The Regulation of Frontal Cortex Cholesterol Metabolism Abnormalities by NR3C1/NRIP1/NR1H2 Is Involved in the Occurrence of Stress-Induced Depression
Abstract
:1. Introduction
2. Results
2.1. Abnormal Cholesterol Levels in the Serum of Depression Patients
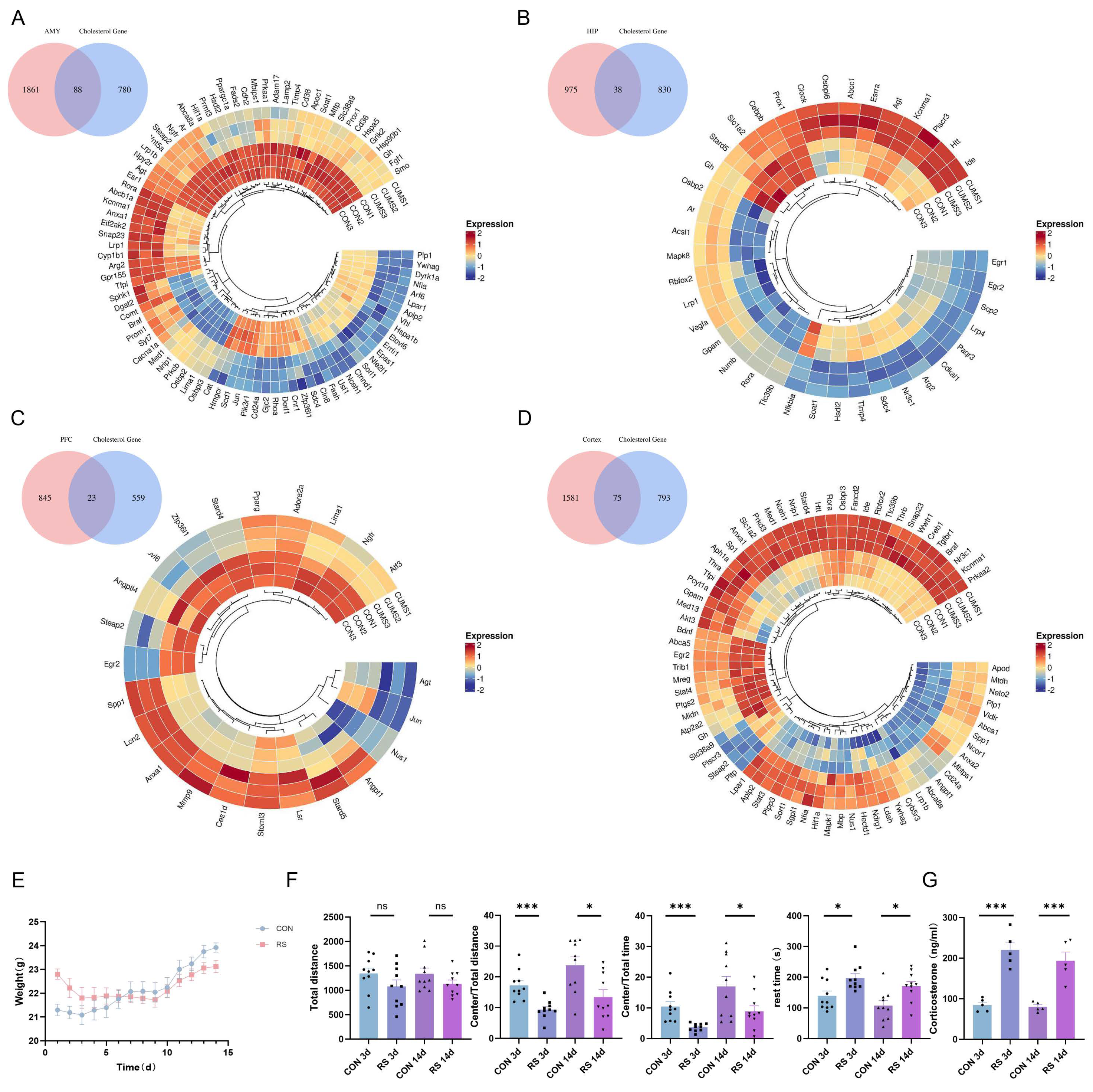
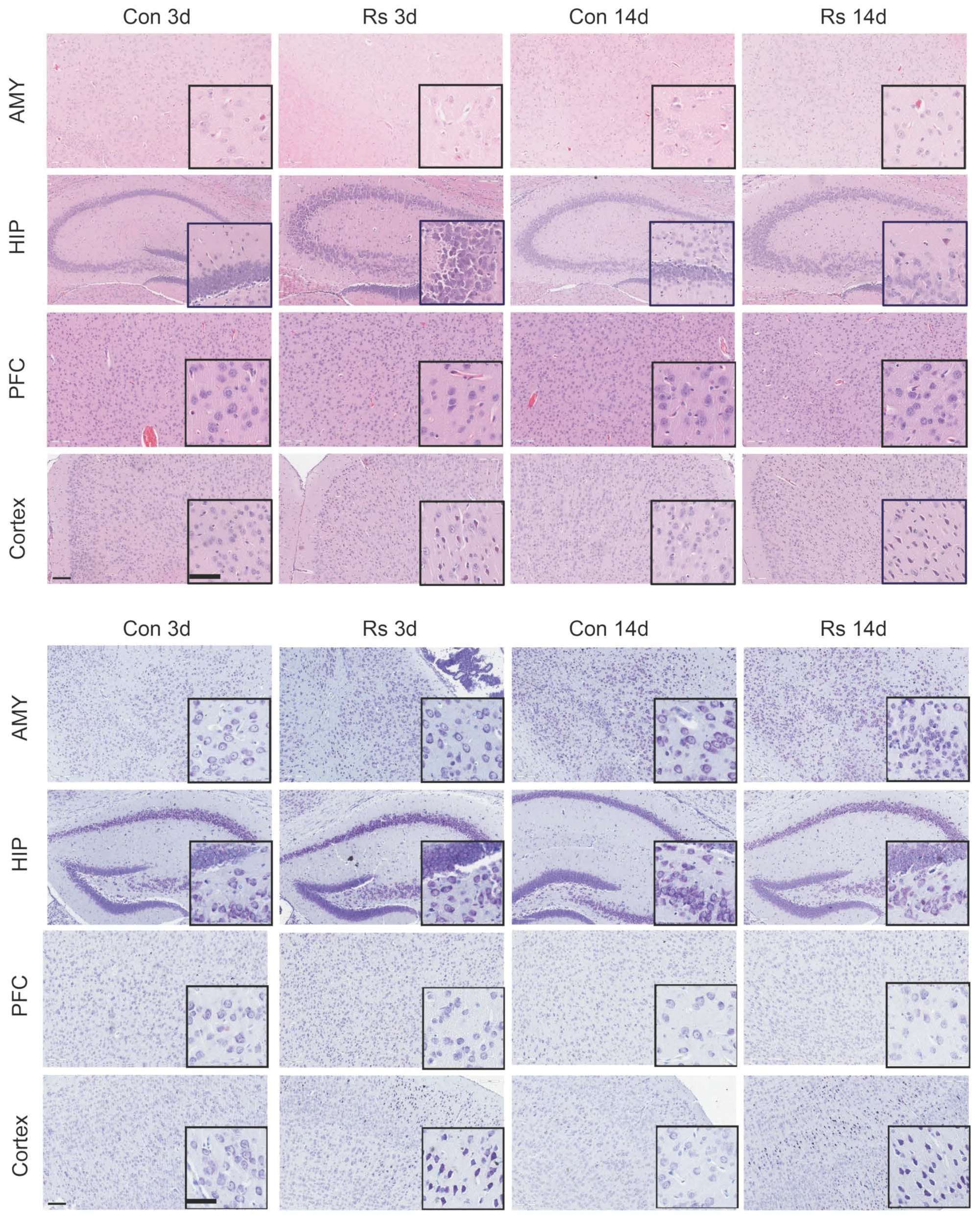
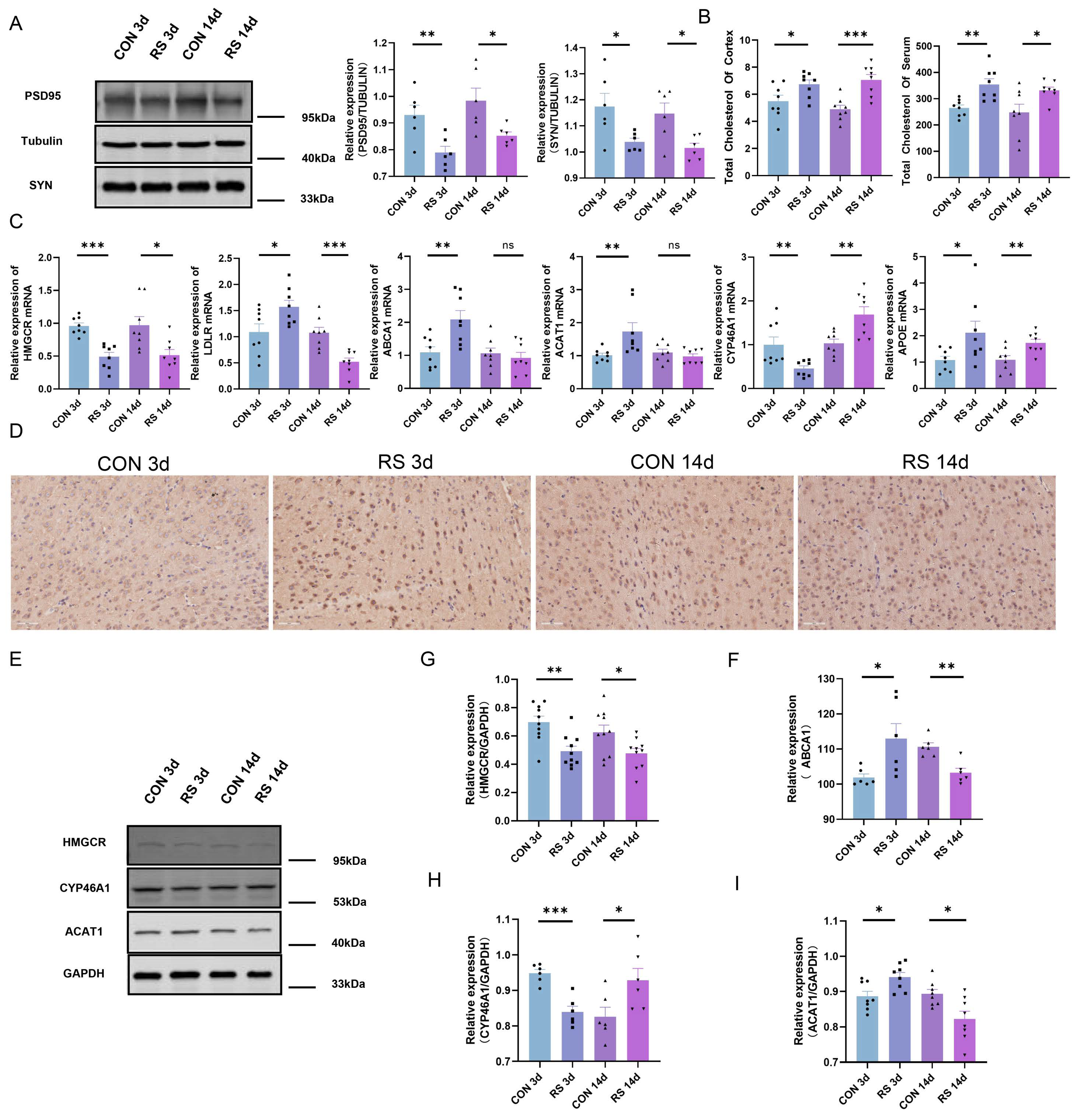
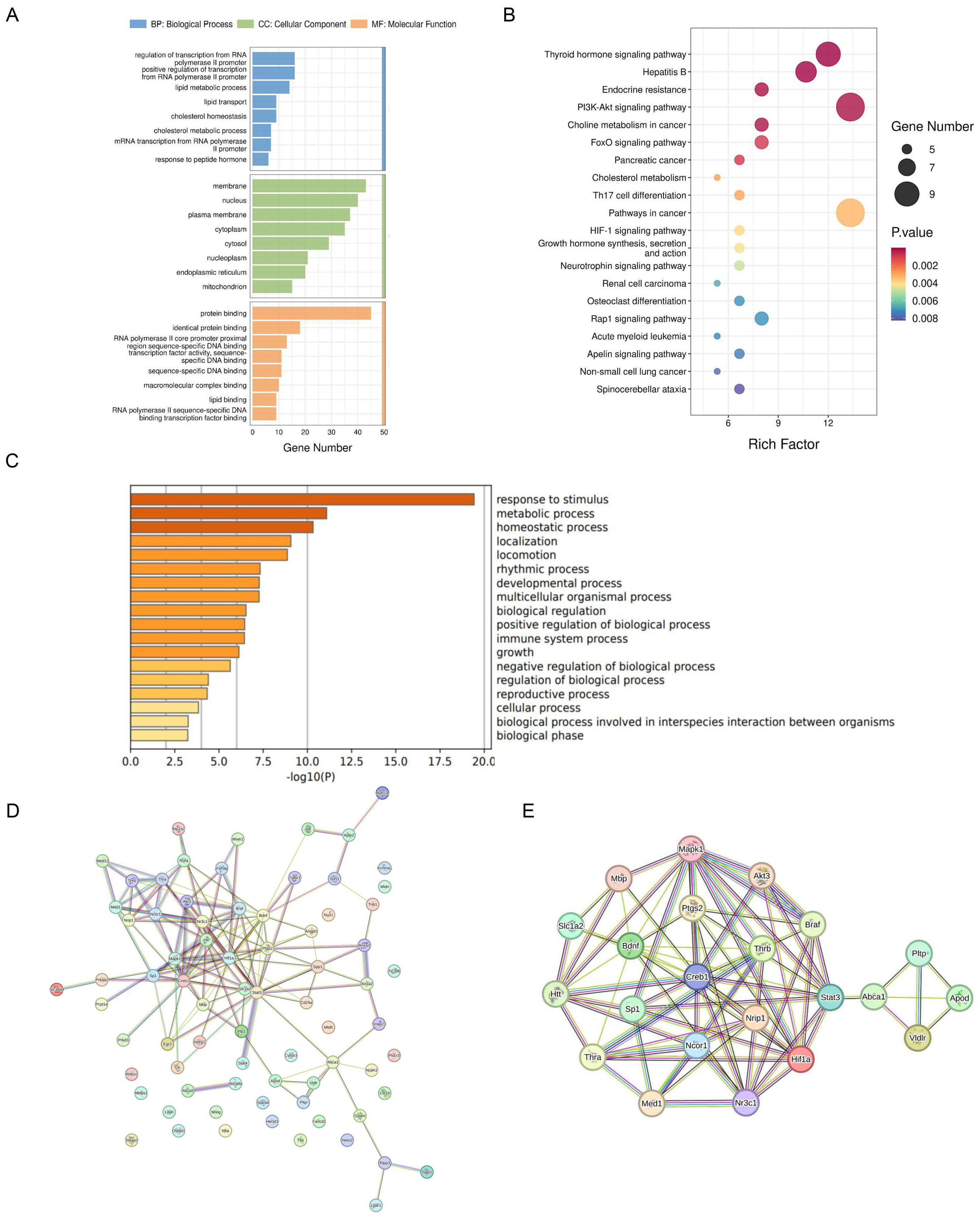
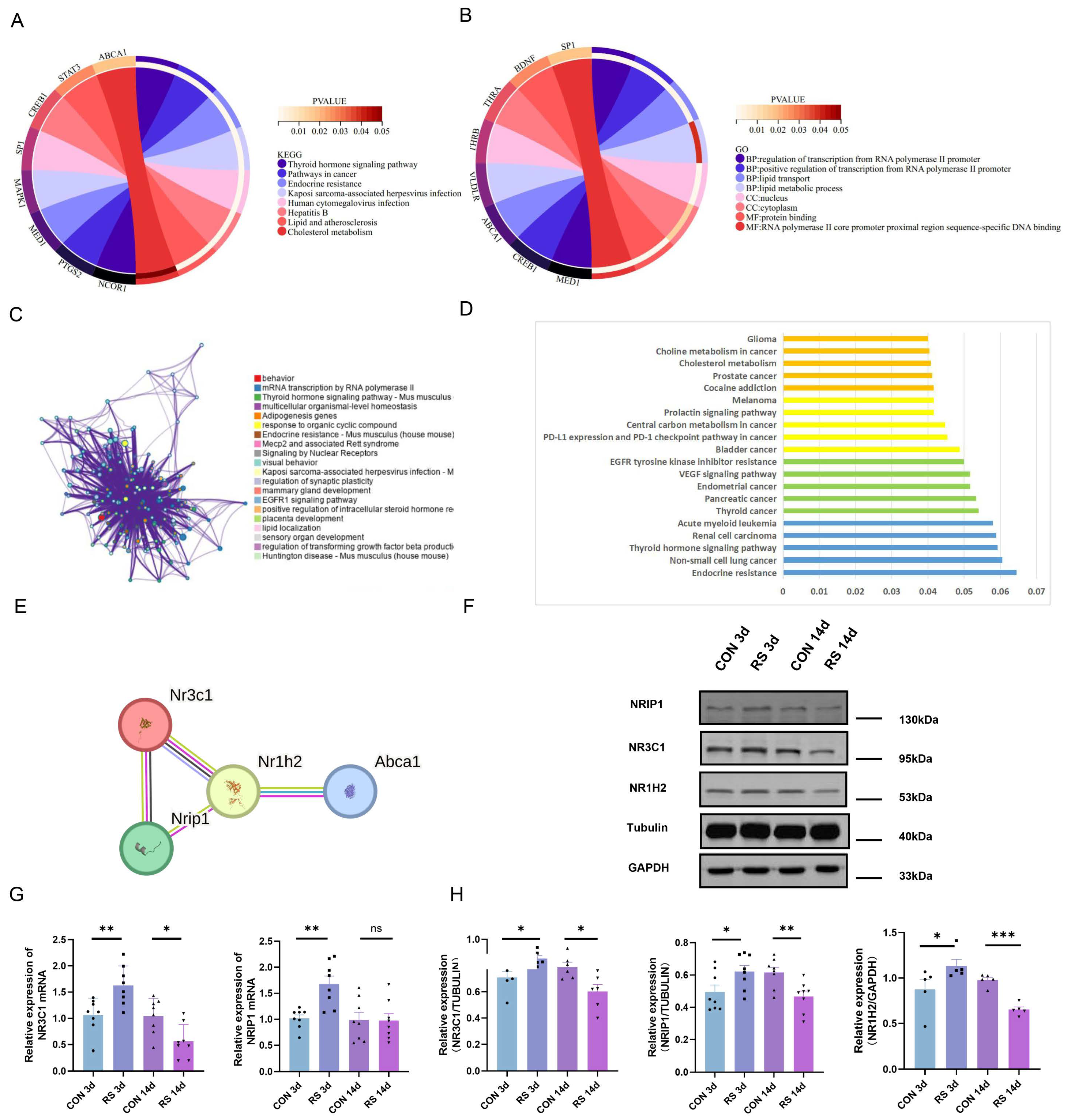
2.2. Dysregulation of Cholesterol Metabolism in the Mouse Brain under Stress
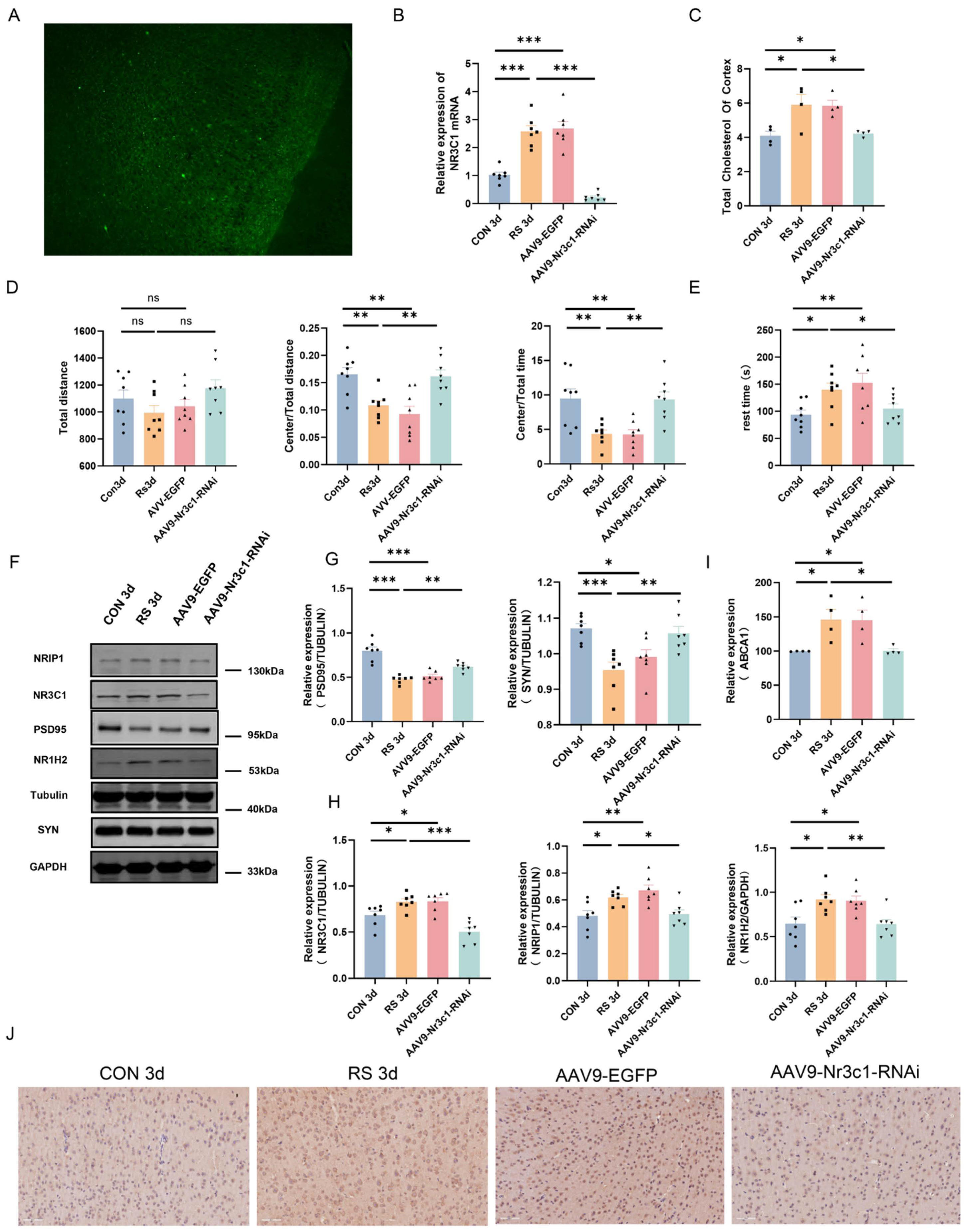
2.3. NR3C1/NRIP1/NR1H2 Pathway Involved in Depression-like Behavior Induced by Short-Term Stress
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Model Establishment
4.2. Open Field Test and Tail Suspension Test
4.3. HE Staining and Thionine Staining
4.4. Enzyme-Linked Immunosorbent Assay
4.5. Bioinformatics Data Analysis
4.6. Immunohistochemical Staining
4.7. Real-Time Fluorescent Quantitative PCR
4.8. Western Blotting
4.9. Statistical Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, J.; Xu, X.; Huang, Y.; Li, T.; Ma, C.; Xu, G.; Yin, H.; Xu, X.; Ma, Y.; Wang, L.; et al. Prevalence of depressive disorders and treatment in China: A cross-sectional epidemiological study. Lancet Psychiatry 2021, 8, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Smith, K. Mental health: A world of depression. Nature 2014, 515, 181. [Google Scholar] [CrossRef] [PubMed]
- Wray, N.R.; Ripke, S.; Mattheisen, M.; Trzaskowski, M.; Byrne, E.M.; Abdellaoui, A.; Adams, M.J.; Agerbo, E.; Air, T.M.; Andlauer, T.M.F.; et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018, 50, 668. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Toups, M.; Nemeroff, C.B. The Bidirectional Relationship of Depression and Inflammation: Double Trouble. Neuron 2020, 107, 234–256. [Google Scholar] [CrossRef] [PubMed]
