Preimplantation Genetic Testing of Spinocerebellar Ataxia Type 3/Machado–Joseph Disease—Robust Tools for Direct and Indirect Detection of the ATXN3 (CAG)n Repeat Expansion
Abstract
1. Introduction
2. Results
2.1. Clinical IVF-PGT-M of SCA3/MJD
2.2. In Silico Mining of Microsatellite Markers for Hexadecaplex PCR
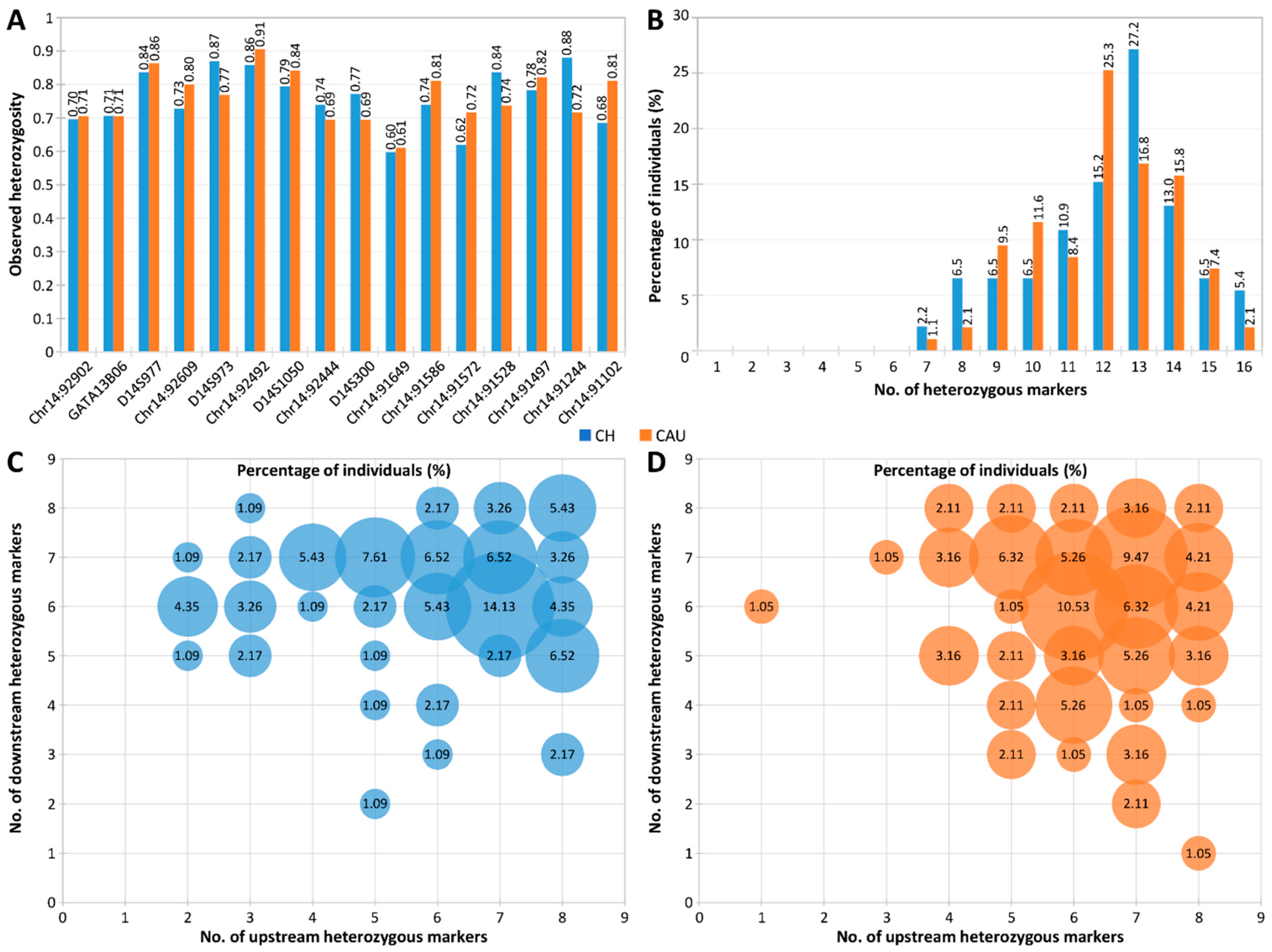
2.3. Evaluation of Marker Heterozygosity and Polymorphism
3. Discussion
4. Materials and Methods
4.1. Biological Samples
4.2. SCA3/MJD PGT-M Combining TP-PCR of ATXN3 (CAG)n and Linked Microsatellite Analysis
4.3. Hexadecaplex Microsatellite Marker Panel Selection
4.4. Hexadecaplex Microsatellite PCR and Genotyping
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paulson, H.; Shakkottai, V. Spinocerebellar ataxia type 3. In GeneReviews(®); Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1998. [Google Scholar]
- Paulson, H.L.; Shakkottai, V.G.; Clark, H.B.; Orr, H.T. Polyglutamine spinocerebellar ataxias—From genes to potential treatments. Nat. Rev. Neurosci. 2017, 18, 613–626. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Okamoto, T.; Taniwaki, M.; Aizawa, M.; Inoue, M.; Katayama, S.; Kawakami, H.; Nakamura, S.; Nishimura, M.; Akiguchi, I.; et al. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat. Genet. 1994, 8, 221–228. [Google Scholar] [CrossRef]
- Klockgether, T.; Mariotti, C.; Paulson, H.L. Spinocerebellar ataxia. Nat. Rev. Dis. Primers 2019, 5, 24. [Google Scholar] [CrossRef]
- Hersheson, J.; Haworth, A.; Houlden, H. The inherited ataxias: Genetic heterogeneity, mutation databases, and future directions in research and clinical diagnostics. Hum. Mutat. 2012, 33, 1324–1332. [Google Scholar] [CrossRef]
- Paulson, H. Machado-Joseph disease/spinocerebellar ataxia type 3. Handb. Clin. Neurol. 2012, 103, 437–449. [Google Scholar] [CrossRef]
- Stoyas, C.A.; La Spada, A.R. The CAG-polyglutamine repeat diseases: A clinical, molecular, genetic, and pathophysiologic nosology. Handb. Clin. Neurol. 2018, 147, 143–170. [Google Scholar] [CrossRef]
- Drüsedau, M.; Dreesen, J.C.; De Die-Smulders, C.; Hardy, K.; Bras, M.; Dumoulin, J.C.; Evers, J.L.; Smeets, H.J.; Geraedts, J.P.; Herbergs, J. Preimplantation genetic diagnosis of spinocerebellar ataxia 3 by (CAG)(n) repeat detection. Mol. Hum. Reprod. 2004, 10, 71–75. [Google Scholar] [CrossRef]
- Melo, A.R.; Ramos, A.; Kazachkova, N.; Raposo, M.; Bettencourt, B.F.; Rendeiro, A.R.; Kay, T.; Vasconcelos, J.; Bruges-Armas, J.; Lima, M. Triplet repeat primed PCR (TP-PCR) in molecular diagnostic testing for spinocerebellar ataxia type 3 (SCA3). Mol. Diagn. Ther. 2016, 20, 617–622. [Google Scholar] [CrossRef]
