Antibiofilm and Antivirulence Potentials of 3,2′-Dihydroxyflavone against Staphylococcus aureus
Abstract
:1. Introduction
2. Results
2.1. Antimicrobial and Antibiofilm Activity of Various Flavonoids against S. aureus
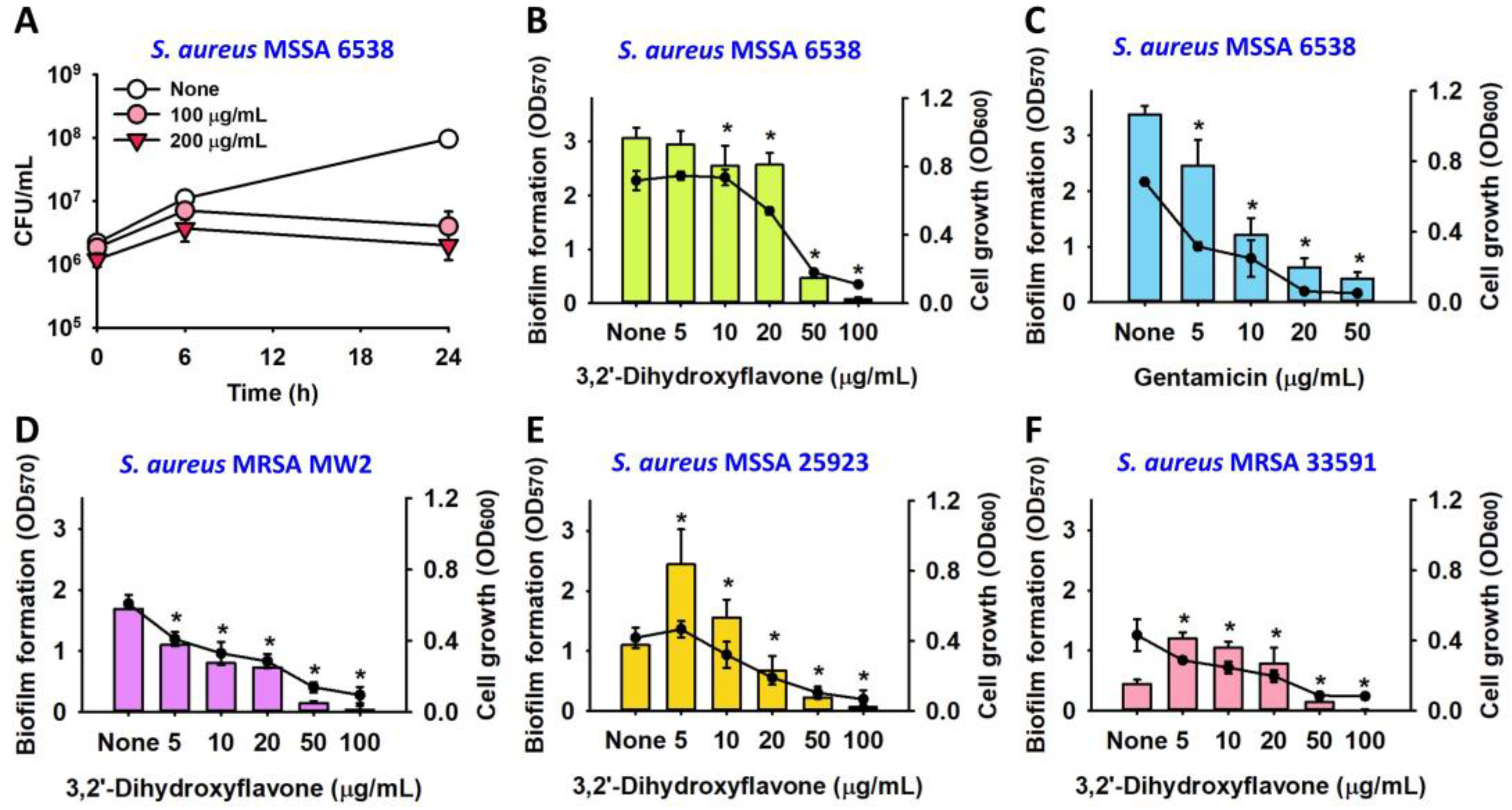
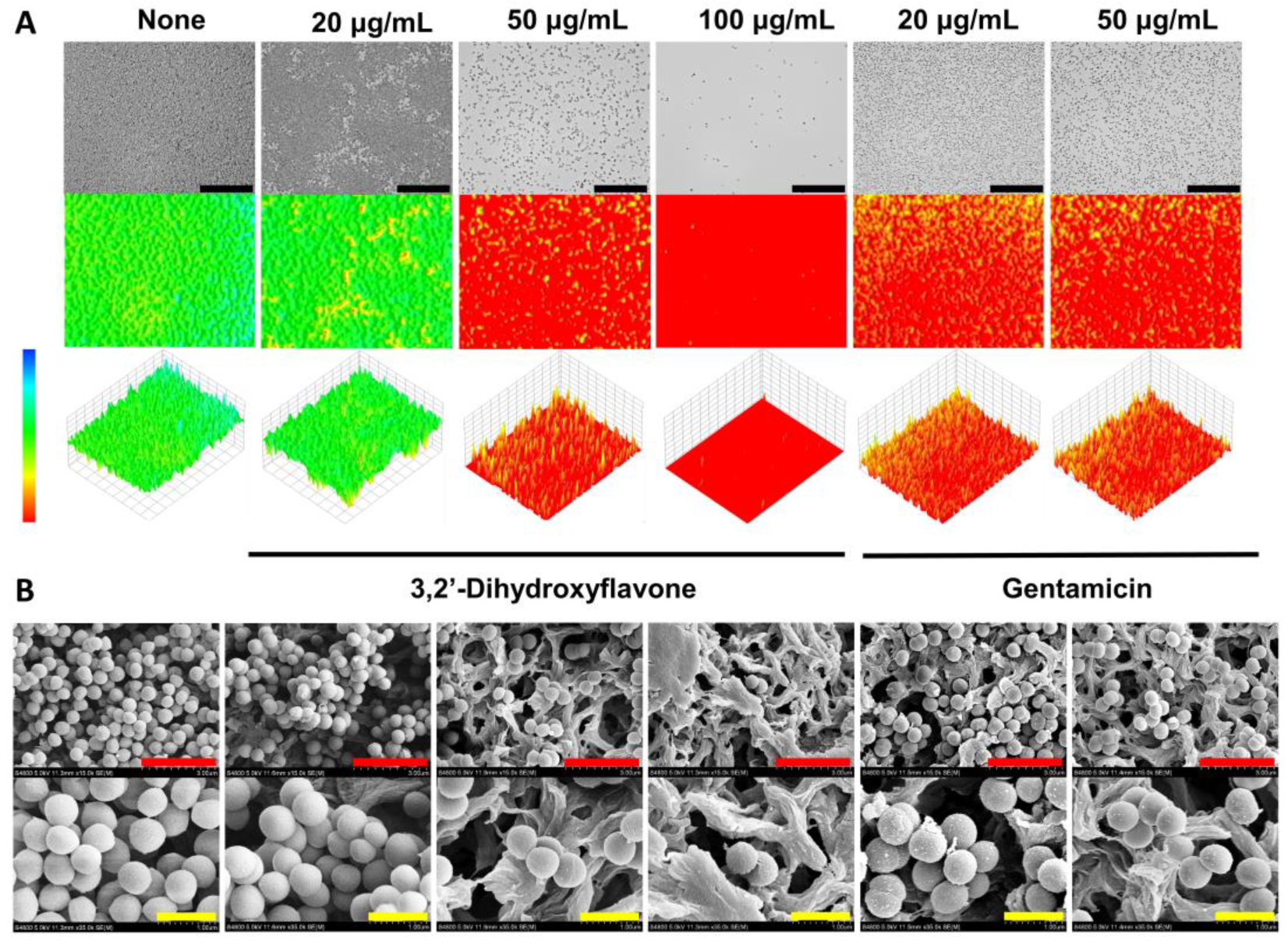
2.2. Observation of the Antibiofilm Effects of 3,2′-DHF
2.3. Antibiofilm Effect of 3,2′-DHF on Dual Biofilms of S. aureus and C. albicans
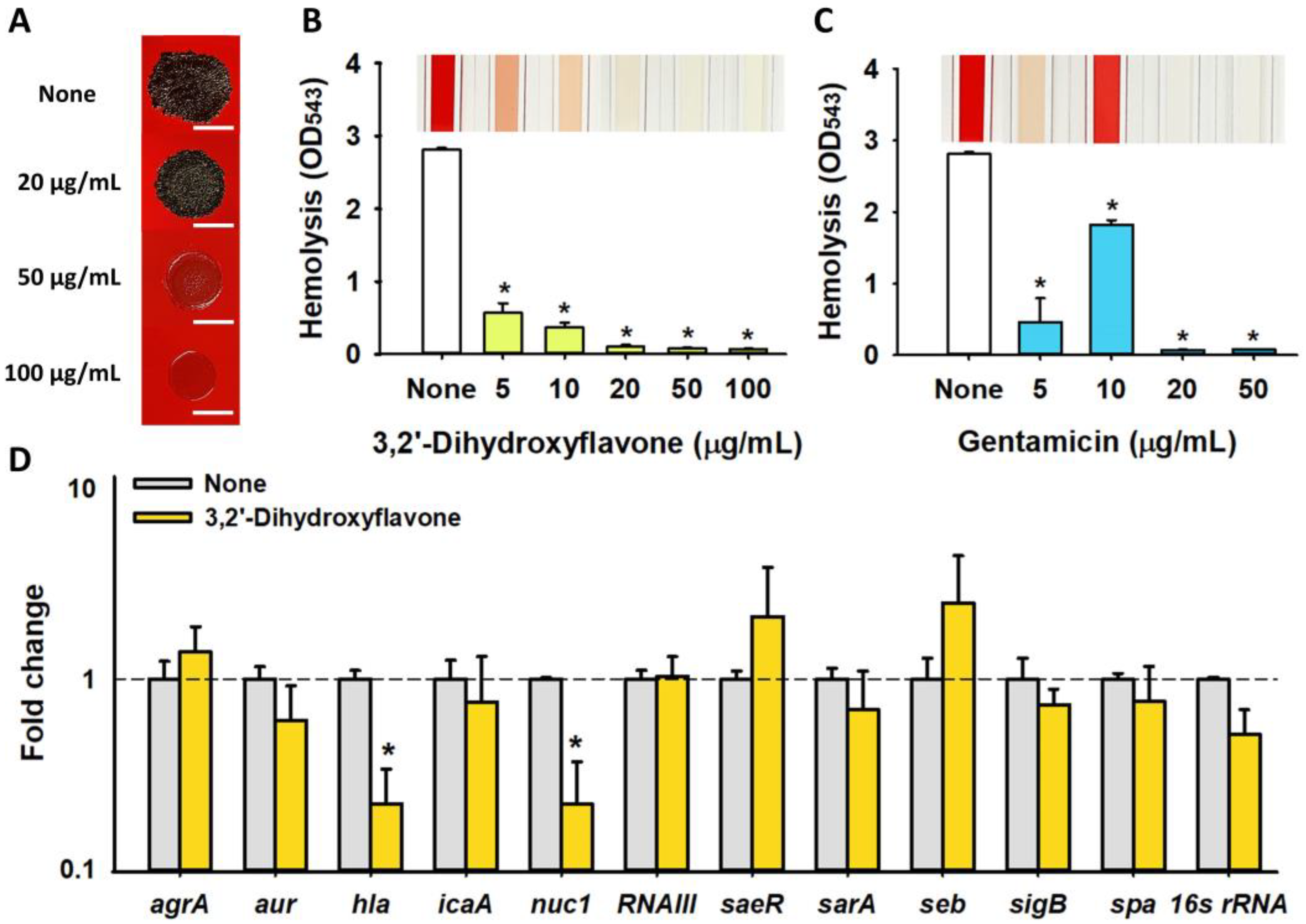
2.4. Effects of 3,2′-DHF on Slime Production and Hemolytic Activity in S. aureus
2.5. Differential Gene Expression Induced by 3,2′-DHF in S. aureus
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Growth Conditions, and Chemicals
4.2. Microtiter Dish Biofilm Formation Assay
4.3. Time–Kill Kinetics Assay
4.4. Biofilm Visualization by Live Microscopy and SEM
4.5. Biofilm Assay of Dual Species of S. aureus and C. albicans
4.6. Slime Production Assay
4.7. Hemolytic Activity Assay
4.8. RNA Isolation and qRT-PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moormeier, D.E.; Bayles, K.W. Staphylococcus aureus biofilm: A complex developmental organism. Mol. Microbiol. 2017, 104, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-resistant Staphylococcus aureus (MRSA): Antibiotic-resistance and the biofilm phenotype. J. Med. Chem. Comm. 2019, 10, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Carevic, T.; Stojkovic, D.; Ivanov, M. Plant flavonoids as reservoirs of therapeutics against microbial virulence traits: A comprehensive review