- Tartt, A.N.; Mariani, M.B.; Hen, R.; Mann, J.J.; Boldrini, M. Dysregulation of adult hippocampal neuroplasticity in major depression: Pathogenesis and therapeutic implications. Mol. Psychiatry 2022, 27, 2689–2699. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.M.; McLoughlin, D.M. Peripheral blood inflammatory markers in depression: Response to electroconvulsive therapy and relationship with cognitive performance. Psychiatry Res. 2022, 315, 114725. [Google Scholar] [CrossRef] [PubMed]
- Hankin, B.L.L.; Demers, C.H.H.; Hennessey, E.P.; Perzow, S.E.D.; Curran, M.C.C.; Gallop, R.J.J.; Hoffman, M.C.; Davis, E.P. Effect of Brief Interpersonal Therapy on Depression During Pregnancy A Randomized Clinical Trial. JAMA Psychiatry 2023, 80, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Schramm, E.; Klein, D.N.; Elsaesser, M.; Furukawa, T.A.; Domschke, K. Review of dysthymia and persistent depressive disorder: History, correlates, and clinical implications. Lancet Psychiatry 2020, 7, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Pereira, J.S.; Rea, K.; Nolan, Y.M.; O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Depression’s Unholy Trinity: Dysregulated Stress, Immunity, and the Microbiome. Annu. Rev. Psychol. 2020, 71, 49–78. [Google Scholar] [CrossRef]
- Lee, S.H.; Jung, E. Adverse effects of early-life stress: Focus on the rodent neuroendocrine system. Neural Regen. Res. 2024, 19, 336–341. [Google Scholar] [CrossRef]
- Alozkan-Sever, C.; Uppendahl, J.R.; Cuijpers, P.; de Vries, R.; Rahman, A.; Mittendorfer-Rutz, E.; Akhtar, A.; Zheng, Z.; Sijbrandij, M. Research Review: Psychological and psychosocial interventions for children and adolescents with depression, anxiety, and post-traumatic stress disorder in low- and middle-income countries—A systematic review and meta-analysis. J Child. Psychol. Psychiatry. 2023, 64, 1776–1788. [Google Scholar] [CrossRef]
