Biosynthesis and Characterization of Aeonium arboreum-Derived Silver Nanoparticles: Antimicrobial Activity, Biofilm Inhibition, Antihemolytic Activity, and In Silico Studies
Abstract
:1. Introduction
2. Results and Discussion
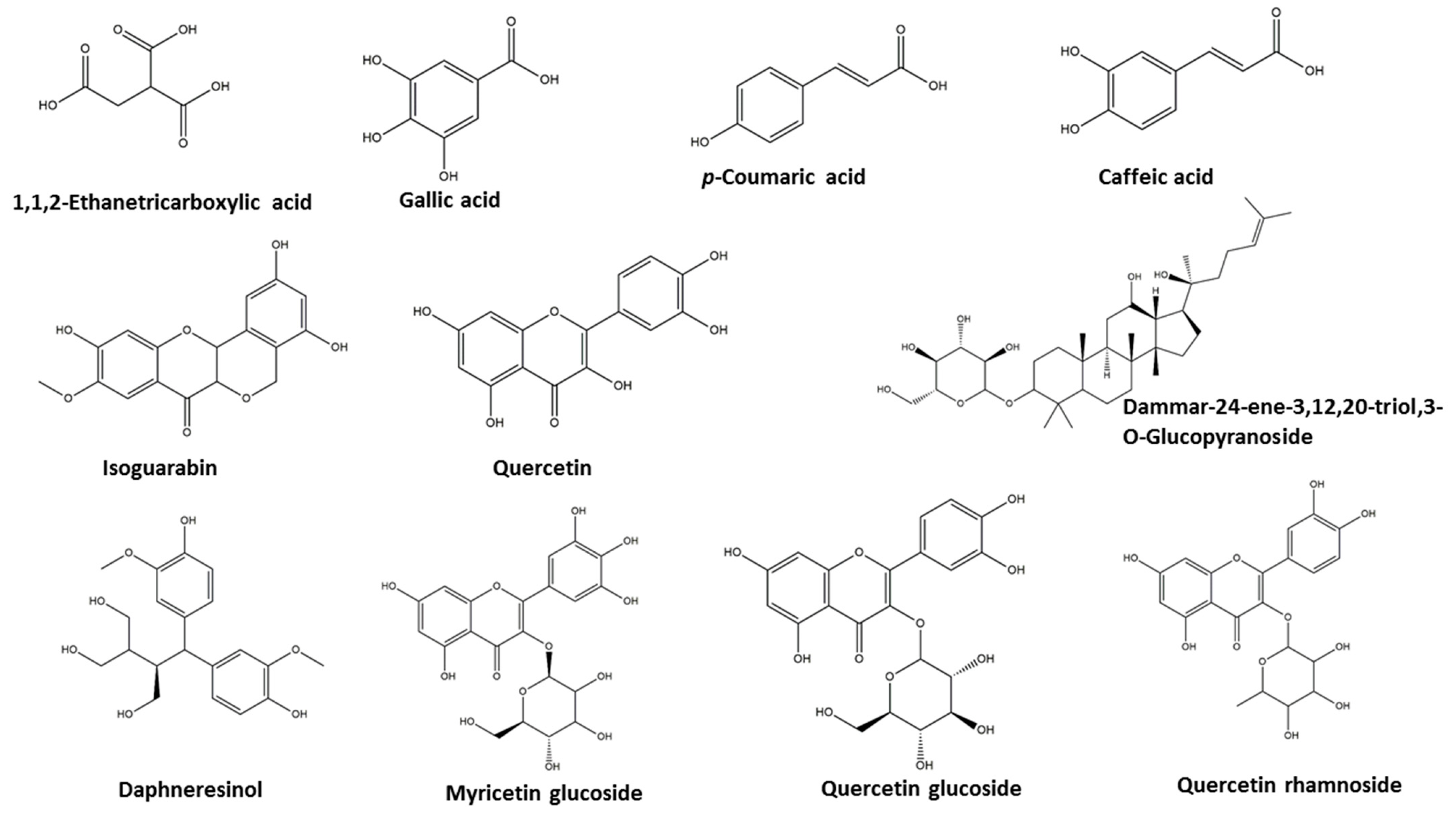
2.1. Metabolomics Analysis of the Crude Extract of Aeonium Arboreum’s Aerial Parts
2.2. Characterization of Silver Nanoparticles
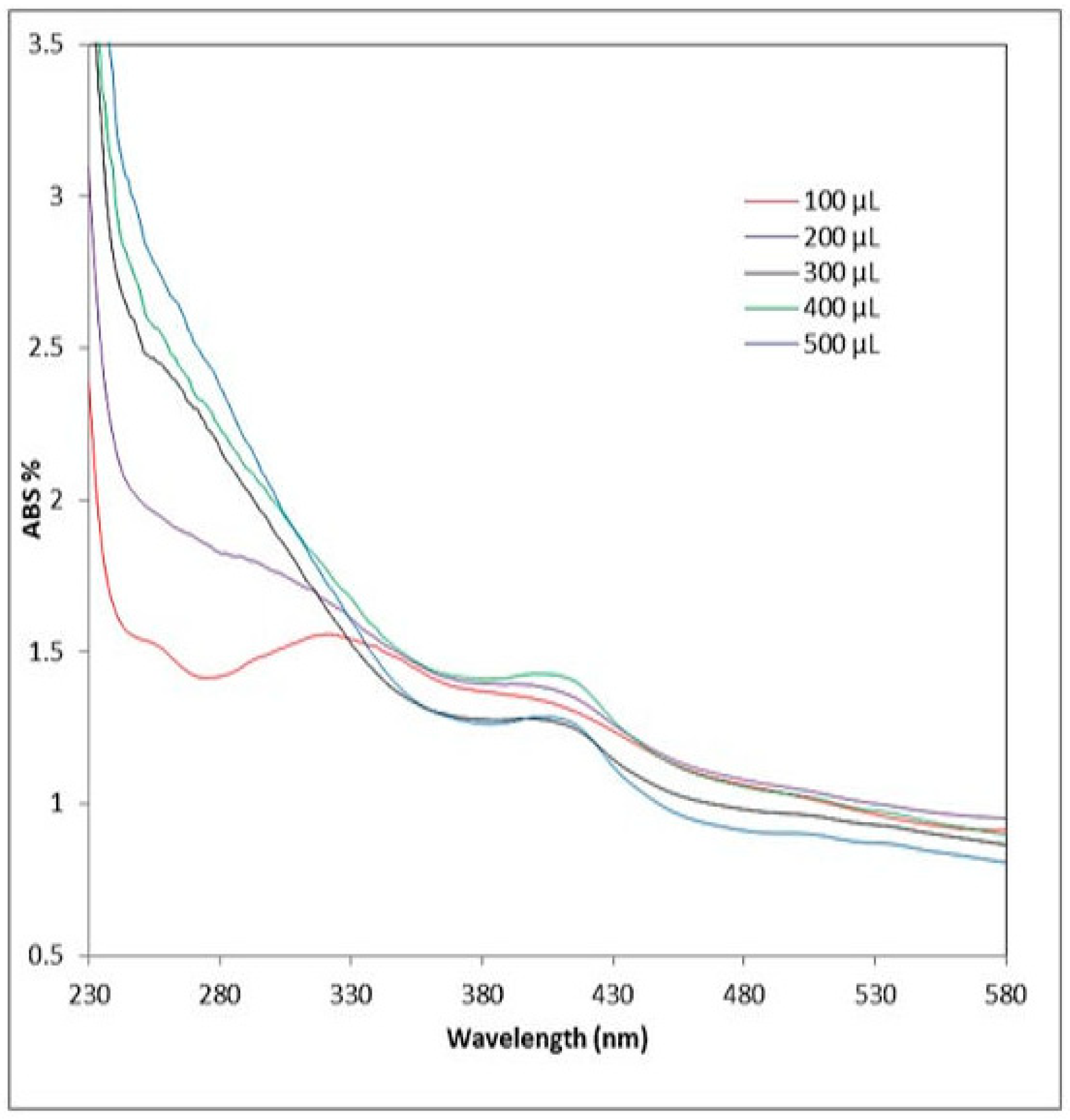
2.2.1. UV Analysis
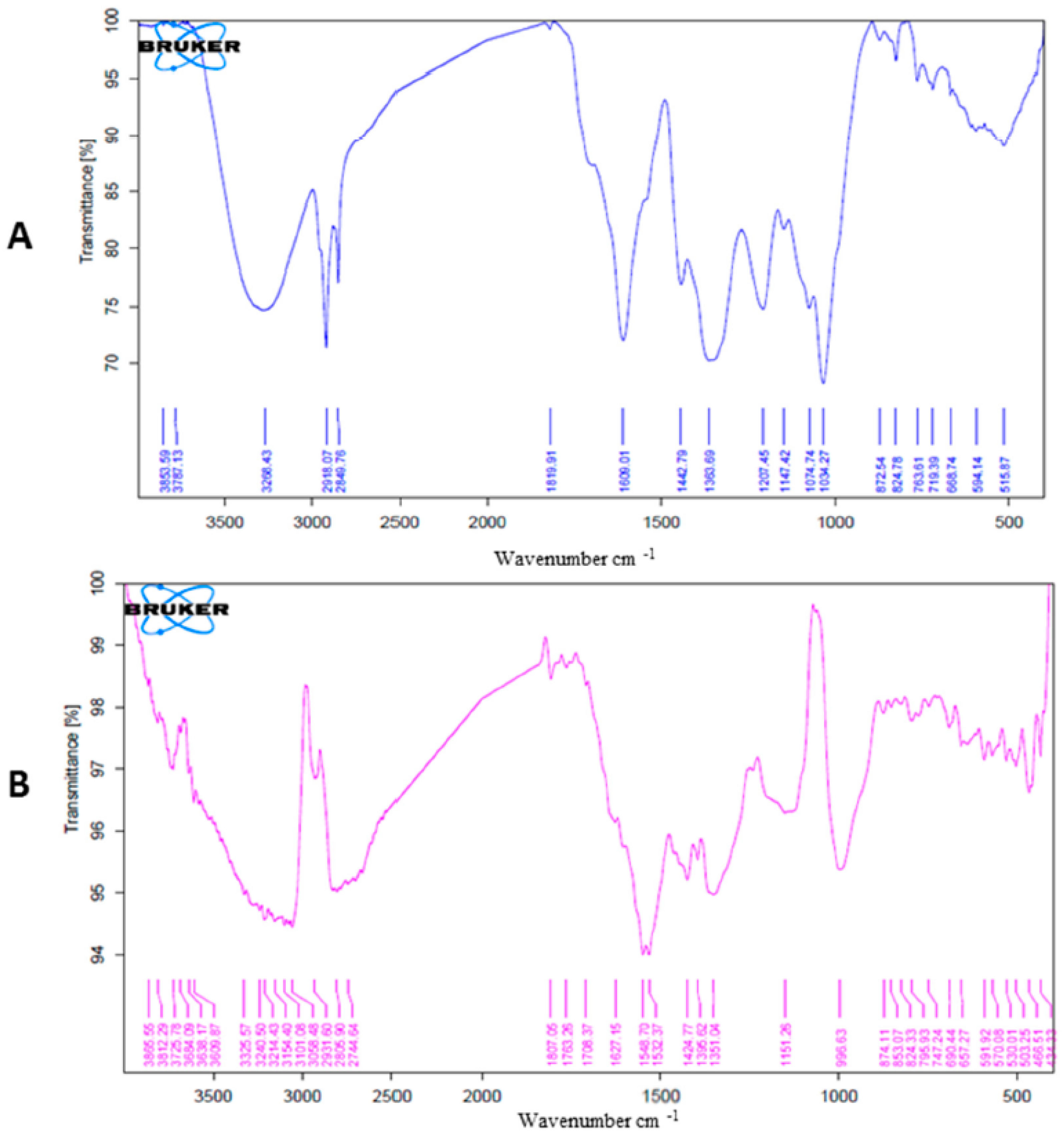
2.2.2. FTIR Analysis of AgNPs and A. arboreum Extract
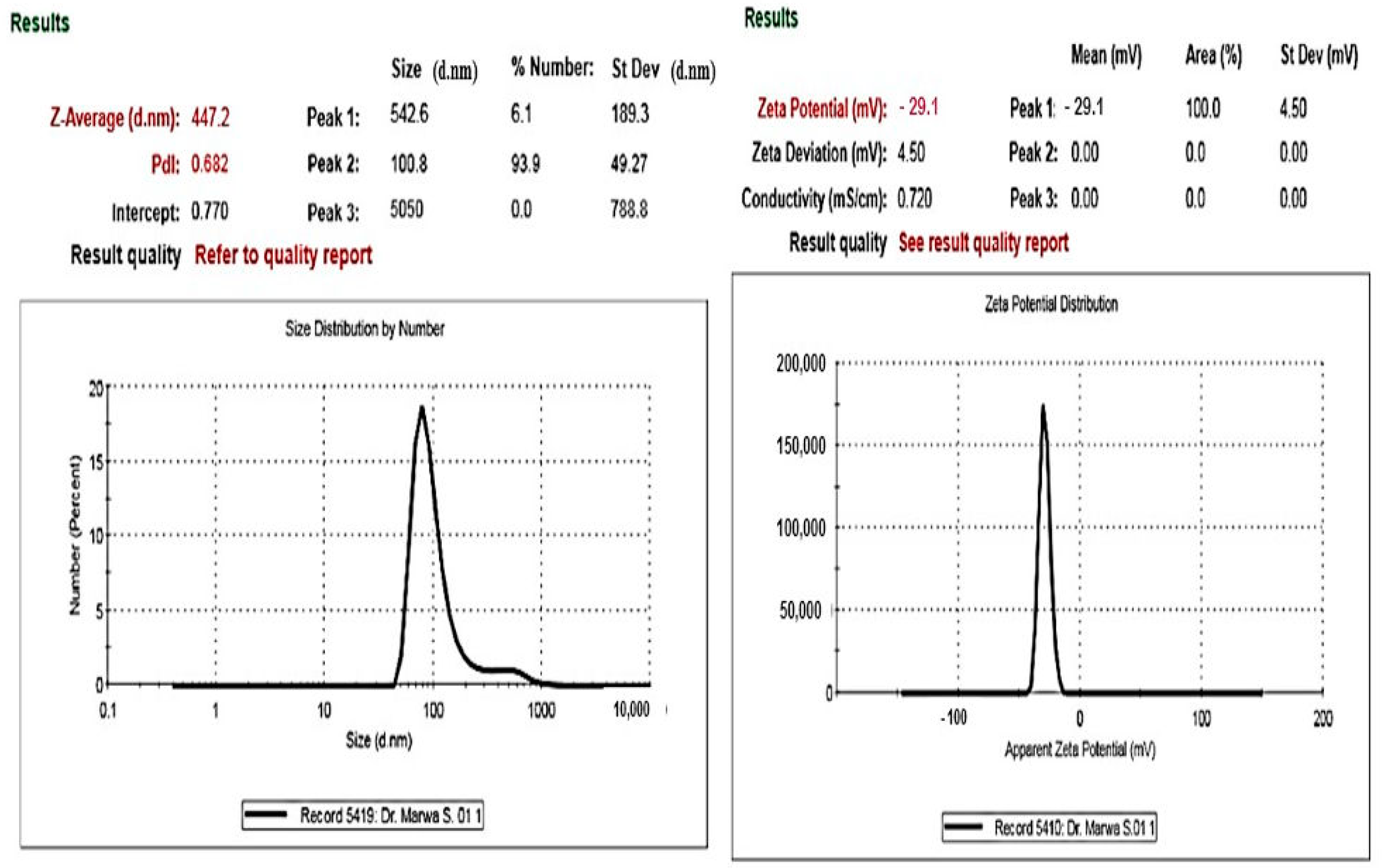
2.2.3. Zeta Potential and Light Scattering Dynamics
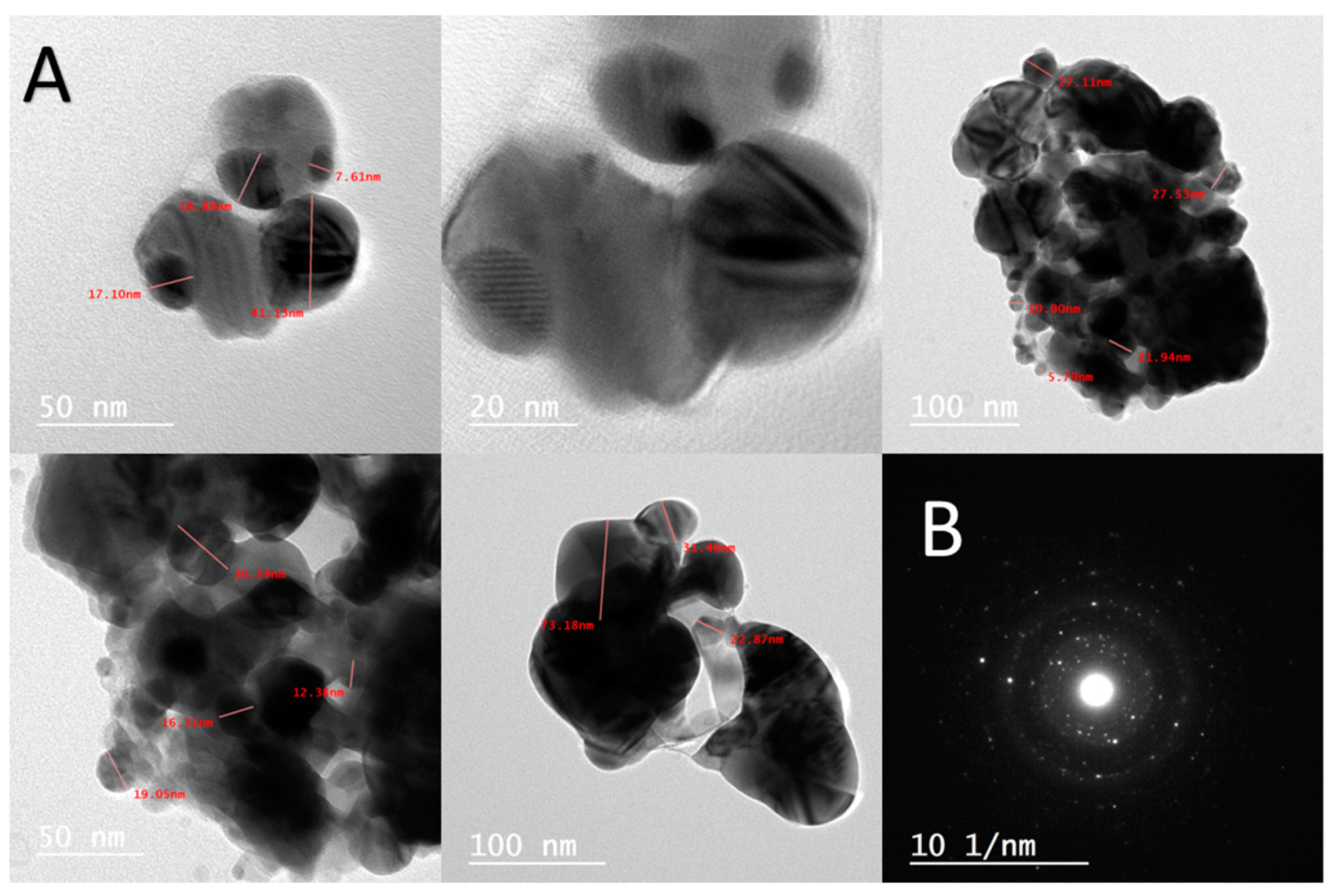
2.2.4. Transmission Electron Microscopy (TEM)
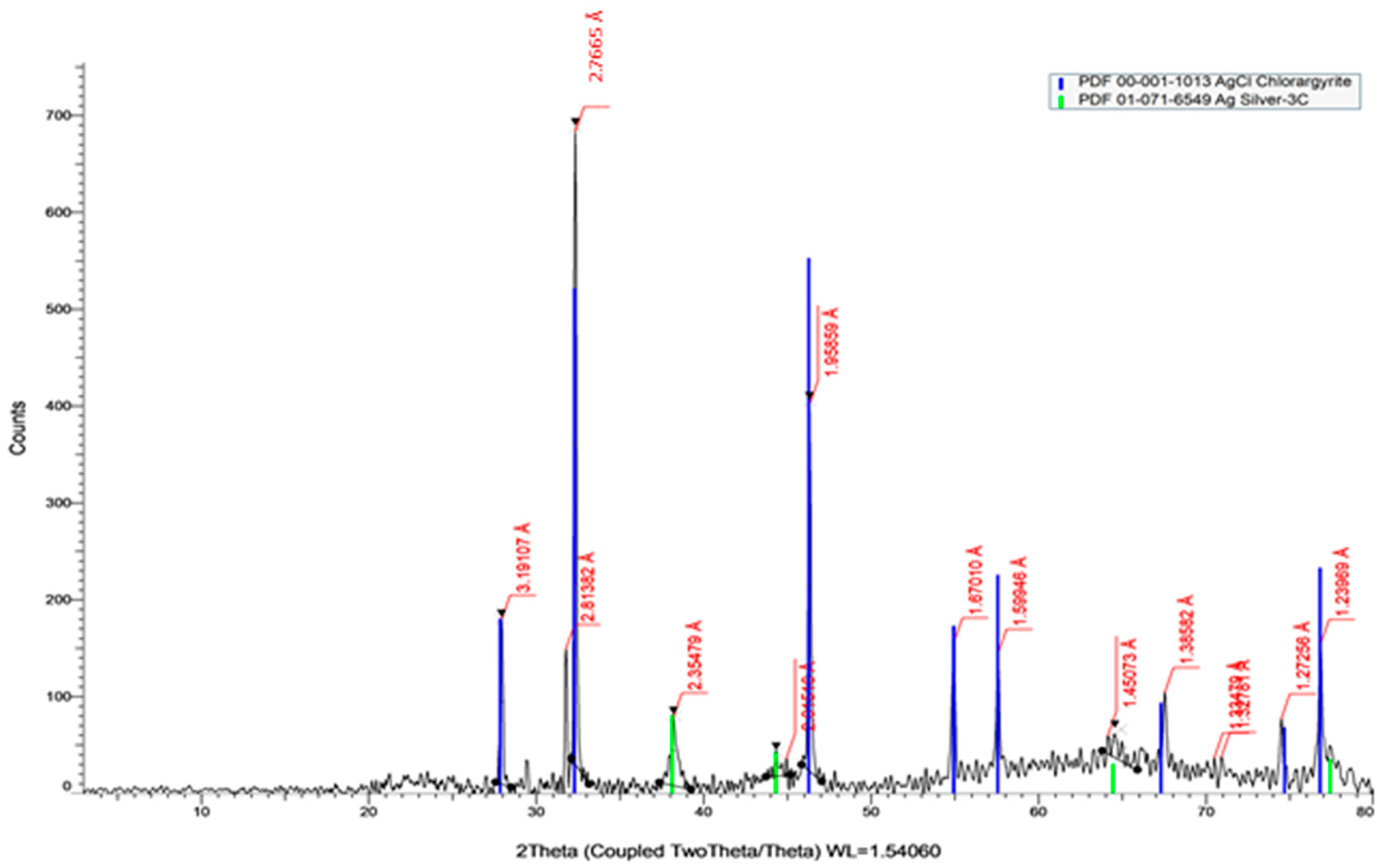
2.2.5. X-ray Diffraction Analysis (XRD)
2.3. Antimicrobial Activity
2.3.1. Antimicrobial Activity of Plant Extract and Its Nanoform
2.3.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
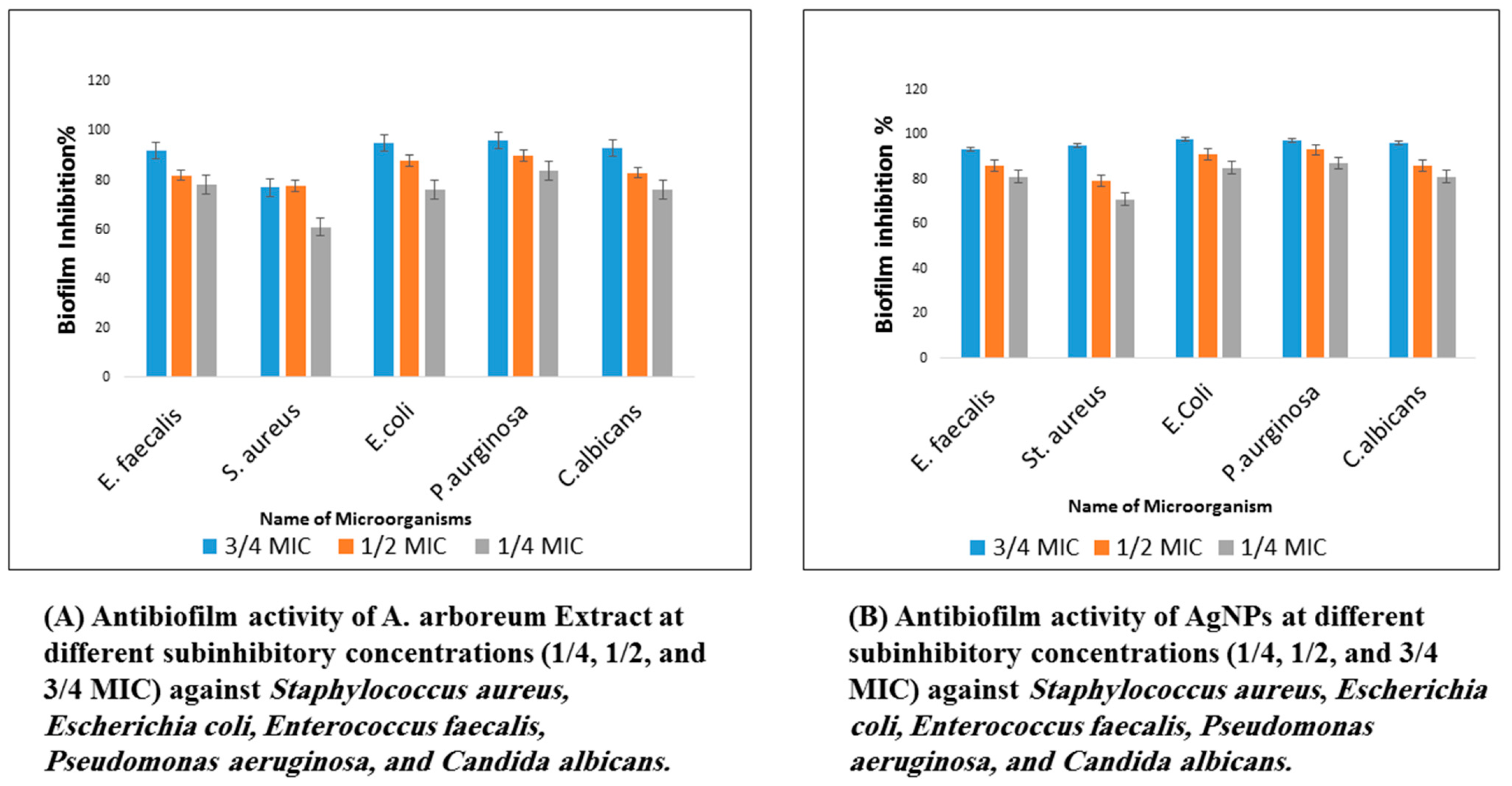
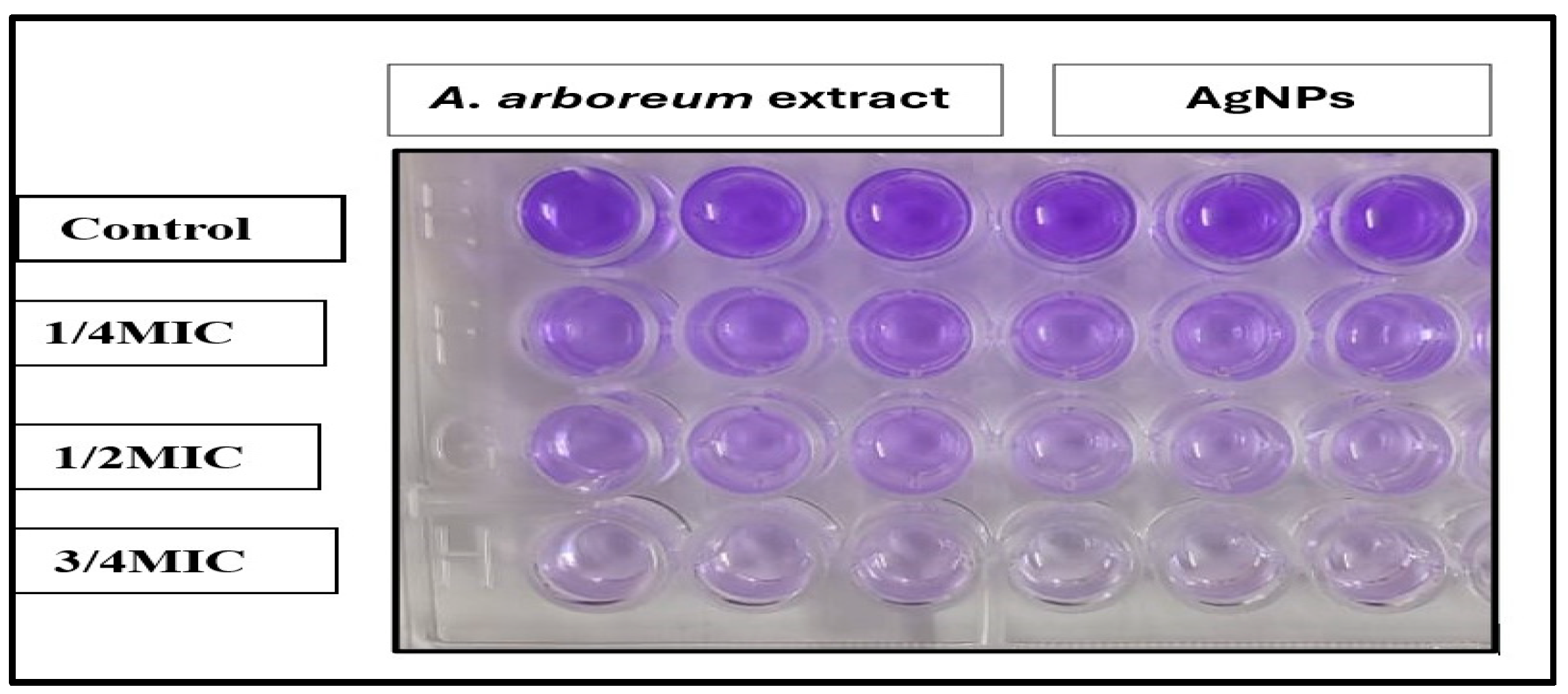
2.3.3. Determination of Biofilm Inhibition Activity
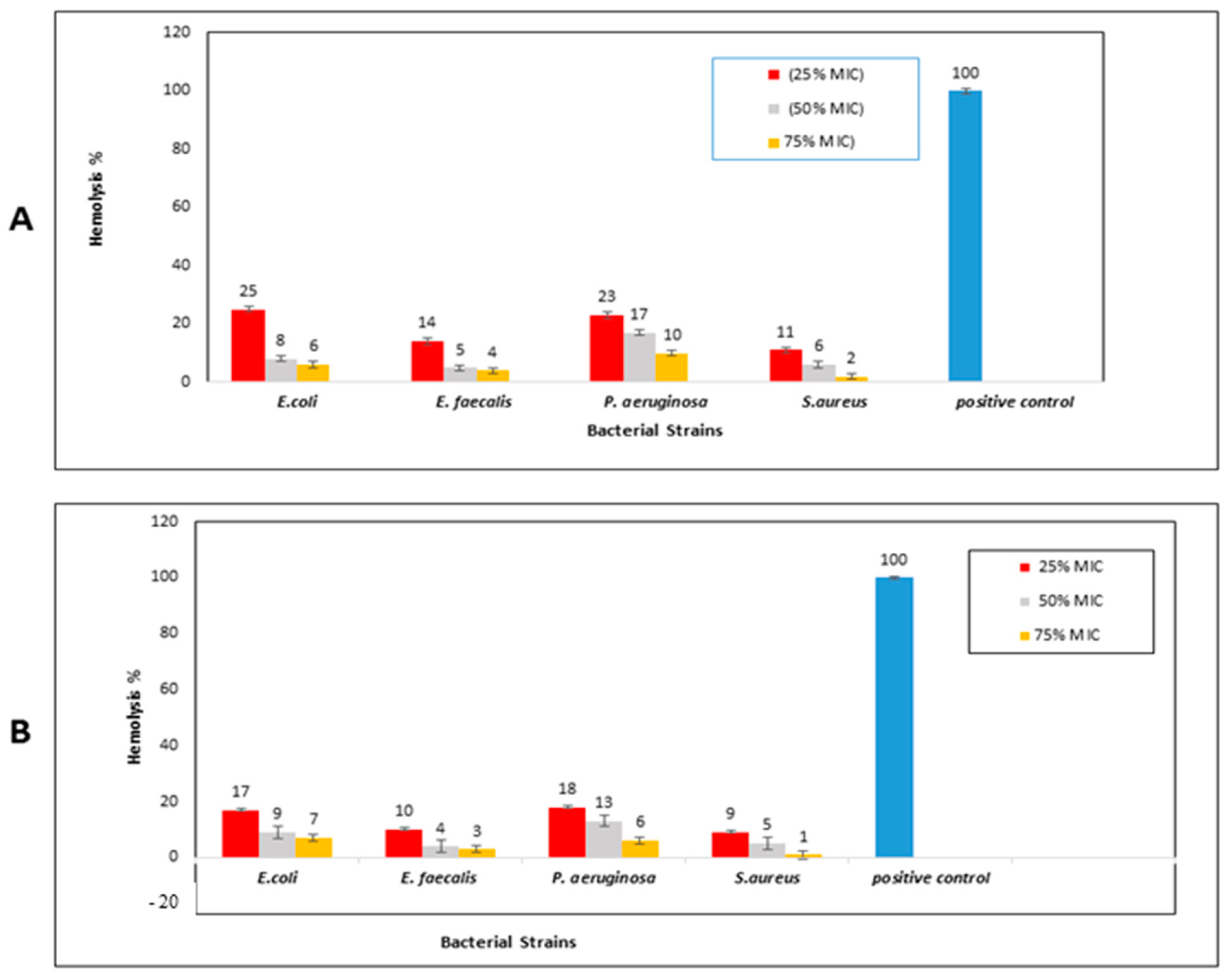
2.3.4. Hemolysis Inhibition Activity
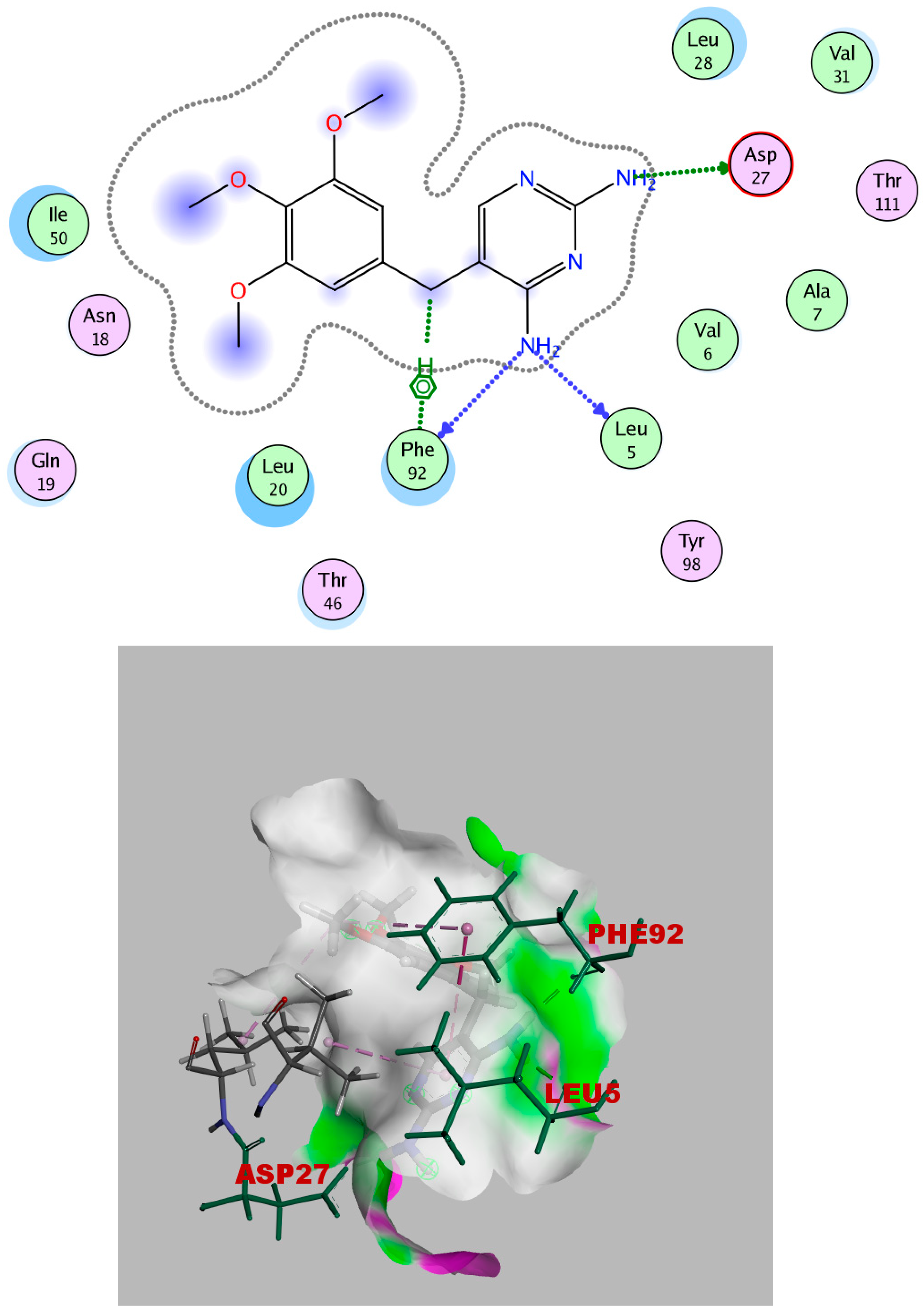
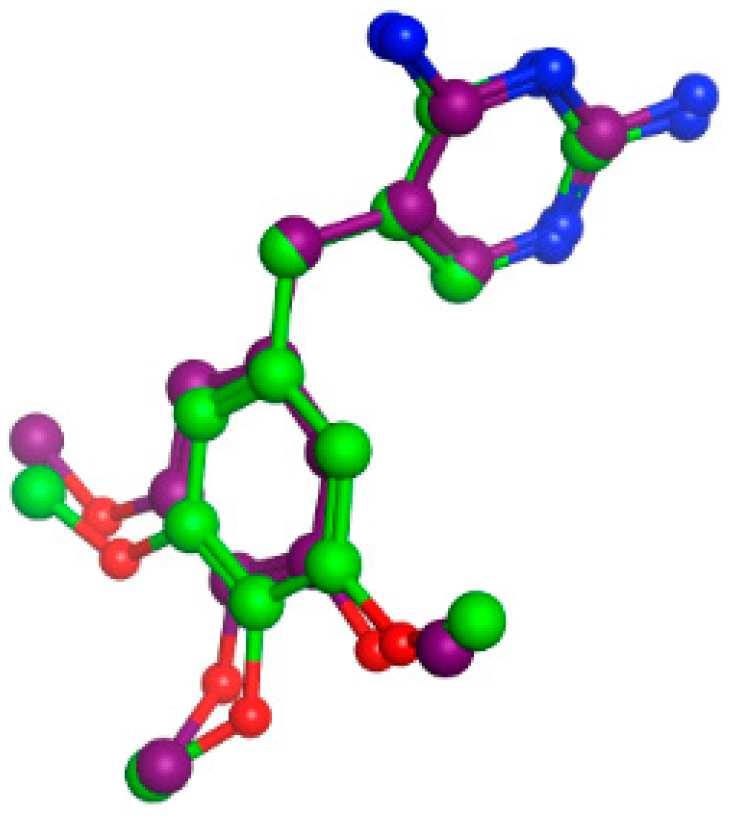
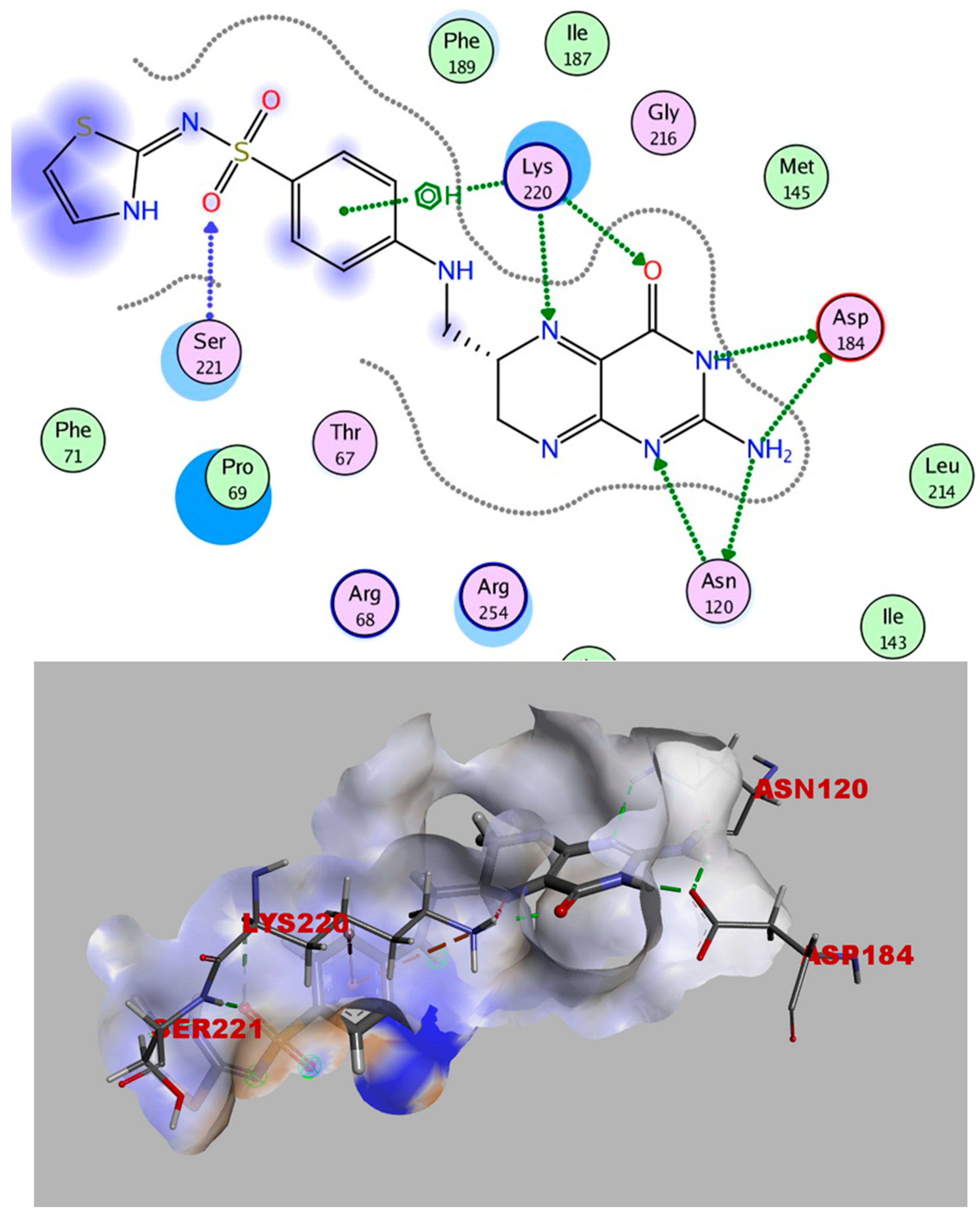
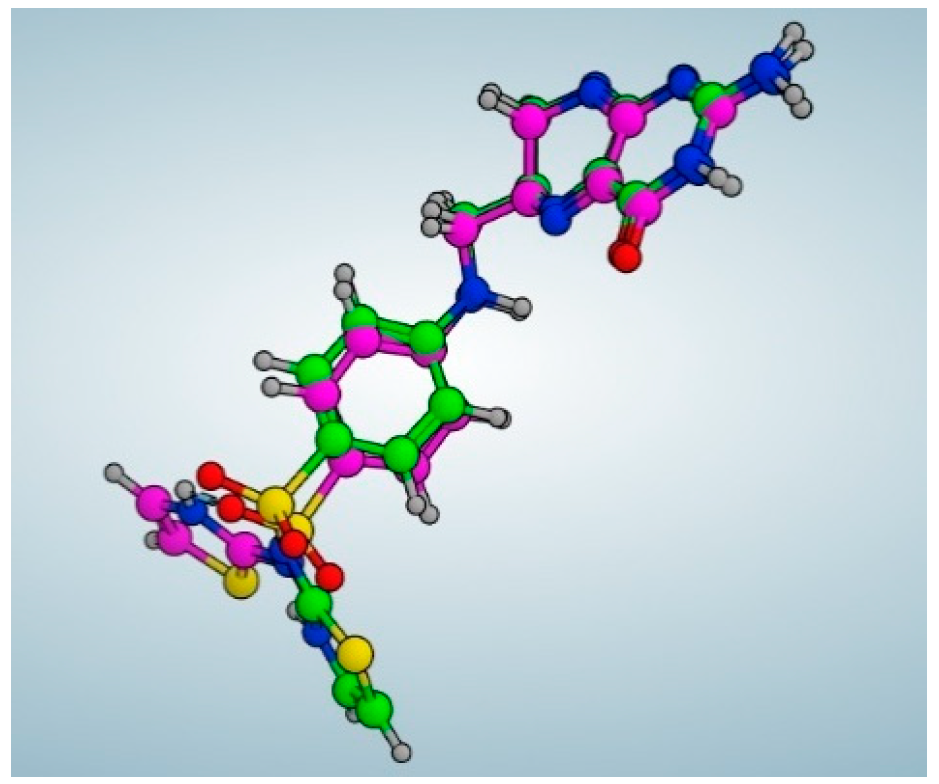
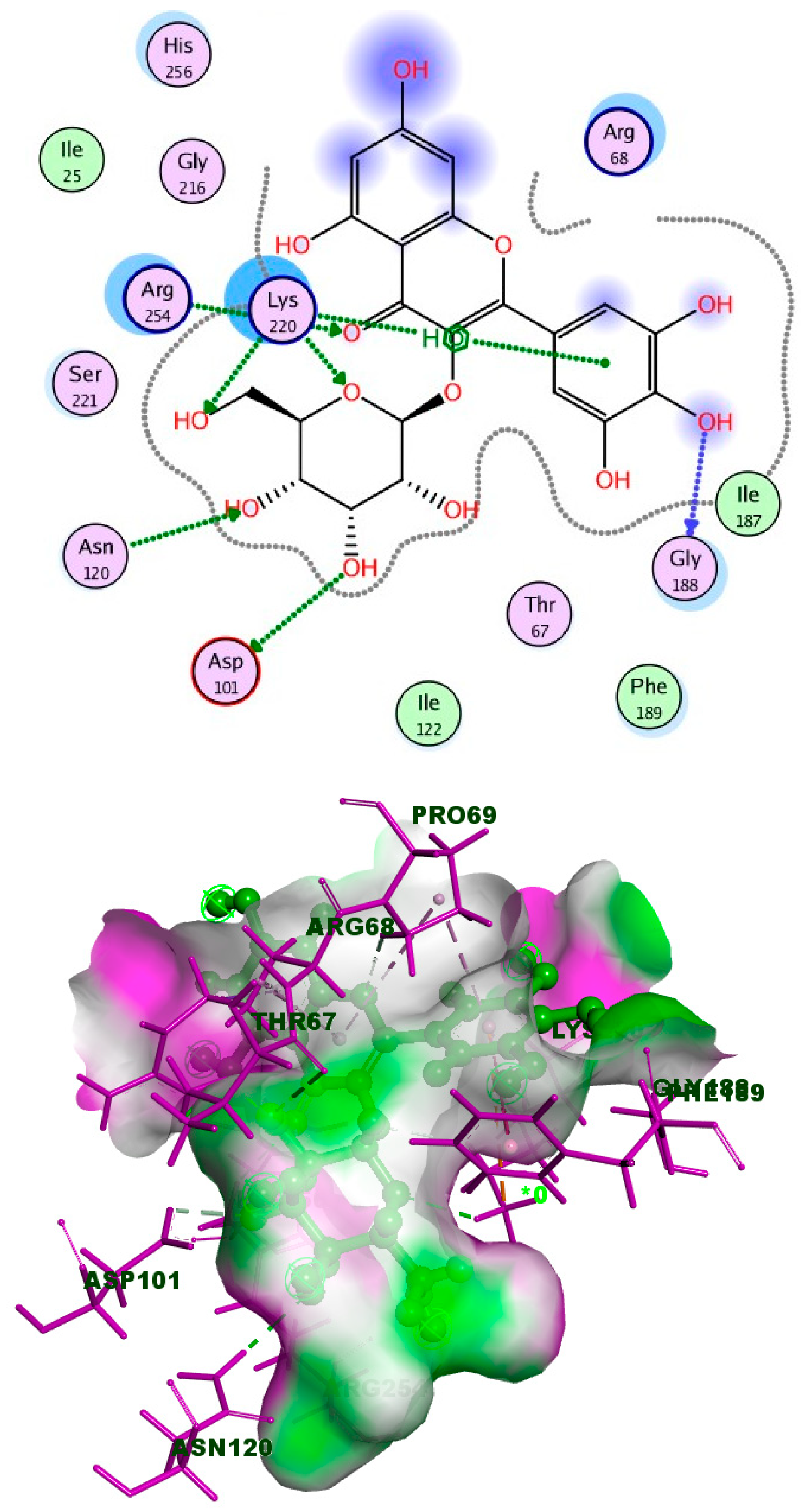
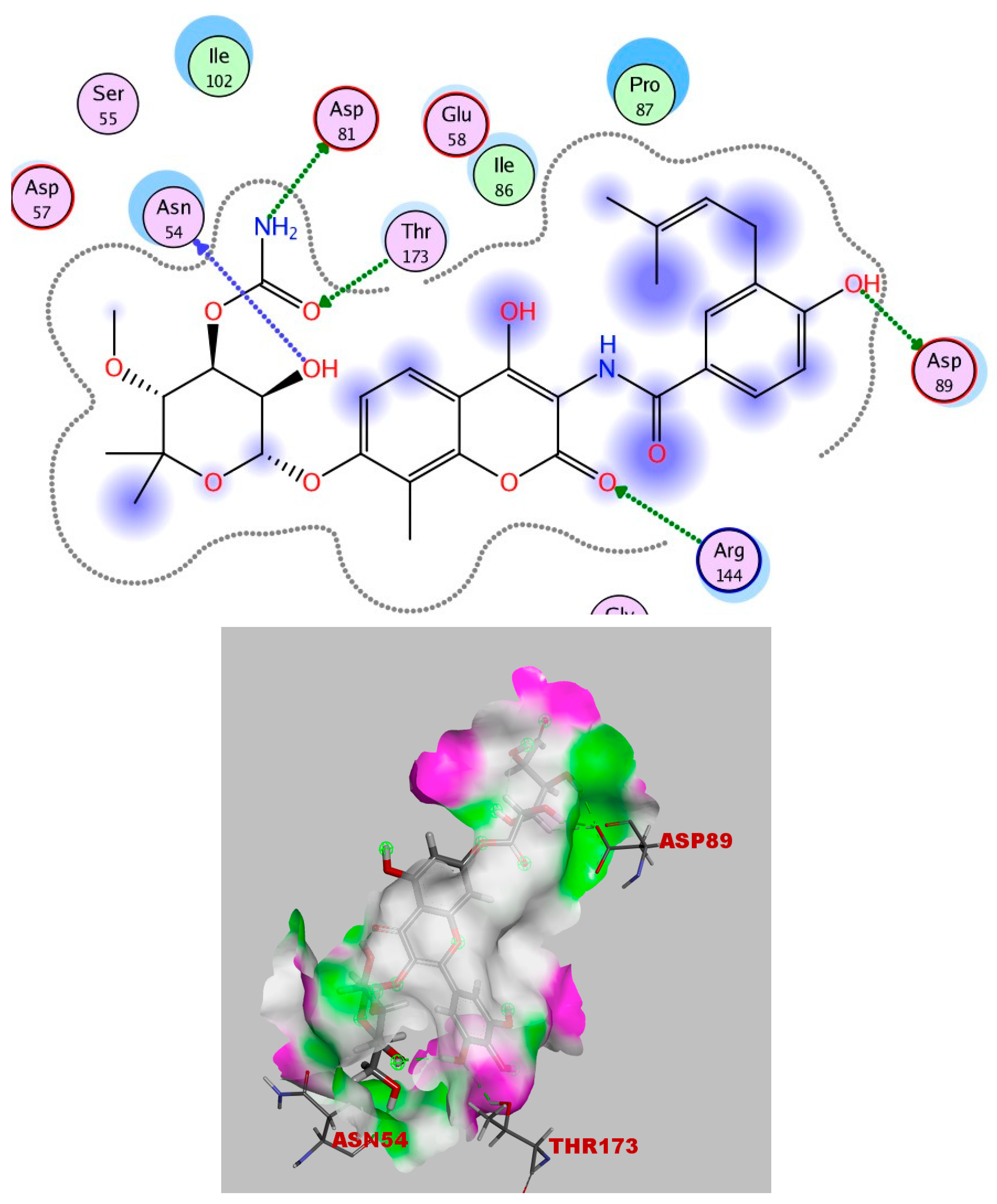
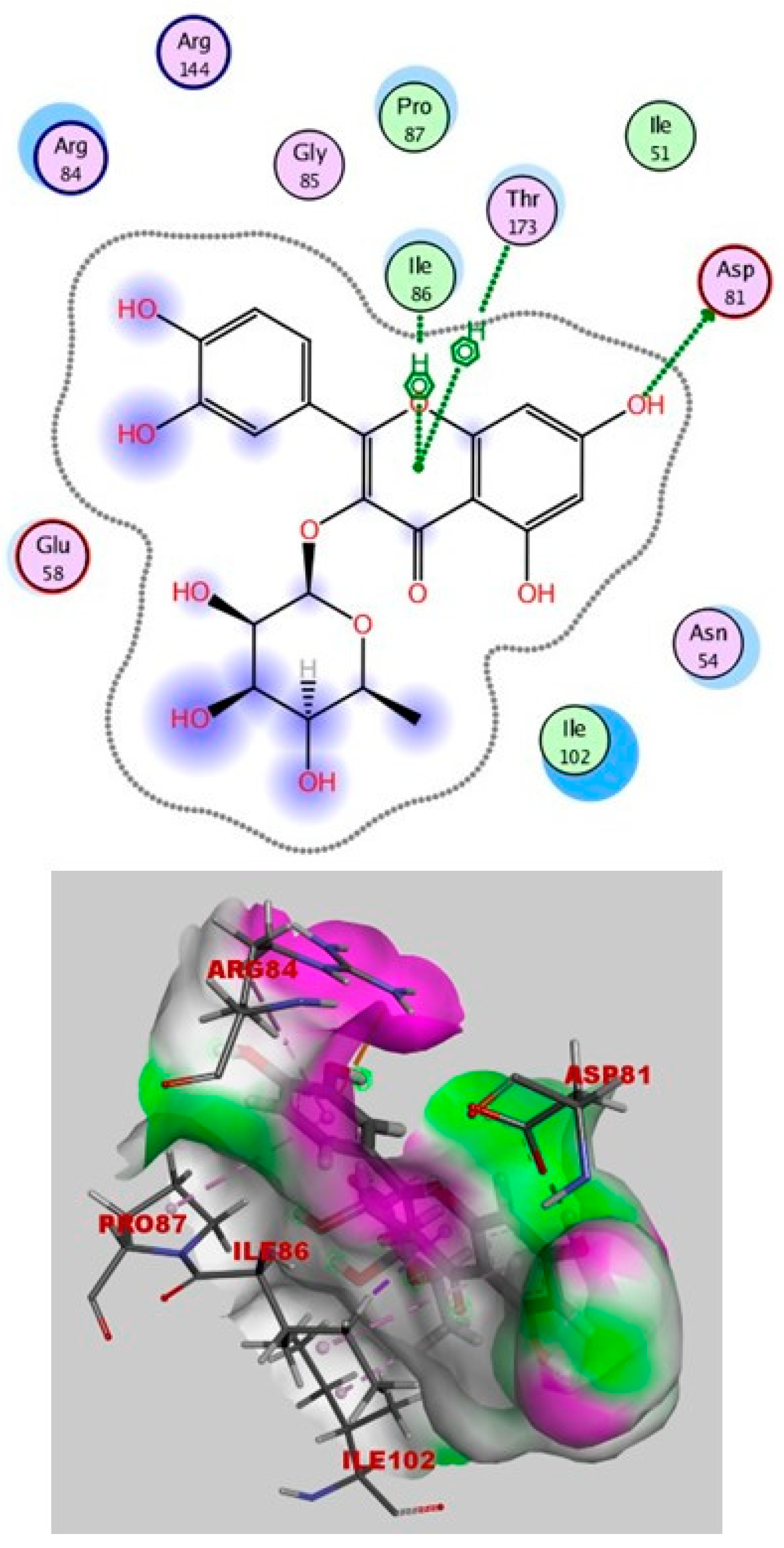
2.4. Molecular Docking
Simulations of Molecular Docking
3. Experimental Design
3.1. Plant Material
3.2. Chemicals
3.3. Extraction Procedure
3.4. Metabolic Profiling of Aeonium arboreum Methanolic Crude Extract
3.5. Green Synthesis of Silver Nanoparticles
3.6. Characterization of Silver Nanoparticles
3.6.1. UV Spectral Analysis
3.6.2. FTIR Analysis
3.6.3. Zeta-Sizer Measurements
3.6.4. Transmission Electron Microscopy (TEM)
3.6.5. X-ray Diffraction (XRD)
3.7. Antimicrobial Activity
3.7.1. Determination of Antimicrobial Activity
3.7.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.7.3. Determination of Antibiofilm Activity
