Identification of the Key Gene DfCCoAOMT1 through Comparative Analysis of Lignification in Dendrocalamus farinosus XK4 and ZPX Bamboo Shoots during Cold Storage
Abstract
:1. Introduction
2. Results
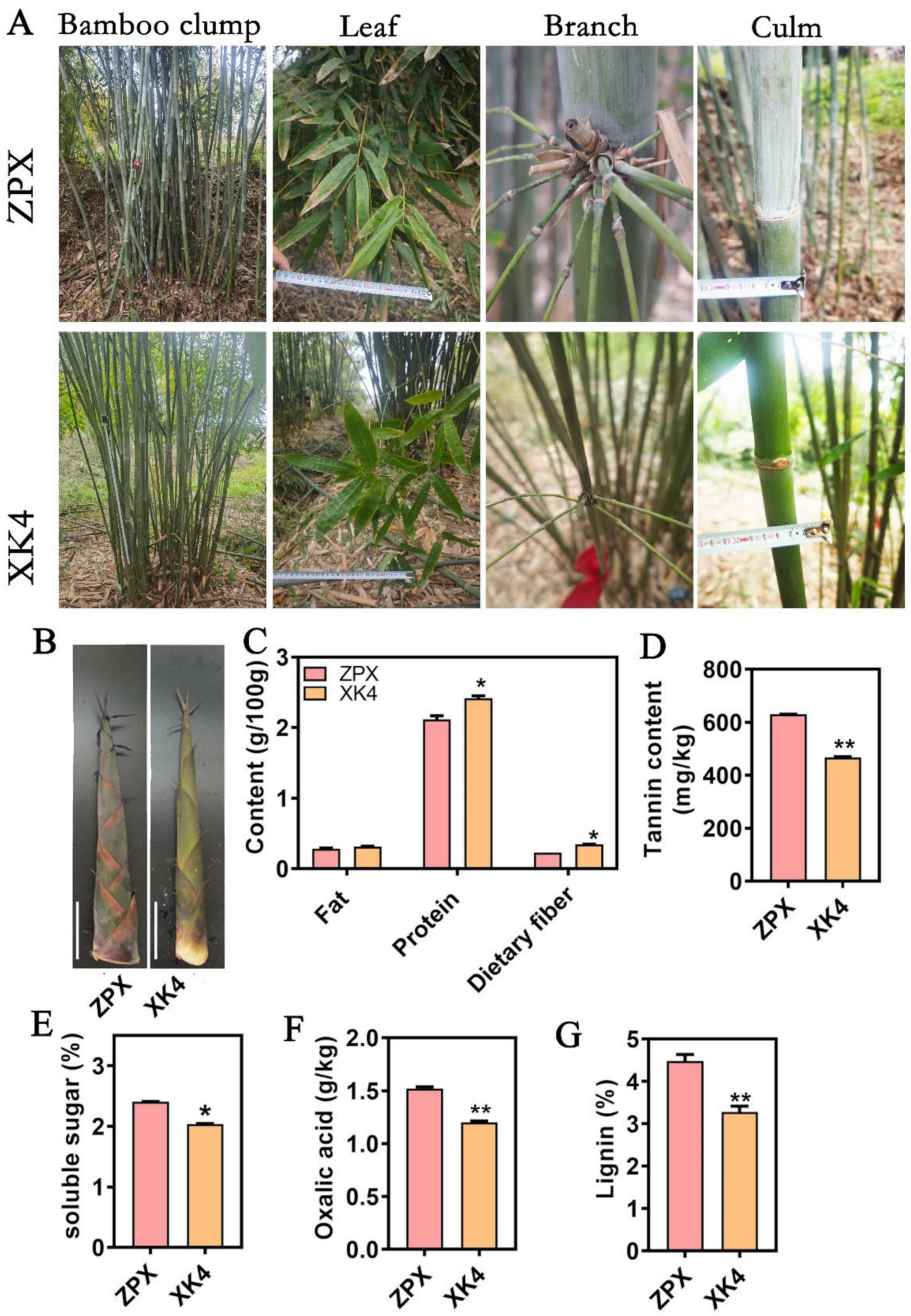
2.1. Analysis of the Main Nutritional Composition of ZPX and XK4 D. farinosus Bamboo Shoots
2.2. Changes in Lignin Content of Bamboo Shoots ZPX and XK4 under Cold Storage Conditions
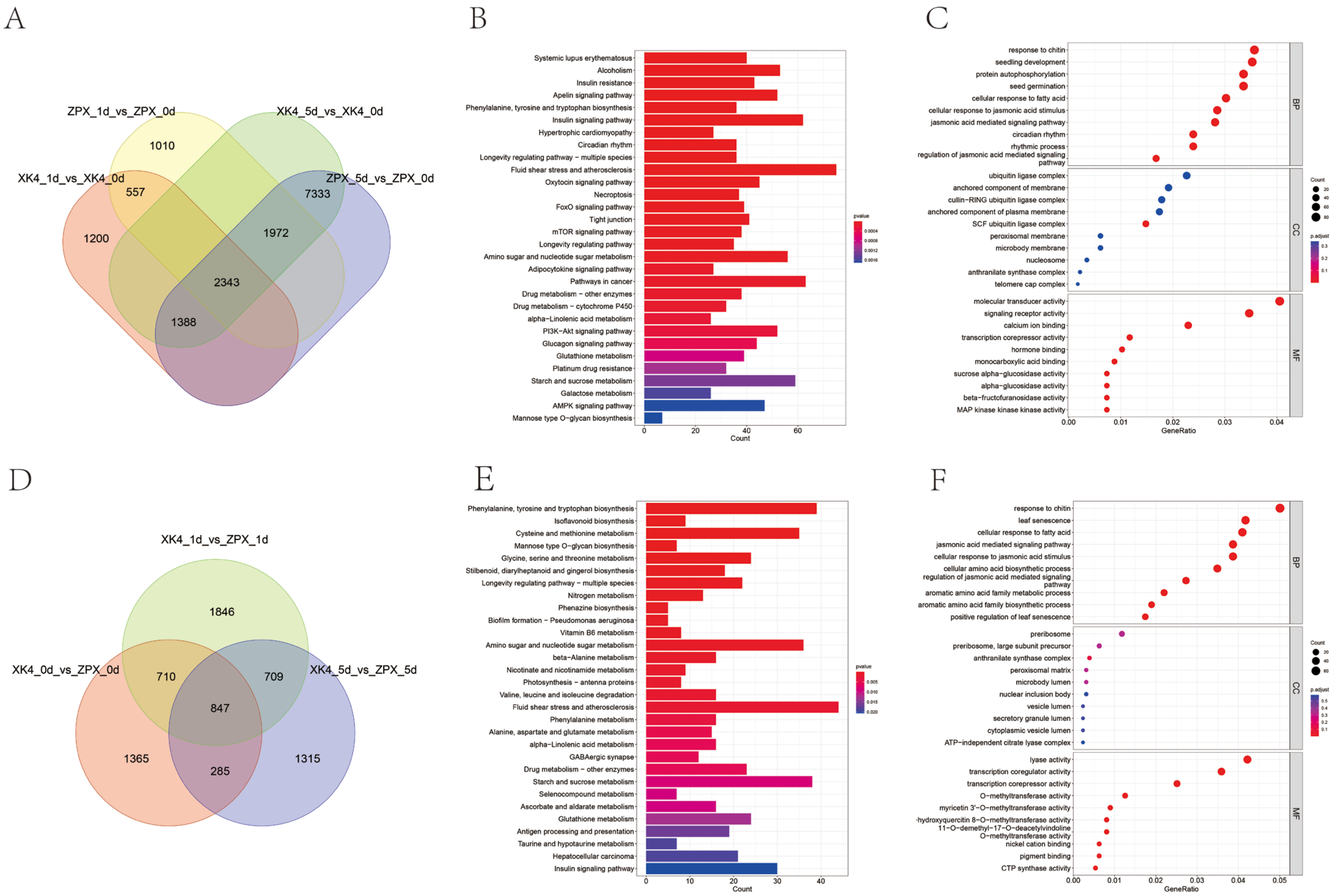
2.3. Transcriptome Sequencing Reveals Differential Gene Expression in Cold-Stored D. farinosus Bamboo Shoots ZPX and XK4
2.4. WGCNA Analysis of DEGs of ZPX and XK4 Bamboo Shoots after Cold Storage for 1d and 5d
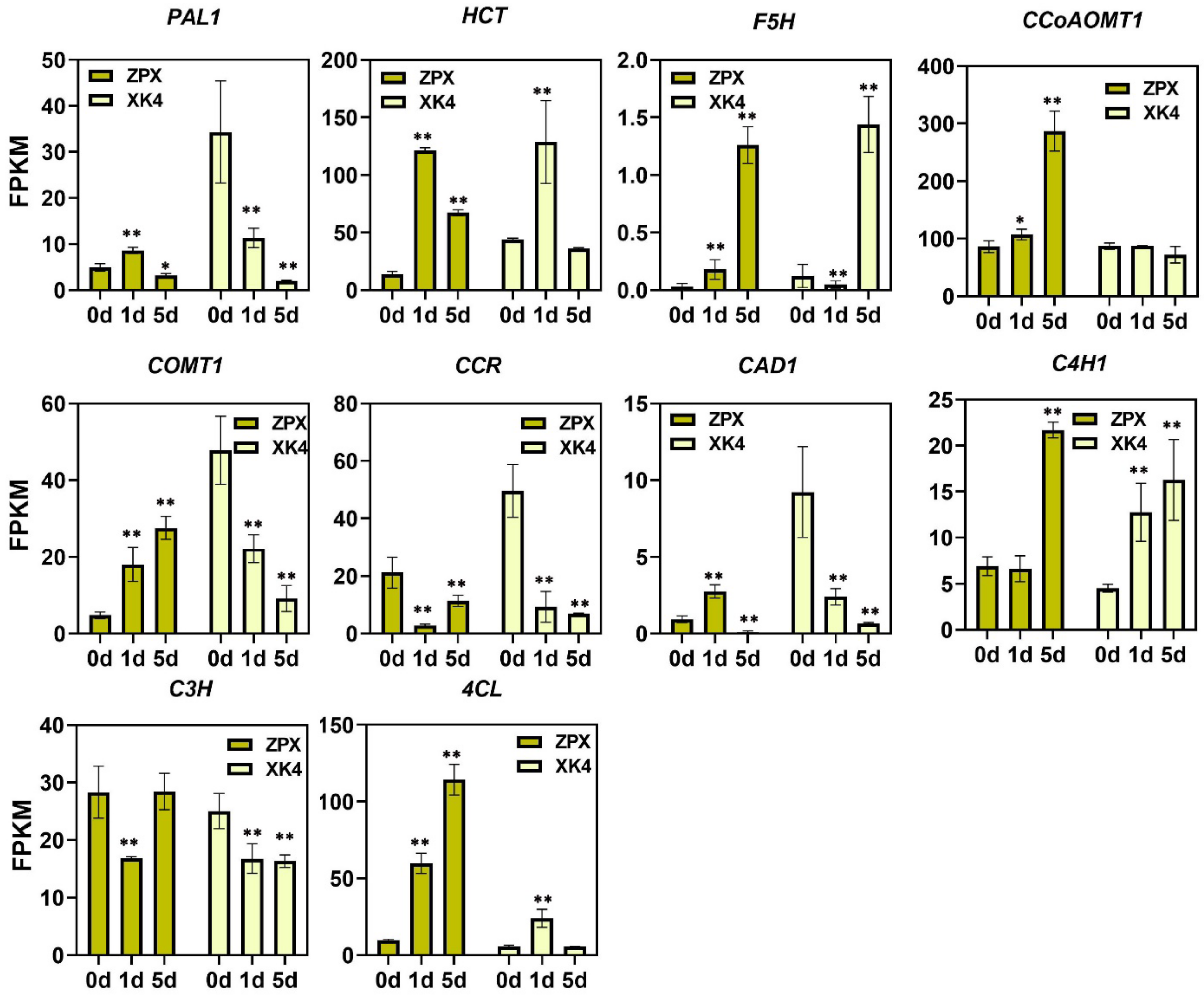
2.5. Expression Levels of Lignin Synthase Genes in Two Bamboo Shoots under Cold Storage Conditions
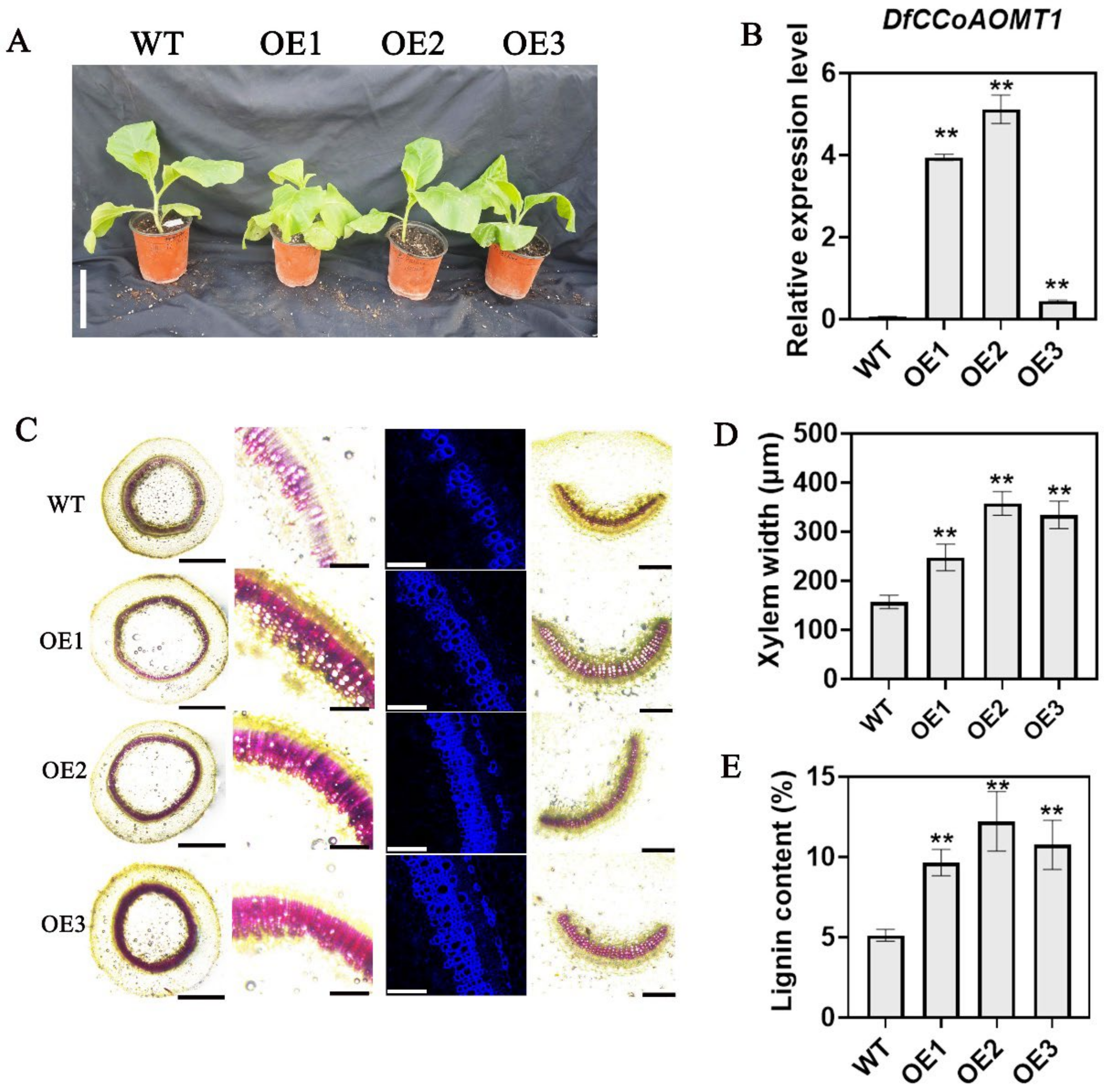
2.6. Heterologous Overexpression of DfCCoAOMT1 in Tobacco Significantly Enhances Lignin Synthesis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Cold Storage Treatments
4.2. RNA Extraction and RT-qPCR
4.3. RNA Sequencing
4.4. Weighted Correlation Network Analysis
4.5. Overexpression of DfCCoAOMT1 in Tobacco
4.6. Lignin Content Analysis
4.7. Histochemical Staining
4.8. Determination of Lignin Autofluorescence
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalita, T.; Dutta, U. A comparative study on indigenous usage of Bamboo shoot in the health care practices in NE India. Clarion 2012, 58, 6949–6954. [Google Scholar]