update. Curr. Pharm. Des. 2023, 29, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial flavonoids as a potential substitute for overcoming antimicrobial resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, F.; Scognamiglio, M.; Fiorentino, A.; Buommino, E.; D’Abrosca, B. Plant derived natural products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm activity and molecular mechanisms. Molecules 2020, 25, 5024. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Lee, J.-H.; Cho, M.H.; Lee, J. Red wines and flavonoids diminish Staphylococcus aureus virulence with anti-biofilm and anti-hemolytic activities. Biofouling 2015, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Manner, S.; Skogman, M.; Goeres, D.; Vuorela, P.; Fallarero, A. Systematic exploration of natural and synthetic flavonoids for the inhibition of Staphylococcus aureus biofilms. Int. J. Mol. Sci. 2013, 14, 19434–19451. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.A.A.; dos Santos Rodrigues, J.B.; Magnani, M.; de Souza, E.L.; de Siqueira-Júnior, J.P. Inhibitory effects of flavonoids on biofilm formation by Staphylococcus aureus that overexpresses efflux protein genes. Microb. Pathog. 2017, 107, 193–197. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, J.-H.; Cho, H.S.; Joo, S.W.; Cho, M.H.; Lee, J. Anti-biofilm activities of quercetin and tannic acid against Staphylococcus aureus. Biofouling 2013, 29, 491–499. [Google Scholar] [CrossRef]
