DNA Sequence Variations Affecting Serotonin Transporter Transcriptional Regulation and Activity: Do They Impact Alcohol Addiction?
Abstract
:1. Introduction
2. Results
2.1. Allelic Frequencies
2.2. Genotipic Frequencies
2.3. Haplotype Analysis
3. Discussion
4. Materials and Methods
4.1. Analyzed Populations and Sampling
4.2. DNA Extraction
4.3. SNP and VNTR Region Genotyping
4.4. Polymerase Chain Reaction Assays
4.5. Mini-Sequencing Assay
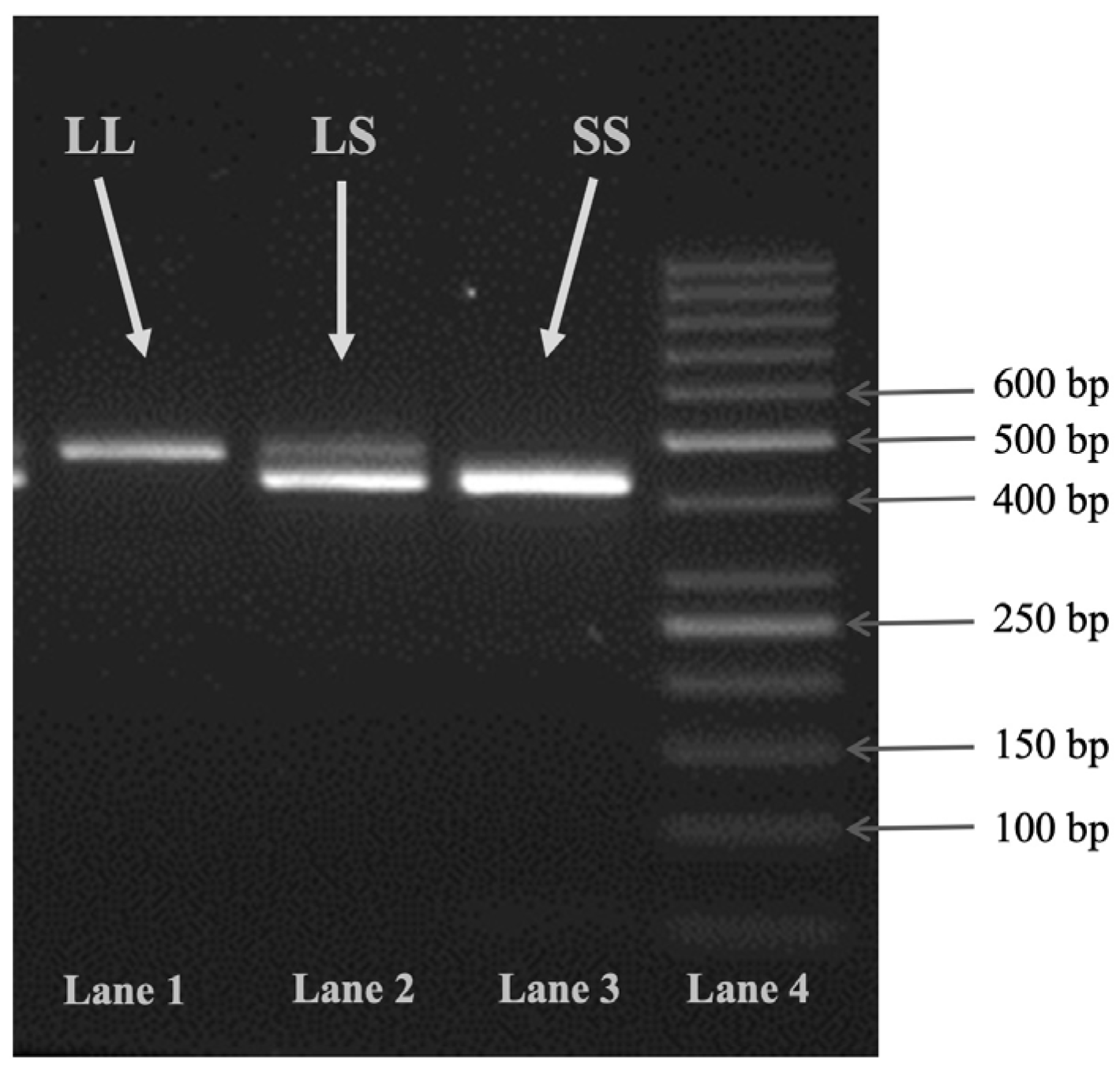
4.6. VNTR Region Genotyping
4.7. Haplotype Analyses
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haley, S.J.; Jardine, S.J.; Kelvin, E.A.; Herrmann, C.; Maroko, A.R. Neighborhood Alcohol Outlet Density, Historical Redlining, and Violent Crime in NYC 2014–2018. Int. J. Environ. Res. Public Health 2023, 20, 3212. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M. Behavioral Predictors of Substance-Use Initiation in Adolescents With and Without Attention-Deficit/Hyperactivity Disorder. Pediatrics 2006, 117, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Slater, M.D.; Hayes, A.F.; Goodall, C.E.; Ewoldsen, D.R. Increasing support for alcohol-control enforcement through news coverage of alcohol’s role in injuries and crime. J. Stud. Alcohol. Drugs 2012, 73, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Schuckit, M.A. Alcohol-use disorders. Lancet 2009, 373, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Tobore, T.O. On the Neurobiological Role of Oxidative Stress in Alcohol-Induced Impulsive, Aggressive and Suicidal Behavior. Subst. Use Misuse 2019, 54, 2290–2303. [Google Scholar] [CrossRef] [PubMed]
- Coriale, G.; Gencarelli, S.; Battagliese, G.; Delfino, D.; Fiorentino, D.; Petrella, C.; Greco, A.; Ralli, M.; Attilia, M.L.; Messina, M.P.; et al. Physiological Responses to Induced Stress in Individuals Affected by Alcohol Use Disorder with Dual Diagnosis and Alexithymia. Clin. Ter. 2020, 171, e120–e129. [Google Scholar] [CrossRef] [PubMed]
- Ledda, R.; Battagliese, G.; Attilia, F.; Rotondo, C.; Pisciotta, F.; Gencarelli, S.; Greco, A.; Fiore, M.; Ceccanti, M.; Attilia, M.L. Drop-out, relapse and abstinence in a cohort of alcoholic people under detoxification. Physiol. Behav. 2019, 198, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Ceccanti, M.; Coriale, G.; Hamilton, D.A.; Carito, V.; Coccurello, R.; Scalese, B.; Ciafrè, S.; Codazzo, C.; Messina, M.P.; Chaldakov, G.N.; et al. Virtual Morris task responses in individuals in an abstinence phase from alcohol. Can. J. Physiol. Pharmacol. 2018, 96, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Bryant, K.J.; Nelson, S.; Braithwaite, R.S.; Roach, D. Integrating HIV/AIDS and alcohol research. Alcohol. Res. Heal. J. Natl. Inst. Alcohol. Abus. Alcohol. 2010, 33, 167–178. [Google Scholar]
- Fama, R.; Le Berre, A.-P.; Sassoon, S.A.; Zahr, N.M.; Pohl, K.M.; Pfefferbaum, A.; Sullivan, E.V. Memory impairment in alcohol use disorder is associated with regional frontal brain volumes. Drug Alcohol. Depend. 2021, 228, 109058. [Google Scholar] [CrossRef]