- Singhal, P.; Bal, L.M.; Satya, S.; Sudhakar, P.; Naik, S.N. Bamboo shoots: A novel source of nutrition and medicine. Crit. Rev. Food Sci. Nutr. 2013, 53, 517–534. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, H.; Liu, R.; Wu, W.; Mu, H.; Han, Y.; Yang, H.; Gao, H. Ameliorating effects of water bamboo shoot (Zizania latifolia) on acute alcoholism in a mice model and its chemical composition. Food Chem. 2022, 378, 132122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Wang, D.; Ye, F.; He, Y.; Hu, Z.; Zhao, G. A systematic review on the composition, storage, processing of bamboo shoots: Focusing the nutritional and functional benefits. J. Funct. Foods 2020, 71, 104015. [Google Scholar] [CrossRef]
- Hai-Hui, Q.; Zhi-Tao, L. The Research Progress of Bamboo Shoot Processing and Preservation. Acad. Period. Farm Prod. Process. 2013, 4, 56–59. [Google Scholar]
- Kleinhenz, V.; Gosbee, M.; Elsmore, S.; Lyall, T.W.; Blackburn, K.; Harrower, K.; Midmore, D.J. Storage methods for extending shelf life of fresh, edible bamboo shoots [Bambusa oldhamii (Munro)]. Postharvest Biol. Technol. 2000, 19, 253–264. [Google Scholar] [CrossRef]
- Luo, Z.; Yan, X.B. Accumulation of lignin and involvement of enzymes in bamboo shoot during storage. Eur. Food Res. Technol. 2008, 226, 635–640. [Google Scholar] [CrossRef]
