Histochemistry for Molecular Imaging in Nanomedicine
Abstract
:1. Introduction
2. Histochemistry: Tradition for Novelty
3. Histochemical Staining Techniques in Nanomedical Studies
4. Immunohistochemistry in Nanomedical Studies
4.1. In Vitro Applications
4.2. Ex Vivo Applications
4.3. Both In Vitro and Ex Vivo Applications
5. Combination of Immunohistochemistry and Histochemical Staining in Nanomedical Studies
5.1. In Vitro Applications
5.2. Ex Vivo Applications
5.3. Both In Vitro and Ex Vivo Applications
5.4. In Vitro, Ex Vivo, and In Vivo Applications
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freitas, R.A., Jr. Nanomedicine, Vol. I: Basic Capabilities; Landes Bioscience: Georgetown, TX, USA, 1999. [Google Scholar]
- Weber, D.O. Nanomedicine. Health Forum J. 1999, 42, 32–36. [Google Scholar] [PubMed]
- Malatesta, M. Transmission Electron Microscopy as a Powerful Tool to Investigate the Interaction of Nanoparticles with Subcellular Structures. Int. J. Mol. Sci. 2021, 22, 12789. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Lanza, G.M.; Wickline, S.A.; Caruthers, S.D. Nanomedicine: Perspective and promises with ligand-directed molecular imaging. Eur. J. Radiol. 2009, 70, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Dearling, J.L.J.; Packard, A.B. Molecular imaging in nanomedicine—A developmental tool and a clinical necessity. J. Control. Release 2017, 261, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Medina, C.; Hak, S.; Reiner, T.; Fayad, Z.A.; Nahrendorf, M.; Mulder, W.J.M. Integrating nanomedicine and imaging. Philos. Trans. A Math. Phys. Eng. Sci. 2017, 375, 20170110. [Google Scholar] [CrossRef] [PubMed]
- Calderan, L.; Malatesta, M. Imaging techniques in nanomedical research. Eur. J. Histochem. 2020, 64, 3151. [Google Scholar] [CrossRef] [PubMed]
- Pellico, J.; Gawne, P.J.; de Rosales, R.T.M. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021, 50, 3355–3423. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, M. Transmission electron microscopy for nanomedicine: Novel applications for long-established techniques. Eur. J. Histochem. 2016, 60, 2751. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, M. Ultrastructural histochemistry in biomedical research: Alive and kicking. Eur. J. Histochem. 2018, 62, 2990. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, M. Histochemistry for nanomedicine: Novelty in tradition. Eur. J. Histochem. 2021, 65, 3376. [Google Scholar] [CrossRef]
- Wick, M.R. Histochemistry as a tool in morphological analysis: A historical review. Ann. Diagn. Pathol. 2012, 16, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Lison, L. Histochimie Animale; Gautier-Villars Publishers: Paris, France, 1936. [Google Scholar]
- Glick, D. Techniques of Histo- and Cyto-Chemistry; Interscience Publishers: New York, NY, USA, 1949. [Google Scholar]
- Feulgen, R.; Rossenbeck, H. Mikroskopisch-chemischer Nachweis einer Nucleinsaure von Typus der Thymonucleinsaure und die darauf beruhende elective Farbung von Zellkernen in Mikroskopischer Praparaten. Hoppe Seyler’s Z Physiol. Chem. 1924, 135, 203–248. [Google Scholar] [CrossRef]
- Kasten, F.H. Robert Feulgen and his histochemical reaction for DNA. Biotech. Histochem. 2003, 78, 45–49. [Google Scholar] [CrossRef]
- Coons, A.H. Fluorescent antibodies as histochemical tools. Fed. Proc. 1951, 10, 558–559. [Google Scholar] [PubMed]
- Ortiz Hidalgo, C. Immunohistochemistry in Historical Perspective: Knowing the Past to Understand the Present. In Immunohistochemistry and Immunocytochemistry. Methods in Molecular Biology; Del Valle, L., Ed.; Humana: New York, NY, USA, 2022; Volume 2422, pp. 17–31. [Google Scholar] [CrossRef]
- Vidal, S.; Horvath, E.; Kovacs, K. Ultrastructural Immunohistochemistry. In Morphology Methods; Lloyd, R.V., Ed.; Humana Press: Totowa, NJ, USA, 2001; pp. 375–402. [Google Scholar] [CrossRef]
- Durrant, I. Nonradioactive In Situ Hybridization for Cells and Tissues. In Basic DNA and RNA Protocols. Methods in Molecular Biology; Harwood, A.J., Ed.; Humana Press: New York, NY, USA, 1996; Volume 58, pp. 155–167. [Google Scholar] [CrossRef]
- Van Noorden, C.J. Imaging enzymes at work: Metabolic mapping by enzyme histochemistry. J. Histochem. Cytochem. 2010, 58, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Pellicciari, C. Histochemistry today: Detection and location of single molecules. Eur. J. Histochem. 2017, 61, 2885. [Google Scholar] [CrossRef]
- Pellicciari, C.; Biggiogera, M.; Malatesta, M. (Eds.) Histochemistry of Single Molecules: Methods and Protocols, 2nd ed.; Humana Press: New York, NY, USA, 2023; Volume 2566. [Google Scholar]
- Lanier, L.L.; Recktenwald, D.J. Multicolor immunofluorescence and flow cytometry. Methods 1991, 2, 192–199. [Google Scholar] [CrossRef]