- Shorey, S.; Ng, E.D.; Wong, C. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br. J. Clin. Psychol. 2022, 61, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.D.; Rizak, J.; Feng, X.L.; Yang, S.C.; Lu, L.B.; Pan, L.; Yin, Y.; Hu, X.T. Prolonged secretion of cortisol as a possible mechanism underlying stress and depressive behaviour. Sci. Rep. 2016, 6, 30187. [Google Scholar] [CrossRef] [PubMed]
- Flouri, E.; Francesconi, M.; Midouhas, E.; Lewis, G. Prenatal and childhood adverse life events, inflammation and depressive symptoms across adolescence. J. Affect. Disord. 2020, 260, 577–582. [Google Scholar] [CrossRef]
- Donaldson, K.R.; Jonas, K.G.; Tian, Y.; Larsen, E.M.; Klein, D.N.; Mohanty, A.; Bromet, E.J.; Kotov, R. Dynamic interplay between life events and course of psychotic disorders: 10-year longitudinal study following first admission. Psychol. Med. 2022, 52, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Musliner, K.L.; Andersen, K.K.; Agerbo, E.; Albinana, C.; Vilhjalmsson, B.J.; Rajagopal, V.M.; Bybjerg-Grauholm, J.; Baekved-Hansen, M.; Pedersen, C.B.; Pedersen, M.G.; et al. Polygenic liability, stressful life events and risk for secondary-treated depression in early life: A nationwide register-based case-cohort study. Psychol. Med. 2023, 53, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Stefanaki, C.; Paltoglou, G.; Mastorakos, G.; Chrousos, G.P. Chronic Stress and Steatosis of Muscles, Bones, Liver, and Pancreas: A Review. Horm. Res. Paediatr. 2023, 96, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Yeagle, P.L. Cholesterol and the cell membrane. Biochim. Biophys. Acta 1985, 822, 267–287. [Google Scholar] [CrossRef]
- Maxfield, F.R.; Tabas, I. Role of cholesterol and lipid organization in disease. Nature 2005, 438, 612–621. [Google Scholar] [CrossRef]
