The Influence of Sex Steroid Hormone Fluctuations on Capsaicin-Induced Pain and TRPV1 Expression
Abstract
:1. Introduction
2. Results
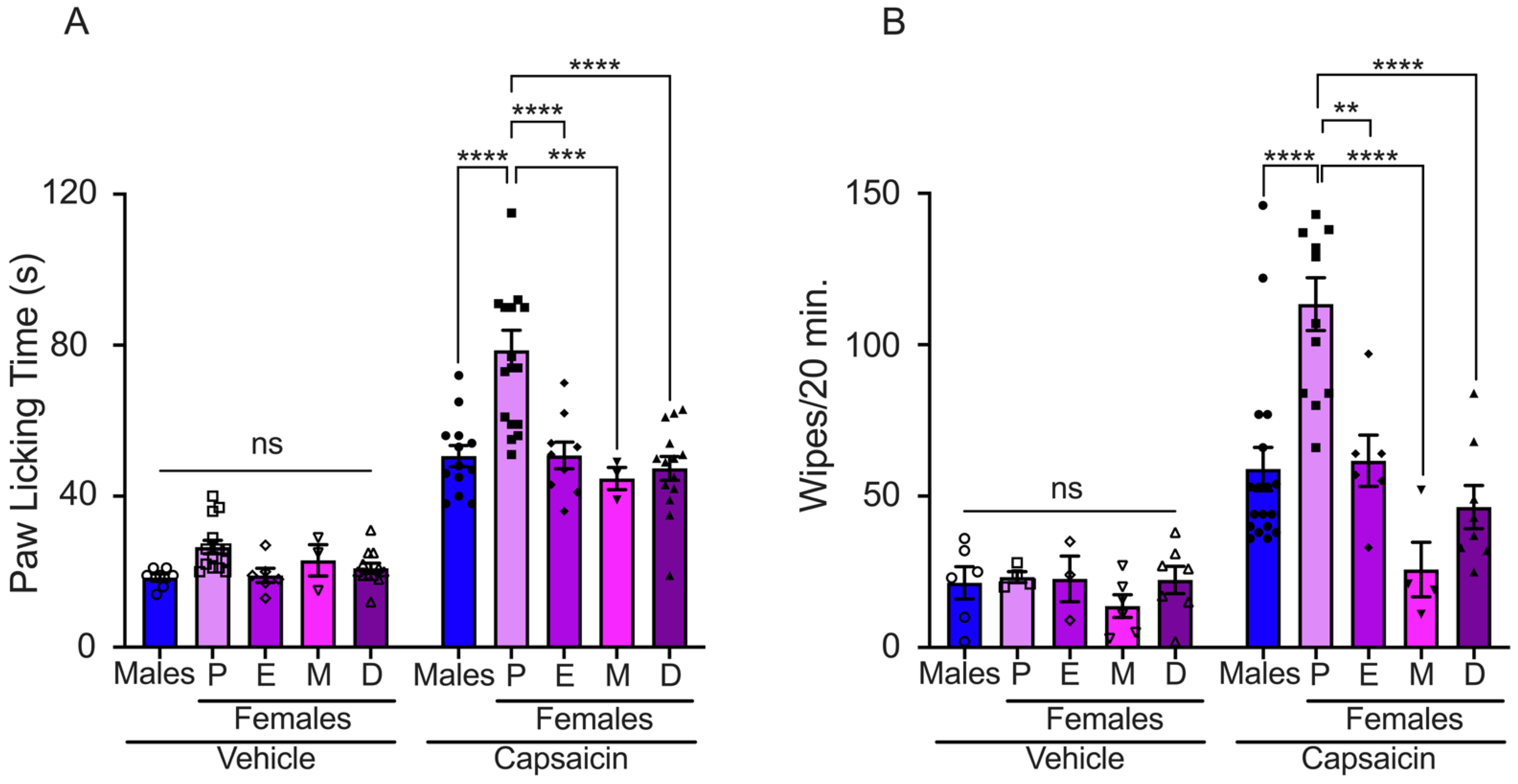
2.1. Effects of Sexual Steroid Hormone Fluctuations on Capsaicin-Induced Pain in Mice
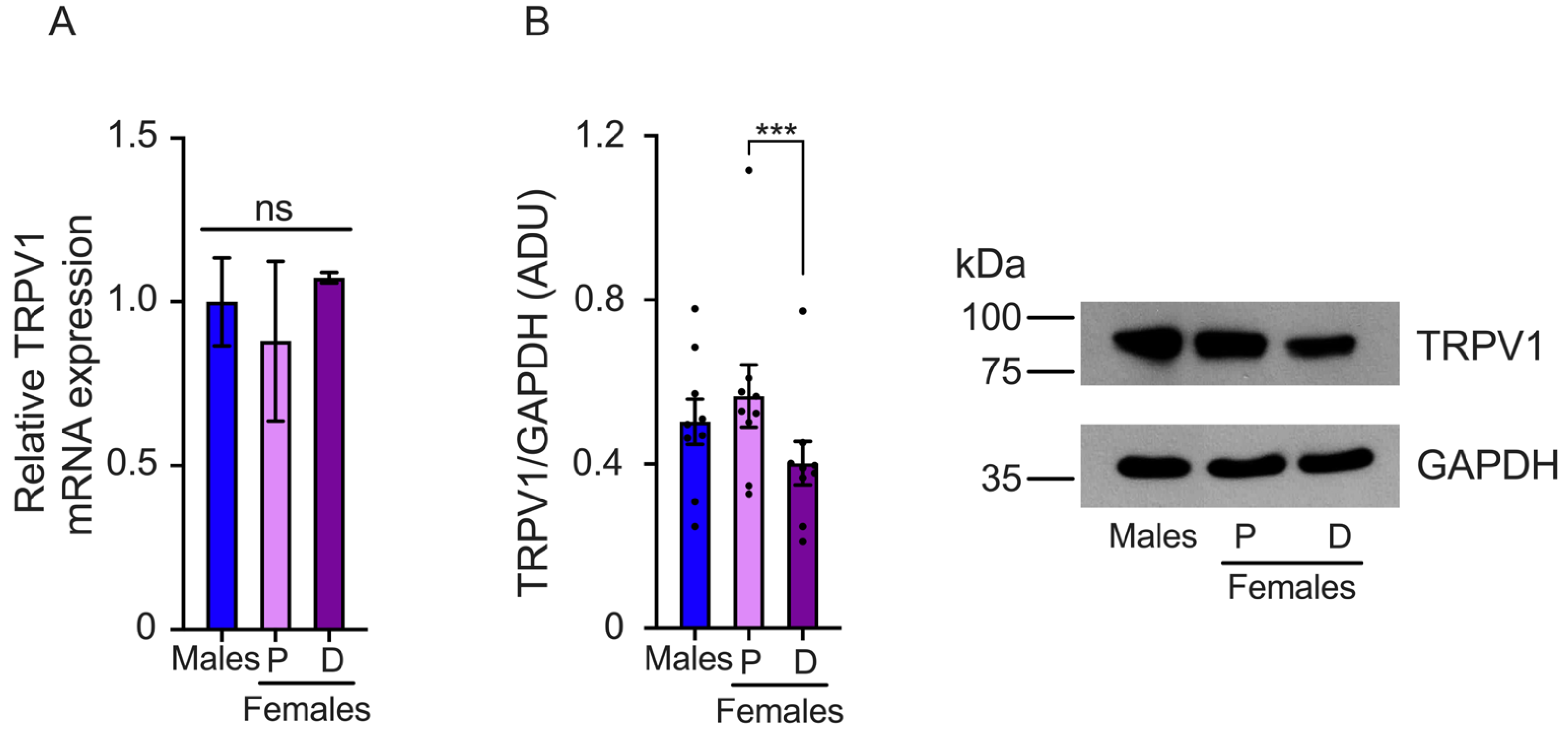
2.2. TRPV1 Gene Expression Is Differentially Regulated during Proestrus and Diestrus
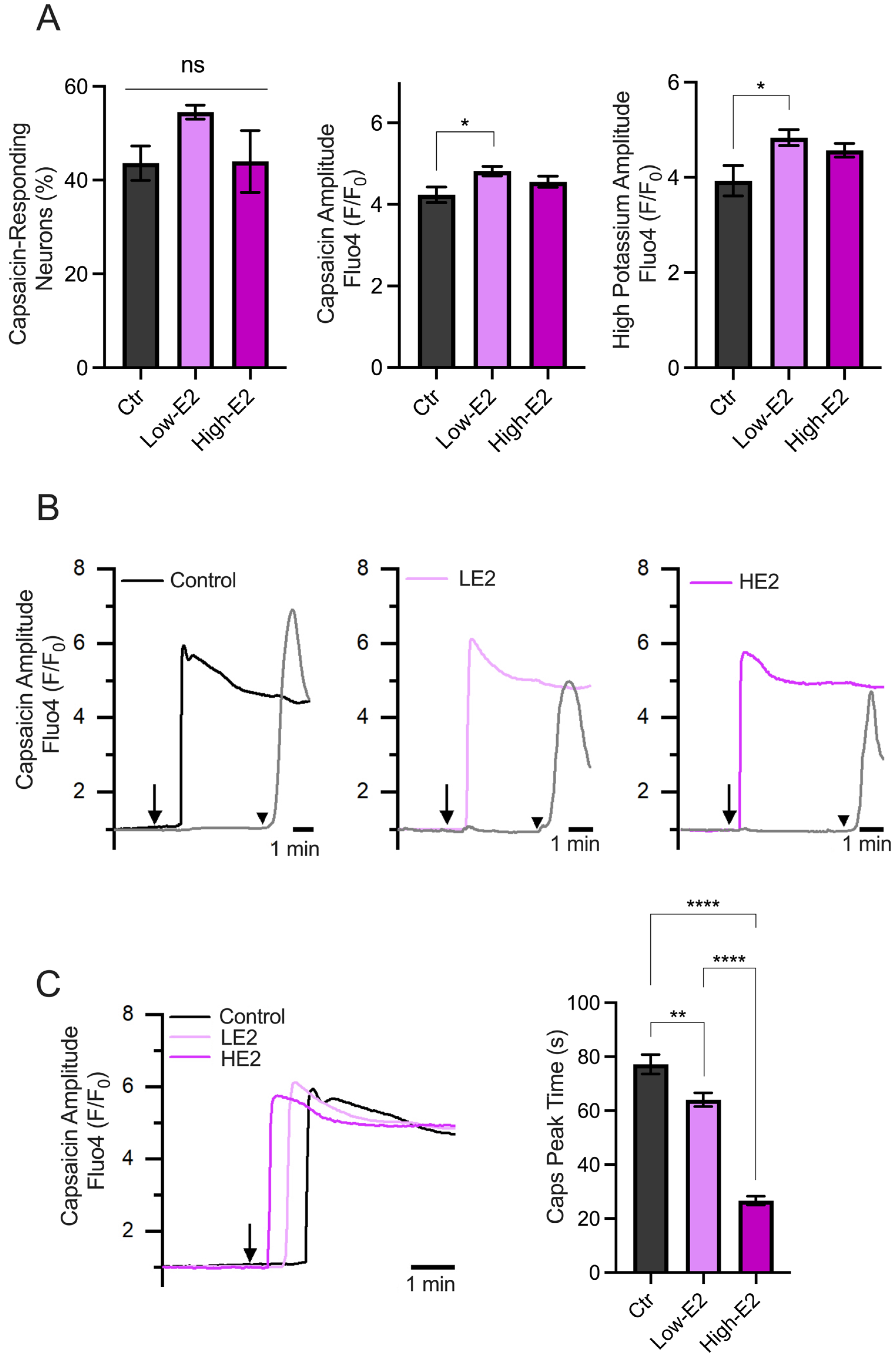
2.3. Effects of 17β-Estradiol on Capsaicin Response on Trigeminal Neurons
2.4. ERα Protein Levels in the TGs Are Higher in the Proestrus Than in the Diestrus Phase
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Determination of the Mouse Estrous Cycle
4.3. Pain Behavior Assays
4.4. DRG and TG Dissection
4.5. TG Primary Cell Culture
4.6. Calcium Influx Measure
4.7. Western Blot Assays
4.8. qPCR
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tracey, W.D., Jr. Nociception. Curr. Biol. 2017, 27, R129–R133. [Google Scholar] [CrossRef] [PubMed]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.Z.; Bautista, D.M. Getting in Touch with Mechanical Pain Mechanisms. Trends Neurosci. 2020, 43, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Cho, H.; Hwang, S.W.; Jung, J.; Shin, C.Y.; Lee, S.Y.; Kim, S.H.; Lee, M.G.; Choi, Y.H.; Kim, J.; et al. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc. Natl. Acad. Sci. USA 2002, 99, 10150–10155. [Google Scholar] [CrossRef]
- Zhang, N.; Inan, S.; Cowan, A.; Sun, R.; Wang, J.M.; Rogers, T.J.; Caterina, M.; Oppenheim, J.J. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc. Natl. Acad. Sci. USA 2005, 102, 4536–4541. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Morales-Lazaro, S.L.; Simon, S.A.; Rosenbaum, T. The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1). J. Physiol. 2013, 591, 3109–3121. [Google Scholar] [CrossRef]