- Buchwalow, I.B.; Minin, E.A.; Boecker, W. A multicolor fluorescence immunostaining technique for simultaneous antigen targeting. Acta Histochem. 2005, 107, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Mazzini, G.; Danova, M. Histochemistry in advanced cytometry: From fluorochromes to mass probes. Methods Mol. Biol. 2023, 2566, 1–25. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Sheng, Z.; Yang, Y.; Ma, G. Dual-modal tracking of transplanted mesenchymal stem cells after myocardial infarction. Int. J. Nanomed. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Mou, Y.; Hou, Y.; Chen, B.; Hua, Z.; Zhang, Y.; Xi, H.; Xia, G.; Wang, Z.; Huang, X.; Han, W.; et al. In vivo migration of dendritic cells labeled with synthetic superparamagnetic iron oxide. Int. J. Nanomed. 2011, 6, 2633–2640. [Google Scholar] [CrossRef]
- Schlachter, E.K.; Widmer, H.R.; Bregy, A.; Lönnfors-Weitzel, T.; Vajtai, I.; Corazza, N.; Bernau, V.J.; Weitzel, T.; Mordasini, P.; Slotboom, J.; et al. Metabolic pathway and distribution of superparamagnetic iron oxide nanoparticles: In vivo study. Int. J. Nanomed. 2011, 6, 1793–1800. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Nitta, N.; Sonoda, A.; Nitta-Seko, A.; Ohta, S.; Otani, H.; Takahashi, M.; Murata, K.; Murase, K.; Nohara, S.; et al. Histological study of the biodynamics of iron oxide nanoparticles with different diameters. Int. J. Nanomed. 2011, 6, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.B.; Zhou, J.X.; Yuan, R.X. Folate-targeted polymeric micelles loaded with ultrasmall superparamagnetic iron oxide: Combined small size and high MRI sensitivity. Int. J. Nanomed. 2012, 7, 2863–2872. [Google Scholar] [CrossRef]
- Hsieh, W.J.; Liang, C.J.; Chieh, J.J.; Wang, S.H.; Lai, I.R.; Chen, J.H.; Chang, F.H.; Tseng, W.K.; Yang, S.Y.; Wu, C.C.; et al. In vivo tumor targeting and imaging with anti-vascular endothelial growth factor antibody-conjugated dextran-coated iron oxide nanoparticles. Int. J. Nanomed. 2012, 7, 2833–2842. [Google Scholar] [CrossRef]
- Huang, K.W.; Chieh, J.J.; Horng, H.E.; Hong, C.Y.; Yang, H.C. Characteristics of magnetic labeling on liver tumors with anti-alpha-fetoprotein-mediated Fe3O4 magnetic nanoparticles. Int. J. Nanomed. 2012, 7, 2987–2996. [Google Scholar] [CrossRef]
- Kenzaoui, B.H.; Vilà, M.R.; Miquel, J.M.; Cengelli, F.; Juillerat-Jeanneret, L. Evaluation of uptake and transport of cationic and anionic ultrasmall iron oxide nanoparticles by human colon cells. Int. J. Nanomed. 2012, 7, 1275–1286. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, G.; Arora, V.; Mewar, S.; Sharma, U.; Jagannathan, N.R.; Sapra, S.; Dinda, A.K.; Kharbanda, S.; Singh, H. Cellular interaction of folic acid conjugated superparamagnetic iron oxide nanoparticles and its use as contrast agent for targeted magnetic imaging of tumor cells. Int. J. Nanomed. 2012, 7, 3503–3516. [Google Scholar] [CrossRef]
- Vaněček, V.; Zablotskii, V.; Forostyak, S.; Růžička, J.; Herynek, V.; Babič, M.; Jendelová, P.; Kubinová, S.; Dejneka, A.; Syková, E. Highly efficient magnetic targeting of mesenchymal stem cells in spinal cord injury. Int. J. Nanomed. 2012, 7, 3719–3730. [Google Scholar] [CrossRef]
- Zhu, X.M.; Wang, Y.X.; Leung, K.C.; Lee, S.F.; Zhao, F.; Wang, D.W.; Lai, J.M.; Wan, C.; Cheng, C.H.; Ahuja, A.T. Enhanced cellular uptake of aminosilane-coated superparamagnetic iron oxide nanoparticles in mammalian cell lines. Int. J. Nanomed. 2012, 7, 953–964. [Google Scholar] [CrossRef]
- Li, X.X.; Li, K.A.; Qin, J.B.; Ye, K.C.; Yang, X.R.; Li, W.M.; Xie, Q.S.; Jiang, M.E.; Zhang, G.X.; Lu, X.W. In vivo MRI tracking of iron oxide nanoparticle-labeled human mesenchymal stem cells in limb ischemia. Int. J. Nanomed. 2013, 8, 1063–1073. [Google Scholar] [CrossRef]
- Hsiao, J.K.; Wu, H.C.; Liu, H.M.; Yu, A.; Lin, C.T. A multifunctional peptide for targeted imaging and chemotherapy for nasopharyngeal and breast cancers. Nanomedicine 2015, 11, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Busato, A.; Bonafede, R.; Bontempi, P.; Scambi, I.; Schiaffino, L.; Benati, D.; Malatesta, M.; Sbarbati, A.; Marzola, P.; Mariotti, R. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: A new method to obtain labeled exosomes. Int. J. Nanomed. 2016, 11, 2481–2490. [Google Scholar] [CrossRef]
- Panseri, S.; Montesi, M.; Iafisco, M.; Adamiano, A.; Ghetti, M.; Cenacchi, G.; Tampieri, A. Magnetic Labelling of Mesenchymal Stem Cells with Iron-Doped Hydroxyapatite Nanoparticles as Tool for Cell Therapy. J. Biomed. Nanotechnol. 2016, 12, 909–921. [Google Scholar] [CrossRef] [PubMed]
- Busato, A.; Bonafede, R.; Bontempi, P.; Scambi, I.; Schiaffino, L.; Benati, D.; Malatesta, M.; Sbarbati, A.; Marzola, P.; Mariotti, R. Labeling and Magnetic Resonance Imaging of Exosomes Isolated from Adipose Stem Cells. Curr. Protoc. Cell Biol. 2017, 75, 3.44.1–3.44.15. [Google Scholar] [CrossRef] [PubMed]
