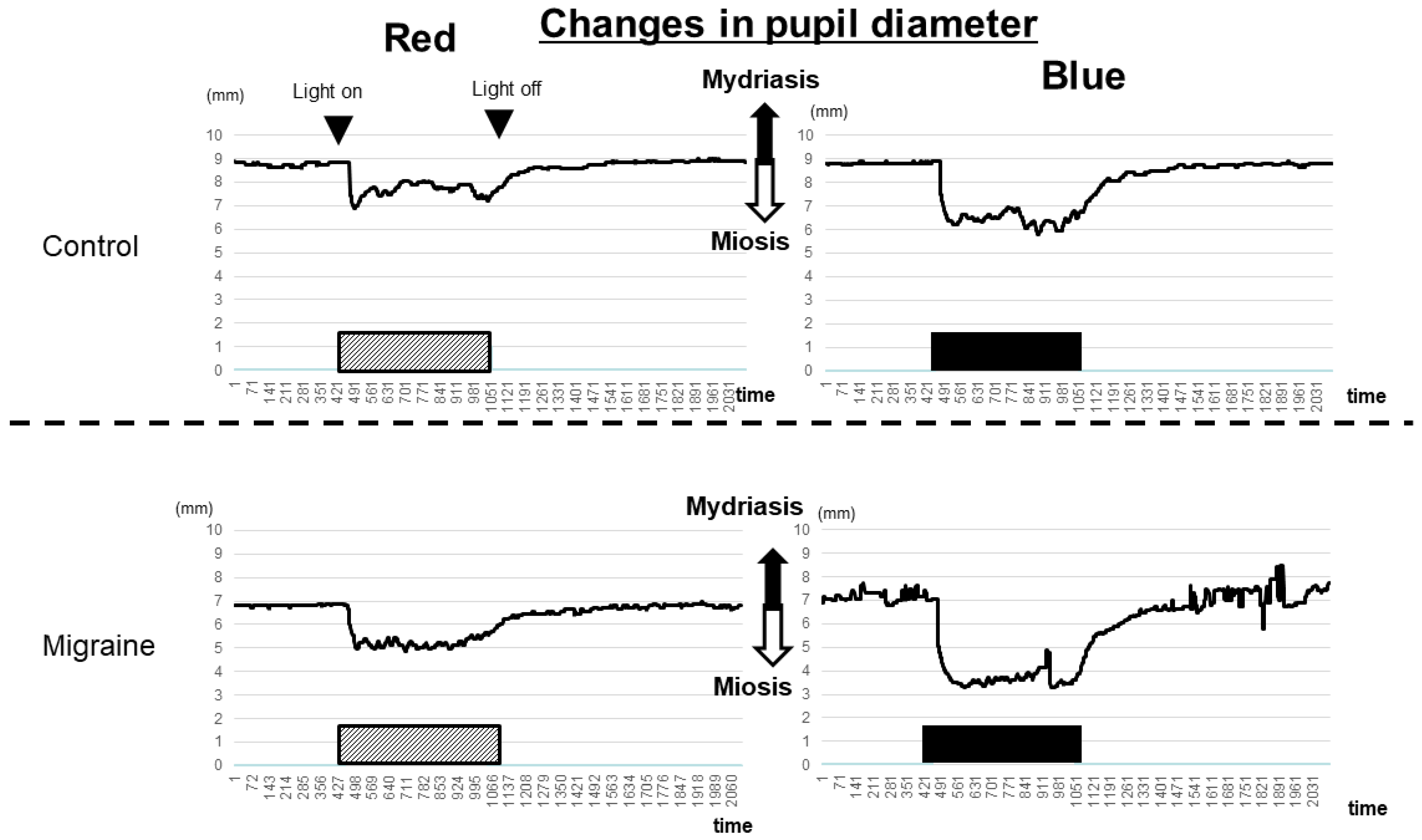
2.1. Hyperreactivity of ipRGCs in Patients with Migraine Compared with that in Healthy Controls
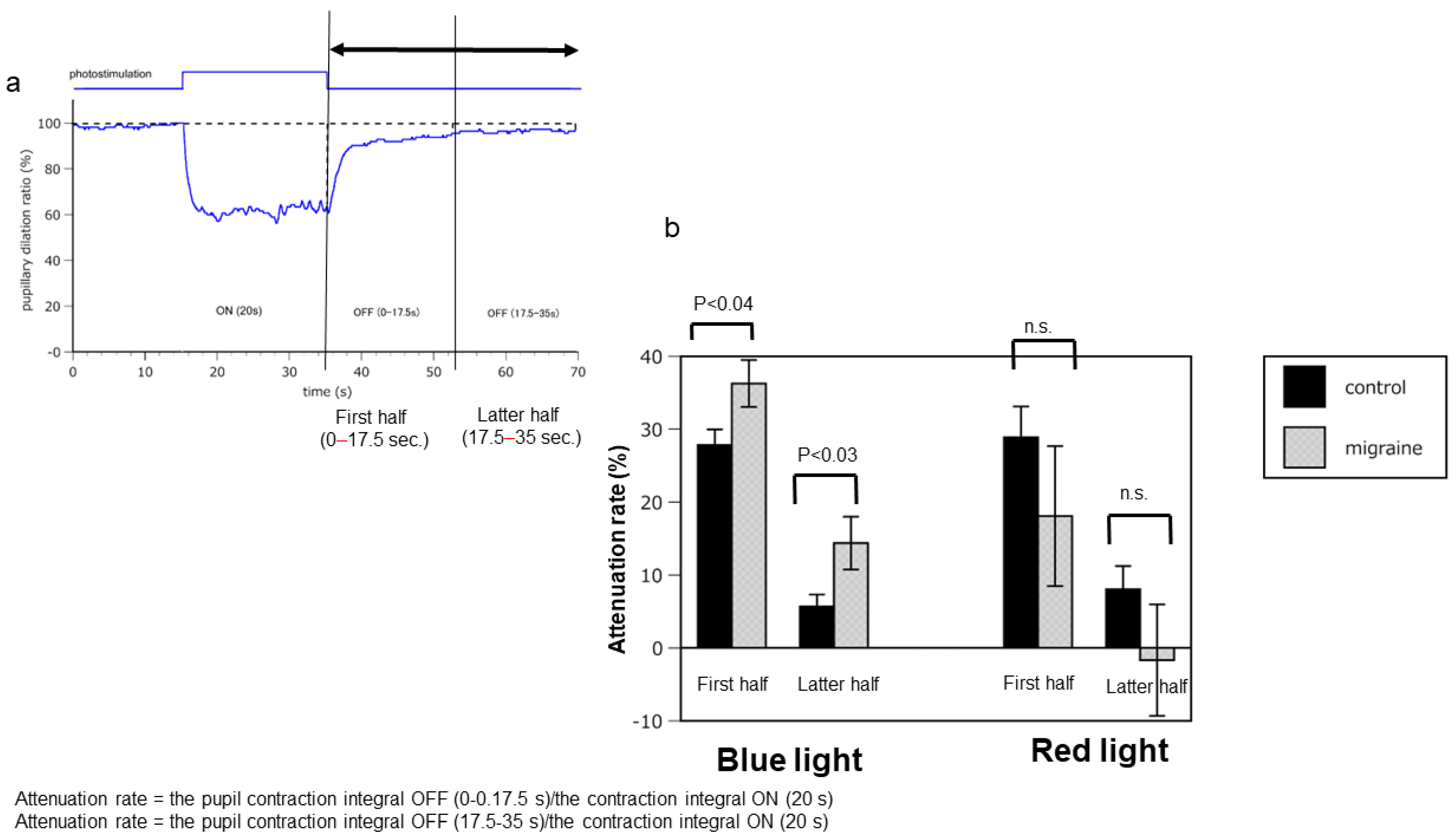