- Akbar, A.; Yiangou, Y.; Facer, P.; Walters, J.R.; Anand, P.; Ghosh, S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut 2008, 57, 923–929. [Google Scholar] [CrossRef]
- Meents, J.E.; Neeb, L.; Reuter, U. TRPV1 in migraine pathophysiology. Trends Mol. Med. 2010, 16, 153–159. [Google Scholar] [CrossRef]
- Qian, H.Y.; Zhou, F.; Wu, R.; Cao, X.J.; Zhu, T.; Yuan, H.D.; Chen, Y.N.; Zhang, P.A. Metformin Attenuates Bone Cancer Pain by Reducing TRPV1 and ASIC3 Expression. Front. Pharmacol. 2021, 12, 713944. [Google Scholar] [CrossRef]
- Engler, A.; Aeschlimann, A.; Simmen, B.R.; Michel, B.A.; Gay, R.E.; Gay, S.; Sprott, H. Expression of transient receptor potential vanilloid 1 (TRPV1) in synovial fibroblasts from patients with osteoarthritis and rheumatoid arthritis. Biochem. Biophys. Res. Commun. 2007, 359, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Bartley, E.J.; Fillingim, R.B. Sex differences in pain: A brief review of clinical and experimental findings. Br. J. Anaesth. 2013, 111, 52–58. [Google Scholar] [CrossRef]
- Fillingim, R.B. Individual differences in pain: Understanding the mosaic that makes pain personal. Pain 2017, 158 (Suppl. S1), S11–S18. [Google Scholar] [CrossRef]
- Casale, R.; Atzeni, F.; Bazzichi, L.; Beretta, G.; Costantini, E.; Sacerdote, P.; Tassorelli, C. Pain in Women: A Perspective Review on a Relevant Clinical Issue that Deserves Prioritization. Pain Ther. 2021, 10, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Mogil, J.S. Qualitative sex differences in pain processing: Emerging evidence of a biased literature. Nat. Rev. Neurosci. 2020, 21, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Fillingim, R.B.; King, C.D.; Ribeiro-Dasilva, M.C.; Rahim-Williams, B.; Riley, J.L., 3rd. Sex, gender, and pain: A review of recent clinical and experimental findings. J. Pain 2009, 10, 447–485. [Google Scholar] [CrossRef] [PubMed]