3.7.4. Determination of the Antihemolytic Activity of Plant Extract and Nano-Formula
3.8. Molecular Docking
3.8.1. Simulations of Molecular Docking
3.8.2. Target Protein Structure Preparation
3.8.3. Investigated Drug Molecules’ Preparation
3.8.4. Docking Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tarek, A.; Abdalla, S.; Dokmak, N.A.; Ahmed, A.A.; El-Mahdy, T.S.; Safwat, N.A. Bacterial Diversity and Antibiotic Resistance Patterns of Community-Acquired Urinary Tract Infections in Mega Size Clinical Samples of Egyptian Patients: A Cross-Sectional Study. Cureus 2024, 16, e51838. [Google Scholar] [CrossRef]
- Behzadi, P.; Gajdács, M.; Pallós, P.; Ónodi, B.; Stájer, A.; Matusovits, D.; Kárpáti, K.; Burián, K.; Battah, B.; Ferrari, M. Relationship between biofilm-formation, phenotypic virulence factors and antibiotic resistance in environmental Pseudomonas aeruginosa. Pathogens 2022, 11, 1015. [Google Scholar] [CrossRef]
- Mogrovejo, D.C.; Perini, L.; Gostinčar, C.; Sepčić, K.; Turk, M.; Ambrožič-Avguštin, J.; Brill, F.H.; Gunde-Cimerman, N. Prevalence of antimicrobial resistance and hemolytic phenotypes in culturable arctic bacteria. Front. Microbiol. 2020, 11, 570. [Google Scholar] [CrossRef]
- Khan, T.; Sankhe, K.; Suvarna, V.; Sherje, A.; Patel, K.; Dravyakar, B. DNA gyrase inhibitors: Progress and synthesis of potent compounds as antibacterial agents. Biomed. Pharmacother. 2018, 103, 923–938. [Google Scholar] [CrossRef]
- He, J.; Qiao, W.; An, Q.; Yang, T.; Luo, Y. Dihydrofolate reductase inhibitors for use as antimicrobial agents. Eur. J. Med. Chem. 2020, 195, 112268. [Google Scholar] [CrossRef]
- Hammoudeh, D.I.; Zhao, Y.; White, S.W.; Lee, R.E. Replacing sulfa drugs with novel DHPS inhibitors. Future Med. Chem. 2013, 5, 1331–1340. [Google Scholar] [CrossRef]
- El-Rafie, H.; El-Rafie, M.; Zahran, M. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr. Polym. 2013, 96, 403–410. [Google Scholar] [CrossRef]