- Huang, T.L.; Chen, J.F. Cholesterol and lipids in depression: Stress, hypothalamo-pituitary-adrenocortical axis, and inflammation/immunity. Adv. Clin. Chem. 2005, 39, 81–105. [Google Scholar]
- Staurenghi, E.; Giannelli, S.; Testa, G.; Sottero, B.; Leonarduzzi, G.; Gamba, P. Cholesterol Dysmetabolism in Alzheimer’s Disease: A Starring Role for Astrocytes? Antioxidants 2021, 10, 1890. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Zhang, M.; Zhang, S.; Bai, S.; Jiang, Z.; Cai, Q.; Cao, K.; Shen, C.; Shi, Y.; Zhang, R.; et al. Stress causes cognitive impairment by affecting cholesterol efflux and reuptake leading to abnormalities in lipid metabolism of rats. J. Integr. Neurosci. 2020, 19, 39–49. [Google Scholar] [CrossRef]
- Chuang, J.; Cui, H.; Mason, B.L.; Mahgoub, M.; Bookout, A.L.; Yu, H.G.; Perello, M.; Elmquist, J.K.; Repa, J.J.; Zigman, J.M.; et al. Chronic social defeat stress disrupts regulation of lipid synthesis. J. Lipid Res. 2010, 51, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Lin, Y.L.; Wei, L.N. Behavioral stress reduces RIP140 expression in astrocyte and increases brain lipid accumulation. Brain. Behav. Immun. 2015, 46, 270–279. [Google Scholar] [CrossRef]
- Noguchi, N.; Urano, Y.; Takabe, W.; Saito, Y. New aspects of 24(S)-hydroxycholesterol in modulating neuronal cell death. Free Radic. Biol. Med. 2015, 87, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Ferrari, C.; Turner, L.; Mariani, N.; Enache, D.; Hastings, C.; Kose, M.; Lombardo, G.; McLaughlin, A.P.; Nettis, M.A.; et al. Whole-blood expression of inflammasome- and glucocorticoid-related mRNAs correctly separates treatment-resistant depressed patients from drug-free and responsive patients in the BIODEP study. Transl. Psychiatry 2020, 10, 232. [Google Scholar] [CrossRef] [PubMed]