- Collett, B.J.; Berkley, K.; Task Force on Fact Sheets for the Global Year Against Pain ‘Pain in Women’. The IASP Global Year against pain in women. Pain 2007, 132 (Suppl. S1), S1–S2. [Google Scholar] [CrossRef]
- Gazerani, P.; Andersen, O.K.; Arendt-Nielsen, L. A human experimental capsaicin model for trigeminal sensitization. Gender-specific differences. Pain 2005, 118, 155–163. [Google Scholar] [CrossRef]
- Lu, Y.C.; Chen, C.W.; Wang, S.Y.; Wu, F.S. 17Beta-estradiol mediates the sex difference in capsaicin-induced nociception in rats. J. Pharmacol. Exp. Ther. 2009, 331, 1104–1110. [Google Scholar] [CrossRef]
- Payrits, M.; Saghy, E.; Cseko, K.; Pohoczky, K.; Bolcskei, K.; Ernszt, D.; Barabas, K.; Szolcsanyi, J.; Abraham, I.M.; Helyes, Z.; et al. Estradiol Sensitizes the Transient Receptor Potential Vanilloid 1 Receptor in Pain Responses. Endocrinology 2017, 158, 3249–3258. [Google Scholar] [CrossRef]
- Yamagata, K.; Sugimura, M.; Yoshida, M.; Sekine, S.; Kawano, A.; Oyamaguchi, A.; Maegawa, H.; Niwa, H. Estrogens Exacerbate Nociceptive Pain via Up-Regulation of TRPV1 and ANO1 in Trigeminal Primary Neurons of Female Rats. Endocrinology 2016, 157, 4309–4317. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, O.; Singh, U.; Goswami, C.; Singru, P.S. Transient receptor potential vanilloid 1-6 (Trpv1-6) gene expression in the mouse brain during estrous cycle. Brain Res. 2018, 1701, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, D.J.; Gibson, E.; Davis, S.; Golebiowski, B.; Walters, K.A.; Desai, R. Ultrasensitive Serum Estradiol Measurement by Liquid Chromatography-Mass Spectrometry in Postmenopausal Women and Mice. J. Endocr. Soc. 2020, 4, bvaa086. [Google Scholar] [CrossRef]