- Cho, K.-H.; Park, J.-E.; Osaka, T.; Park, S.-G. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim. Acta 2005, 51, 956–960. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Haider, A.; Kang, I.-K. Preparation of silver nanoparticles and their industrial and biomedical applications: A comprehensive review. Adv. Mater. Sci. Eng. 2015, 2015, 165257. [Google Scholar] [CrossRef]
- Pugliara, A.; Makasheva, K.; Despax, B.; Bayle, M.; Carles, R.; Benzo, P.; BenAssayag, G.; Pécassou, B.; Sancho, M.C.; Navarro, E. Assessing bio-available silver released from silver nanoparticles embedded in silica layers using the green algae Chlamydomonas reinhardtii as bio-sensors. Sci. Total Environ. 2016, 565, 863–871. [Google Scholar] [CrossRef]
- Alam, K.; Al Farraj, D.A.; Mah-e-Fatima, S.; Yameen, M.A.; Elshikh, M.S.; Alkufeidy, R.M.; Mustafa, A.E.-Z.M.; Bhasme, P.; Alshammari, M.K.; Alkubaisi, N.A. Anti-biofilm activity of plant derived extracts against infectious pathogen-Pseudomonas aeruginosa PAO1. J. Infect. Public Health 2020, 13, 1734–1741. [Google Scholar] [CrossRef]
- Salem, W.; Leitner, D.R.; Zingl, F.G.; Schratter, G.; Prassl, R.; Goessler, W.; Reidl, J.; Schild, S. Antibacterial activity of silver and zinc nanoparticles against Vibrio cholerae and enterotoxic Escherichia coli. Int. J. Med. Microbiol. 2015, 305, 85–95. [Google Scholar] [CrossRef]
- Nasr-Eldin, M.A.; Abdelhamid, A.; Baraka, D. Antibiofilm and antiviral potential of leaf extracts from Moringa oleifera and rosemary (Rosmarinus officinalis Lam.). Egypt. J. Microbiol. 2017, 52, 129–139. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Rodriguez-Sanchez, L.; Blanco, M.C.; López-Quintela, M.A. Electrochemical synthesis of silver nanoparticles. J. Phys. Chem. B 2000, 104, 9683–9688. [Google Scholar] [CrossRef]
- Essghaier, B.; Dridi, R.; Mottola, F.; Rocco, L.; Zid, M.F.; Hannachi, H. Biosynthesis and characterization of silver nanoparticles from the extremophile plant aeonium haworthii and their antioxidant, antimicrobial and anti-diabetic capacities. Nanomaterials 2022, 13, 100. [Google Scholar] [CrossRef]
- Melk, M.M.; El-Hawary, S.S.; Melek, F.R.; Saleh, D.O.; Ali, O.M.; El Raey, M.A.; Selim, N.M. Nano zinc oxide green-synthesized from Plumbago auriculata lam. alcoholic extract. Plants 2021, 10, 2447. [Google Scholar] [CrossRef]