- Ratushna, O.O. Glucose deprivation affects the expression of genes encoding cAMP-activated protein kinase and related proteins in U87 glioma cells in ERN1 dependent manner. Endocr. Regul. 2020, 54, 244–254. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, L.; Li, Z.; Gao, H.; Yue, Z.; Liu, Z.; Liu, X.; Feng, X.; Liu, P. RIP140 triggers foam-cell formation by repressing ABCA1/G1 expression and cholesterol efflux via liver X receptor. FEBS Lett. 2015, 589, 455–460. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [CrossRef]
- Keller, J.; Gomez, R.; Williams, G.; Lembke, A.; Lazzeroni, L.; Murphy, G.J.; Schatzberg, A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry 2017, 22, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.M.; Antomagesh, F.; Comesana, S.; Soengas, J.L.; Vijayan, M.M. Chronic cortisol stimulation enhances hypothalamus-specific enrichment of metabolites in the rainbow trout brain. Am. J. Physiol.-Endocrinol. Metab. 2024, 326, E382–E397. [Google Scholar] [CrossRef] [PubMed]
- Perez-Leighton, C.; Kerr, B.; Scherer, P.E.; Baudrand, R.; Cortes, V. The interplay between leptin, glucocorticoids, and GLP1 regulates food intake and feeding behaviour. Biol. Rev. Camb. Philos. Soc. 2024, 99, 653–674. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.J.; Musenbichler, C.; Bohm, L.; Farber, K.; Fischer, A.I.; von Nippold, F.; Winkelmann, M.; Richter-Schmidinger, T.; Muhle, C.; Kornhuber, J.; et al. LDL cholesterol relates to depression, its severity, and the prospective course. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 92, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Wang, S.; Huang, Q.; Chen, X.; Qiu, L.; Ouyang, K.; Chen, Y. The association between non-high-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio (NHHR) and risk of depression among US adults: A cross-sectional NHANES study. J. Affect. Disord. 2024, 344, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zou, L.; Meng, L.; Qiang, G.; Yan, M.; Zhang, Z. Cholesterol Metabolism in Neurodegenerative Diseases: Molecular Mechanisms and Therapeutic Targets. Mol. Neurobiol. 2021, 58, 2183–2201. [Google Scholar] [CrossRef] [PubMed]