- Bradshaw, H.; Miller, J.; Ling, Q.; Malsnee, K.; Ruda, M.A. Sex differences and phases of the estrous cycle alter the response of spinal cord dynorphin neurons to peripheral inflammation and hyperalgesia. Pain 2000, 85, 93–99. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Maixner, W.; Girdler, S.S.; Light, K.C.; Harris, M.B.; Sheps, D.S.; Mason, G.A. Ischemic but not thermal pain sensitivity varies across the menstrual cycle. Psychosom. Med. 1997, 59, 512–520. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, X.; Zhou, Q. Effect of Testosterone on TRPV1 Expression in a Model of Orofacial Myositis Pain in the Rat. J. Mol. Neurosci. 2018, 64, 93–101. [Google Scholar] [CrossRef]
- Mendez-Resendiz, K.A.; Enciso-Pablo, O.; Gonzalez-Ramirez, R.; Juarez-Contreras, R.; Rosenbaum, T.; Morales-Lazaro, S.L. Steroids and TRP Channels: A Close Relationship. Int. J. Mol. Sci. 2020, 21, 3819. [Google Scholar] [CrossRef]
- Ortiz-Renteria, M.; Juarez-Contreras, R.; Gonzalez-Ramirez, R.; Islas, L.D.; Sierra-Ramirez, F.; Llorente, I.; Simon, S.A.; Hiriart, M.; Rosenbaum, T.; Morales-Lazaro, S.L. TRPV1 channels and the progesterone receptor Sig-1R interact to regulate pain. Proc. Natl. Acad. Sci. USA 2018, 115, E1657–E1666. [Google Scholar] [CrossRef]
- Goffaux, P.; Michaud, K.; Gaudreau, J.; Chalaye, P.; Rainville, P.; Marchand, S. Sex differences in perceived pain are affected by an anxious brain. Pain 2011, 152, 2065–2073. [Google Scholar] [CrossRef] [PubMed]
- Harriott, A.M.; Waruinge, A.; Appiah-Danquah, V.; Berhanu, L.; Morais, A.; Ayata, C. The effect of sex and estrus cycle stage on optogenetic spreading depression induced migraine-like pain phenotypes. J. Headache Pain 2023, 24, 85. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.; Hao, T.; Kou, X.X.; Gan, Y.H.; Ma, X.C. Synovial TRPV1 is upregulated by 17-beta-estradiol and involved in allodynia of inflamed temporomandibular joints in female rats. Arch. Oral Biol. 2015, 60, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Taleghany, N.; Sarajari, S.; DonCarlos, L.L.; Gollapudi, L.; Oblinger, M.M. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J. Neurosci. Res. 1999, 57, 603–615. [Google Scholar] [CrossRef]