- Faleye, O.S.; Lee, J.-H.; Lee, J. Selected flavonoids exhibit antibiofilm and antibacterial effects against Vibrio by disrupting membrane integrity, virulence and metabolic activities. Biofilm 2023, 6, 100165. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Teixeira, J.A.; Pereira, M.O.; Rocha, C.M.; Sousa, A.M. Evolving biofilm inhibition and eradication in clinical settings through plant-based antibiofilm agents. Phytomedicine 2023, 119, 154973. [Google Scholar] [CrossRef] [PubMed]
- Harriott, M.M.; Noverr, M.C. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob. Agents Chemother. 2009, 53, 3914–3922. [Google Scholar] [CrossRef]
- Podbielska, A.; Galkowska, H.; Stelmach, E.; Mlynarczyk, G.; Olszewski, W.L. Slime production by Staphylococcus aureus and Staphylococcus epidermidis strains isolated from patients with diabetic foot ulcers. Arch. Immunol. Ther. Exp. 2010, 58, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, P.; Lv, H.; Deng, X.; Wang, J. A natural dietary flavone myricetin as an α-hemolysin inhibitor for controlling Staphylococcus aureus infection. Front. Cell Infect. Microbiol. 2020, 10, 330. [Google Scholar] [CrossRef]
- Mohd Nasuha, N.A.; Choo, Y.-M. A new flavone from Malaysia Borneo Marsdenia tinctoria. Nat. Prod. Res. 2016, 30, 1532–1536. [Google Scholar] [CrossRef]
- Kim, S.; Shin, Y.; Choi, Y.; Lim, K.-M.; Jeong, Y.; Dayem, A.A.; Lee, Y.; An, J.; Song, K.; Jang, S.B. Improved wound healing and skin regeneration ability of 3,2′-Dihydroxyflavone-treated mesenchymal stem cell-derived extracellular vesicles. Int. J. Mol. Sci. 2023, 24, 6964. [Google Scholar] [CrossRef]
