Western Diet Modifies Platelet Activation Profiles in Male Mice
Abstract
1. Introduction
2. Results
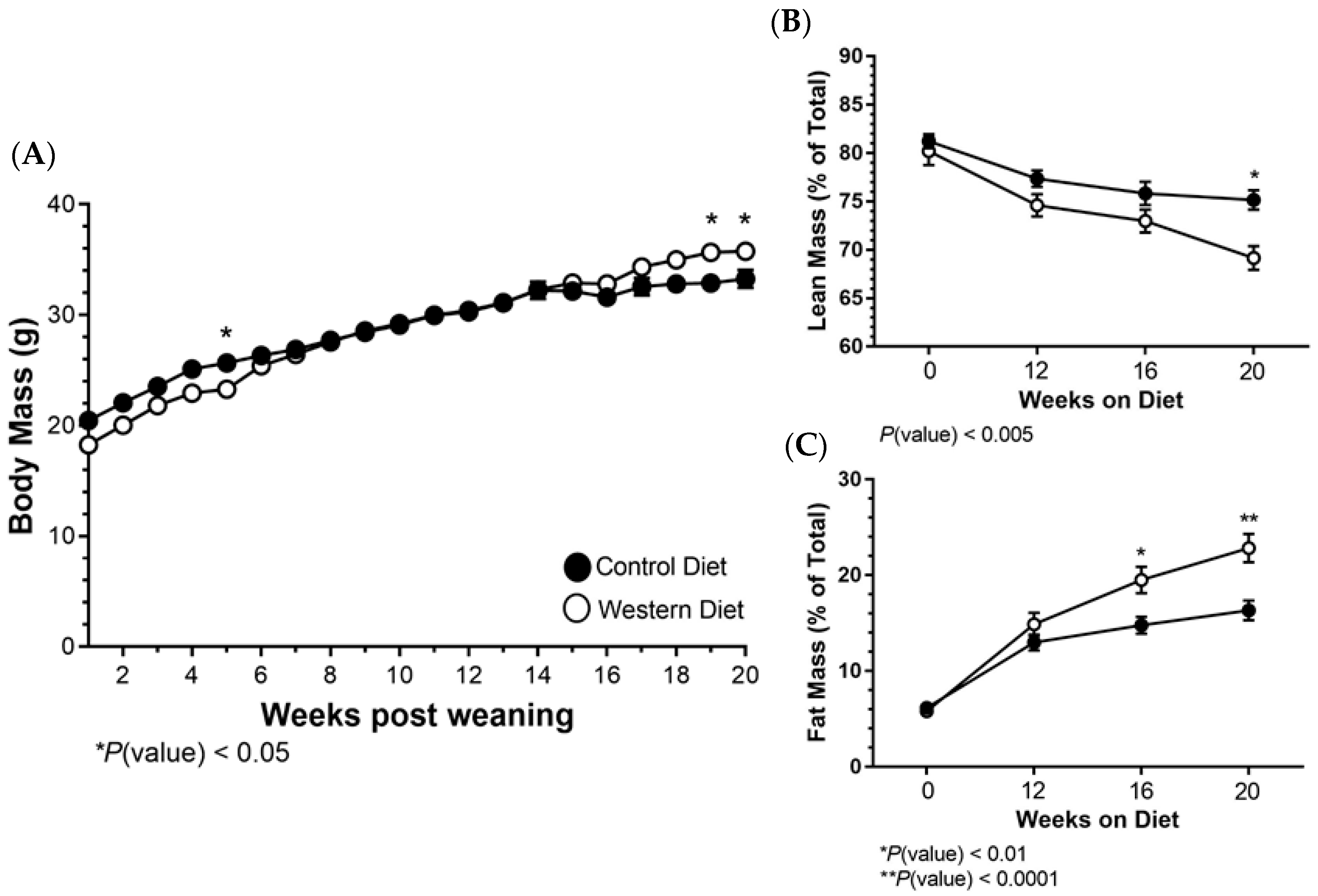
2.1. Body Mass and Composition Changes Resulting from a Western Diet
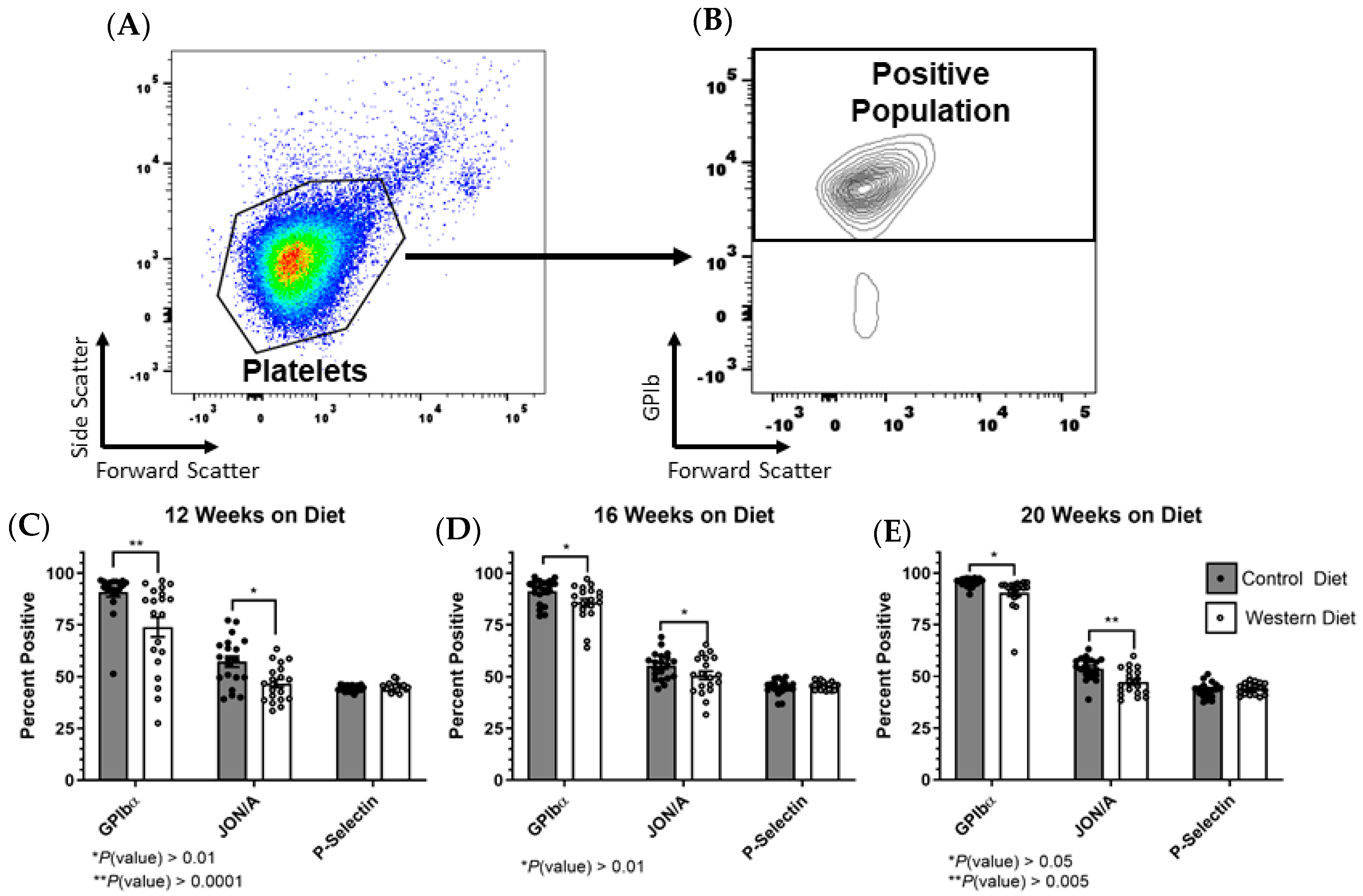
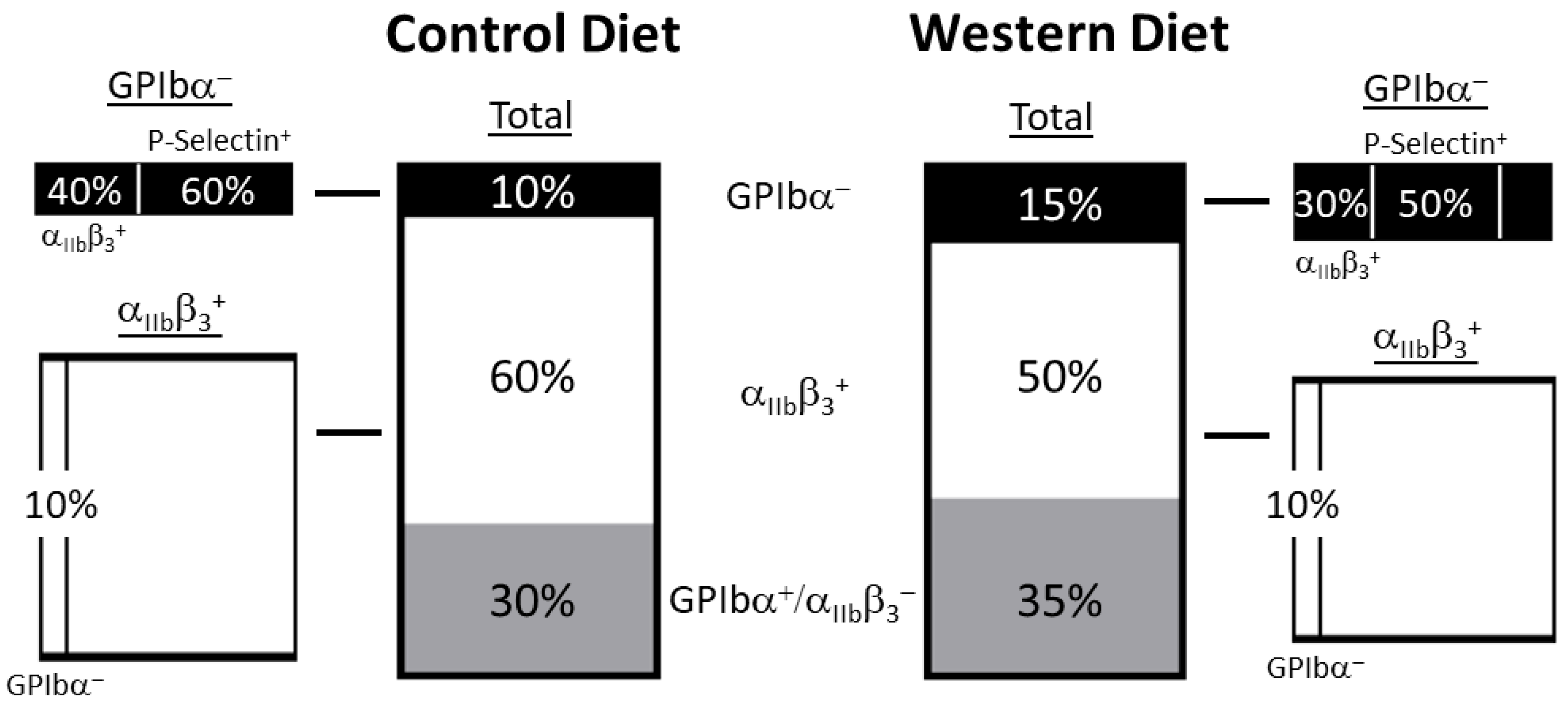
2.2. Platelet Receptor Expression Changes in the Total Population as a Result of the Western Diet
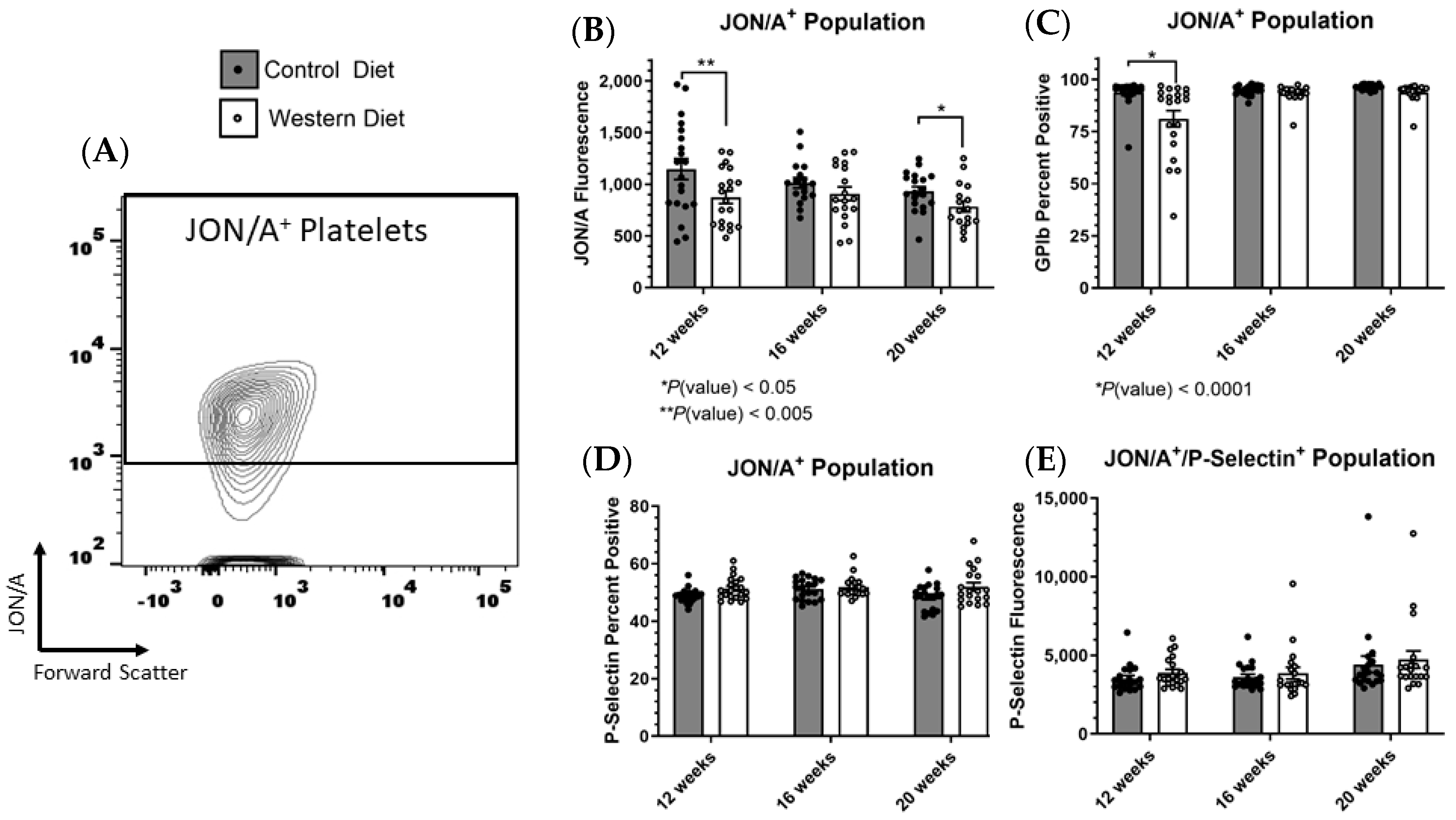
2.3. Receptor Expression within Stimulated Platelet Subpopulations Influenced by Western Diet
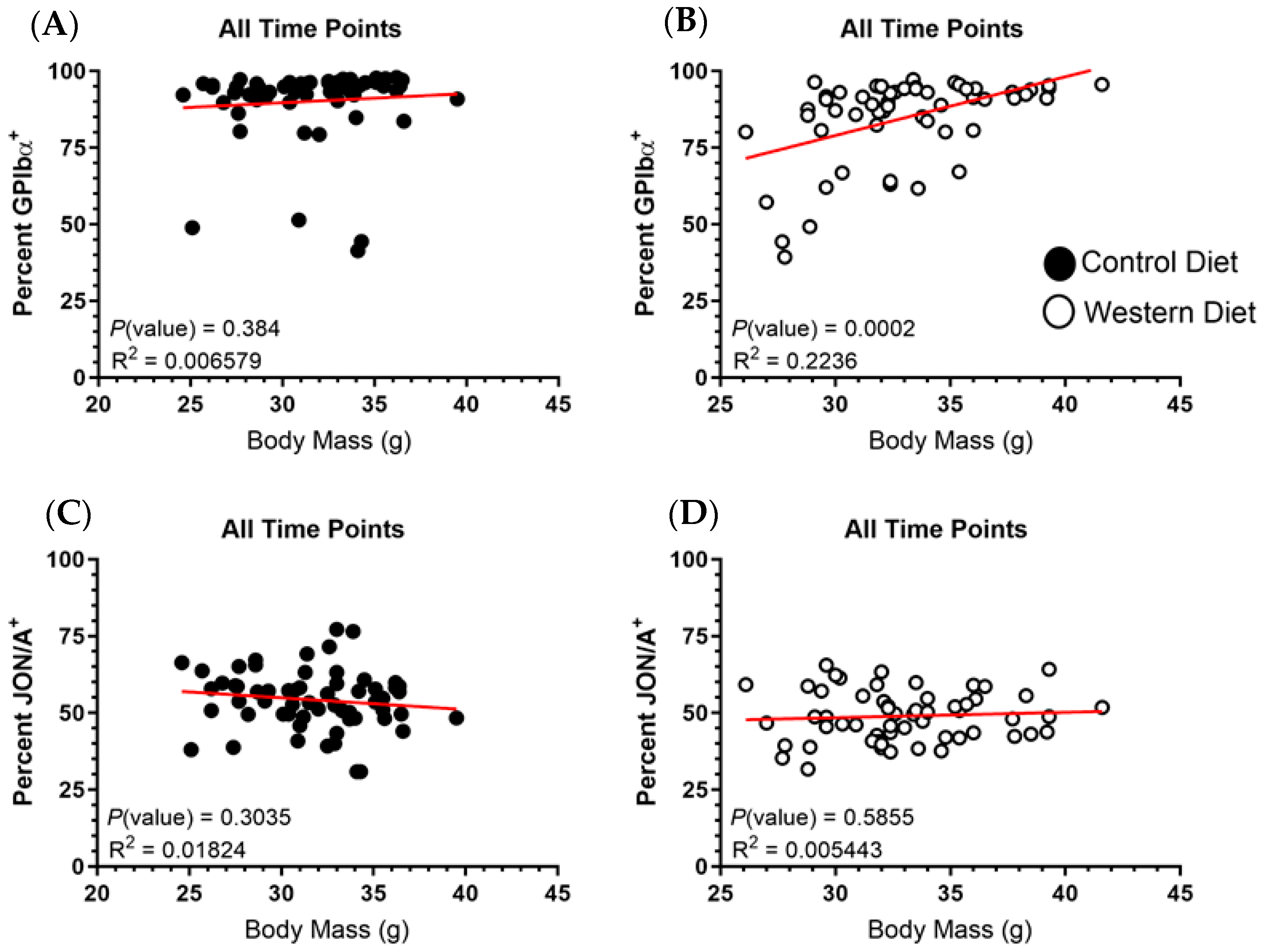
2.4. Body Mass Demonstrates No Correlation with GPIbα or αIIbβ3 Expression
2.5. Fat Mass Does Not Correlate with Platelet Receptor Expression
3. Discussion
4. Materials and Methods
4.1. Mouse Model and Diet Administration
4.2. Blood Collection
4.3. Platelet Rich Plasma Generation and Stimulation
4.4. Flow Cytometry Preparation, Acquisition and Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cercato, C.; Fonseca, F.A. Cardiovascular risk and obesity. Diabetol. Metab. Syndr. 2019, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Lopez-Jimenez, F.; Almahmeed, W.; Bays, H.; Cuevas, A.; Di Angelantonio, E.; le Roux, C.W.; Sattar, N.; Sun, M.C.; Wittert, G.; Pinto, F.J.; et al. Obesity and cardiovascular disease: Mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. Eur. J. Prev. Cardiol. 2022, 29, 2218–2237. [Google Scholar] [CrossRef] [PubMed]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J. The Role of Nutrition in Obesity. Nutrients 2023, 15, 2556. [Google Scholar] [CrossRef] [PubMed]