- Ministry of Health. Relazione del Ministro della Salute al Parlamento Sugli Interventi Realizzati ai Sensi Della Legge 30.3.2001 N.125 “Legge Quadro in Materia di Alcol e Problemi Alcolcorrelati” Anni 2007–2008 e Successive Modifiche 2009. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_1451_allegato.pdf (accessed on 28 May 2024).
- Ciafrè, S.; Carito, V.; Ferraguti, G.; Greco, A.; Chaldakov, G.N.; Fiore, M.; Ceccanti, M. How alcohol drinking affects our genes: An epigenetic point of view. Biochem. Cell Biol. 2019, 97, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Tulisiak, C.T.; Harris, R.A.; Ponomarev, I. DNA modifications in models of alcohol use disorders. Alcohol 2017, 60, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Carito, V.; Ceccanti, M.; Ferraguti, G.; Coccurello, R.; Ciafrè, S.; Tirassa, P.; Fiore, M. NGF and BDNF Alterations by Prenatal Alcohol Exposure. Curr. Neuropharmacol. 2019, 17, 308–317. [Google Scholar] [CrossRef]
- Ciafrè, S.; Ferraguti, G.; Greco, A.; Polimeni, A.; Ralli, M.; Ceci, F.M.; Fiore, M. Alcohol as an early life stressor: Epigenetics, metabolic, neuroendocrine and neurobehavioral implications. Neurosci. Biobehav. Rev. 2020, 118, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Haan, E.; Westmoreland, K.E.; Schellhas, L.; Sallis, H.M.; Taylor, G.; Zuccolo, L.; Munafò, M.R. Prenatal smoking, alcohol and caffeine exposure and offspring externalizing disorders: A systematic review and meta-analysis. Addiction 2022, 117, 2602–2613. [Google Scholar] [CrossRef] [PubMed]
- Oei, J.L. Alcohol use in pregnancy and its impact on the mother and child. Addiction 2020, 115, 2148–2163. [Google Scholar] [CrossRef]
- Ceci, F.M.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Iannitelli, A.; Carito, V.; Tirassa, P.; Chaldakov, G.N.; Messina, M.P.; et al. Nerve Growth Factor in Alcohol Use Disorders. Curr. Neuropharmacol. 2020, 19, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Terracina, S.; Ferraguti, G.; Tarani, L.; Messina, M.P.; Lucarelli, M.; Vitali, M.; De Persis, S.; Greco, A.; Minni, A.; Polimeni, A.; et al. Transgenerational Abnormalities Induced by Paternal Preconceptual Alcohol Drinking. Findings from Humans and Animal Models. Curr. Neuropharmacol. 2021, 19, 1158–1173. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.C.; Wang, H.; Bedi, Y.; Golding, M.C. Preconception paternal alcohol exposure exerts sex-specific effects on offspring growth and long-term metabolic programming. Epigenetics Chromatin 2019, 12, 1–17. [Google Scholar] [CrossRef]
- Roach, A.N.; Zimmel, K.N.; Thomas, K.N.; Basel, A.; Bhadsavle, S.S.; Golding, M.C. Preconception paternal alcohol exposure decreases IVF embryo survival and pregnancy success rates in a mouse model. Mol. Hum. Reprod. 2023, 29, gaad002. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Lesch, O.M.; Walter, H. Subtypes of alcoholism and their role in therapy. Alcohol Alcohol. 1996, 31, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Malcolm, R.; Anton, R.F.; Randall, C.L.; Johnston, A.; Brady, K.; Thevos, A. A Placebo-Controlled Trial of Buspirone in Anxious Inpatient Alcoholics. Alcohol. Clin. Exp. Res. 1992, 16, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Giannouli, V. Lesch Type III Alcoholism in Bulgarian Women: Implications and Recommendations for Psychotherapy. Int. J. Caring Sci. 2017, 10, 1569–1576. [Google Scholar]