- Han, D.; Kim, H.J.; Choi, H.Y.; Kim, B.; Yang, G.; Han, J.; Dayem, A.A.; Lee, H.-R.; Kim, J.H.; Lee, K.-M. 3,2′-Dihydroxyflavone-treated pluripotent stem cells show enhanced proliferation, pluripotency marker expression, and neuroprotective properties. Cell Transplant. 2015, 24, 1511–1532. [Google Scholar] [CrossRef]
- Kim, K.; Abdal Dayem, A.; Gil, M.; Yang, G.-M.; Lee, S.B.; Kwon, O.-H.; Choi, S.; Kang, G.-H.; Lim, K.M.; Kim, D. 3,2′-Dihydroxyflavone improves the proliferation and survival of human pluripotent stem cells and their differentiation into hematopoietic progenitor cells. J. Clin. Med. 2020, 9, 669. [Google Scholar] [CrossRef]
- Guo, Y.; Ding, S.-J.; Ding, X.; Liu, Z.; Wang, J.-L.; Chen, Y.; Liu, P.-P.; Li, H.-X.; Zhou, G.-H.; Tang, C.-B. Effects of selected flavonoids on cell proliferation and differentiation of porcine muscle stem cells for cultured meat production. Food Res. Int. 2022, 160, 111459. [Google Scholar] [CrossRef]
- Cotelle, N.; Bernier, J.-L.; Catteau, J.-P.; Pommery, J.; Wallet, J.-C.; Gaydou, E.M. Medicine Antioxidant properties of hydroxy-flavones. Free Radic. Biol. Med. 1996, 20, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Caiazza, N.C.; O’Toole, G.A. Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J. Bacteriol. 2003, 185, 3214–3217. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Jiang, F.; Zhang, F.; Hamushan, M.; Du, J.; Mao, Y.; Wang, Q.; Han, P.; Tang, J.; Shen, H. Thermonucleases contribute to Staphylococcus aureus biofilm formation in implant-associated infections—A redundant and complementary story. Front. Microbiol. 