- Faruque, H.A.; Choi, E.S.; Kim, J.H.; Kim, S.; Kim, E. In vivo removal of radioactive cesium compound using Prussian blue-deposited iron oxide nanoparticles. Nanomedicine 2019, 14, 3143–3158. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Qian, X.; Kang, L.; Wang, K.; Zhang, H.; Yang, S.M.; Zhang, W. A step towards glucose control with a novel nanomagnetic-insulin for diabetes care. Int. J. Pharm. 2021, 601, 120587. [Google Scholar] [CrossRef] [PubMed]
- Carton, F.; Chevalier, Y.; Nicoletti, L.; Tarnowska, M.; Stella, B.; Arpicco, S.; Malatesta, M.; Jordheim, L.P.; Briançon, S.; Lollo, G. Rationally designed hyaluronic acid-based nano-complexes for pentamidine delivery. Int. J. Pharm. 2019, 568, 118526. [Google Scholar] [CrossRef] [PubMed]
- Carton, F.; Repellin, M.; Lollo, G.; Malatesta, M. Alcian blue staining to track the intracellular fate of hyaluronic-acid-based nanoparticles at transmission electron microscopy. Eur. J. Histochem. 2019, 63, 3086. [Google Scholar] [CrossRef]
- Repellin, M.; Carton, F.; Boschi, F.; Galiè, M.; Perduca, M.; Calderan, L.; Jacquier, A.; Carras, J.; Schaeffer, L.; Briançon, S.; et al. Repurposing pentamidine using hyaluronic acid-based nanocarriers for skeletal muscle treatment in myotonic dystrophy. Nanomedicine 2023, 47, 102623. [Google Scholar] [CrossRef]
- Gromnicova, R.; Yilmaz, C.U.; Orhan, N.; Kaya, M.; Davies, H.; Williams, P.; Romero, I.A.; Sharrack, B.; Male, D. Localization and mobility of glucose-coated gold nanoparticles within the brain. Nanomedicine 2016, 11, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, M.; Giagnacovo, M.; Costanzo, M.; Conti, B.; Genta, I.; Dorati, R.; Galimberti, V.; Biggiogera, M.; Zancanaro, C. Diaminobenzidine photoconversion is a suitable tool for tracking the intracellular location of fluorescently labelled nanoparticles at transmission electron microscopy. Eur. J. Histochem. 2012, 56, e20. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, M.; Grecchi, S.; Chiesa, E.; Cisterna, B.; Costanzo, M.; Zancanaro, C. Internalized chitosan nanoparticles persist for long time in cultured cells. Eur. J. Histochem. 2015, 59, 2492. [Google Scholar] [CrossRef] [PubMed]
- Pansieri, J.; Plissonneau, M.; Stransky-Heilkron, N.; Dumoulin, M.; Heinrich-Balard, L.; Rivory, P.; Morfin, J.F.; Toth, E.; Saraiva, M.J.; Allémann, E.; et al. Multimodal imaging Gd-nanoparticles functionalized with Pittsburgh compound B or a nanobody for amyloid plaques targeting. Nanomedicine 2017, 12, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Leve, F.; Bonfim, D.P.; Fontes, G.; Morgado-Díaz, J.A. Gold nanoparticles regulate tight junctions and improve cetuximab effect in colon cancer cells. Nanomedicine 2019, 14, 1565–1578. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, L.; Soldani, C.; Bottone, M.G.; Malatesta, M.; Martin, T.E.; Rothblum, L.I.; Pellicciari, C.; Biggiogera, M. DADLE induces a reversible hibernation-like state in HeLa cells. Histochem. Cell Biol. 2006, 125, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Malatesta, M.; Galimberti, V.; Cisterna, B.; Costanzo, M.; Biggiogera, M.; Zancanaro, C. Chitosan nanoparticles are efficient carriers for delivering biodegradable drugs to neuronal cells. Histochem. Cell Biol. 2014, 141, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Balkundi, S.; Nowacek, A.S.; Veerubhotla, R.S.; Chen, H.; Martinez-Skinner, A.; Roy, U.; Mosley, R.L.; Kanmogne, G.; Liu, X.; Kabanov, A.V.; et al. Comparative manufacture and cell-based delivery of antiretroviral nanoformulations. Int. J. Nanomed. 2011, 6, 3393–3404. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Murata, Y.; Sugi, T.; Kato, K. Inorganic nanoparticles kill Toxoplasma gondii via changes in redox status and mitochondrial membrane potential. Int. J. Nanomed. 2017, 12, 1647–1661. [Google Scholar] [CrossRef]
- Raman, J.; Lakshmanan, H.; John, P.A.; Zhijian, C.; Periasamy, V.; David, P.; Naidu, M.; Sabaratnam, V. Neurite outgrowth stimulatory effects of myco synthesized AuNPs from Hericium erinaceus (Bull.: Fr.) Pers. on pheochromocytoma (PC-12) cells. Int. J. Nanomed. 2015, 10, 5853–5863. [Google Scholar] [CrossRef]
- Neacsu, P.; Mazare, A.; Schmuki, P.; Cimpean, A. Attenuation of the macrophage inflammatory activity by TiO2 nanotubes via inhibition of MAPK and NF-kappaB pathways. Int. J. Nanomed. 2015, 10, 6455–6467. [Google Scholar] [CrossRef]