- Giannouli, V. Violence in severe mental illness: Is cognition missing in the associations with ethnicity, cannabis and alcohol? Australas Psychiatry 2017, 25, 633. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.T.; Noronha, A.; Goldman, D.; Koob, G.F. Genetic studies of alcohol dependence in the context of the addiction cycle. Neuropharmacology 2017, 122, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Stickel, F.; Moreno, C.; Hampe, J.; Morgan, M.Y. The genetics of alcohol dependence and alcohol-related liver disease. J. Hepatol. 2017, 66, 195–211. [Google Scholar] [CrossRef] [PubMed]
- Dick, D.M.; Balcke, E.; McCutcheon, V.; Francis, M.; Kuo, S.; Salvatore, J.; Meyers, J.; Bierut, L.J.; Schuckit, M.; Hesselbrock, V.; et al. The collaborative study on the genetics of alcoholism: Sample and clinical data. Genes. Brain Behav. 2023, 22, e12860. [Google Scholar] [CrossRef] [PubMed]
- Farris, S.P.; Wolen, A.R.; Miles, M.F. Using expression genetics to study the neurobiology of ethanol and alcoholism. Int. Rev. Neurobiol. 2010, 91, 95–128. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Johnson, E.C.; Colbert, S.; Pandey, G.; Chan, G.; Bauer, L.; Francis, M.W.; Hesselbrock, V.; Kamarajan, C.; Kramer, J.; et al. Evaluating risk for alcohol use disorder: Polygenic risk scores and family history. Alcohol. Clin. Exp. Res. 2022, 46, 374–383. [Google Scholar] [CrossRef]
- Johnson, E.C.; Salvatore, J.E.; Lai, D.; Merikangas, A.K.; Nurnberger, J.I.; Tischfield, J.A.; Xuei, X.; Kamarajan, C.; Wetherill, L.; Rice, J.P.; et al. The collaborative study on the genetics of alcoholism: Genetics. Genes. Brain Behav. 2023, 22, e12856. [Google Scholar] [CrossRef]
- D’Angelo, A.; Petrella, C.; Greco, A.; Ralli, M.; Vitali, M.; Giovagnoli, R.; De Persis, S.; Fiore, M.; Ceccanti, M.; Messina, M.P. Acute alcohol intoxication: A clinical overview. Clin. Ter. 2022, 173, 280–291. [Google Scholar] [CrossRef]
- Edenberg, H.J.; Foroud, T. Genetics of alcoholism. Handb. Clin. Neurol. 2014, 125, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.; Dick, D.M.; King, A.; Ray, L.A.; Sher, K.J.; Vena, A.; Vendruscolo, L.F.; Acion, L. Mechanisms of Alcohol Addiction: Bridging Human and Animal Studies. Alcohol. Alcohol. 2021, 55, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Neurocircuitry of alcohol addiction: Synthesis from animal models. Handb. Clin. Neurol. 2014, 125, 33–54. [Google Scholar] [CrossRef]
- Ferraguti, G.; Pascale, E.; Lucarelli, M. Alcohol Addiction: A Molecular Biology Perspective. Curr. Med. Chem. 2015, 22, 670–684. [Google Scholar] [CrossRef]
- Margoob, M.A.; Mushtaq, D. Serotonin transporter gene polymorphism and psychiatric disorders: Is there a link? Indian J. Psychiatry 2011, 53, 289–299. [Google Scholar] [CrossRef]
- Lovinger, D.M. Communication networks in the brain: Neurons, receptors, neurotransmitters, and alcohol. Alcohol. Res. Heal. J. Natl. Inst. Alcohol. Abus. Alcohol. 2008, 31, 196–214. [Google Scholar]
