Single-Ion Magnetism of the [DyIII(hfac)4]− Anions in the Crystalline Semiconductor {TSeT1.5}●+[DyIII(hfac)4]− Containing Weakly Dimerized Stacks of Tetraselenatetracene
Abstract
:1. Introduction
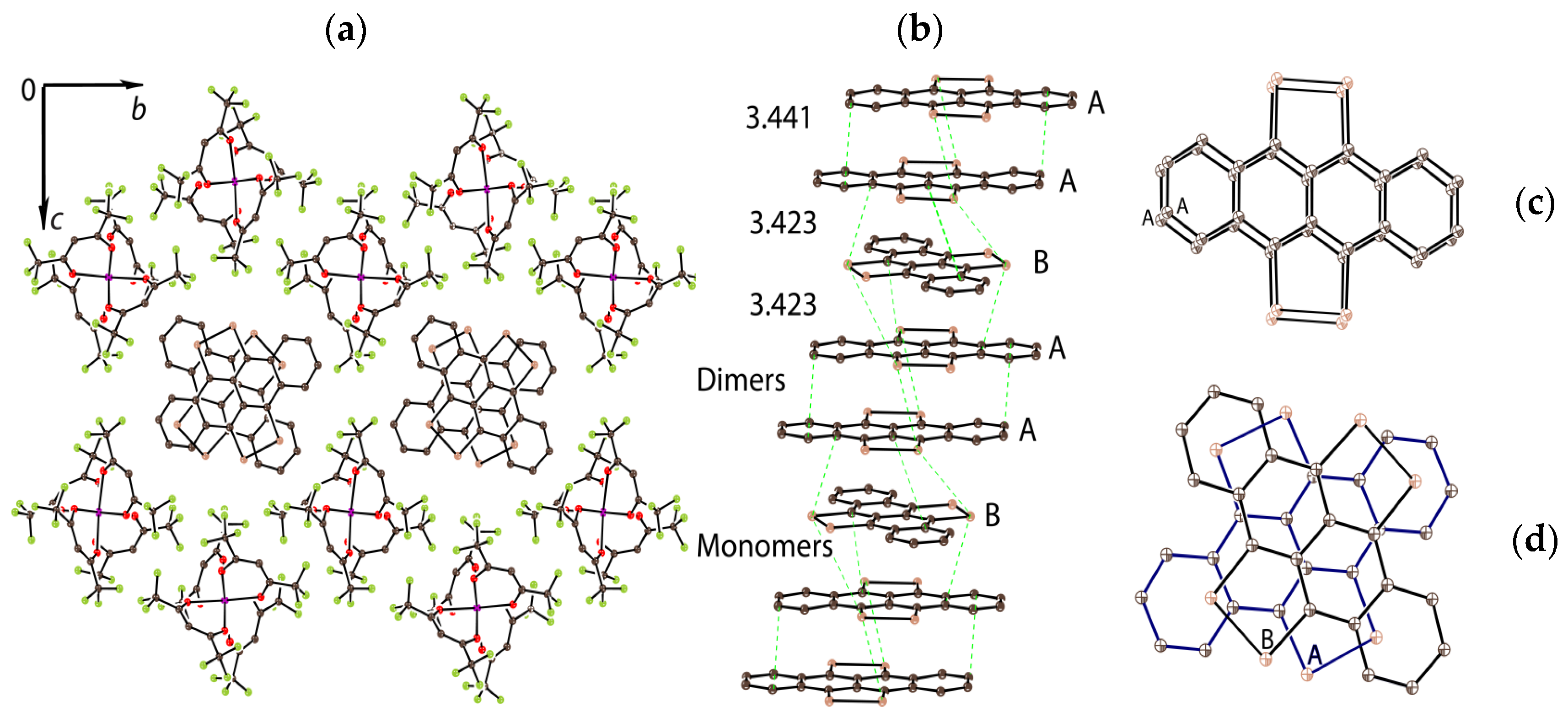
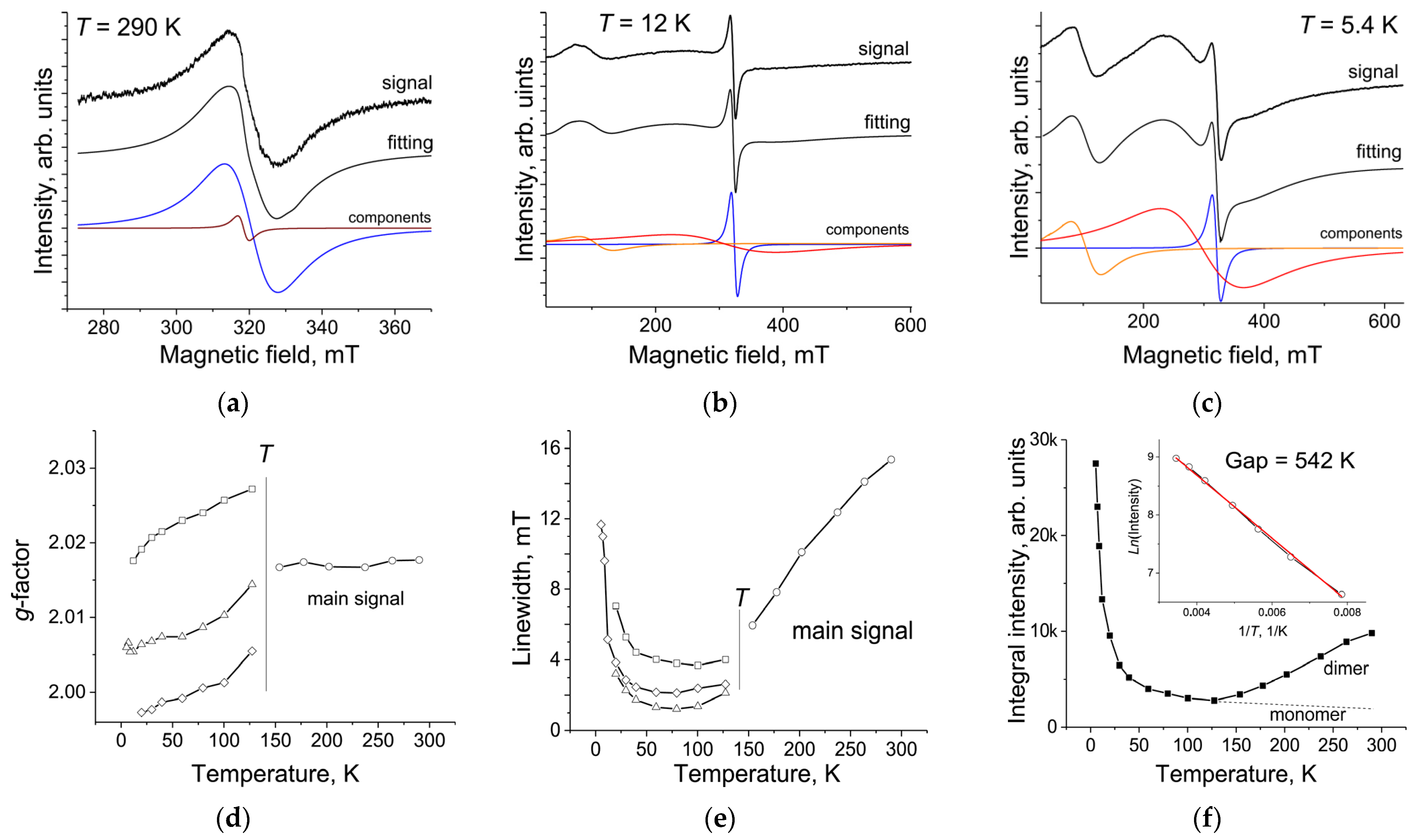
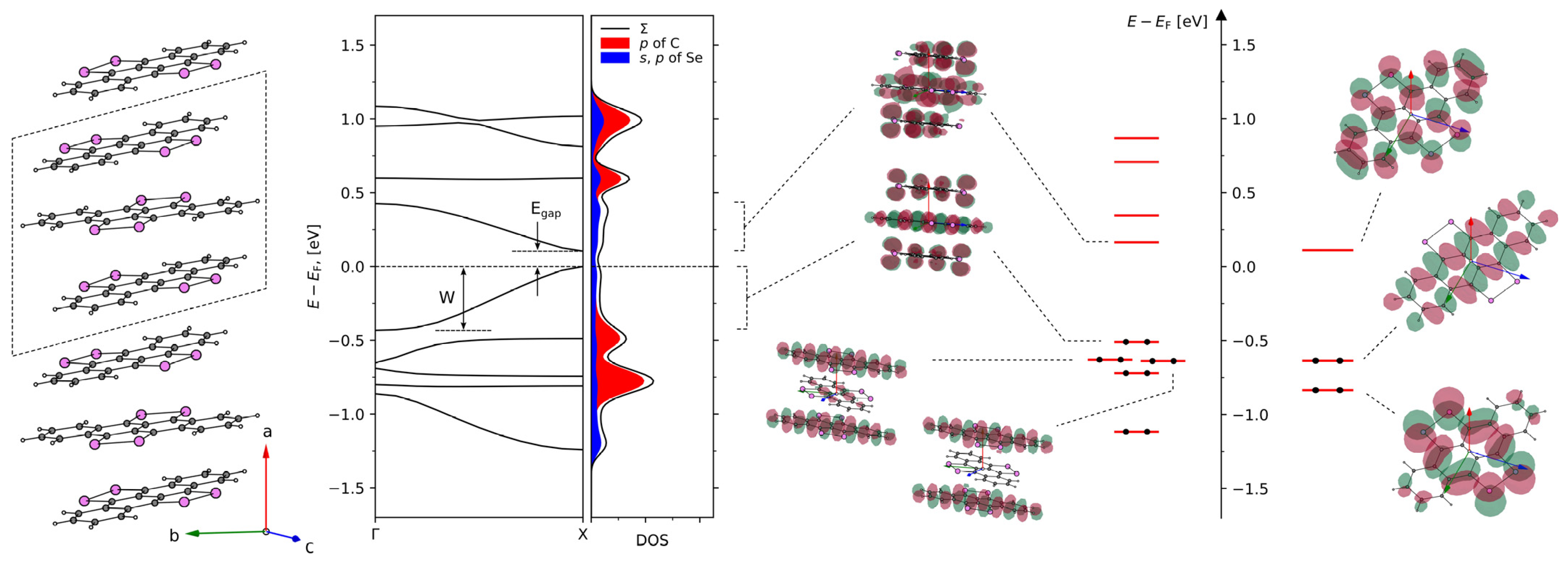
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. General
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Laukhin, V. Coexistence of Ferromagnetism and Metallic Conductivity in a Molecule-Based Layered Compound. Nature 2000, 408, 447–449. [Google Scholar] [CrossRef]
- Gonzalez, M.I.; Turkiewicz, A.B.; Darago, L.E.; Oktawiec, J.; Bustillo, K.; Grandjean, F.; Long, G.J.; Long, J.R. Confinement of Atomically Defined Metal Halide Sheets in a Metal–Organic Framework. Nature 2020, 577, 64–68. [Google Scholar] [CrossRef]
- Naito, T. Modern History of Organic Conductors: An Overview. Crystals 2021, 11, 838. [Google Scholar] [CrossRef]
- Williams, J.M. (Ed.) Organic Superconductors (Including Fullerenes): Synthesis, Structure, Properties, and Theory; Prentice Hall Inorganic and Organometallic Chemistry Series; Prentice Hall: Englewood Cliffs, NJ, USA, 1992; ISBN 978-0-13-640566-5. [Google Scholar]
- Saito, G.; Yoshida, Y. Development of Conductive Organic Molecular Assemblies: Organic Metals, Superconductors, and Exotic Functional Materials. Bull. Chem. Soc. Jpn. 2007, 80, 1–137. [Google Scholar] [CrossRef]
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic Bistability in a Metal-Ion Cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Fang, M.; Evans, W.J.; Long, J.R. A N23– Radical-Bridged Terbium Complex Exhibiting Magnetic Hysteresis at 14 K. J. Am. Chem. Soc. 2011, 133, 14236–14239. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Day, B.M.; Chen, Y.; Tong, M.; Mansikkamäki, A.; Layfield, R.A. A Dysprosium Metallocene Single-Molecule Magnet Functioning at the Axial Limit. Angew. Chem. Int. Ed. 2017, 56, 11445–11449. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.A.P.; Ortu, F.; Reta, D.; Chilton, N.F.; Mills, D.P. Molecular Magnetic Hysteresis at 60 Kelvin in Dysprosocenium. Nature 2017, 548, 439–442. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic Hysteresis up to 80 Kelvin in a Dysprosium Metallocene Single-Molecule Magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.; Kaizu, Y. Lanthanide Double-Decker Complexes Functioning as Magnets at the Single-Molecular Level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef]
- Liu, F.; Krylov, D.S.; Spree, L.; Avdoshenko, S.M.; Samoylova, N.A.; Rosenkranz, M.; Kostanyan, A.; Greber, T.; Wolter, A.U.B.; Büchner, B.; et al. Single Molecule Magnet with an Unpaired Electron Trapped between Two Lanthanide Ions inside a Fullerene. Nat. Commun. 2017, 8, 16098. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Dong, B.-W.; Liu, Z.; Liu, J.-J.; Su, J.; Yu, C.; Xiong, J.; Shi, D.-E.; Wang, Y.; Wang, B.-W.; et al. Endohedral Metallofullerene as Molecular High Spin Qubit: Diverse Rabi Cycles in Gd2@C79N. J. Am. Chem. Soc. 2018, 140, 1123–1130. [Google Scholar] [CrossRef]
- Feng, M.; Tong, M. Single Ion Magnets from 3d to 5f: Developments and Strategies. Chem. Eur. J. 2018, 24, 7574–7594. [Google Scholar] [CrossRef]
- Hanasaki, N.; Tajima, H.; Matsuda, M.; Naito, T.; Inabe, T. Giant Negative Magnetoresistance in Quasi-One-Dimensional Conductor TPP[Fe(Pc)(CN)2]2: Interplay between Local Moments and One-Dimensional Conduction Electrons. Phys. Rev. B 2000, 62, 5839–5842. [Google Scholar] [CrossRef]