- Zhang, L.; Li, R.; Chen, H.; Wei, J.; Qian, H.; Su, S.; Shao, J.; Wang, L.; Qian, X.; Liu, B. Human cytotoxic T-lymphocyte membrane-camouflaged nanoparticles combined with low-dose irradiation: A new approach to enhance drug targeting in gastric cancer. Int. J. Nanomed. 2017, 12, 2129–2142. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Zhang, Y. Endostar-loaded PEG-PLGA nanoparticles: In vitro and in vivo evaluation. Int. J. Nanomed. 2010, 5, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Abedini, F.; Hosseinkhani, H.; Ismail, M.; Domb, A.J.; Omar, A.R.; Chong, P.P.; Hong, P.D.; Yu, D.S.; Farbr, I.Y. Cationized dextran nanoparticle-encapsulated CXCR4-siRNA enhanced correlation between CXCR4 expression and serum alkaline phosphatase in a mouse model of colorectal cancer. Int. J. Nanomed. 2012, 7, 4159–4168. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, S.; You, H.; Zhang, Y.; Yang, B.; Sun, X.; Yang, L.; Chen, Y.; Fu, S.; Wu, J. In vivo synergistic anti-tumor effect of paclitaxel nanoparticles combined with radiotherapy on human cervical carcinoma. Drug Deliv. 2017, 24, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, B.; Uehara, M.; Ordikhani, F.; Li, X.; Jiang, L.; Banouni, N.; Ichimura, T.; Kasinath, V.; Eskandari, S.K.; Annabi, N.; et al. Ectopic high endothelial venules in pancreatic ductal adenocarcinoma: A unique site for targeted delivery. eBioMedicine 2018, 38, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Karmali, P.P.; Kotamraju, V.R.; Kastantin, M.; Black, M.; Missirlis, D.; Tirrell, M.; Ruoslahti, E. Targeting of albumin-embedded paclitaxel nanoparticles to tumors. Nanomedicine 2009, 5, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Oppermann, E.; Tran, A.; Imlau, U.; Qian, K.; Vogl, T.J. Transarterial administration of integrin inhibitor loaded nanoparticles combined with transarterial chemoembolization for treating hepatocellular carcinoma in a rat model. World J. Gastroenterol. 2016, 22, 5042–5049. [Google Scholar] [CrossRef] [PubMed]
- Simón, M.; Jørgensen, J.T.; Norregaard, K.; Kjaer, A. (18)F-FDG positron emission tomography and diffusion-weighted magnetic resonance imaging for response evaluation of nanoparticle-mediated photothermal therapy. Sci. Rep. 2020, 10, 7595. [Google Scholar] [CrossRef]
- Perry, J.L.; Reuter, K.G.; Luft, J.C.; Pecot, C.V.; Zamboni, W.; DeSimone, J.M. Mediating Passive Tumor Accumulation through Particle Size, Tumor Type, and Location. Nano Lett. 2017, 17, 2879–2886. [Google Scholar] [CrossRef]
- Aly, A.E.; Harmon, B.; Padegimas, L.; Sesenoglu-Laird, O.; Cooper, M.J.; Yurek, D.M.; Waszczak, B.L. Intranasal delivery of hGDNF plasmid DNA nanoparticles results in long-term and widespread transfection of perivascular cells in rat brain. Nanomedicine 2019, 16, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.Y.; Yang, T.C.; Wang, Y.C.; Lee, W.H.; Chang, T.M.; Kau, Y.C.; Liu, S.J. Targeted concurrent and sequential delivery of chemotherapeutic and antiangiogenic agents to the brain by using drug-loaded nanofibrous membranes. Int. J. Nanomed. 2017, 12, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Somagoni, J.; Boakye, C.H.; Godugu, C.; Patel, A.R.; Mendonca Faria, H.A.; Zucolotto, V.; Singh, M. Nanomiemgel—A novel drug delivery system for topical application--in vitro and in vivo evaluation. PLoS ONE 2014, 9, e115952. [Google Scholar] [CrossRef] [PubMed]
- Almer, G.; Wernig, K.; Saba-Lepek, M.; Haj-Yahya, S.; Rattenberger, J.; Wagner, J.; Gradauer, K.; Frascione, D.; Pabst, G.; Leitinger, G.; et al. Adiponectin-coated nanoparticles for enhanced imaging of atherosclerotic plaques. Int. J. Nanomed. 2011, 6, 1279–1290. [Google Scholar] [CrossRef]
- Prow, T.W.; Bhutto, I.; Kim, S.Y.; Grebe, R.; Merges, C.; McLeod, D.S.; Uno, K.; Mennon, M.; Rodriguez, L.; Leong, K.; et al. Ocular nanoparticle toxicity and transfection of the retina and retinal pigment epithelium. Nanomedicine 2008, 4, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kambhampati, S.P.; Clunies-Ross, A.J.; Bhutto, I.; Mishra, M.K.; Edwards, M.; McLeod, D.S.; Kannan, R.M.; Lutty, G. Systemic and Intravitreal Delivery of Dendrimers to Activated Microglia/Macrophage in Ischemia/Reperfusion Mouse Retina. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4413–4424. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yao, L.; Li, J.; Zhang, W.; Wu, X.; Liu, Y.; Lin, M.; Su, W.; Li, Y.; Liang, D. Celastrol nanoparticles inhibit corneal neovascularization induced by suturing in rats. Int. J. Nanomed. 2012, 7, 1163–1173. [Google Scholar] [CrossRef]
- Yoon, J.; Korkmaz Zirpel, N.; Park, H.J.; Han, S.; Hwang, K.H.; Shin, J.; Cho, S.W.; Nam, C.H.; Chung, S. Angiogenic Type I Collagen Extracellular Matrix Integrated with Recombinant Bacteriophages Displaying Vascular Endothelial Growth Factors. Adv. Healthc. Mater. 2016, 5, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Ali, A.; Giteru, S.G.; Shavandi, A.; Clarkson, A.N. Silk fibroin nanoscaffolds for neural tissue engineering. J. Mater. Sci. Mater. Med. 2020, 31, 81. [Google Scholar] [CrossRef]
- Horie, R.T.; Sakamoto, T.; Nakagawa, T.; Ishihara, T.; Higaki, M.; Ito, J. Stealth-nanoparticle strategy for enhancing the efficacy of steroids in mice with noise-induced hearing loss. Nanomedicine 2010, 5, 1331–1340. [Google Scholar] [CrossRef]