- Rakhra, V.; Galappaththy, S.L.; Bulchandani, S.; Cabandugama, P.K. Obesity and the Western Diet: How We Got Here. Mo. Med. 2020, 117, 536–538. [Google Scholar] [PubMed]
- Kleinbongard, P.; Andreadou, I.; Vilahur, G. The platelet paradox of injury versus protection in myocardial infarction—Has it been overlooked? Basic Res. Cardiol. 2021, 116, 37. [Google Scholar] [CrossRef]
- Shaik, N.F.; Regan, R.F.; Naik, U.P. Platelets as drivers of ischemia/reperfusion injury after stroke. Blood Adv. 2021, 5, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Baaten, C.C.F.M.J.; Nagy, M.; Bergmeier, W.; Spronk, H.M.H.; van der Meijden, P.E.J. Platelet biology and function: Plaque erosion vs. rupture. Eur. Heart J. 2023, 45, 18–31. [Google Scholar] [CrossRef]
- Lievens, D.; von Hundelshausen, P. Platelets in atherosclerosis. Thromb. Haemost. 2011, 106, 827–838. [Google Scholar] [CrossRef]
- Barrachina, M.N.; Morán, L.A.; Izquierdo, I.; Casanueva, F.F.; Pardo, M.; García, Á. Analysis of platelets from a diet-induced obesity rat model: Elucidating platelet dysfunction in obesity. Sci. Rep. 2020, 10, 13104. [Google Scholar] [CrossRef]