- Hiraga, H.; Miyasaka, H.; Nakata, K.; Kajiwara, T.; Takaishi, S.; Oshima, Y.; Nojiri, H.; Yamashita, M. Hybrid Molecular Material Exhibiting Single-Molecule Magnet Behavior and Molecular Conductivity. Inorg. Chem. 2007, 46, 9661–9671. [Google Scholar] [CrossRef]
- Hiraga, H.; Miyasaka, H.; Clérac, R.; Fourmigué, M.; Yamashita, M. [MIII(dmit)2]−-Coordinated MnIII Salen-Type Dimers (MIII = NiIII, AuIII; dmit2− = 1,3-Dithiol-2-thione-4,5-dithiolate): Design of Single-Component Conducting Single-Molecule Magnet-Based Materials. Inorg. Chem. 2009, 48, 2887–2898. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Miyasaka, H.; Yamashita, M. Hybridized Molecular Materials Based on [MnIII2] Single Molecule Magnets with Molecular Conductors [Ni(Dmit)2]n−. Phys. B Condens. Matter 2010, 405, S313–S316. [Google Scholar] [CrossRef]
- Kubo, K.; Shiga, T.; Yamamoto, T.; Tajima, A.; Moriwaki, T.; Ikemoto, Y.; Yamashita, M.; Sessini, E.; Mercuri, M.L.; Deplano, P.; et al. Electronic State of a Conducting Single Molecule Magnet Based on Mn-Salen Type and Ni-Dithiolene Complexes. Inorg. Chem. 2011, 50, 9337–9344. [Google Scholar] [CrossRef]
- Shen, Y.; Cosquer, G.; Ito, H.; Izuogu, D.C.; Thom, A.J.W.; Ina, T.; Uruga, T.; Yoshida, T.; Takaishi, S.; Breedlove, B.K.; et al. An Organic–Inorganic Hybrid Exhibiting Electrical Conduction and Single-Ion Magnetism. Angew. Chem. Int. Ed. 2020, 59, 2399–2406. [Google Scholar] [CrossRef]
- Shen, Y.; Ito, H.; Zhang, H.; Yamochi, H.; Katagiri, S.; Yoshina, S.K.; Otsuka, A.; Ishikawa, M.; Cosquer, G.; Uchida, K.; et al. Simultaneous Manifestation of Metallic Conductivity and Single-Molecule Magnetism in a Layered Molecule-Based Compound. Chem. Sci. 2020, 11, 11154–11161. [Google Scholar] [CrossRef]