- Lian, M.; Zhao, M.; Phang, G.P.; Soong, Y.T.; Yoon, C.S.; Lee, C.G.; Law, H.Y.; Chong, S.S. Rapid molecular screen of spinocerebellar ataxia types 1, 2, and 3 by triplet-primed PCR and melting curve analysis. J. Mol. Diagn. 2021, 23, 565–576. [Google Scholar] [CrossRef]
- Lian, M.; Limwongse, C.; Yoon, C.S.; Lee, C.G.; Law, H.Y.; Chong, S.S. Single-tube screen for rapid detection of repeat expansions in seven common spinocerebellar ataxias. Clin. Chem. 2022, 68, 794–802. [Google Scholar] [CrossRef]
- Laitinen-Forsblom, P.; Poost, B.; Noss, D.; Handyside, A.; Nevinny-Stickel-Hinzpeter, C. Pregnancy following exclusion testing for SCA3 on polar bodies in a fresh cycle: A comparison of STR-based targeted haplotyping and genome-wide SNP analysis and meiomapping. Reprod. BioMedicine Online 2019, 38, e14. [Google Scholar] [CrossRef]
- Spits, C.; Le Caignec, C.; De Rycke, M.; Van Haute, L.; Van Steirteghem, A.; Liebaers, I.; Sermon, K. Whole-genome multiple displacement amplification from single cells. Nat. Protoc. 2006, 1, 1965–1970. [Google Scholar] [CrossRef]
- Harton, G.L.; De Rycke, M.; Fiorentino, F.; Moutou, C.; SenGupta, S.; Traeger-Synodinos, J.; Harper, J.C. ESHRE PGD consortium best practice guidelines for amplification-based PGD. Hum. Reprod. 2011, 26, 33–40. [Google Scholar] [CrossRef]
- Rajan-Babu, I.S.; Lian, M.; Cheah, F.S.H.; Chen, M.; Tan, A.S.C.; Prasath, E.B.; Loh, S.F.; Chong, S.S. FMR1 CGG repeat expansion mutation detection and linked haplotype analysis for reliable and accurate preimplantation genetic diagnosis of fragile X syndrome. Expert Rev. Mol. Med. 2017, 19, e10. [Google Scholar] [CrossRef]
- Lian, M.; Lee, C.G.; Chong, S.S. Robust Preimplantation Genetic Testing Strategy for Myotonic Dystrophy Type 1 by Bidirectional Triplet-Primed Polymerase Chain Reaction Combined With Multi-microsatellite Haplotyping Following Whole-Genome Amplification. Front. Genet. 2019, 10, 589. [Google Scholar] [CrossRef]
- Zhao, M.; Cheah, F.S.H.; Tan, A.S.C.; Lian, M.; Phang, G.P.; Agarwal, A.; Chong, S.S. Robust preimplantation genetic testing of Huntington Disease by combined triplet-primed PCR analysis of the HTT CAG repeat and multi-microsatellite haplotyping. Sci. Rep. 2019, 9, 16481. [Google Scholar] [CrossRef]
- Machado, F.B.; Medina-Acosta, E. High-resolution combined linkage physical map of short tandem repeat loci on human chromosome band Xq28 for indirect haemophilia A carrier detection. Haemophilia 2009, 15, 297–308. [Google Scholar] [CrossRef]