- Barrachina, M.N.; Sueiro, A.M.; Izquierdo, I.; Hermida-Nogueira, L.; Guitián, E.; Casanueva, F.F.; Farndale, R.W.; Moroi, M.; Jung, S.M.; Pardo, M.; et al. GPVI surface expression and signalling pathway activation are increased in platelets from obese patients: Elucidating potential anti-atherothrombotic targets in obesity. Atherosclerosis 2019, 281, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Senis, Y.A.; Mazharian, A.; Mori, J. Src family kinases: At the forefront of platelet activation. Blood 2014, 124, 2013–2024. [Google Scholar] [CrossRef]
- Senis, Y.A.; Nagy, Z.; Mori, J.; Lane, S.; Lane, P. Platelet Src family kinases: A tale of reversible phosphorylation. Res. Pract. Thromb. Haemost. 2021, 5, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.E.; Karunakaran, D.; Shen, Y.; Arthur, J.F.; Andrews, R.K.; Berndt, M.C. Controlled shedding of platelet glycoprotein (GP)VI and GPIb–IX–V by ADAM family metalloproteinases. J. Thromb. Haemost. 2007, 5, 1530–1537. [Google Scholar] [CrossRef]
- Fullard, J.F. The role of the platelet glycoprotein IIb/IIIa in thrombosis and haemostasis. Curr. Pharm. Des. 2004, 10, 1567–1576. [Google Scholar] [CrossRef] [PubMed]
- Merten, M.; Thiagarajan, P. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation 2000, 102, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Nurden, A.; Combrié, R.; Pasquet, J.M. Redistribution of glycoprotein Ib within platelets in response to protease-activated receptors 1 and 4: Roles of cytoskeleton and calcium. J. Thromb. Haemost. 2003, 1, 2206–2215. [Google Scholar] [CrossRef]