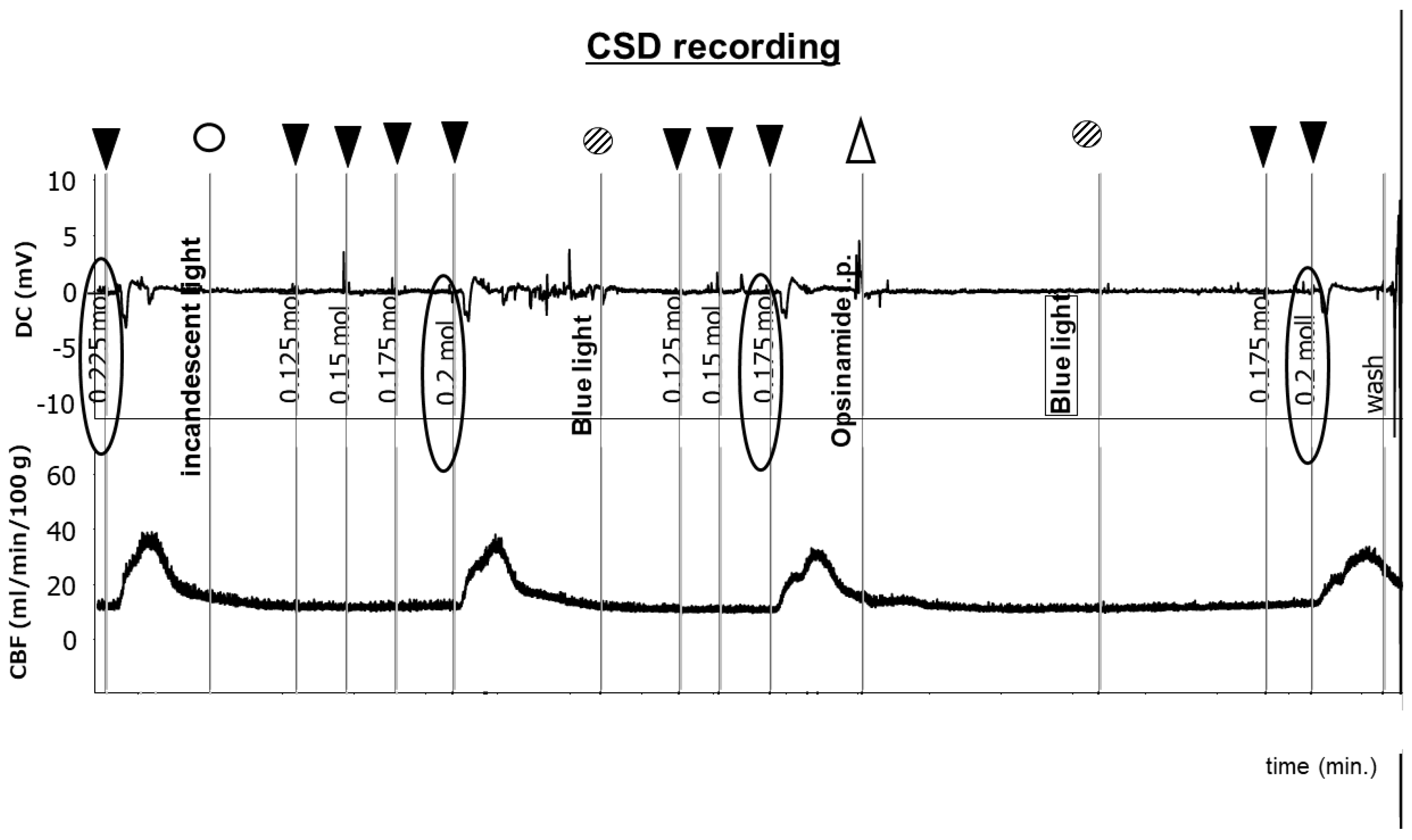
2.2. Sensitivity of ipRGCs Affects CSD
3. Discussion
4. Materials and Methods
4.1. Analysis of Function of ipRGCs Associated with Pupillary Light Responses in