- Li, D.; Limwachiranon, J.; Li, L.; Zhang, L.; Xu, Y.; Fu, M.; Luo, Z. Hydrogen peroxide accelerated the lignification process of bamboo shoots by activating the phenylpropanoid pathway and programmed cell death in postharvest storage. Postharvest Biol. Technol. 2019, 153, 79–86. [Google Scholar] [CrossRef]
- Barros, J.; Serk, H.; Granlund, I.; Pesquet, E. The cell biology of lignification in higher plants. Ann. Bot. 2015, 115, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, C.; Zhang, H.; Ying, Y.; Hu, Y.; Song, L. Comparative analysis of the lignification process of two bamboo shoots stored at room temperature. Plants 2020, 9, 1399. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Choi, H.; An, G. Roles of lignin biosynthesis and regulatory genes in plant development. J. Integr. Plant Biol. 2015, 57, 902–912. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and Biological Functions in Plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhao, X.; Gu, X.; Peng, J.; Song, W.; Deng, B.; Cao, Y.; Hu, S. Genome-Wide Identification and Expression Analysis of Dendrocalamus farinosus CCoAOMT Gene Family and the Role of DfCCoAOMT14 Involved in Lignin Synthesis. Int. J. Mol. Sci. 2023, 24, 8965. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.N.; Wu, G.B.; Zhang, S.; Kuang, F.Y.; Chen, F.H. The identification and functional verification of the cinnamate 4-hydroxylase gene from wax apple fruit and its role in lignin biosynthesis during nitric oxide-delayed postharvest cottony softening—ScienceDirect. Postharvest Biol. Technol. 