- Ritchie, J.L.; Alexander, H.D.; Rea, I.M. Flow cytometry analysis of platelet P-selectin expression in whole blood--methodological considerations. Clin. Lab. Haematol. 2000, 22, 359–363. [Google Scholar] [CrossRef]
- Vardon Bounes, F.; Mémier, V.; Marcaud, M.; Jacquemin, A.; Hamzeh-Cognasse, H.; Garcia, C.; Series, J.; Sié, P.; Minville, V.; Gratacap, M.-P.; et al. Platelet activation and prothrombotic properties in a mouse model of peritoneal sepsis. Sci. Rep. 2018, 8, 13536. [Google Scholar] [CrossRef]
- Pethaperumal, S.; Hung, S.-C.; Lien, T.-S.; Sun, D.-S.; Chang, H.-H. P-Selectin is a Critical Factor for Platelet-Mediated Protection on Restraint Stress-Induced Gastrointestinal Injury in Mice. Int. J. Mol. Sci. 2022, 23, 11909. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.L.; Nguyen, N.; Tablin, F. Canine platelets express functional Toll-like receptor-4: Lipopolysaccharide-triggered platelet activation is dependent on adenosine diphosphate and thromboxane A2 in dogs. BMC Vet. Res. 2019, 15, 245. [Google Scholar] [CrossRef] [PubMed]
- Waller, A.K.; Lantos, L.; Sammut, A.; Salgin, B.; McKinney, H.; Foster, H.R.; Kriek, N.; Gibbins, J.M.; Stanworth, S.J.; Garner, S.F.; et al. Flow cytometry for near-patient testing in premature neonates reveals variation in platelet function: A novel approach to guide platelet transfusion. Pediatr. Res. 2019, 85, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.A.; Hermundstad, B.; Flesland, A.K.; Llohn, A.H.; Saether, P.C. Variation