Patients with Migraine and Controls
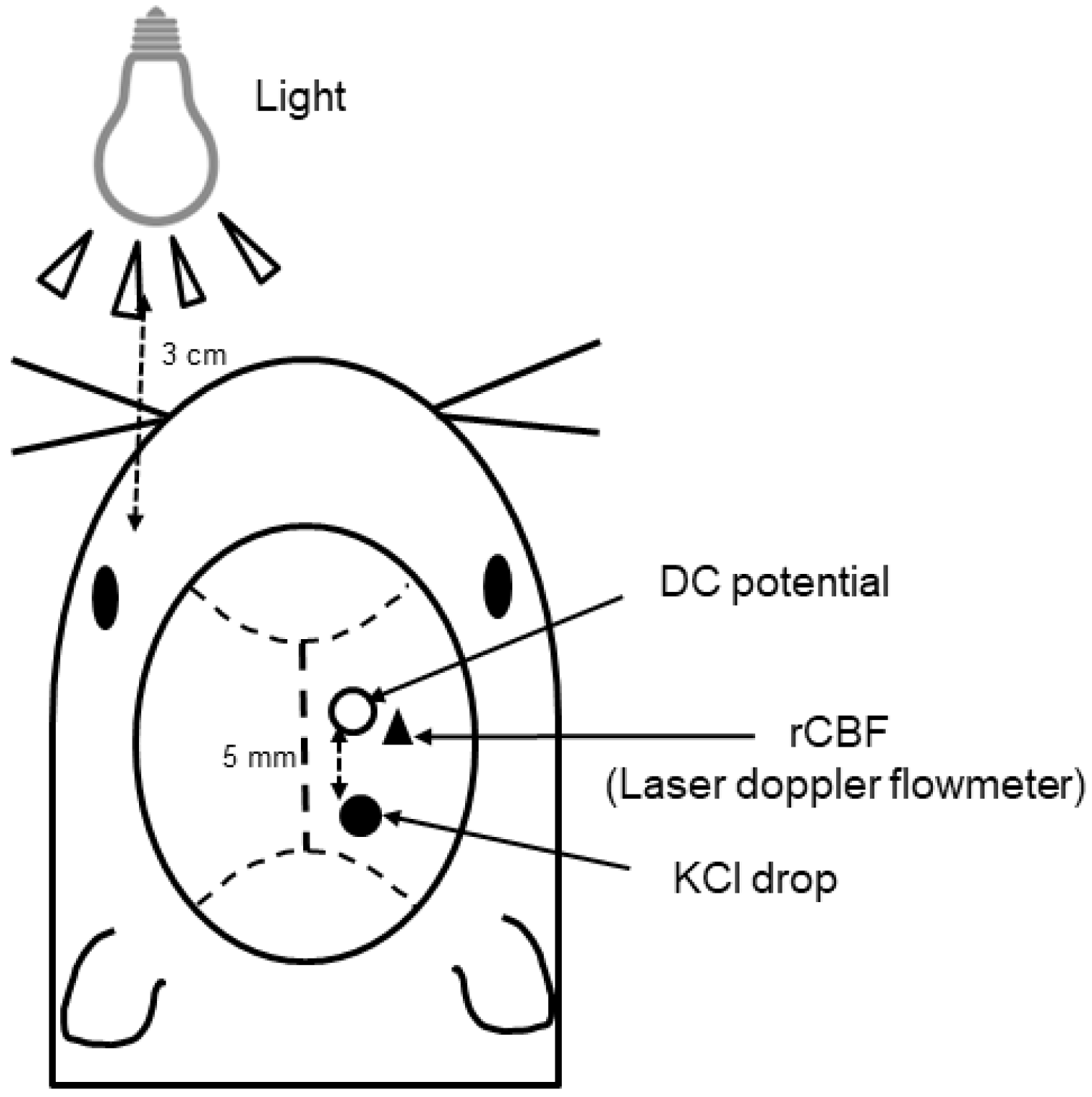
4.2. Analysis of Changes in CSD Response to Light in Mice
4.3. Evaluation of the CSD Threshold
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Atlas: Country Resources for Neurological Disorders, 2nd ed.; WHO: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241565509 (accessed on 31 January 2024).