- Furukawa, T.A.; Cipriani, A.; Cowen, P.J.; Leucht, S.; Egger, M.; Salanti, G. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: A systematic review and dose-response meta-analysis. Lancet Psychiatry 2019, 6, 601–609. [Google Scholar] [CrossRef]
- Philibert, R.A.; Sandhu, H.; Hollenbeck, N.; Gunter, T.; Adams, W.; Madan, A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2008, 147B, 543–549. [Google Scholar] [CrossRef]
- Tahara, T.; Shibata, T.; Okubo, M.; Sumi, K.; Ishizuka, T.; Nakamura, M.; Nagasaka, M.; Nakagawa, Y.; Ohmiya, N.; Arisawa, T.; et al. Change in DNA methylation patterns of SLC6A4 gene in the gastric mucosa in functional dyspepsia. PLoS ONE 2014, 9, e105565. [Google Scholar] [CrossRef]
- Iurescia, S.; Seripa, D.; Rinaldi, M. Looking Beyond the 5-HTTLPR Polymorphism: Genetic and Epigenetic Layers of Regulation Affecting the Serotonin Transporter Gene Expression. Mol. Neurobiol. 2017, 54, 8386–8403. [Google Scholar] [CrossRef] [PubMed]
- Kranzler, H.R.; Armeli, S.; Tennen, H.; Covault, J.; Feinn, R.; Arias, A.J.; Pettinati, H.M.; Oncken, C. A double-blind, randomized trial of sertraline for alcohol dependence: Moderation by age of onset [corrected] and 5-hydroxytryptamine transporter-linked promoter region genotype. J. Clin. Psychopharmacol. 2011, 31, 22–30. [Google Scholar] [CrossRef]
- Mandal, T.; Bairy, L.K.; Sharma, P.S.V.N. Association between functional polymorphisms in serotonin transporter gene (SLC6A4) and escitalopram treatment response in depressive patients in a South Indian population. Eur. J. Clin. Pharmacol. 2020, 76, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Ma, Y.; Zhu, X.; Guo, R.; Wang, J.; He, L. Pharmacogenetic association of bi- and triallelic polymorphisms of SLC6A4 with antidepressant response in major depressive disorder. J. Affect. Disord. 2020, 273, 254–264. [Google Scholar] [CrossRef]
- Pascale, E.; Ferraguti, G.; Codazzo, C.; Passarelli, F.; Mancinelli, R.; Bonvicini, C.; Bruno, S.M.; Lucarelli, M.; Ceccanti, M. Alcohol dependence and serotonin transporter functional polymorphisms 5-HTTLPR and rs25531 in an Italian population. Alcohol Alcohol. 2015, 50, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Fratelli, C.; Siqueira, J.; Silva, C.; Ferreira, E.; Silva, I. 5httlpr genetic variant and major depressive disorder: A review. Genes 2020, 11, 1–21. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Fan, Q.-Y.; He, J.-H.; Zhu, S.-G.; Huang, C.-P.; Zhang, X.; Zhu, J.-H. SLC6A4 Repeat and Single-Nucleotide Polymorphisms Are Associated With Depression and Rest Tremor in Parkinson’s Disease: An Exploratory Study. Front. Neurol. 2019, 10, 333. [Google Scholar] [CrossRef]
- Tanahashi, S.; Tanii, H.; Konishi, Y.; Otowa, T.; Sasaki, T.; Tochigi, M.; Okazaki, Y.; Kaiya, H.; Okada, M. Association of Serotonin Transporter Gene (5-HTTLPR/rs25531) Polymorphism with Comorbidities of Panic Disorder. Neuropsychobiology 2021, 80, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-A.; Fang, W.-H.; Liu, Y.-P.; Tzeng, N.-S.; Shyu, J.-F.; Wan, F.-J.; Huang, S.-Y.; Chang, T.-C.; Chang, C.-C. Sex-specific pathways among tri-allelic serotonin transporter polymorphism, trait neuroticism and generalized anxiety disorder. J. Psychiatry Neurosci. 2020, 45, 379–386. [Google Scholar] [CrossRef]