- Bram, E.; Javanmardi, K.; Nicholson, K.; Culp, K.; Thibert, J.R.; Kemppainen, J.; Le, V.; Schlageter, A.; Hadd, A.; Latham, G.J. Comprehensive genotyping of the C9orf72 hexanucleotide repeat region in 2095 ALS samples from the NINDS collection using a two-mode, long-read PCR assay. Amyotroph. Lateral Scler. Front. Degener. 2019, 20, 107–114. [Google Scholar] [CrossRef]
- Lan, X.; Li, N.; Wan, H.; Luo, L.; Wu, Y.; Li, S.; An, Y.; Wu, B.L. Developing a one-step triplet-repeat primed PCR assay for diagnosing myotonic dystrophy. J. Genet. Genom. 2018, 45, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Jama, M.; Millson, A.; Miller, C.E.; Lyon, E. Triplet repeat primed PCR simplifies testing for huntington disease. J. Mol. Diagn. 2013, 15, 255–262. [Google Scholar] [CrossRef]
- Sermon, K.; Seneca, S.; De Rycke, M.; Goossens, V.; Van de Velde, H.; De Vos, A.; Platteau, P.; Lissens, W.; Van Steirteghem, A.; Liebaers, I. PGD in the lab for triplet repeat diseases—Myotonic dystrophy, Huntington’s disease and Fragile-X syndrome. Mol. Cell. Endocrinol. 2001, 183 (Suppl. S1), S77–S85. [Google Scholar] [CrossRef]
- Zhao, M.; Lee, C.G.; Law, H.Y.; Chong, S.S. Enhanced detection and sizing of the HTT CAG repeat expansion in Huntington disease using an improved triplet-primed PCR assay. Neurodegener. Dis. 2016, 16, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Falk, M.; Vojtiskova, M.; Lukas, Z.; Kroupova, I.; Froster, U. Simple procedure for automatic detection of unstable alleles in the myotonic dystrophy and Huntington’s disease loci. Genet. Test 2006, 10, 85–97. [Google Scholar] [CrossRef]
- Scott, R.T., Jr.; Upham, K.M.; Forman, E.J.; Zhao, T.; Treff, N.R. Cleavage-stage biopsy significantly impairs human embryonic implantation potential while blastocyst biopsy does not: A randomized and paired clinical trial. Fertil. Steril. 2013, 100, 624–630. [Google Scholar] [CrossRef]
- Carvalho, F.; Moutou, C.; Dimitriadou, E.; Dreesen, J.; Giménez, C.; Goossens, V.; Kakourou, G.; Vermeulen, N.; Zuccarello, D.; De Rycke, M. ESHRE PGT Consortium good practice recommendations for the detection of monogenic disorders. Hum. Reprod. Open 2020, 2020, hoaa018. [Google Scholar] [CrossRef]
- Broman, K.W.; Murray, J.C.; Sheffield, V.C.; White, R.L.; Weber, J.L. Comprehensive human genetic maps: Individual and sex-specific variation in recombination. Am. J. Hum. Genet. 1998, 63, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar] [PubMed]
- Nei, M.; Roychoudhury, A.K. Sampling variances of heterozygosity and genetic distance. Genetics 1974, 76, 379–390. [Google Scholar] [CrossRef]
- Weir, B.S.; Reynolds, J.; Dodds, K.G. The variance of sample heterozygosity. Theor. Popul. Biol. 1990, 37, 235–253. [Google Scholar] [CrossRef]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]