- Li, X.Y.; Yang, C.H.; Lv, J.J.; Liu, H.; Zhang, L.Y.; Yin, M.Y.; Guo, Z.L.; Zhang, R.H. Global, regional, and national epidemiology of migraine and tension-type headache in youths and young adults aged 15–39 years from 1990 to 2019: Findings from the global burden of disease study 2019. J. Headache Pain 2023, 24, 126. [Google Scholar] [CrossRef] [PubMed]
- Sakai, F.; Igarashi, H. Prevalence of migraine in Japan: A nationwide survey. Cephalalgia 1997, 17, 15–22. [Google Scholar] [CrossRef]
- Takeshima, T.; Ishizaki, K.; Fukuhara, Y.; Ijiri, T.; Kusumi, M.; Wakutani, Y.; Mori, M.; Kawashima, M.; Kowa, H.; Adachi, Y.; et al. Population-based door-to-door survey of migraine in Japan: The Daisen study. Headache 2004, 44, 8–19. [Google Scholar] [CrossRef]
- Hirata, K.; Ueda, K.; Komori, M.; Zagar, A.J.; Selzler, K.J.; Nelson, A.M.; Han, Y.; Jaffe, D.H.; Matsumori, Y.; Takeshima, T. Comprehensive population-based survey of migraine in Japan: Results of the Observational survey of the Epidemiology, treatment, and Care of Migraine. (OVERCOME [Japan]) study. Curr. Med. Res. Opin. 2021, 37, 1945–1955. [Google Scholar] [CrossRef]
- Shimizu, T.; Sakai, F.; Miyake, H.; Sone, T.; Sato, M.; Tanabe, S.; Azuma, Y.; Dodick, D.W. Disability, quality of life, productivity impairment and employer costs of migraine in the workplace. J. Headache Pain 2021, 22, 29. [Google Scholar] [CrossRef]
- Dodick, D.W. Migraine. Lancet 2018, 391, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Albilali, A.; Dilli, E. Photophobia: When light hurts, a review. Curr. Neurol. Neurosci. Rep. 2018, 18, 62. [Google Scholar] [CrossRef]
- Martin, L.F.; Patwardhan, A.M.; Jain, S.V.; Salloum, M.M.; Freeman, J.; Khanna, R.; Gannala, P.; Goel, V.; Jones-MacFarland, F.N.; Killgore, W.D.; et al. Evaluation of green light exposure on headache frequency and quality of life in migraine patients: A preliminary one-way cross-over clinical trial. Cephalalgia 2021, 41, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Tatsumoto, M.; Suzuki, E.; Nagata, M.; Suzuki, K.; Hirata, K. Prophylactic treatment for patients with migraine using blue cut for night glass. Intern. Med. 2023, 62, 849–854. [Google Scholar] [CrossRef]
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by retinal ganglion cells that set the circadian clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef]
- Ksendzovsky, A.; Pomeraniec, I.J.; Zaghloul, K.A.; Provencio, J.J.; Provencio, I. Clinical implications of the melanopsin-based non-image-forming visual system. Neurology 2017, 88, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Mure, L.S. Intrinsically photosensitive retinal ganglion cells of the human retina. Front. Neurol. 2021, 12, 636330. [Google Scholar] [CrossRef] [PubMed]
- Gamlin, P.D.; McDougal, D.H.; Pokorny, J.; Smith, V.C.; Yau, K.W.; Dacey, D.M. Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vision Res. 2007, 47, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, P.; Zele, A.J.; Feigl, B. The post-illumination pupil response (PIPR). Investig. Ophthalmol. Vis. Sci. 2015, 56, 3838–3849. [Google Scholar] [CrossRef] [PubMed]
- van der Meijden, W.P.; te Lindert, B.H.; Bijlenga, D.; Coppens, J.E.; Gómez-Herrero, G.; Bruijel, J.; Kooij, J.J.; Cajochen, C.; Bourgin, P.; Van Someren, E.J. Post-illumination pupil response after blue light: Reliability of optimized melanopsin-based phototransduction assessment. Exp. Eye Res. 2015, 139, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Bonmati-Carrion, M.A.; Hild, K.; Isherwood, C.M.; Sweeney, S.J.; Revell, V.L.; Madrid, J.A.; Rol, M.A.; Skene, D.J. Effect of single and combined monochromatic light on the human pupillary light response. Front. Neurol. 2018, 9, 1019. [Google Scholar]
- Hughes, S.; Jagannath, A.; Rodgers, J.; Hankins, M.W.; Peirson, S.N.; Foster, R.G. Signalling by melanopsin (OPN4) expressing photosensitive retinal ganglion cells. Eye 2016, 30, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Do, M.T.H. Melanopsin and the intrinsically photosensitive retinal ganglion cells: Biophysics to behavior. Neuron 2019, 104, 205–226. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Kainz, V.; Jakubowski, M.; Gooley, J.J.; Saper, C.B.; Digre, K.; Burstein, R. A neural mechanism for exacerbation of headache by light. Nat. Neurosci. 2010, 13, 239–245. [Google Scholar] [CrossRef]