- Shen, Y.; Ito, H.; Zhang, H.; Yamochi, H.; Cosquer, G.; Herrmann, C.; Ina, T.; Yoshina, S.K.; Breedlove, B.K.; Otsuka, A.; et al. Emergence of Metallic Conduction and Cobalt(II)-Based Single-Molecule Magnetism in the Same Temperature Range. J. Am. Chem. Soc. 2021, 143, 4891–4895. [Google Scholar] [CrossRef] [PubMed]
- Kushch, N.D.; Buravov, L.I.; Kushch, P.P.; Shilov, G.V.; Yamochi, H.; Ishikawa, M.; Otsuka, A.; Shakin, A.A.; Maximova, O.V.; Volkova, O.S.; et al. Multifunctional Compound Combining Conductivity and Single-Molecule Magnetism in the Same Temperature Range. Inorg. Chem. 2018, 57, 2386–2389. [Google Scholar] [CrossRef] [PubMed]
- Flakina, A.M.; Zhilyaeva, E.I.; Shilov, G.V.; Faraonov, M.A.; Torunova, S.A.; Konarev, D.V. Layered Organic Conductors Based on BEDT-TTF and Ho, Dy, Tb Chlorides. Magnetochemistry 2022, 8, 142. [Google Scholar] [CrossRef]
- Shen, Y.; Cosquer, G.; Breedlove, B.; Yamashita, M. Hybrid Molecular Compound Exhibiting Slow Magnetic Relaxation and Electrical Conductivity. Magnetochemistry 2016, 2, 44. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, H.; Ballesteros-Rivas, M.; Woods, T.J.; Dunbar, K.R. Conducting Molecular Nanomagnet of DyIII with Partially Charged TCNQ Radicals. Chem. Eur. J. 2017, 23, 7448–7452. [Google Scholar] [CrossRef]
- Shen, Y.; Cosquer, G.; Zhang, H.; Breedlove, B.K.; Cui, M.; Yamashita, M. 4f−π Molecular Hybrid Exhibiting Rich Conductive Phases and Slow Relaxation of Magnetization. J. Am. Chem. Soc. 2021, 143, 9543–9550. [Google Scholar] [CrossRef]
- Wan, Q.; Wakizaka, M.; Zhang, H.; Shen, Y.; Funakoshi, N.; Che, C.-M.; Takaishi, S.; Yamashita, M. A New Organic Conductor of Tetramethyltetraselenafulvalene (TMTSF) with a Magnetic Dy(III) Complex. Magnetochemistry 2023, 9, 77. [Google Scholar] [CrossRef]