2019, 158, 110964. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, F.; Xing, H.; Mao, K.; Chen, J. Correlation Analysis of Lignin Accumulation and Expression of Key Genes Involved in Lignin Biosynthesis of Ramie (Boehmeria nivea). Genes 2019, 10, 389. [Google Scholar] [CrossRef]
- Hu, S.L.; Zhou, J.Y.; Cao, Y.; Lu, X.Q.; Duan, N.; Ren, P.; Chen, K. In vitro callus induction and plant regeneration from mature seed embryo and young shoots in a giant sympodial bamboo, Dendrocalamus farinosus (Keng et Keng f.) Chia et H.L. Fung. Afr. J. Biotechnol. 2011, 10, 3210–3215. [Google Scholar]
- Xiao, L.; Jiang, X.; Deng, Y.; Xu, K.; Duan, X.; Wan, K.; Tang, X. Study on characteristics and lignification mechanism of postharvest banana fruit during chilling injury. Foods 2023, 12, 1097. [Google Scholar] [CrossRef]
- Li, C.; Suo, J.; Xuan, L.; Ding, M.; Zhang, H.; Song, L.; Ying, Y. Bamboo shoot-lignification delay by melatonin during low temperature storage. Postharvest Biol. Technol. 2019, 156, 110933. [Google Scholar] [CrossRef]
- Miao, M.; Wang, Q.; Zhang, T.; Jiang, B. Effect of high hydrostatic pressure (HHP) treatment on texture changes of water bamboo shoots cultivated in China. Postharvest Biol. Technol. 2011, 59, 327–329. [Google Scholar] [CrossRef]
- Li, D.; Qin, X.; Tian, P.; Wang, J. Toughening and its association with the postharvest quality of king oyster mushroom (Pleurotus eryngii) stored at low temperature. Food Chem. 2016, 196, 1092–1100. [Google Scholar] [CrossRef]