- Gerasimova, E.; Burkhanova, G.; Chernova, K.; Zakharov, A.; Enikeev, D.; Khaertdinov, N.; Giniatullin, R.; Sitdikova, G. Hyperhomocysteinemia increases susceptibility to cortical spreading depression associated with photophobia, mechanical allodynia, and anxiety in rats. Behav. Brain Res. 2021, 409, 113324. [Google Scholar] [CrossRef]
- Charles, A.C.; Baca, S.M. Cortical spreading depression and migraine. Nat. Rev. Neurol. 2013, 9, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, M. Cerebral blood flow in migraine and cortical spreading depression. Acta Neurol. Scand. Suppl. 1987, 113, 1–40. [Google Scholar] [CrossRef]
- Lauritzen, M. Cortical spreading depression in migraine. Cephalalgia 2001, 21, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Karatas, H.; Erdener, S.E.; Gursoy-Ozdemir, Y.; Lule, S.; Eren-Koçak, E.; Sen, Z.D.; Dalkara, T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 2013, 339, 1092–1095. [Google Scholar] [CrossRef]
- Noseda, R.; Burstein, R. Advances in understanding the mechanisms of migraine-type photophobia. Curr. Opin. Neurol. 2011, 24, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Noseda, R.; Bernstein, C.A.; Nir, R.R.; Lee, A.J.; Fulton, A.B.; Bertisch, S.M.; Hovaguimian, A.; Cestari, D.M.; Saavedra-Walker, R.; Borsook, D.; et al. Migraine photophobia originating in cone-driven retinal pathways. Brain 2016, 139, 1971–1986. [Google Scholar] [CrossRef]
- Noseda, R.; Copenhagen, D.; Burstein, R. Current understanding of photophobia, visual networks and headaches. Cephalalgia 2019, 39, 1623–1634. [Google Scholar] [CrossRef]
- Mathew, A.A.; Panonnummal, R. Cortical spreading depression: Culprits and mechanisms. Exp. Brain Res. 2022, 240, 733–749. [Google Scholar] [CrossRef]
- Higuchi, S.; Hida, A.; Tsujimura, S.; Mishima, K.; Yasukouchi, A.; Lee, S.I.; Kinjyo, Y.; Miyahira, M. Melanopsin gene polymorphism I394T is associated with pupillary light responses in a dose-dependent manner. PLoS ONE 2013, 8, e60310. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Unekawa, M.; Tomita, M.; Tomita, Y.; Toriumi, H.; Suzuki, N. Sustained decrease and remarkable increase in red blood cell velocity in intraparenchymal capillaries associated with potassium-induced cortical spreading depression. Microcirculation 2012, 19, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Unekawa, M.; Kitagawa, S.; Takizawa, T.; Kayama, Y.; Nakahara, J.; Shibata, M. Cortical spreading depolarisation-induced facial hyperalgesia, photophobia and hypomotility are ameliorated by sumatriptan and olcegepant. Sci. Rep. 2020, 10, 11408. [Google Scholar] [CrossRef] [PubMed]
- Ebine, T.; Toriumi, H.; Shimizu, T.; Unekawa, M.; Takizawa, T.; Kayama, Y.; Shibata, M.; Suzuki, N. Alterations in the threshold of the potassium concentration to evoke cortical spreading depression during the natural estrous cycle in mice. Neurosci. Res. 2016, 112, 57–62. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagata, E.; Takao, M.; Toriumi, H.; Suzuki, M.; Fujii, N.; Kohara, S.; Tsuda, A.; Nakayama, T.; Kadokura, A.; Hadano, M. Hypersensitivity of Intrinsically Photosensitive Retinal Ganglion Cells in Migraine Induces Cortical Spreading Depression. Int. J. Mol. Sci. 2024, 25, 7980. https://doi.org/10.3390/ijms25147980
Nagata E, Takao M, Toriumi H, Suzuki M, Fujii N, Kohara S, Tsuda A, Nakayama T, Kadokura A, Hadano M. Hypersensitivity of Intrinsically Photosensitive Retinal Ganglion Cells in Migraine Induces Cortical Spreading Depression. International Journal of Molecular Sciences. 2024; 25(14):7980. https://doi.org/10.3390/ijms25147980
Chicago/Turabian StyleNagata, Eiichiro, Motoharu Takao, Haruki Toriumi, Mari Suzuki, Natsuko Fujii, Saori Kohara, Akio Tsuda, Taira Nakayama, Ayana Kadokura, and Manaka Hadano. 2024. "Hypersensitivity of Intrinsically Photosensitive Retinal Ganglion Cells in Migraine Induces Cortical Spreading Depression" International Journal of Molecular Sciences 25, no. 14: 7980. https://doi.org/10.3390/ijms25147980