- Sato, T.; Breedlove, B.K.; Yamashita, M.; Katoh, K. Electro-Conductive Single-Molecule Magnet Composed of a Dysprosium(III)-Phthalocyaninato Double-Decker Complex with Magnetoresistance. Angew. Chem. Int. Ed. 2021, 60, 21179–21183. [Google Scholar] [CrossRef]
- Galangau, O.; Flores Gonzalez, J.; Montigaud, V.; Dorcet, V.; Le Guennic, B.; Cador, O.; Pointillart, F. Dysprosium Single-Molecule Magnets Involving 1,10-Phenantroline-5,6-Dione Ligand. Magnetochemistry 2020, 6, 19. [Google Scholar] [CrossRef]
- Raebiger, J.W.; Miller, J.S. Magnetic Ordering in the Rare Earth Molecule-Based Magnets, Ln(TCNE)3 (Ln = Gd, Dy; TCNE = Tetracyanoethylene). Inorg. Chem. 2002, 41, 3308–3312. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. Program SHAPE, University of Barcelona, Spain (version 2.1, March 2013). Available online: https://www.shapesoftware.com/00_Website_Homepage/ (accessed on 17 July 2024).
- Pinsky, M.; Avnir, D. Continuous Symmetry Measures. 5. The Classical Polyhedra. Inorg. Chem. 1998, 37, 5575–5582. [Google Scholar] [CrossRef]
- Shibaeva, R.P.; Kaminskii, V.F. Structure of conducting complexes based on cation-radicals. II. Crystal and molecular structure of the 2:1 complex of tetraselenotetracene and chlorine, TSeT2•+C1−. Kristallografiya 1977, 23, 1183–1188. [Google Scholar]
- Shibaeva, R.P.; Kaminskii, V.F.; Kotov, A.I.; Yagubskii, E.B.; Khidekel, M.L. Structure of electroconducting complexes based on radical cations III. Thiocyanate of tetraselenotetracene (TSeT2)+(SCN)−. Kristallografiya 1979, 24, 271–275. [Google Scholar]
- Kaminskii, V.F.; Kostuchenko, E.E.; Shibaeva, R.P.; Yagubskii, E.B.; Zvarykina, A.V. Quasi-one-dimensional conductors based on tetrathiatetracene (TTT) and tetraselenatetracene (TSeT) with metal complex mercury anions:synthesis, structure, properties. J. Phys. Colloq. 1983, 44, C3-1167–C3-1181. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A Powerful New Program for the Analysis of Anisotropic Monomeric and Exchange-coupled Polynuclear d- and f-block Complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Abragam, A.; Bleaney, B. Electron Paramagnetic Resonance of Transition Ions; Oxford Classic Texts in the Physical Sciences; Oxford University Press: Oxford, UK, 2012; ISBN 978-0-19-965152-8. [Google Scholar]
- Chen, Y.; Ma, F.; Chen, X.; Dong, B.; Wang, K.; Jiang, S.; Wang, C.; Chen, X.; Qi, D.; Sun, H.; et al. Novel Bis(Phthalocyaninato) Rare Earth Complexes with the Bulky and Strong Electron-Donating Dibutylamino Groups: Synthesis, Spectroscopy, and SMM Properties. Inorg. Chem. Front. 2017, 4, 1465–1471. [Google Scholar] [CrossRef]
- Shintoyo, S.; Murakami, K.; Fujinami, T.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Watanabe, M.; Tsuchimoto, M.; Mrozinski, J.; et al. Crystal Field Splitting of the Ground State of Terbium(III) and Dysprosium(III) Complexes with a Triimidazolyl Tripod Ligand and an Acetate Determined by Magnetic Analysis and Luminescence. Inorg. Chem. 2014, 53, 10359–10369. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, C.A.P. Blocking like It’s Hot: A Synthetic Chemists’ Path to High-Temperature Lanthanide Single Molecule Magnets. Dalton Trans. 2020, 49, 14320–14337. [Google Scholar] [CrossRef] [PubMed]
- Bruker. APEX3, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 2016. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Perez-Albuerne, E.A.; Johnson, H., Jr.; Trevoy, D. Highly Conducting Ion-Radical Salts of Tetrathiotetracene. J. Chem. Phys. 1971, 55, 1547–1554. [Google Scholar] [CrossRef]