- He, X.; Zhu, Y.; Wang, M.; Jing, G.; Zhu, R.; Wang, S. Antidepressant effects of curcumin and HU-211 coencapsulated solid lipid nanoparticles against corticosterone-induced cellular and animal models of major depression. Int. J. Nanomed. 2016, 11, 4975–4990. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Nguyen, M.; Foote, H.P.; Caster, J.M.; Roche, K.C.; Peters, C.G.; Wu, P.; Jayaraman, L.; Garmey, E.G.; Tepper, J.E.; et al. CRLX101, a Nanoparticle-Drug Conjugate Containing Camptothecin, Improves Rectal Cancer Chemoradiotherapy by Inhibiting DNA Repair and HIF1alpha. Cancer Res. 2017, 77, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Yuan, C.; Shen, S.; Yi, X.; Gong, H.; Yang, K.; Liu, Z. Bottom-Up Synthesis of Metal-Ion-Doped WS2 Nanoflakes for Cancer Theranostics. ACS Nano 2015, 9, 11090–11101. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.U.; Kim, J.; Bokara, K.K.; Kim, J.Y.; Khang, D.; Webster, T.J.; Lee, J.E. Carbon nanotubes impregnated with subventricular zone neural progenitor cells promotes recovery from stroke. Int. J. Nanomed. 2012, 7, 2751–2765. [Google Scholar] [CrossRef] [PubMed]
- Boyoglu, S.; Vig, K.; Pillai, S.; Rangari, V.; Dennis, V.A.; Khazi, F.; Singh, S.R. Enhanced delivery and expression of a nanoencapsulated DNA vaccine vector for respiratory syncytial virus. Nanomedicine 2009, 5, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Kılıçay, E.; Demirbilek, M.; Türk, M.; Güven, E.; Hazer, B.; Denkbas, E.B. Preparation and characterization of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBHHX) based nanoparticles for targeted cancer therapy. Eur. J. Pharm. Sci. 2011, 44, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Yu, H.; Jia, Z.Y.; Yao, Q.L.; Teng, G.J. Efficient nano iron particle-labeling and noninvasive MR imaging of mouse bone marrow-derived endothelial progenitor cells. Int. J. Nanomed. 2011, 6, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Mocan, L.; Matea, C.; Tabaran, F.A.; Mosteanu, O.; Pop, T.; Mocan, T.; Iancu, C. Photothermal treatment of liver cancer with albumin-conjugated gold nanoparticles initiates Golgi Apparatus-ER dysfunction and caspase-3 apoptotic pathway activation by selective targeting of Gp60 receptor. Int. J. Nanomed. 2015, 10, 5435–5445. [Google Scholar] [CrossRef]
- Yoshida, S.; Duong, C.; Oestergaard, M.; Fazio, M.; Chen, C.; Peralta, R.; Guo, S.; Seth, P.P.; Li, Y.; Beckett, L.; et al. MXD3 antisense oligonucleotide with superparamagnetic iron oxide nanoparticles: A new targeted approach for neuroblastoma. Nanomedicine 2020, 24, 102127. [Google Scholar] [CrossRef]
- Kadiu, I.; Nowacek, A.; McMillan, J.; Gendelman, H.E. Macrophage endocytic trafficking of antiretroviral nanoparticles. Nanomedicine 2011, 6, 975–994. [Google Scholar] [CrossRef]
- Chen, M.; Le, D.Q.; Hein, S.; Li, P.; Nygaard, J.V.; Kassem, M.; Kjems, J.; Besenbacher, F.; Bünger, C. Fabrication and characterization of a rapid prototyped tissue engineering scaffold with embedded multicomponent matrix for controlled drug release. Int. J. Nanomed. 2012, 7, 4285–4297. [Google Scholar] [CrossRef] [PubMed]
- Filova, E.; Fojt, J.; Kryslova, M.; Moravec, H.; Joska, L.; Bacakova, L. The diameter of nanotubes formed on Ti-6Al-4V alloy controls the adhesion and differentiation of Saos-2 cells. Int. J. Nanomed. 2015, 10, 7145–7163. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, M.; Musilkova, J.; Riedel, T.; Stranska, D.; Brynda, E.; Zaloudkova, M.; Bacakova, L. The potential applications of fibrin-coated electrospun polylactide nanofibers in skin tissue engineering. Int. J. Nanomed. 2016, 11, 771–789. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lü, S.; Jiang, X.X.; Li, X.; Li, H.; Lin, Q.; Mou, Y.; Zhao, Y.; Han, Y.; Zhou, J.; et al. Carbon nanotubes enhance intercalated disc assembly in cardiac myocytes via the beta1-integrin-mediated signaling pathway. Biomaterials 2015, 55, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Rauti, R.; Lozano, N.; León, V.; Scaini, D.; Musto, M.; Rago, I.; Ulloa Severino, F.P.; Fabbro, A.; Casalis, L.; Vázquez, E.; et al. Graphene Oxide Nanosheets Reshape Synaptic Function in Cultured Brain Networks. ACS Nano 2016, 10, 4459–4471. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gong, Y.; Zhou, X.; Jin, H.; Yan, H.; Wang, S.; Liu, J. Facile synthesis of soybean phospholipid-encapsulated MoS2 nanosheets for efficient in vitro and in vivo photothermal regression of breast tumor. Int. J. Nanomed. 2016, 11, 1819–1833. [Google Scholar] [CrossRef]
- El-Far, M.; Salah, N.; Essam, A.; Abd El-Azim, A.; Karam, M.; El-Sherbiny, I.M. Potential anticancer activity and mechanism of action of nanoformulated curcumin in experimental Ehrlich ascites carcinoma-bearing animals. Nanomedicine 2019, 14, 553–573. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zang, Z.; Chen, Z.; Cui, L.; Chang, Z.; Ma, A.; Yin, T.; Liang, R.; Han, Y.; Wu, Z.; et al. Nanophotosensitizer-engineered Salmonella bacteria with hypoxia targeting and photothermal-assisted mutual bioaccumulation for solid tumor therapy. Biomaterials 2019, 214, 119226. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Park, J.W.; Cha, H.R.; Jung, S.Y.; Lee, J.E.; Jung, S.S.; Kim, J.O.; Kim, S.Y.; Lee, C.S.; Park, H.S. Silver nanoparticles modify VEGF signaling pathway and mucus hypersecretion in allergic airway inflammation. Int. J. Nanomed. 2012, 7, 1329–1343. [Google Scholar] [CrossRef]