in Platelet Activation State in Pre-Donation Whole Blood: Effect of Time of Day and ABO Blood Group. J. Blood Med. 2022, 13, 283–292. [Google Scholar] [CrossRef]
- Johnson, L.; Lei, P.; Waters, L.; Padula, M.P.; Marks, D.C. Identification of platelet subpopulations in cryopreserved platelet components using multi-colour imaging flow cytometry. Sci. Rep. 2023, 13, 1221. [Google Scholar] [CrossRef] [PubMed]
- Moroi, M.; Farndale, R.W.; Jung, S.M. Activation-induced changes in platelet surface receptor expression and the contribution of the large-platelet subpopulation to activation. Res. Pract. Thromb. Haemost. 2020, 4, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Six, K.R.; Debaene, C.; Van den Hauwe, M.; De Rycke, R.; Gardiner, E.E.; Compernolle, V.; Feys, H.B. GPIbα shedding in platelets is controlled by strict intracellular containment of both enzyme and substrate. J. Thromb. Haemost. 2023, 21, 2223–2235. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, W.; Schulte, V.; Brockhoff, G.; Bier, U.; Zirngibl, H.; Nieswandt, B. Flow cytometric detection of activated mouse integrin alphaIIbbeta3 with a novel monoclonal antibody. Cytometry 2002, 48, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Deppermann, C.; Kubes, P. Start a fire, kill the bug: The role of platelets in inflammation and infection. Innate Immun. 2018, 24, 335–348. [Google Scholar] [CrossRef]