2021, 12, 687888. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Horswill, A.R. Micrococcal nuclease regulates biofilm formation and dispersal in methicillin-resistant Staphylococcus aureus USA300. Msphere 2024, 9, e00126-24. [Google Scholar] [CrossRef] [PubMed]
- Matilla-Cuenca, L.; Gil, C.; Cuesta, S.; Rapún-Araiz, B.; Žiemytė, M.; Mira, A.; Lasa, I.; Valle, J. Antibiofilm activity of flavo-noids on staphylococcal biofilms through targeting BAP amyloids. Sci. Rep. 2020, 10, 18968. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.-H.; Wang, R.; Zhao, S.-Q.; Chen, B.-R.; Zeng, X.-A. Inhibition of biofilm formation of foodborne Staphylococcus aureus by the citrus flavonoid naringenin. Foods 2021, 10, 2614. [Google Scholar] [CrossRef] [PubMed]
- Veiko, A.G.; Olchowik-Grabarek, E.; Sekowski, S.; Roszkowska, A.; Lapshina, E.A.; Dobrzynska, I.; Zamaraeva, M.; Za-vodnik, I.B. Antimicrobial activity of quercetin, naringenin and catechin: Flavonoids inhibit Staphylococcus aureus-induced hemolysis and modify membranes of bacteria and erythrocytes. Molecules 2023, 28, 1252. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Kannappan, A.; Gowrishankar, S.; Srinivasan, R.; Pandian, S.K.; Ravi, A.V. Antibiofilm activity of Vetiveria zizanioides root extract against methicillin-resistant Staphylococcus aureus. Microb. Pathog. 2017, 110, 313–324. [Google Scholar] [CrossRef]