- Tong, F.; Dong, B.; Chai, R.; Tong, K.; Wang, Y.; Chen, S.; Zhou, X.; Liu, D. Simvastatin nanoparticles attenuated intestinal ischemia/reperfusion injury by downregulating BMP4/COX-2 pathway in rats. Int. J. Nanomed. 2017, 12, 2477–2488. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Sheikh, B.Y.; Taha, M.M.; How, C.W.; Abdullah, R.; Yagoub, U.; El-Sunousi, R.; Eid, E.E. Thymoquinone-loaded nanostructured lipid carriers: Preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration. Int. J. Nanomed. 2013, 8, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Wang, Q.; Li, J.; Hu, M.; Shi, S.J.; Li, Z.W.; Wu, G.L.; Cui, H.H.; Li, Y.Y.; Zhang, Q.; et al. Silver nanoparticles/chitosan oligosaccharide/poly(vinyl alcohol) nanofiber promotes wound healing by activating TGFbeta1/Smad signaling pathway. Int. J. Nanomed. 2016, 11, 373–386. [Google Scholar] [CrossRef]
- Plencner, M.; East, B.; Tonar, Z.; Otáhal, M.; Prosecká, E.; Rampichová, M.; Krejčí, T.; Litvinec, A.; Buzgo, M.; Míčková, A.; et al. Abdominal closure reinforcement by using polypropylene mesh functionalized with poly-epsilon-caprolactone nanofibers and growth factors for prevention of incisional hernia formation. Int. J. Nanomed. 2014, 9, 3263–3277. [Google Scholar] [CrossRef] [PubMed]
- Harms, C.; Helms, K.; Taschner, T.; Stratos, I.; Ignatius, A.; Gerber, T.; Lenz, S.; Rammelt, S.; Vollmar, B.; Mittlmeier, T. Osteogenic capacity of nanocrystalline bone cement in a weight-bearing defect at the ovine tibial metaphysis. Int. J. Nanomed. 2012, 7, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Fahmy, T.M.; Metcalfe, S.M.; Morton, S.L.; Dong, X.; Inverardi, L.; Adams, D.B.; Gao, W.; Wang, H. Immuno-isolation of pancreatic islet allografts using pegylated nanotherapy leads to long-term normoglycemia in full MHC mismatch recipient mice. PLoS ONE 2012, 7, e50265. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mundra, V.; Mahato, R.I. Nanomedicines of Hedgehog inhibitor and PPAR-gamma agonist for treating liver fibrosis. Pharm. Res. 2014, 31, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Coluccia, D.; Figueiredo, C.A.; Wu, M.Y.; Riemenschneider, A.N.; Diaz, R.; Luck, A.; Smith, C.; Das, S.; Ackerley, C.; O’Reilly, M.; et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomedicine 2018, 14, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cui, W.; Li, B.; Hong, Z. Targeted therapy for glioma using cyclic RGD-entrapped polyionic complex nanomicelles. Int. J. Nanomed. 2012, 7, 2853–2862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, X.; Men, K.; Wang, B.; Zhang, S.; Qiu, J.; Huang, M.; Gou, M.; Huang, N.; Qian, Z.; et al. Gene therapy for C-26 colon cancer using heparin-polyethyleneimine nanoparticle-mediated survivin T34A. Int. J. Nanomed. 2011, 6, 2419–2427. [Google Scholar] [CrossRef]
- Fan, R.; Tong, A.; Li, X.; Gao, X.; Mei, L.; Zhou, L.; Zhang, X.; You, C.; Guo, G. Enhanced antitumor effects by docetaxel/LL37-loaded thermosensitive hydrogel nanoparticles in peritoneal carcinomatosis of colorectal cancer. Int. J. Nanomed. 2015, 10, 7291–7305. [Google Scholar] [CrossRef]
- Xu, B.; Xia, S.; Wang, F.; Jin, Q.; Yu, T.; He, L.; Chen, Y.; Liu, Y.; Li, S.; Tan, X.; et al. Polymeric Nanomedicine for Combined Gene/Chemotherapy Elicits Enhanced Tumor Suppression. Mol. Pharm. 2016, 13, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zheng, Y.; Wang, R.; Huang, W.; Liu, L.; Hu, X.; Liu, S.; Yue, J.; Tong, T.; Jing, X. Antitumor activity of folate-targeted, paclitaxel-loaded polymeric micelles on a human esophageal EC9706 cancer cell line. Int. J. Nanomed. 2012, 7, 3487–3502. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, W.; Lian, G.; Qian, C.; Wang, L.; Zeng, L.; Liao, C.; Liang, B.; Huang, B.; Huang, K.; et al. Development of an MRI-visible nonviral vector for siRNA delivery targeting gastric cancer. Int. J. Nanomed. 2012, 7, 359–368. [Google Scholar] [CrossRef]
- Tang, B.; Tang, F.; Wang, Z.; Qi, G.; Liang, X.; Li, B.; Yuan, S.; Liu, J.; Yu, S.; He, S. Upregulation of Akt/NF-kappaB-regulated inflammation and Akt/Bad-related apoptosis signaling pathway involved in hepatic carcinoma process: Suppression by carnosic acid nanoparticle. Int. J. Nanomed. 2016, 11, 6401–6420. [Google Scholar] [CrossRef]
- Revuri, V.; Cherukula, K.; Nafiujjaman, M.; Vijayan, V.; Jeong, Y.Y.; Park, I.K.; Lee, Y.K. In Situ Oxygenic Nanopods Targeting Tumor Adaption to Hypoxia Potentiate Image-Guided Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11, 19782–19792. [Google Scholar] [CrossRef] [PubMed]