- Hou, D.; Lu, H.; Zhao, Z.; Pei, J.; Yang, H.; Wu, A.; Yu, X.; Lin, X. Integrative transcriptomic and metabolomic data provide insights into gene networks associated with lignification in postharvest Lei bamboo shoots under low temperature. Food Chem. 2022, 368, 130822. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Fang, X.; Han, Y.; Liu, R.; Chen, H.; Gao, H. Analysis of lignin metabolism in water bamboo shoots during storage. Postharvest Biol. Technol. 2022, 192, 111989. [Google Scholar] [CrossRef]
- Lwin, W.W.; Pongprasert, N.; Boonyaritthongchai, P.; Wongs-Aree, C.; Srilaong, V. Synergistic effect of vacuum packaging and cold shock reduce lignification of asparagus. J. Food Biochem. 2020, 44, e13479. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Chen, L.; Li, X.; Liu, K. Lignin accumulation and biosynthetic enzyme activities in relation to postharvest firmness of fresh waxy corn. J. Food Process. Preserv. 2018, 42, e13333. [Google Scholar] [CrossRef]
- Yang, B.; Han, Y.; Gao, H.; Liu, R.; Xu, F.; Liu, R.; Xiao, S.; Li, B.; Chen, H. Application of melatonin delays lignification in postharvest water bamboo shoots in association with energy metabolism. Postharvest Biol. Technol. 2023, 196, 112149. [Google Scholar] [CrossRef]