- Alshogran, O.Y.; Al-Eitan, L.N.; Altawalbeh, S.M.; Aman, H.A. Association of DRD4 exon III and 5-HTTLPR VNTR genetic polymorphisms with psychiatric symptoms in hemodialysis patients. PLoS ONE 2021, 16, e0249284. [Google Scholar] [CrossRef]
- Hu, X.-Z.; Lipsky, R.H.; Zhu, G.; Akhtar, L.A.; Taubman, J.; Greenberg, B.D.; Xu, K.; Arnold, P.D.; Richter, M.A.; Kennedy, J.L.; et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 2006, 78, 815–826. [Google Scholar] [CrossRef]
- Martin, J.; Cleak, J.; Willis-Owen, S.A.G.; Flint, J.; Shifman, S. Mapping regulatory variants for the serotonin transporter gene based on allelic expression imbalance. Mol. Psychiatry 2007, 12, 421–422. [Google Scholar] [CrossRef]
- Seneviratne, C.; Huang, W.; Ait-Daoud, N.; Li, M.D.; Johnson, B.A. Characterization of a functional polymorphism in the 3’ UTR of SLC6A4 and its association with drinking intensity. Alcohol. Clin. Exp. Res. 2009, 33, 332–339. [Google Scholar] [CrossRef]
- Pinto, C.; Souza, R.P.; Lioult, D.; Semeralul, M.; Kennedy, J.L.; Warsh, J.J.; Wong, A.H.; De Luca, V. Parent of origin effect and allelic expression imbalance of the serotonin transporter in bipolar disorder and suicidal behaviour. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 533–538. [Google Scholar] [CrossRef]
- Hui, P.; Yang, J.; Wang, J.; Zhao, L.; Wang, X.; Su, X.; Wang, J.; Ma, W.; Fan, J.; Chen, W.; et al. Association between 5-hydroxytryptamine gene polymorphism rs140700 and primary insomnia in Chinese population. Intern. Med. J. 2021, 51, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Prasad, H.C.; Zhu, C.-B.; McCauley, J.L.; Samuvel, D.J.; Ramamoorthy, S.; Shelton, R.C.; Hewlett, W.A.; Sutcliffe, J.S.; Blakely, R.D. Human serotonin transporter variants display altered sensitivity to protein kinase G and p38 mitogen-activated protein kinase. Proc. Natl. Acad. Sci. USA 2005, 102, 11545–11550. [Google Scholar] [CrossRef] [PubMed]
- Prasad, H.C.; Steiner, J.A.; Sutcliffe, J.S.; Blakely, R.D. Enhanced activity of human serotonin transporter variants associated with autism. Philos. Trans. R Soc. London Ser. B Biol. Sci. 2009, 364, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Sutcliffe, J.S.; Delahanty, R.J.; Prasad, H.C.; McCauley, J.L.; Han, Q.; Jiang, L.; Li, C.; Folstein, S.E.; Blakely, R.D. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am. J. Hum. Genet. 2005, 77, 265–279. [Google Scholar] [CrossRef]
- Wendland, J.R.; Moya, P.R.; Kruse, M.R.; Ren-Patterson, R.F.; Jensen, C.L.; Timpano, K.R.; Murphy, D.L. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum. Mol. Genet. 2008, 17, 717–723. [Google Scholar] [CrossRef]