- Divyakolu, S.; Chikkala, R.; Ratnakar, K.S.; Sritharan, V. Hemolysins of Staphylococcus aureus—An update on their biology, role in pathogenesis and as targets for anti-virulence therapy. Adv. Infect. Dis. 2019, 9, 80–104. [Google Scholar] [CrossRef]
- Jeon, H.; Boya, B.R.; Kim, G.; Lee, J.-H.; Lee, J. Inhibitory effects of bromoindoles on Escherichia coli O157: H7 biofilms. Biotechnol. Bioproc. Eng. 2024, 29, 1–10. [Google Scholar] [CrossRef]
- Paramanya, S.; Lee, J.-H.; Lee, J. Antibiofilm activity of carotenoid crocetin against Staphylococcal strains. Front. Cell Infect. Microbiol. 2024, 14, 1404960. [Google Scholar] [CrossRef]
- Park, I.; Lee, J.-H.; Ma, J.Y.; Tan, Y.; Lee, J. Antivirulence activities of retinoic acids against Staphylococcus aureus. Front. Microbiol. 2023, 14, 1224085. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Lee, J.-H.; Park, S.; Kim, S.; Lee, J. Inhibition of polymicrobial biofilm formation by saw palmetto oil, lauric acid and myristic acid. Microb. Biotechnol. 2022, 15, 590–602. [Google Scholar] [CrossRef] [PubMed]
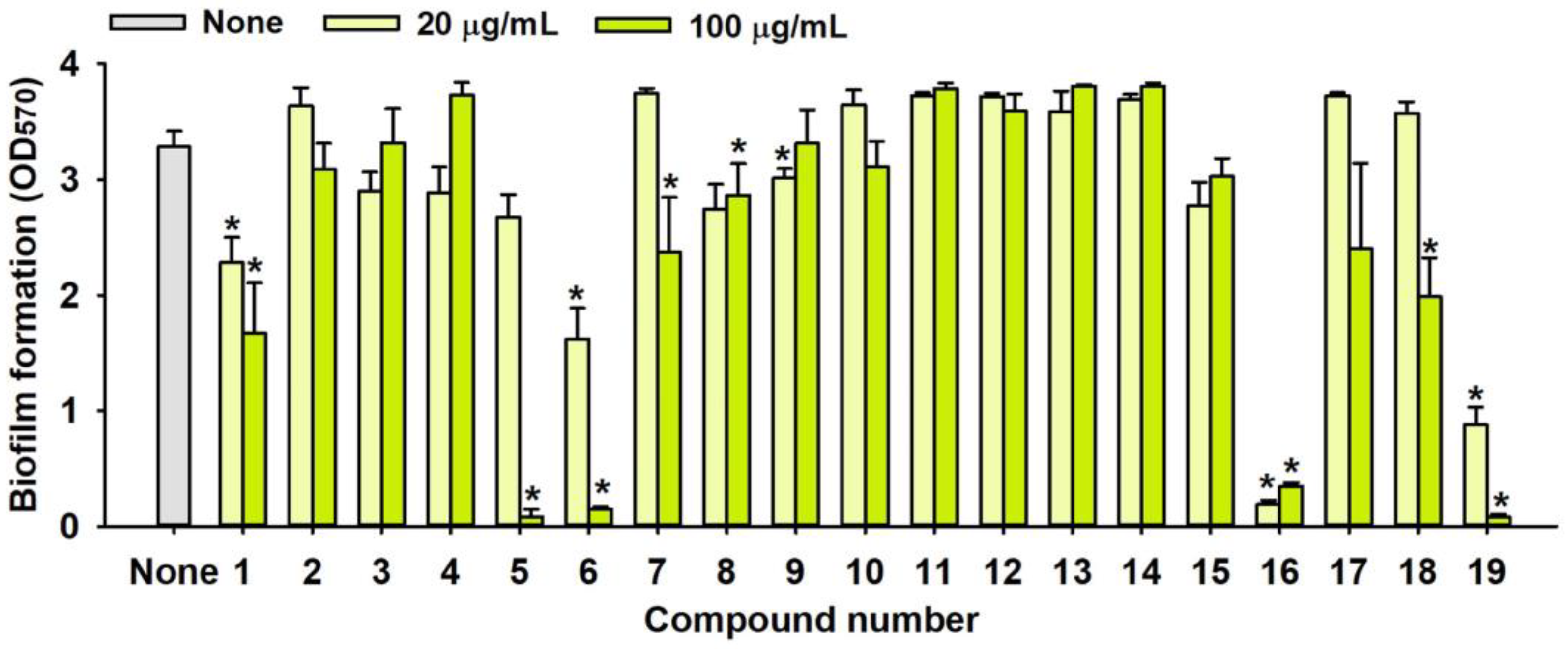

| No. | Material | Structure | MIC (μg/mL) | No. | Material | Structure | MIC (μg/mL) |
|---|---|---|---|---|---|---|---|
| 1 | Apigenin |  | >400 | 11 | Flavanone | 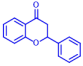 | >400 |
| 2 | 7-Hydroxyflavone | 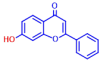 | >400 | 12 | 6-Hydroxyflavone |  | >400 |
| 3 | Epicatechin | 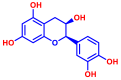 | >400 | 13 | 6-Aminoflavone | 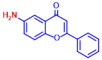 | >400 |