- Loera-Valencia, R.; Goikolea, J.; Parrado-Fernandez, C.; Merino-Serraisa, P.; Maioli, S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: Potential novel targets for treatment. J. Steroid. Biochem. Mol. Biol. 2019, 190, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.; Park, S.J.; Park, S.M. Cholesterol Metabolism in the Brain and Its Association with Parkinson’s Disease. Exp. Neurobiol. 2019, 28, 554–567, Erratum in Exp. Neurobiol. 2020, 29, 324. [Google Scholar] [CrossRef]
- Valenza, M.; Chen, J.Y.; Di Paolo, E.; Ruozi, B.; Belletti, D.; Bardile, C.F.; Leoni, V.; Caccia, C.; Brilli, E.; Di Donato, S.; et al. Cholesterol-loaded nanoparticles ameliorate synaptic and cognitive function in Huntington’s disease mice. EMBO Mol. Med. 2015, 7, 1547–1564. [Google Scholar] [CrossRef]
- Aguzzoli, H.B.; Brandon, J.A.; Page, M.L.; Nations, K.A.; Dikobe, K.I.; White, B.J.; Gordon, L.A.; Fox, G.A.; Wadsworth, M.E.; Doyle, P.H.; et al. Mapping medically relevant RNA isoform diversity in the aged human frontal cortex with deep long-read RNA-seq. Nat. Biotechnol. 2024. [Google Scholar] [CrossRef]
- Cheng, W.; Rolls, E.T.; Qiu, J.; Liu, W.; Tang, Y.; Huang, C.C.; Wang, X.; Zhang, J.; Lin, W.; Zheng, L.; et al. Medial reward and lateral non-reward orbitofrontal cortex circuits change in opposite directions in depression. Brain 2016, 139, 3296–3309. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Q. Cholesterol metabolism and homeostasis in the brain. Protein Cell 2015, 6, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Howe, V.; Sharpe, L.J.; Prabhu, A.V.; Brown, A.J. New insights into cellular cholesterol acquisition: Promoter analysis of human HMGCR and SQLE, two key control enzymes in cholesterol synthesis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 647–657. [Google Scholar] [CrossRef]