- Ozaki, N.; Goldman, D.; Kaye, W.H.; Plotnicov, K.; Greenberg, B.D.; Lappalainen, J.; Rudnick, G.; Murphy, D.L. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol. Psychiatry 2003, 8, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.N.; Plenge, P.; Bay, T.; Egebjerg, J.; Gether, U. A single nucleotide polymorphism in the human serotonin transporter introduces a new site for N-linked glycosylation. Neuropharmacology 2009, 57, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Mia, M.A.; Uddin, M.N.; Akter, Y.; Jesmin Wal Marzan, L. Exploring the Structural and Functional Effects of Nonsynonymous SNPs in the Human Serotonin Transporter Gene Through In Silico Approaches. Bioinform. Biol. Insights 2022, 16, 11779322221104308. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, J.D.; Speed, W.C.; Pakstis, A.J.; Heffelfinger, C.E.; Kidd, K.K. Worldwide population variation and haplotype analysis at the serotonin transporter gene SLC6A4 and implications for association studies. Biol. Psychiatry 2013, 74, 879–889. [Google Scholar] [CrossRef]
- Lovejoy, E.A.; Scott, A.C.; Fiskerstrand, C.E.; Bubb, V.J.; Quinn, J.P. The serotonin transporter intronic VNTR enhancer correlated with a predisposition to affective disorders has distinct regulatory elements within the domain based on the primary DNA sequence of the repeat unit. Eur. J. Neurosci. 2003, 17, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Voisey, J.; Swagell, C.D.; Hughes, I.P.; Lawford, B.R.; Young, R.M.D.; Morris, C.P. A novel SNP in COMT is associated with alcohol dependence but not opiate or nicotine dependence: A case control study. Behav. Brain Funct. 2011, 7, 51. [Google Scholar] [CrossRef]
- Boyd, S.J.; Schacht, J.P.; Prisciandaro, J.J.; Voronin, K.; Anton, R.F. Alcohol-Induced Stimulation Mediates the Effect of a GABRA2 SNP on Alcohol Self-Administrated among Alcohol-Dependent Individuals. Alcohol Alcohol. 2016, 51, 549–554. [Google Scholar] [CrossRef]
- Oo, K.Z.; Aung, Y.K.; Jenkins, M.A.; Win, A.K. Associations of 5HTTLPR polymorphism with major depressive disorder and alcohol dependence: A systematic review and meta-analysis. Aust. N. Z. J. Psychiatry 2016, 50, 842–857. [Google Scholar] [CrossRef]
- Haddley, K.; Bubb, V.J.; Breen, G.; Parades-Esquivel, U.M.; Quinn, J.P. Behavioural genetics of the serotonin transporter. Curr. Top. Behav. Neurosci. 2012, 12, 503–535. [Google Scholar] [CrossRef]
- Iurescia, S.; Seripa, D.; Rinaldi, M. Role of the 5-HTTLPR and SNP Promoter Polymorphisms on Serotonin Transporter Gene Expression: A Closer Look at Genetic Architecture and In Vitro Functional Studies of Common and Uncommon Allelic Variants. Mol. Neurobiol. 2016, 53, 5510–5526. [Google Scholar] [CrossRef]
- Wray, N.R.; James, M.R.; Gordon, S.D.; Dumenil, T.; Ryan, L.; Coventry, W.L.; Statham, D.J.; Pergadia, M.L.; Madden, P.A.; Heath, A.C.; et al. Accurate, Large-Scale Genotyping of 5HTTLPR and Flanking Single Nucleotide Polymorphisms in an Association Study of Depression, Anxiety, and Personality Measures. Biol. Psychiatry 2009, 66, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Herold, C.; Becker, T. Genetic association analysis with FAMHAP: A major program update. Bioinformatics 2009, 25, 134–136. [Google Scholar] [CrossRef] [PubMed]


| Variation | Position | Base Shift | Amino Acid Change | Function | References |
|---|---|---|---|---|---|
| rs25531 | Promoter | SNP A/G | Loss (G allele) | [54] | |
| rs2020933 | Intron 1 | SNP T/A | Gain (A allele) | [55] | |