- Hassan, M.H.; Elwekeel, A.; Moawad, A.; Afifi, N.; Amin, E.; El Amir, D. Phytochemical constituents and biological activity of selected genera of family Crassulaceae: A review. S. Afr. J. Bot. 2021, 141, 383–404. [Google Scholar] [CrossRef]
- Castillo, A. Efecto antihipertensivo del extracto hidroalcohólico de las hojas de Aeonium arboreum (L) Webb. & Berth “rosa verde” Ayacucho-2015. Undergraduate Thesis, National University of San Cristobal de Huamanga, Ayacucho, Ayacucho Region, Peru, 2015. [Google Scholar]
- Affes, S.; Ben Younes, A.; Frikha, D.; Allouche, N.; Treilhou, M.; Tene, N.; Mezghani-Jarraya, R. ESI-MS/MS analysis of phenolic compounds from Aeonium arboreum leaf extracts and evaluation of their antioxidant and antimicrobial activities. Molecules 2021, 26, 4338. [Google Scholar] [CrossRef]
- Alfeqy, M.M.; El-Hawary, S.S.; El-Halawany, A.M.; Rabeh, M.A.; Alshehri, S.A.; Serry, A.M.; Fahmy, H.A.; Ezzat, M.I. Effect of Phenolics from Aeonium arboreum on Alpha Glucosidase, Pancreatic Lipase, and Oxidative Stress; a Bio-Guided Approach. Pharmaceutics 2023, 15, 2541. [Google Scholar] [CrossRef]
- Abd-Elal, R.M.; Shamma, R.N.; Rashed, H.M.; Bendas, E.R. Trans-nasal zolmitriptan novasomes: In-vitro preparation, optimization and in-vivo evaluation of brain targeting efficiency. Drug Deliv. 2016, 23, 3374–3386. [Google Scholar] [CrossRef]
- Abd-Elal, R.M.; Elosaily, G.H.; Gad, S.; Khafagy, E.-S.; Mostafa, Y. Full factorial design, optimization, in vitro and ex vivo studies of ocular timolol-loaded microsponges. J. Pharm. Innov. 2020, 15, 651–663. [Google Scholar] [CrossRef]
- Fernández-Villa, D.; Aguilar, M.R.; Rojo, L. Folic acid antagonists: Antimicrobial and immunomodulating mechanisms and applications. Int. J. Mol. Sci. 2019, 20, 4996. [Google Scholar] [CrossRef]
- Al-Wahaibi, L.H.; Mahmoud, M.A.; Alzahrani, H.A.; Abou-Zied, H.A.; Gomaa, H.A.; Youssif, B.G.; Bräse, S.; Rabea, S.M. Discovery of new Schiff bases of the disalicylic acid scaffold as DNA gyrase and topoisomerase IV inhibitors endowed with antibacterial properties. Front. Chem. 2024, 12, 1419242. [Google Scholar] [CrossRef]