- Valdez, C.M.; Smith, M.A.; Perry, G.; Phelix, C.F.; Santamaria, F. Cholesterol Homeostasis Markers are Localized to Mouse Hippocampal Pyramidal and Granule Layers. Hippocampus 2010, 20, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Kusumo, H.; Costa, L.G.; Guizzetti, M. Cholesterol efflux is differentially regulated in neurons and astrocytes: Implications for brain cholesterol homeostasis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2013, 1831, 263–275. [Google Scholar] [CrossRef]
- Djelti, F.; Braudeau, J.; Hudry, E.; Dhenain, M.; Varin, J.; Bieche, I.; Marquer, C.; Chali, F.; Ayciriex, S.; Auzeil, N.; et al. CYP46A1 inhibition, brain cholesterol accumulation and neurodegeneration pave the way for Alzheimer’s disease. Brain 2015, 138, 2383–2398. [Google Scholar] [CrossRef]
- Di Paolo, G.; Kim, T. Erratum: Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nat. Rev. Neurosci. 2011, 12, 484. [Google Scholar] [CrossRef]
- Riabovol, O.O.; Tsymbal, D.O.; Minchenko, D.O.; Lebid-Biletska, K.M.; Sliusar, M.Y.; Rudnytska, O.V.; Minchenko, O.H. Effect of glucose deprivation on the expression of genes encoding glucocorticoid receptor and some related factors in ERN1-knockdown U87 glioma cells. Endocr. Regul. 2019, 53, 237–249. [Google Scholar] [CrossRef]
- Tian, X.; Li, Y.; Lei, L.; Feng, X.; Xin, H.; Chen, H.; Zhang, G.; Zuo, M.; Shi, W.; Cong, B. The TF/Nrf2/GSTP1 pathway is involved in stress-induced hepatocellular injury through ferroptosis. J. Cell. Mol. Med. 2024, 28, e18494. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Y.; Li, M.; Liu, J.; Zhang, G.; Ma, X.; Shi, W.; Cong, B. Bioinformatics Analysis of Molecular Interactions between Endoplasmic Reticulum Stress and Ferroptosis under Stress Exposure. Anal. Cell. Pathol. 2023, 2023, 9979291. [Google Scholar] [CrossRef] [PubMed]