- Scherlinger, M.; Richez, C.; Tsokos, G.C.; Boilard, E.; Blanco, P. The role of platelets in immune-mediated inflammatory diseases. Nat. Rev. Immunol. 2023, 23, 495–510. [Google Scholar] [CrossRef]
- Hildebrandt, X.; Ibrahim, M.; Peltzer, N. Cell death and inflammation during obesity: “Know my methods, WAT(son)”. Cell Death Differ. 2023, 30, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus 2022, 14, e22711. [Google Scholar] [CrossRef] [PubMed]
- Bye, A.P.; Unsworth, A.J.; Gibbins, J.M. Platelet signaling: A complex interplay between inhibitory and activatory networks. J. Thromb. Haemost. 2016, 14, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Estevez, B.; Du, X. New Concepts and Mechanisms of Platelet Activation Signaling. Physiology (Bethesda) 2017, 32, 162–177. [Google Scholar] [CrossRef] [PubMed]
- DeCortin, M.E.; Brass, L.F.; Diamond, S.L. Core and shell platelets of a thrombus: A new microfluidic assay to study mechanics and biochemistry. Res. Pract. Thromb. Haemost. 2020, 4, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Stalker, T.J.; Traxler, E.A.; Wu, J.; Wannemacher, K.M.; Cermignano, S.L.; Voronov, R.; Diamond, S.L.; Brass, L.F. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood 2013, 121, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Antoniades, C.; Bakogiannis, C.; Tousoulis, D.; Antonopoulos, A.S.; Stefanadis, C. The CD40/CD40 ligand system: Linking inflammation with atherothrombosis. J. Am. Coll. Cardiol. 