- Haggag, E.; Elshamy, A.; Rabeh, M.; Gabr, N.; Salem, M.; Youssif, K.; Samir, A.; Bin Muhsinah, A.; Alsayari, A.; Abdelmohsen, U.R. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int. J. Nanomed 2019, 14, 6217–6229. [Google Scholar] [CrossRef]
- Tawfik, N.F.; Abdo, R.; Abbott, G.; Abdelmohsen, U.; Edrada-Ebel, R.; Haggag, E. Metabolomics and dereplication study of the endophytic fungus Aspergillus chevelieri in search of bioactive natural compounds. J. Adv. Pharm. Res. 2017, 1, 100–109. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Cheng, C.; Viegelmann, C.; Zhang, T.; Grkovic, T.; Ahmed, S.; Quinn, R.J.; Hentschel, U.; Edrada-Ebel, R. Dereplication strategies for targeted isolation of new antitrypanosomal actinosporins A and B from a marine sponge associated-Actinokineospora sp. EG49. Mar. Drugs 2014, 12, 1220–1244. [Google Scholar] [CrossRef]
- Emam, M.; El Raey, M.A.; Eisa, W.H.; El-Haddad, A.E.; Osman, S.M.; El-Ansari, M.A.; Rabie, A.-G.M. Green synthesis of silver nanoparticles from Caesalpinia gilliesii (Hook) leaves: Antimicrobial activity and in vitro cytotoxic effect against BJ-1 and MCF-7 cells. J. Appl. Pharm. Sci. 2017, 7, 226–233. [Google Scholar]
- Emam, M.; Soliman, M.M.; Eisa, W.H.; Hasanin, M. Solid and liquid green Ag nanoparticles based on banana peel extract as an eco-friendly remedy for ringworm in pets. Surf. Interface Anal. 2022, 54, 607–618. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Emam, M.; Soliman, M.M.; Latif, R.R.A.; Salem, M.M.; El Raey, M.A.; Eisa, W.H. Green silver nanoparticles based on Lavandula coronopifolia aerial parts extract against mycotic mastitis in cattle. Biocatal. Agric. Biotechnol. 2022, 42, 102350. [Google Scholar] [CrossRef]