| Non-Adjusted Model | OR | 95% CI | p-Value |
|---|---|---|---|
| Age | 0.334 | ||
| Gender | 1.749 | 1.592~1.921 | 0.000 |
| Race | 0.101 | ||
| Educational Level | 0.867 | 0.836~0.899 | 0.000 |
| Body mass index (BMI) | 1.027 | 1.021~1.032 | 0.000 |
| Family income | 0.773 | 0.749~0.798 | 0.000 |
| High-Density Lipoprotein (HDL) | 0.994 | 0.991~0.997 | 0.000 |
| Total Cholesterol | 1.002 | 1.001~1.003 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, R.; Li, Y.; Zhu, W.; Xin, H.; Yang, H.; Feng, X.; Wang, Z.; Li, S.; Cong, B.; Shi, W. The Regulation of Frontal Cortex Cholesterol Metabolism Abnormalities by NR3C1/NRIP1/NR1H2 Is Involved in the Occurrence of Stress-Induced Depression. Int. J. Mol. Sci. 2024, 25, 8075. https://doi.org/10.3390/ijms25158075
Shi R, Li Y, Zhu W, Xin H, Yang H, Feng X, Wang Z, Li S, Cong B, Shi W. The Regulation of Frontal Cortex Cholesterol Metabolism Abnormalities by NR3C1/NRIP1/NR1H2 Is Involved in the Occurrence of Stress-Induced Depression. International Journal of Molecular Sciences. 2024; 25(15):8075. https://doi.org/10.3390/ijms25158075
Chicago/Turabian StyleShi, Rui, Yingmin Li, Weihao Zhu, Hongjian Xin, Huihuang Yang, Xiaowei Feng, Zhen Wang, Shujin Li, Bin Cong, and Weibo Shi. 2024. "The Regulation of Frontal Cortex Cholesterol Metabolism Abnormalities by NR3C1/NRIP1/NR1H2 Is Involved in the Occurrence of Stress-Induced Depression" International Journal of Molecular Sciences 25, no. 15: 8075. https://doi.org/10.3390/ijms25158075
APA StyleShi, R., Li, Y., Zhu, W., Xin, H., Yang, H., Feng, X., Wang, Z., Li, S., Cong, B., & Shi, W. (2024). The Regulation of Frontal Cortex Cholesterol Metabolism Abnormalities by NR3C1/NRIP1/NR1H2 Is Involved in the Occurrence of Stress-Induced Depression. International Journal of Molecular Sciences, 25(15), 8075. https://doi.org/10.3390/ijms25158075