| Primer | Primer Sequence (5′-3′) | GenBank ID: Nucleotides | Concentration (µmol/L) | Expected TP-PCR Product Size |
|---|---|---|---|---|
| SCA3-F | 6-Fam-ATGTCTAGATTTCCTAAGATCAGCACTTCC | NG_008198.2: 40371–40400 | 0.50 | 240 bp + (CAG)n |
| TP-R | GTTTCGGCGTTACGAGTGGACCCCTGCTGCTGCTGCTG | - | 0.05 | |
| Tail | GTTTCGGCGTTACGAGTGGA | - | 0.50 |
| Microsatellite Marker | Repeat Motif | Primer Sequence (5′-3′) a | Concentration (µmol/L) | Amplicon Size Range (bp) f | He f | Ho f | PIC f | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CH | CAU | CH | CAU | CH | CAU | ||||||
| Chr14:92902 | (GAG)n | F | M13−2GCTAAGACCAAAATGTGAGCCAG | 0.3 | 190–213 | 0.64 | 0.69 | 0.70 | 0.71 | 0.57 | 0.64 |
| R | bCCCATGAATTTCGAGTGTGTAAGG | 0.3 | |||||||||
| GATA13B06 | (CTAT)n | F | M13−1CAGACAGCCATGGTGGTTCTTG | 0.3 | 368–402 | 0.75 | 0.72 | 0.71 | 0.71 | 0.71 | 0.67 |
| R | cTGTAAGGGTCTGGATGATTCTGG | 0.3 | |||||||||
| D14S977 | (CA)n | F | M13−2CTTATAGGATCTGGGGTGTACGC | 0.2 | 102–128 | 0.84 | 0.85 | 0.84 | 0.86 | 0.82 | 0.84 |
| R | dTTTCTCTCCATGCACTCCCTG | 0.2 | |||||||||
| Chr14:92609 | (AC)n | F | M13−1CCAGCGCCTTGATCTTGGAAG | 0.3 | 263–287 | 0.78 | 0.78 | 0.73 | 0.80 | 0.75 | 0.75 |
| R | bACTGTCTCCGCTTACAGAAGTTG | 0.3 | |||||||||
| D14S973 | (AC)n | F | M13−2CCAGCAAAGGACAGTTCTGC | 0.3 | 218–254 | 0.83 | 0.81 | 0.87 | 0.77 | 0.81 | 0.78 |
| R | bAGCCGGAAGAATGGAGAATAGC | 0.3 | |||||||||
| Chr14:92492 | (TG)n | F | M13−1TTTCCAAGGCTCCTATGGACCC | 0.2 | 126–158 | 0.85 | 0.86 | 0.86 | 0.91 | 0.83 | 0.85 |
| R | bCCGTGCTTTAACCCTCTACCC | 0.2 | |||||||||
| D14S1050 | (GT)n | F | M13−3TCTCTTAGGGCACCTGTGG | 0.3 | 193–215 | 0.77 | 0.82 | 0.79 | 0.84 | 0.73 | 0.80 |
| R | bCATTGCTGGGGCAAGGTAAGG | 0.3 | |||||||||
| Chr14:92444 | (GT)n | F | M13−1GCCTCCCCTTATTCATGGAGTAG | 0.2 | 169–191 | 0.70 | 0.72 | 0.74 | 0.69 | 0.64 | 0.69 |
| R | bCCTCCAACAACACTTGCACACC | 0.2 | |||||||||
| D14S300 | (GT)n | F | M13−3TACTCTGCCACAGACACCTTC | 0.3 | 238–252 | 0.72 | 0.74 | 0.77 | 0.69 | 0.67 | 0.69 |
| R | bACCTTACTAAAGGGCTGCCATG | 0.3 | |||||||||
| Chr14:91649 | (AC)n | F | M13−1CAGCCGGGGCAACAATCTC | 0.2 | 213–225 | 0.60 | 0.65 | 0.60 | 0.61 | 0.52 | 0.60 |
| R | bGGTGATAACTGTAGACCAGCTC | 0.2 | |||||||||
| Chr14:91586 | (TC)n | F | M13−1CCTCCTTTTGGCTGTTTGAACTG | 0.2 | 329–361 | 0.75 | 0.83 | 0.74 | 0.81 | 0.71 | 0.81 |
| R | eTTATTGGAGCTGGCCTTAGAGC | 0.2 | |||||||||
| Chr14:91572 | (GT)n | F | M13−2GAACTCAAAATAAGAGGCTTTGGGG | 0.5 | 317–331 | 0.68 | 0.73 | 0.62 | 0.72 | 0.63 | 0.70 |
| R | bGGCAGCAAGCTCTTCATGTTAC | 0.5 | |||||||||