- Hamed, A.; Abdel-Razek, A.S.; Araby, M.; Abu-Elghait, M.; El-Hosari, D.G.; Frese, M.; Soliman, H.S.; Stammler, H.G.; Sewald, N.; Shaaban, M. Meleagrin from marine fungus Emericella dentata Nq45: Crystal structure and diverse biological activity studies. Nat. Prod. Res. 2021, 35, 3830–3838. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Huang, X.-J.; Xiong, N.; Chen, B.C.; Luo, F.; Huang, M.; Ding, Z.S.; Qian, C.D. The Antibacterial Properties of 4,8,4′,8′-Tetramethoxy (1,1′-biphenanthrene)-2,7,2′,7′-Tetrol from Fibrous Roots of Bletilla striata. Indian J. Microbiol. 2021, 61, 195–202. [Google Scholar] [CrossRef]
- Wu, S.; Liu, G.; Jin, W.; Xiu, P.; Sun, C. Antibiofilm and anti-infection of a marine bacterial exopolysaccharide against Pseudomonas aeruginosa. Front. Microbiol. 2016, 7, 178739. [Google Scholar] [CrossRef]
- Uddin, M.N.; Banu, H.; Rahman, M.H.; Farhana, J. Antimicrobial, antioxidant and anti-hemolytic effect of different fractions of ethanolic extracts of Tamarindus indica L. bark. J. Pharmacogn. Phytochem. 2016, 5, 83–87. [Google Scholar]
- Chemical Computing Group ULC. Molecular Operating Environment; 1010 Sherbrooke St. West, Suite# 910; Chemical Computing Group Inc.: Montreal, QC, Canada, 2019. [Google Scholar]
- Bylund, D.; Norström, S.H.; Essén, S.A.; Lundström, U.S. Analysis of low molecular mass organic acids in natural waters by ion exclusion chromatography tandem mass spectrometry. J. Chromatogr. A 2007, 1176, 89–93. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, M.; Wang, L. HPLC-DAD-ESIMS analysis of phenolic compounds in bayberries (Myrica rubra Sieb. et Zucc.). Food Chem. 2007, 100, 845–852. [Google Scholar] [CrossRef]
- Milad, R.; El-Ahmady, S.; Singab, A.N. Genus Kalanchoe (Crassulaceae): A review of its ethnomedicinal, botanical, chemical and pharmacological properties. Eur. J. Med. Plants 2013, 4, 86–104. [Google Scholar] [CrossRef]
- Bensouici, C.; Kabouche, A.; Karioti, A.; Öztürk, M.; Duru, M.E.; Bilia, A.R.; Kabouche, Z. Compounds from Sedum caeruleum with antioxidant, anticholinesterase, and antibacterial activities. Pharm. Biol. 2016, 54, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Gaind, K.; Singla, A.K.; Wallace, J.W. Flavonoid glycosides of Kalanchoe spathulata. Phytochemistry 1981, 20, 530–531. [Google Scholar] [CrossRef]