| rs1042173 | 3′-UTR | SNP T/G | Gain (G allele) | [56,57] | |
| rs140700 | Intron 6 | SNP G/A | Loss (A allele) | [58] | |
| rs199909202 | Exon 7 | SNP C/T | Ser293Phe | Gain (T allele) | [59] |
| rs755973197 | Exon 9 | SNP C/A | Leu362Met | Gain (A allele) | [59] |
| rs28914834 | Exon 13 | SNP C/G | Leu550Val | Gain (G allele) | [60,61] |
| rs25532 | Promoter | SNP C/T | Loss (T allele) | [62] | |
| rs16965628 | Intron 1 | SNP C/G | Gain (G allele) | [62] | |
| rs28914832 | Exon 10 | SNP A/G | Ile425Val | Gain (G allele) | [59,63] |
| rs2228673 | Exon 5 | SNP G/T | Lys201Asn | Gain (T allele) | [64] |
| rs200850098 | Exon 8 | SNP C/T | Pro339Leu | Loss (T allele) | [59,65] |
| rs765035150 | Exon 3 | SNP A/G | Thr4Ala | Gain (G allele) | [59] |
| rs6355 | Exon 3 | SNP G/C | Gly56Ala | Gain (C allele) | [59,61,66] |
| rs28914833 | Exon 11 | SNP T/C | Phe465Leu | Gain (C allele) | [60] |
| 5-HTTLPR | Promoter | VNTR 43 bp | 14S-16L | Loss (S allele) | [49] |
| STin2 | Intron 2 | VNTR 17 bp | 9/10/12 repeats | Loss (9 and 10 repeats) | [67] |
| Polymorphisms | n Ctrls | n AUD | Allele | Ctrls n alleles (freq.) | AUD n alleles (freq.) | p Value | CI 95% |
|---|---|---|---|---|---|---|---|
| rs1799971 | 440 | 1447 | A | 747 (0.849) | 2486 (0.859) | 0.4853 | 0.8775 to 1.341 |
| G | 133 (0.151) | 408 (0.141) | |||||
| rs2020933 | 438 | 1008 | T | 807 (0.921) | 1851 (0.918) | 0.8378 | 0.7156 to 1.286 |
| A | 69 (0.079) | 165 (0.082) | |||||
| rs1042173 | 440 | 1391 | T | 439 (0.499) | 1481 (0.532) | 0.0901 | 0.7514 to 1.018 |
| G | 441 (0.501) | 1301 (0.468) | |||||
| rs140700 | 441 | 1008 | C | 812 (0.921) | 1861 (0.923) | 0.8775 | 0.7713 to 1.389 |
| T | 70 (0.079) | 155 (0.077) | |||||
| rs199909202 | 441 | 1008 | C | 882 (1.0) | 2016 (1.0) | na | na |
| T | 0 (0.0) | 0 (0.0) | |||||
| rs755973197 | 441 | 968 | C | 882 (1.0) | 1936 (1.0) | na | na |
| A | 0 (0.0) | 0 (0.0) | |||||
| rs28914834 | 441 | 968 | C | 882 (1.0) | 1936 (1.0) | na | na |
| G | 0 (0.0) | 0 (0.0) | |||||
| rs25532 | 168 | 215 | C | 309 (0.92) | 388 (0.902) | 0.4467 | 0.4866 to 1.339 |
| T | 27 (0.08) | 42 (0.098) | |||||
| rs16965628 | 440 | 982 | C | 805 (0.915) | 1768 (0.90) | 0.2484 | 0.6358 to 1.111 |
| G | 75 (0.085) | 196 (0.10) | |||||
| rs28914832 | 440 | 983 | T | 879 (0.999) | 1965 (0.999) | 0.8562 | 0.1396 to 35.81 |
| C | 1 (0.001) | 1 (0.001) | |||||
| rs2228673 | 441 | 984 | C | 882 (1.0) | 1968 (1.0) | na | na |
| A | 0 (0.0) | 0 (0.0) | |||||
| rs200850098 | 440 | 984 | C | 880 (1.0) | 1968 (1.0) | na | na |
| T | 0 (0.0) | 0 (0.0) | |||||
| rs765035150 | 441 | 944 | A | 882 (1.0) | 1888 (1.0) | na | na |
| G | 0 (0.0) | 0 (0.0) | |||||
| rs6355 | 441 | 944 | C | 874 (0.982) | 1875 (0.986) | 0.7021 | 0.5451 to 3.198 |
| G | 8 (0.018) | 13 (0.014) | |||||
| rs28914833 | 441 | 944 | A | 882 (1.0) | 1888 (1.0) | na | na |
| G | 0 (0.0) | 0 (0.0) | |||||
| rs25531+5-HTTLPR | 434 | 1049 | La | 447 (0.515) | 967 (0.461) | 0.0083 ** | 0.6873 to 0.9435 |
| Lg-S | 421 (0.485) | 1131 (0.539) | |||||
| STin2 | 439 | 1028 | 9 | 5 (0.006) | 19 (0.009) | 0.5273 | na |
| 10 | 293 (0.334) | 706 (0.343) | |||||