- Do, C.T.; Pollet, B.; Thevenin, J.; Sibout, R.; Denoue, D.; Barriere, Y.; Lapierre, C.; Jouanin, L. Both caffeoyl Coenzyme A 3-O-methyltransferase 1 and caffeic acid O-methyltransferase 1 are involved in redundant functions for lignin, flavonoids and sinapoyl malate biosynthesis in Arabidopsis. Planta 2007, 226, 1117–1129. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Cho, D.J.; Lee, H.; Nam, M.H.; Kwon, C.; Yun, H.S. CCoAOMT1, a candidate cargo secreted via VAMP721/722 secretory vesicles in Arabidopsis. Biochem. Biophys. Res. Commun. 2020, 524, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Lim, L.H.; Cheong, M.S.; Baek, D.; Park, M.S.; Cho, H.M.; Lee, S.H.; Jin, B.J.; No, D.H.; Cha, Y.J.; et al. Arabidopsis CCoAOMT1 Plays a Role in Drought Stress Response via ROS- and ABA-Dependent Manners. Plants 2021, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Chao, F.; Zhuo, Z.; Ma, Z.; Li, W.; Chen, G. Identification of hub genes in prostate cancer using robust rank aggregation and weighted gene co-expression network analysis. Aging 2019, 11, 4736–4756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, X.; Zhi, Y.; Li, X.; Zhang, Q.; Niu, J.; Wang, J.; Zhai, H.; Zhao, N.; Li, J.; et al. A non-tandem CCCH-type zinc-finger protein, IbC3H18, functions as a nuclear transcriptional activator and enhances abiotic stress tolerance in sweet potato. New Phytol. 2019, 223, 1918–1936. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, L.; Lei, J.; Chai, S.; Jin, X.; Zou, Y.; Sun, X.; Mei, Y.; Cheng, X.; Yang, X.; et al. IbMYB308, a Sweet Potato R2R3-MYB Gene, Improves Salt Stress Tolerance in Transgenic Tobacco. Genes 2022, 13, 1476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kim, J.I.; Pysh, L.; Chapple, C. Four Isoforms of Arabidopsis 4-Coumarate:CoA Ligase Have Overlapping yet Distinct Roles in Phenylpropanoid Metabolism. Plant Physiol. 2015, 169, 2409–2421. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Song, W.; Chen, S.; Xu, G.; Long, Z.; Yang, H.; Cao, Y.; Hu, S. Identification of the Key Gene DfCCoAOMT1 through Comparative Analysis of Lignification in Dendrocalamus farinosus XK4 and ZPX Bamboo Shoots during Cold Storage. Int. J. Mol. Sci. 2024, 25, 8065. https://doi.org/10.3390/ijms25158065
Zhao X, Song W, Chen S, Xu G, Long Z, Yang H, Cao Y, Hu S. Identification of the Key Gene DfCCoAOMT1 through Comparative Analysis of Lignification in Dendrocalamus farinosus XK4 and ZPX Bamboo Shoots during Cold Storage. International Journal of Molecular Sciences. 2024; 25(15):8065. https://doi.org/10.3390/ijms25158065
Chicago/Turabian StyleZhao, Xin, Wenjuan Song, Sen Chen, Gang Xu, Zhijian Long, Heyi Yang, Ying Cao, and Shanglian Hu. 2024. "Identification of the Key Gene DfCCoAOMT1 through Comparative Analysis of Lignification in Dendrocalamus farinosus XK4 and ZPX Bamboo Shoots during Cold Storage" International Journal of Molecular Sciences 25, no. 15: 8065. https://doi.org/10.3390/ijms25158065