- Zolotukhin, S.P.; Kaminskii, V.F.; Kotov, A.I.; Khidekel’, M.L.; Shibaeva, R.P.; Yagubskii, E.B. Studies in the field of organic metals 3. Nonstoichiometric tetraselenotetracene iodides. Bull. Acad. Sci. USSR Div. Chem. Sci. 1978, 27, 1590–1595. [Google Scholar] [CrossRef]
- Kachapina, L.M.; Kaplunov, M.G.; Kotov, A.I.; Eremenko, O.N.; Yagubskii, É.B.; Borod’ko, Y.G. Vibrational spectra of tetrathiotetracene, tetraselenotetracene, and their cations. J. Appl. Spectrosc. 1979, 30, 54–58. [Google Scholar] [CrossRef]
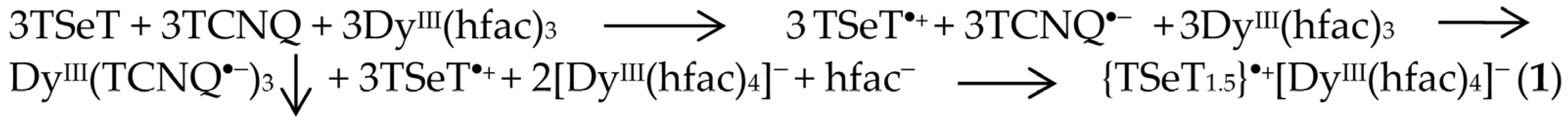

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flakina, A.M.; Nazarov, D.I.; Faraonov, M.A.; Yakushev, I.A.; Kuzmin, A.V.; Khasanov, S.S.; Zverev, V.N.; Otsuka, A.; Yamochi, H.; Kitagawa, H.; et al. Single-Ion Magnetism of the [DyIII(hfac)4]− Anions in the Crystalline Semiconductor {TSeT1.5}●+[DyIII(hfac)4]− Containing Weakly Dimerized Stacks of Tetraselenatetracene. Int. J. Mol. Sci. 2024, 25, 8068. https://doi.org/10.3390/ijms25158068
Flakina AM, Nazarov DI, Faraonov MA, Yakushev IA, Kuzmin AV, Khasanov SS, Zverev VN, Otsuka A, Yamochi H, Kitagawa H, et al. Single-Ion Magnetism of the [DyIII(hfac)4]− Anions in the Crystalline Semiconductor {TSeT1.5}●+[DyIII(hfac)4]− Containing Weakly Dimerized Stacks of Tetraselenatetracene. International Journal of Molecular Sciences. 2024; 25(15):8068. https://doi.org/10.3390/ijms25158068
Chicago/Turabian StyleFlakina, Alexandra M., Dmitry I. Nazarov, Maxim A. Faraonov, Ilya A. Yakushev, Alexey V. Kuzmin, Salavat S. Khasanov, Vladimir N. Zverev, Akihiro Otsuka, Hideki Yamochi, Hiroshi Kitagawa, and et al. 2024. "Single-Ion Magnetism of the [DyIII(hfac)4]− Anions in the Crystalline Semiconductor {TSeT1.5}●+[DyIII(hfac)4]− Containing Weakly Dimerized Stacks of Tetraselenatetracene" International Journal of Molecular Sciences 25, no. 15: 8068. https://doi.org/10.3390/ijms25158068