2009, 54, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Davì, G.; Guagnano, M.T.; Ciabattoni, G.; Basili, S.; Falco, A.; Marinopiccoli, M.; Nutini, M.; Sensi, S.; Patrono, C. Platelet Activation in Obese WomenRole of Inflammation and Oxidant Stress. JAMA 2002, 288, 2008–2014. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Pannacciulli, N.; Coviello, M.; Scarangella, A.; Di Roma, P.; Caringella, M.; Venneri, M.T.; Quaranta, M.; Giorgino, R. sP-selectin plasma levels in obesity: Association with insulin resistance and related metabolic and prothrombotic factors. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 227–232. [Google Scholar] [CrossRef]
- Ezzaty Mirhashemi, M.; Shah, R.V.; Kitchen, R.R.; Rong, J.; Spahillari, A.; Pico, A.R.; Vitseva, O.; Levy, D.; Demarco, D.; Shah, S.; et al. The Dynamic Platelet Transcriptome in Obesity and Weight Loss. Arter. Thromb. Vasc. Biol. 2021, 41, 854–864. [Google Scholar] [CrossRef]
- Weyrich, A.S.; Schwertz, H.; Kraiss, L.W.; Zimmerman, G.A. Protein synthesis by platelets: Historical and new perspectives. J. Thromb. Haemost. 2009, 7, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Barrachina, M.N.; Hermida-Nogueira, L.; Moran, L.A.; Casas, V.; Hicks, S.M.; Sueiro, A.M.; Di, Y.; Andrews, R.K.; Watson, S.P.; Gardiner, E.E.; et al. Phosphoproteomic Analysis of Platelets in Severe Obesity Uncovers