- Qiao, C.; Zhang, K.; Jin, H.; Miao, L.; Shi, C.; Liu, X.; Yuan, A.; Liu, J.; Li, D.; Zheng, C.; et al. Using poly(lactic-co-glycolic acid) microspheres to encapsulate plasmid of bone morphogenetic protein 2/polyethylenimine nanoparticles to promote bone formation in vitro and in vivo. Int. J. Nanomed. 2013, 8, 2985–2995. [Google Scholar] [CrossRef]
- Tan, Q.; Tang, H.; Hu, J.; Hu, Y.; Zhou, X.; Tao, Y.; Wu, Z. Controlled release of chitosan/heparin nanoparticle-delivered VEGF enhances regeneration of decellularized tissue-engineered scaffolds. Int. J. Nanomed. 2011, 6, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, N.; Yamamoto-Fukuda, T.; Takahashi, H.; Koji, T. In situ tissue engineering with synthetic self-assembling peptide nanofiber scaffolds, PuraMatrix, for mucosal regeneration in the rat middle-ear. Int. J. Nanomed. 2013, 8, 2629–2640. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Liu, R.; Qiao, C.; Lu, Z.; Shi, Y.; Fan, Z.; Zhang, Z.; Zhang, X. Dual-targeting Theranostic System with Mimicking Apoptosis to Promote Myocardial Infarction Repair via Modulation of Macrophages. Theranostics 2017, 7, 4149–4167. [Google Scholar] [CrossRef]
- Guo, J.; Su, H.; Zeng, Y.; Liang, Y.X.; Wong, W.M.; Ellis-Behnke, R.G.; So, K.F.; Wu, W. Reknitting the injured spinal cord by self-assembling peptide nanofiber scaffold. Nanomedicine 2007, 3, 311–321. [Google Scholar] [CrossRef]
- Guo, J.; Leung, K.K.; Su, H.; Yuan, Q.; Wang, L.; Chu, T.H.; Zhang, W.; Pu, J.K.; Ng, G.K.; Wong, W.M.; et al. Self-assembling peptide nanofiber scaffold promotes the reconstruction of acutely injured brain. Nanomedicine 2009, 5, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Liaw, K.; Sharma, R.; Zhang, Z.; Kannan, S.; Kannan, R.M. Targeting Mitochondrial Dysfunction and Oxidative Stress in Activated Microglia using Dendrimer-Based Therapeutics. Theranostics 2018, 8, 5529–5547. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Niu, D.; Zhang, J.; Zhang, W.; Yao, Y.; Li, P.; Gong, J. Amphiphilic core-shell nanoparticles containing dense polyethyleneimine shells for efficient delivery of microRNA to Kupffer cells. Int. J. Nanomed. 2016, 11, 2785–2797. [Google Scholar] [CrossRef]
- Southworth, R.; Kaneda, M.; Chen, J.; Zhang, L.; Zhang, H.; Yang, X.; Razavi, R.; Lanza, G.; Wickline, S.A. Renal vascular inflammation induced by Western diet in ApoE-null mice quantified by (19)F NMR of VCAM-1 targeted nanobeacons. Nanomedicine 2009, 5, 359–367. [Google Scholar] [CrossRef]
- He, Y.; Xu, H.; Chen, C.; Peng, J.; Tang, H.; Zhang, Z.; Li, Y.; Pang, D. In situ spectral imaging of marker proteins in gastric cancer with near-infrared and visible quantum dots probes. Talanta 2011, 85, 136–141. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, F.J.; Tang, H.; Zhao, C.; Cao, Y.A.; Lv, X.Q.; Chen, D.; Li, Y.D. In-vivo imaging of oral squamous cell carcinoma by EGFR monoclonal antibody conjugated near-infrared quantum dots in mice. Int. J. Nanomed. 2011, 6, 1739–1745. [Google Scholar] [CrossRef]
- Yang, X.Q.; Chen, C.; Peng, C.W.; Hou, J.X.; Liu, S.P.; Qi, C.B.; Gong, Y.P.; Zhu, X.B.; Pang, D.W.; Li, Y. Quantum dot-based quantitative immunofluorescence detection and spectrum analysis of epidermal growth factor receptor in breast cancer tissue arrays. Int. J. Nanomed. 2011, 6, 2265–2273. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Chen, G.; He, C.; Zhu, W.; Feng, S.; Xi, G.; Chen, R.; Lan, F.; Zeng, H. Immunoassay for LMP1 in nasopharyngeal tissue based on surface-enhanced Raman scattering. Int. J. Nanomed. 2012, 7, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Mane, V.; Muro, S. Biodistribution and endocytosis of ICAM-1-targeting antibodies versus nanocarriers in the gastrointestinal tract in mice. Int. J. Nanomed. 2012, 7, 4223–4237. [Google Scholar] [CrossRef]
- Shen, M.; Gong, F.; Pang, P.; Zhu, K.; Meng, X.; Wu, C.; Wang, J.; Shan, H.; Shuai, X. An MRI-visible non-viral vector for targeted Bcl-2 siRNA delivery to neuroblastoma. Int. J. Nanomed. 2012, 7, 3319–3332. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Du, Z.; Wu, M.; Zhang, G. Detection of micrometastases in lung cancer with magnetic nanoparticles and quantum dots. Int. J. Nanomed. 2012, 7, 2315–2324. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Johnston, A.H.; Newman, T.A.; Pyykkö, I.; Zou, J. Targeted delivery of Tet1 peptide functionalized polymersomes to the rat cochlear nerve. Int. J. Nanomed. 2012, 7, 1015–1022. [Google Scholar] [CrossRef]
- Andrade, C.G.; Cabral Filho, P.E.; Tenório, D.P.; Santos, B.S.; Beltrão, E.I.; Fontes, A.; Carvalho, L.B., Jr. Evaluation of glycophenotype in breast cancer by quantum dot-lectin histochemistry. Int. J. Nanomed. 2013, 8, 4623–4629. [Google Scholar] [CrossRef]