- Ablajan, K.; Abliz, Z.; Shang, X.Y.; He, J.M.; Zhang, R.P.; Shi, J.G. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Mateos, R.; Baeza, G.; Sarriá, B.; Bravo, L. Improved LC-MSn characterization of hydroxycinnamic acid derivatives and flavonols in different commercial mate (Ilex paraguariensis) brands. Quantification of polyphenols, methylxanthines, and antioxidant activity. Food Chem. 2018, 241, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Dewi, R.T.; Ekapratiwi, Y.; Sundowo, A.; Ariani, N.; Yolanda, T.; Filaila, E. Bioconversion of quercetin glucosides from Dendrophthoe pentandra leaf using Aspergillus acueletus LS04-3. In AIP Publishing, Proceedings of the 5TH International Symposium on Applied Chemistry 2019; Tangerang, Indonesia, 23–24 October 2019, AIP Publishing: Melville, NY, USA, 2019. [Google Scholar]
- Lin, Y.; Xu, W.; Huang, M.; Xu, W.; Li, H.; Ye, M.; Zhang, X.; Chu, K. Qualitative and quantitative analysis of phenolic acids, flavonoids and iridoid glycosides in Yinhua Kanggan tablet by UPLC-QqQ-MS/MS. Molecules 2015, 20, 12209–12228. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Qin, M.; Xu, C. Research progress on the distribution and structure of chelidonoids in plants. Mod. Drugs Clin. 2011, 26, 174–180. [Google Scholar]
- Liang, S.; Shen, Y.-H.; Tian, J.-M.; Wu, Z.-J.; Jin, H.-Z.; Zhang, W.-D.; Yan, S.-K. Phenylpropanoids from Daphne feddei and their inhibitory activities against NO production. J. Nat. Prod. 2008, 71, 1902–1905. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, H.; Turdu, G. Traditional Chinese medicinal herbs as potential AChE inhibitors for anti-Alzheimer’s disease: A review. Bioorganic Chem. 2017, 75, 50–61. [Google Scholar] [CrossRef]
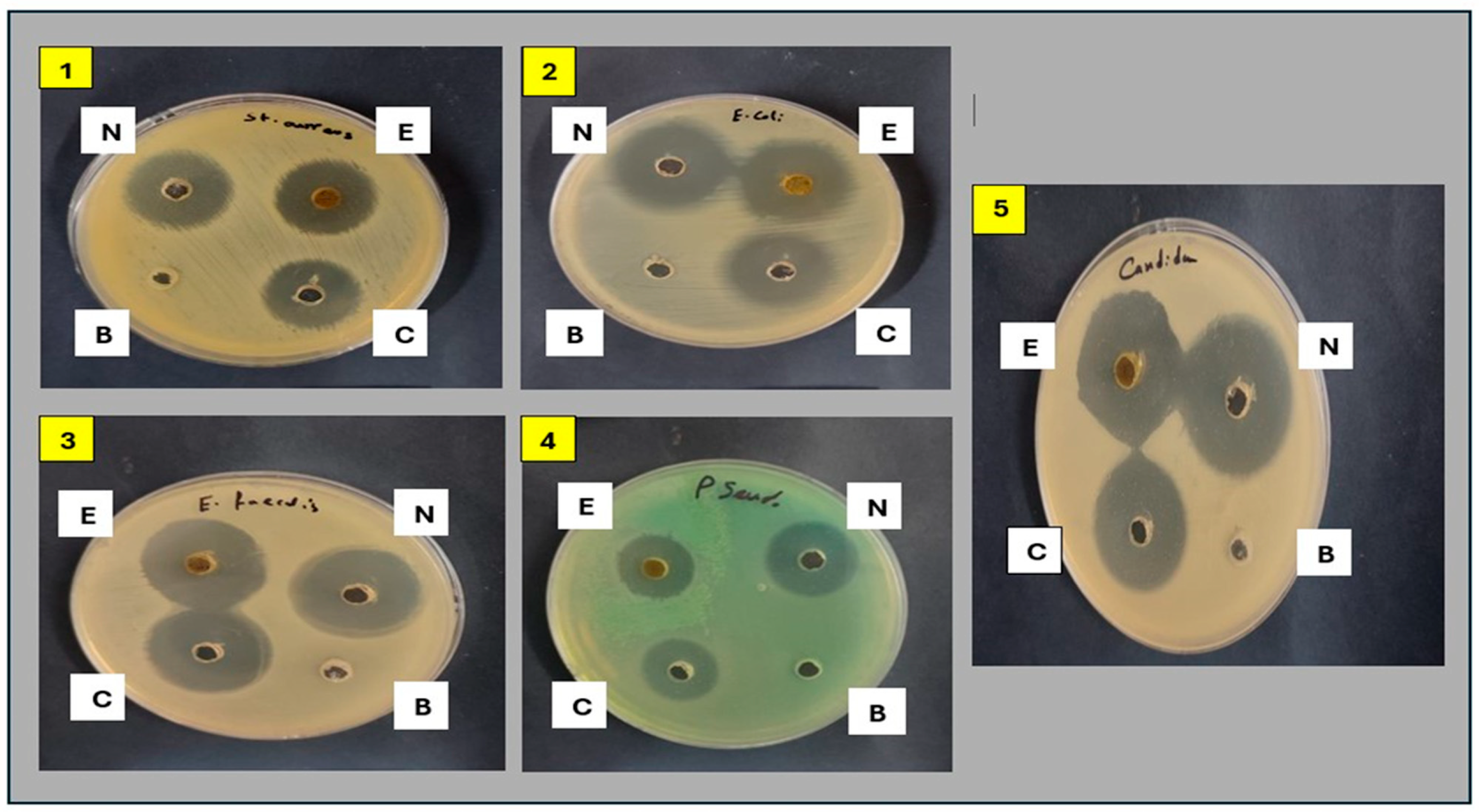


| Test Strains | Zone of Inhibition (mm) | |||
|---|---|---|---|---|
| A. arboreum | AgNPs | Control (Gentamycin) | ||
| Gram-positive | Staphylococcus. aureus (ATCC 6538) | 23 ± 0.2 | 25 ± 0.1 | 22 ± 0.3 |
| Enterococcus faecalis (ATCC 10541) | 27 ± 0.1 | 30 ± 0.1 | 28 ± 0.1 | |
| Gram-negative | Escherichia coli (ATCC 8739) | 27 ± 0.1 | 30 ± 0.3 | 24 ± 0.2 |