- Chen, S.C.; Chang, T.J.; Wu, F.S. Competitive inhibition of the capsaicin receptor-mediated current by dehydroepiandrosterone in rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 2004, 311, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Barrantes, R.; Carvajal-Zamorano, K.; Rodriguez, B.; Cordova, C.; Lozano, C.; Simon, F.; Diaz, P.; Munoz, P.; Marchant, I.; Latorre, R.; et al. TRPV1-Estradiol Stereospecific Relationship Underlies Cell Survival in Oxidative Cell Death. Front. Physiol. 2020, 11, 444. [Google Scholar] [CrossRef] [PubMed]
- McLean, A.C.; Valenzuela, N.; Fai, S.; Bennett, S.A. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J. Vis. Exp. 2012, 67, 4389. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Willems, E.; Leyns, L.; Vandesompele, J. Standardization of real-time PCR gene expression data from independent biological replicates. Anal. Biochem. 2008, 379, 127–129. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota-Carrillo, E.; Juárez-Contreras, R.; González-Ramírez, R.; Luis, E.; Morales-Lázaro, S.L. The Influence of Sex Steroid Hormone Fluctuations on Capsaicin-Induced Pain and TRPV1 Expression. Int. J. Mol. Sci. 2024, 25, 8040. https://doi.org/10.3390/ijms25158040
Mota-Carrillo E, Juárez-Contreras R, González-Ramírez R, Luis E, Morales-Lázaro SL. The Influence of Sex Steroid Hormone Fluctuations on Capsaicin-Induced Pain and TRPV1 Expression. International Journal of Molecular Sciences. 2024; 25(15):8040. https://doi.org/10.3390/ijms25158040
Chicago/Turabian StyleMota-Carrillo, Edgardo, Rebeca Juárez-Contreras, Ricardo González-Ramírez, Enoch Luis, and Sara Luz Morales-Lázaro. 2024. "The Influence of Sex Steroid Hormone Fluctuations on Capsaicin-Induced Pain and TRPV1 Expression" International Journal of Molecular Sciences 25, no. 15: 8040. https://doi.org/10.3390/ijms25158040