Platelet Reactivity and Signaling Pathways Alterations. Arter. Thromb. Vasc. Biol. 2021, 41, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Moroi, M. Activation of the platelet collagen receptor integrin alpha(2)beta(1): Its mechanism and participation in the physiological functions of platelets. Trends Cardiovasc. Med. 2000, 10, 285–292. [Google Scholar] [CrossRef]
- De Kock, L.; Freson, K. The (Patho)Biology of SRC Kinase in Platelets and Megakaryocytes. Medicina 2020, 56, 633. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corken, A.; Wahl, E.C.; Sikes, J.D.; Thakali, K.M. Western Diet Modifies Platelet Activation Profiles in Male Mice. Int. J. Mol. Sci. 2024, 25, 8019. https://doi.org/10.3390/ijms25158019
Corken A, Wahl EC, Sikes JD, Thakali KM. Western Diet Modifies Platelet Activation Profiles in Male Mice. International Journal of Molecular Sciences. 2024; 25(15):8019. https://doi.org/10.3390/ijms25158019
Chicago/Turabian StyleCorken, Adam, Elizabeth C. Wahl, James D. Sikes, and Keshari M. Thakali. 2024. "Western Diet Modifies Platelet Activation Profiles in Male Mice" International Journal of Molecular Sciences 25, no. 15: 8019. https://doi.org/10.3390/ijms25158019
APA StyleCorken, A., Wahl, E. C., Sikes, J. D., & Thakali, K. M. (2024). Western Diet Modifies Platelet Activation Profiles in Male Mice. International Journal of Molecular Sciences, 25(15), 8019. https://doi.org/10.3390/ijms25158019