| 4 | Catechin | 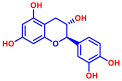 | >400 | 14 | Flavone | 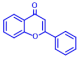 | >400 |
| 5 | 3,2’-Dihydroxyflavone | 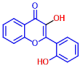 | 75 | 15 | Naringin |  | >400 |
| 6 | Curcumin | 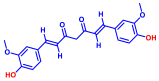 | 50 | 16 | Quercetin | 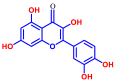 | >400 |
| 7 | 2,2’-Dihydroxy-4-methoxybenzophenone |  | 200 | 17 | Genistein | 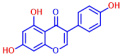 | >400 |
| 8 | 2,2’-Dihydroxy-4,4’-dimethoxybenzophenone | 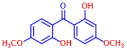 | >400 | 18 | Phloretin | 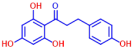 | >400 |
| 9 | Daidzein |  | >400 | 19 | Fisetin | 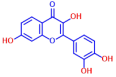 | 200 |
| 10 | Chrysin | 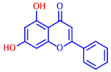 | >400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, I.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Antibiofilm and Antivirulence Potentials of 3,2′-Dihydroxyflavone against Staphylococcus aureus. Int. J. Mol. Sci. 2024, 25, 8059. https://doi.org/10.3390/ijms25158059
Park I, Kim Y-G, Lee J-H, Lee J. Antibiofilm and Antivirulence Potentials of 3,2′-Dihydroxyflavone against Staphylococcus aureus. International Journal of Molecular Sciences. 2024; 25(15):8059. https://doi.org/10.3390/ijms25158059
Chicago/Turabian StylePark, Inji, Yong-Guy Kim, Jin-Hyung Lee, and Jintae Lee. 2024. "Antibiofilm and Antivirulence Potentials of 3,2′-Dihydroxyflavone against Staphylococcus aureus" International Journal of Molecular Sciences 25, no. 15: 8059. https://doi.org/10.3390/ijms25158059