| Pseudomonas aeruginosa (ATCC 90274) | 17 ± 0.2 | 22 ± 0.1 | 18 ± 0.1 | |
| Yeast | Candida albicans (ATCC 10221) | 28 ± 0.3 | 33 ± 0.1 | 27 ± 0.3 |
| Test Strains | A. arboreum | AgNPs | Control (Gentamycin) | |||||
|---|---|---|---|---|---|---|---|---|
| MIC μg/mL | MBC μg/mL | MBC/MIC | MIC μg/mL | MBC μg/mL | MBC/MIC | MIC μg/mL | MBC μg/mL | |
| Staphylococcus aureus | 15.62 | 31.25 | 2 | 7.8 | 15.62 | 2 | 15.62 | 15.62 |
| Enterococcus faecalis | 7.8 | 15.62 | 2 | 3.9 | 7.8 | 2 | 7.8 | 7.8 |
| Escherichia coli | 15.62 | 15.62 | 1 | 7.8 | 7.8 | 1 | 31.25 | 31.25 |
| Pseudomonas aeruginosa | 62.5 | 250 | 4 | 31.25 | 31.25 | 1 | 31.25 | 62.5 |
| Candida albicans | 31.25 | 62.5 | 2 | 15.62 | 15.62 | 1 | 15.62 | 31.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfeqy, M.M.; El-Hawary, S.S.; El-Halawany, A.M.; Rabeh, M.A.; Alshehri, S.A.; Abdelmohsen, U.R.; Safwat, N.A.; Serry, A.M.; Fahmy, H.A.; Ezzat, M.I. Biosynthesis and Characterization of Aeonium arboreum-Derived Silver Nanoparticles: Antimicrobial Activity, Biofilm Inhibition, Antihemolytic Activity, and In Silico Studies. Int. J. Mol. Sci. 2024, 25, 8039. https://doi.org/10.3390/ijms25158039
Alfeqy MM, El-Hawary SS, El-Halawany AM, Rabeh MA, Alshehri SA, Abdelmohsen UR, Safwat NA, Serry AM, Fahmy HA, Ezzat MI. Biosynthesis and Characterization of Aeonium arboreum-Derived Silver Nanoparticles: Antimicrobial Activity, Biofilm Inhibition, Antihemolytic Activity, and In Silico Studies. International Journal of Molecular Sciences. 2024; 25(15):8039. https://doi.org/10.3390/ijms25158039
Chicago/Turabian StyleAlfeqy, Marwah M., Seham S. El-Hawary, Ali M. El-Halawany, Mohamed A. Rabeh, Saad A. Alshehri, Usama Ramadan Abdelmohsen, Nesreen A. Safwat, Aya M. Serry, Heba A. Fahmy, and Marwa I. Ezzat. 2024. "Biosynthesis and Characterization of Aeonium arboreum-Derived Silver Nanoparticles: Antimicrobial Activity, Biofilm Inhibition, Antihemolytic Activity, and In Silico Studies" International Journal of Molecular Sciences 25, no. 15: 8039. https://doi.org/10.3390/ijms25158039