- Qu, Y.G.; Zhang, Q.; Pan, Q.; Zhao, X.D.; Huang, Y.H.; Chen, F.C.; Chen, H.L. Quantum dots immunofluorescence histochemical detection of EGFR gene mutations in the non-small cell lung cancers using mutation-specific antibodies. Int. J. Nanomed. 2014, 9, 5771–5778. [Google Scholar] [CrossRef]
- Sun, J.Z.; Chen, C.; Jiang, G.; Tian, W.Q.; Li, Y.; Sun, S.R. Quantum dot-based immunofluorescent imaging of Ki67 and identification of prognostic value in HER2-positive (non-luminal) breast cancer. Int. J. Nanomed. 2014, 9, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hu, R.; Lian, H.; Liu, Y.; Liu, J.; Liu, J.; Lin, G.; Liu, L.; Duan, X.; Yong, K.T.; et al. Dual-color immunofluorescent labeling with quantum dots of the diabetes-associated proteins aldose reductase and Toll-like receptor 4 in the kidneys of diabetic rats. Int. J. Nanomed. 2015, 10, 3651–3662. [Google Scholar] [CrossRef] [PubMed]
- Mansur, H.S.; Mansur, A.A.; Soriano-Araújo, A.; Lobato, Z.I.; de Carvalho, S.M.; Leite Mde, F. Water-soluble nanoconjugates of quantum dot-chitosan-antibody for in vitro detection of cancer cells based on "enzyme-free" fluoroimmunoassay. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Roy, U.; Ding, H.; Pilakka-Kanthikeel, S.; Raymond, A.D.; Atluri, V.; Yndart, A.; Kaftanovskaya, E.M.; Batrakova, E.; Agudelo, M.; Nair, M. Preparation and characterization of anti-HIV nanodrug targeted to microfold cell of gut-associated lymphoid tissue. Int. J. Nanomed. 2015, 10, 5819–5835. [Google Scholar] [CrossRef]
- Zheng, H.; Li, X.; Chen, C.; Chen, J.; Sun, J.; Sun, S.; Jin, L.; Li, J.; Sun, S.; Wu, X. Quantum dot-based immunofluorescent imaging and quantitative detection of TOP2A and prognostic value in triple-negative breast cancer. Int. J. Nanomed. 2016, 11, 5519–5529. [Google Scholar] [CrossRef]
- Peng, C.; Liu, J.; Yang, G.; Li, Y. Lysyl oxidase activates cancer stromal cells and promotes gastric cancer progression: Quantum dot-based identification of biomarkers in cancer stromal cells. Int. J. Nanomed. 2017, 13, 161–174. [Google Scholar] [CrossRef]
- Woiski, T.D.; de Castro Poncio, L.; de Moura, J.; Orsato, A.; Bezerra, A.G., Jr.; Minozzo, J.C.; de Figueiredo, B.C. Anti-hMC2RL1 Functionalized Gold Nanoparticles for Adrenocortical Tumor Cells Targeting and Imaging. J. Biomed. Nanotechnol. 2017, 13, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Hu, D.; Wang, C.; Chen, S.; Zhao, Z.; Xu, X.; Yao, Y.; Liu, T. A surface-enhanced Raman scattering-based probe method for detecting chromogranin A in adrenal tumors. Nanomedicine 2020, 15, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Mocan, L.; Tabaran, F.A.; Mocan, T.; Bele, C.; Orza, A.I.; Lucan, C.; Stiufiuc, R.; Manaila, I.; Iulia, F.; Dana, I.; et al. Selective ex-vivo photothermal ablation of human pancreatic cancer with albumin functionalized multiwalled carbon nanotubes. Int. J. Nanomed. 2011, 6, 915–928. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, Y.; Xu, K.; Ren, K.; Sun, W. Mouse lymphatic endothelial cell targeted probes: Anti-LYVE-1 antibody-based magnetic nanoparticles. Int. J. Nanomed. 2013, 8, 2273–2284. [Google Scholar] [CrossRef] [PubMed]
- Baiu, D.C.; Artz, N.S.; McElreath, M.R.; Menapace, B.D.; Hernando, D.; Reeder, S.B.; Grüttner, C.; Otto, M. High specificity targeting and detection of human neuroblastoma using multifunctional anti-GD2 iron-oxide nanoparticles. Nanomedicine 2015, 10, 2973–2988. [Google Scholar] [CrossRef] [PubMed]
- Appelbe, O.K.; Zhang, Q.; Pelizzari, C.A.; Weichselbaum, R.R.; Kron, S.J. Image-Guided Radiotherapy Targets Macromolecules through Altering the Tumor Microenvironment. Mol. Pharm. 2016, 13, 3457–3467. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Tao, W.; Ling, X.; Wang, J.; Xiao, Y.; Shi, S.; Ji, X.; Shajii, A.; Gan, S.T.; Kim, N.Y.; et al. Synthetic mRNA nanoparticle-mediated restoration of p53 tumor suppressor sensitizes p53-deficient cancers to mTOR inhibition. Sci. Transl. Med. 2019, 11, eaaw1565. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhu, P.; Yu, L.; Yang, L.; Chen, Y. Ultrasound/Acidity-Triggered and Nanoparticle-Enabled Analgesia. Adv. Healthc. Mater. 2019, 8, e1801350. [Google Scholar] [CrossRef]
- Coleman, R. The impact of histochemistry—A historical perspective. Acta Histochem. 2000, 102, 5–14. [Google Scholar] [CrossRef]
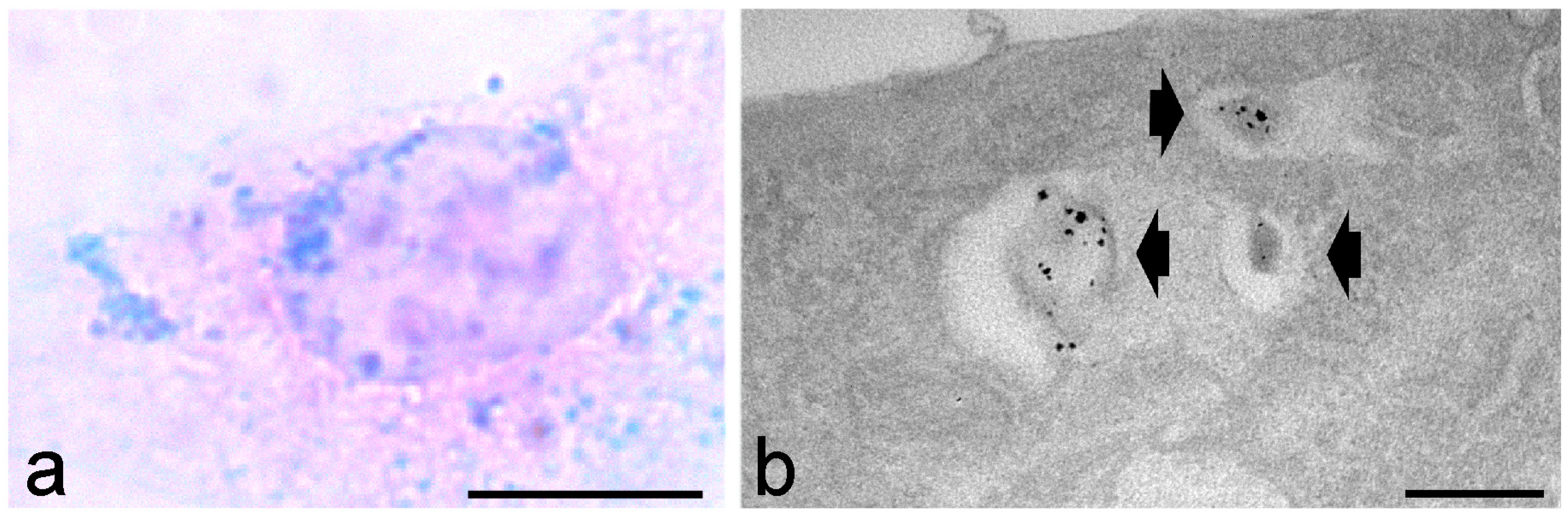
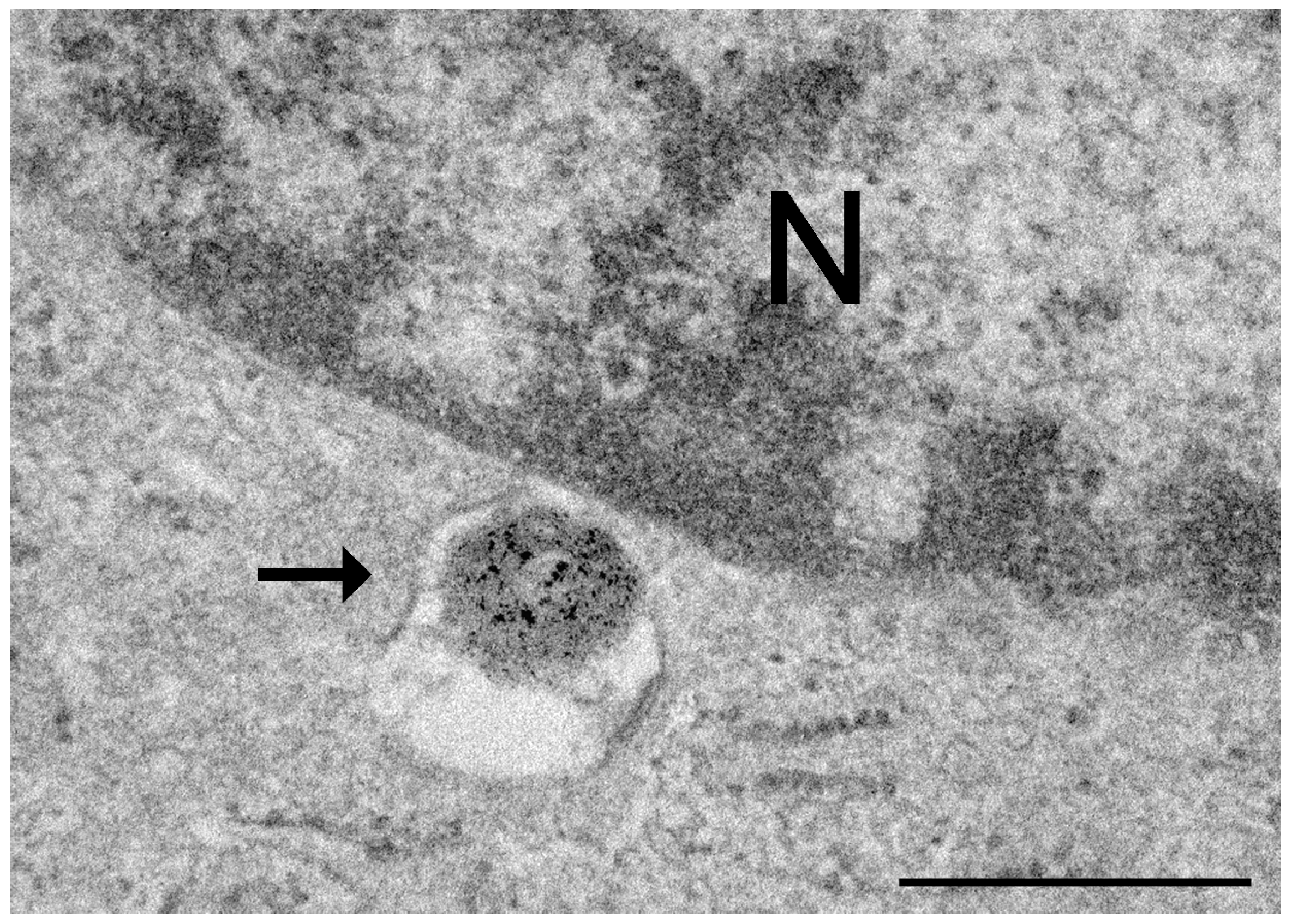
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malatesta, M. Histochemistry for Molecular Imaging in Nanomedicine. Int. J. Mol. Sci. 2024, 25, 8041. https://doi.org/10.3390/ijms25158041
Malatesta M. Histochemistry for Molecular Imaging in Nanomedicine. International Journal of Molecular Sciences. 2024; 25(15):8041. https://doi.org/10.3390/ijms25158041
Chicago/Turabian StyleMalatesta, Manuela. 2024. "Histochemistry for Molecular Imaging in Nanomedicine" International Journal of Molecular Sciences 25, no. 15: 8041. https://doi.org/10.3390/ijms25158041