| Chr14:91528 | (AC)n | F | M13−3GGGTCTGTTAGGAAGTGAGATAAGG | 0.3 | 285–317 | 0.76 | 0.78 | 0.84 | 0.74 | 0.73 | 0.75 |
| R | bGCTAAAGTGACCCTCTTGCCTC | 0.3 | |||||||||
| Chr14:91497 | (GT)n | F | bCAATGTCTTTCTCAGATGTGTGGG | 0.2 | 122–154 | 0.71 | 0.75 | 0.78 | 0.82 | 0.68 | 0.72 |
| R | M13−3CTCTCCAATGAAACAGATGCCC | 0.2 | |||||||||
| Chr14:91244 | (TG)n | F | M13−1CAACTTCATAGCTCATGACCTGC | 0.2 | 310–328 | 0.79 | 0.71 | 0.88 | 0.72 | 0.76 | 0.68 |
| R | bAGGCTCAAACTCACACAGTCAG | 0.2 | |||||||||
| Chr14:91102 | (AC)n | F | dTTTCAAGATCAAGAACGGAGGGG | 0.4 | 410–446 | 0.72 | 0.86 | 0.68 | 0.81 | 0.67 | 0.85 |
| R | M13−3CATCTGCTTCTGGGGATTGGAG | 0.4 | |||||||||
| Oocytes recovered | 11 |
| Oocytes fertilized with two pronuclei | 4 |
| Embryos biopsied | 3 |
| Unaffected embryos | 3 |
| Embryos transferred during the same cycle | 0 |
| Embryos frozen | 3 |
| Frozen thawed embryos transferred at a subsequent cycle | 1 |
| Positive hCG | 1 |
| Pregnancy with fetal heartbeat | 1 |
| Live birth | 1 |
| Affected embryos | 0 |
| Embryos with no diagnosis | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, M.; Tan, V.J.; Taguchi, R.; Zhao, M.; Phang, G.-P.; Tan, A.S.; Liu, S.; Lee, C.G.; Chong, S.S. Preimplantation Genetic Testing of Spinocerebellar Ataxia Type 3/Machado–Joseph Disease—Robust Tools for Direct and Indirect Detection of the ATXN3 (CAG)n Repeat Expansion. Int. J. Mol. Sci. 2024, 25, 8073. https://doi.org/10.3390/ijms25158073
Lian M, Tan VJ, Taguchi R, Zhao M, Phang G-P, Tan AS, Liu S, Lee CG, Chong SS. Preimplantation Genetic Testing of Spinocerebellar Ataxia Type 3/Machado–Joseph Disease—Robust Tools for Direct and Indirect Detection of the ATXN3 (CAG)n Repeat Expansion. International Journal of Molecular Sciences. 2024; 25(15):8073. https://doi.org/10.3390/ijms25158073
Chicago/Turabian StyleLian, Mulias, Vivienne J. Tan, Riho Taguchi, Mingjue Zhao, Gui-Ping Phang, Arnold S. Tan, Shuling Liu, Caroline G. Lee, and Samuel S. Chong. 2024. "Preimplantation Genetic Testing of Spinocerebellar Ataxia Type 3/Machado–Joseph Disease—Robust Tools for Direct and Indirect Detection of the ATXN3 (CAG)n Repeat Expansion" International Journal of Molecular Sciences 25, no. 15: 8073. https://doi.org/10.3390/ijms25158073
APA StyleLian, M., Tan, V. J., Taguchi, R., Zhao, M., Phang, G.-P., Tan, A. S., Liu, S., Lee, C. G., & Chong, S. S. (2024). Preimplantation Genetic Testing of Spinocerebellar Ataxia Type 3/Machado–Joseph Disease—Robust Tools for Direct and Indirect Detection of the ATXN3 (CAG)n Repeat Expansion. International Journal of Molecular Sciences, 25(15), 8073. https://doi.org/10.3390/ijms25158073