| 12 | 580 (0.660) | 1331 (0.648) |
| Polymorphisms | n Ctrls | n AUD | Genotype | Ctrls n (freq.) | AUD n (freq.) | p Value | CI 95% |
|---|---|---|---|---|---|---|---|
| rs1799971 | 440 | 1447 | AA | 319 (0.725) | 1069 (0.739) | 0.6676 | na |
| GA | 109 (0.248) | 348 (0.240) | |||||
| GG | 12 (0.0270) | 30 (0.021) | |||||
| rs2020933 | 438 | 1008 | TT | 370 (0.845) | 853 (0.846) | 0.2843 | na |
| AT | 67 (0.153) | 145 (0.144) | |||||
| AA | 1 (0.020) | 10 (0.010) | |||||
| rs1042173 | 440 | 1391 | TT | 117 (0.266) | 402 (0.289) | 0.1596 | na |
| TG | 205 (0.466) | 677 (0.487) | |||||
| GG | 118 (0.268) | 312 (0.224) | |||||
| rs140700 | 441 | 1008 | CC | 374 (0.848) | 858 (0.851) | 0.9068 | na |
| CT | 64 (0.145) | 145 (0.144) | |||||
| TT | 3 (0.007) | 5 (0.005) | |||||
| rs199909202 | 441 | 1008 | CC | 441 (1.0) | 1008 (1.0) | na | na |
| CT | 0 (0.0) | 0 (0.0) | |||||
| TT | 0 (0.0) | 0 (0.0) | |||||
| rs755973197 | 441 | 968 | CC | 441 (1.0) | 968 (1.0) | na | na |
| CA | 0 (0.0) | 0 (0.0) | |||||
| AA | 0 (0.0) | 0 (0.0) | |||||
| rs28914834 | 441 | 968 | CC | 441(1.0) | 968 (1.0) | na | na |
| CG | 0 (0.0) | 0 (0.0) | |||||
| GG | 0 (0.0) | 0 (0.0) | |||||
| rs25532 | 168 | 215 | CC | 142 (0.845) | 174 (0.809) | 0.6223 | na |
| CT | 25 (0.149) | 40 (0.186) | |||||
| TT | 1 (0.006) | 1 (0.005) | |||||
| rs16965628 | 440 | 982 | CC | 366 (0.832) | 798 (0.813) | 0.1673 | na |
| CG | 73 (0.166) | 172 (0.175) | |||||
| GG | 1 (0.002) | 12 (0.012) | |||||
| rs28914832 | 440 | 983 | TT | 439 (0.998) | 982 (0.999) | na | na |
| TC | 1 (0.002) | 1 (0.001) | |||||
| CC | 0 (0.0) | 0 (0.0) | |||||
| rs2228673 | 441 | 984 | CC | 441 (1.0) | 984 (1.0) | na | na |
| CA | 0 (0.0) | 0 (0.0) | |||||
| AA | 0 (0.0) | 0 (0.0) | |||||
| rs200850098 | 440 | 984 | CC | 440 (1.0) | 984 (1.0) | na | na |
| CT | 0 (0.0) | 0 (0.0) | |||||
| TT | 0 (0.0) | 0 (0.0) | |||||
| rs765035150 | 441 | 944 | AA | 944 (1.0) | 441 (1.0) | na | na |
| AG | 0 (0.0) | 0 (0.0) | |||||
| GG | 0 (0.0) | 0 (0.0) | |||||
| rs6355 | 441 | 944 | CC | 433 (0.982) | 931 (0.986) | na | na |
| CG | 8 (0.018) | 13 (0.014) | |||||
| GG | 0 (0.0) | 0 (0.0) | |||||
| rs28914833 | 441 | 944 | AA | 441 (1.0) | 944 (1.0) | na | na |
| AG | 0 (0.0) | 0 (0.0) | |||||
| GG | 0 (0.0) | 0 (0.0) | |||||
| rs25531+5-HTTLPR | 434 | 1049 | La/La | 116 (0.267) | 245 (0.234) | 0.0151 * | na |
| La/Lg–La/S | 215 (0.496) | 477 (0.454) | |||||
| Lg/Lg–Lg/S–S/S | 103 (0.237) | 327 (0.312) | |||||
| STin2 | 439 | 1028 | 9/9 | 0 (0.0) | 1 (0.001) | 0.8319 | na |
| 10/10 | 53 (0.121) | 120 (0.117) | |||||
| 12/12 | 195 (0.444) | 427 (0.415) | |||||
| 9/10 | 1 (0.002) | 3 (0.003) | |||||
| 9/12 | 4 (0.009) | 14 (0.014) | |||||
| 10/12 | 186 (0.424) | 463 (0.450) |
| Specific Haplotype * [vs All the Other Haplotypes] | Ctrls n (Frequency) | AUD n (Frequency) | Χ, df | p | OR | CI |
|---|---|---|---|---|---|---|
| H1: G T T C C C C C C T C C A C A 14 12 | 30 (0.033) | 106 (0.056) | 6.662, 1 | 0.0098 ** | 0.5836 | 0.3860 to 0.8825 |
| all the other haplotypes | 868 (0.967) | 1790 (0.944) | ||||
| H2: A T G C C C C C C T C C A C A 16 12 | 57 (0.063) | 261 (0.138) | 33.25, 1 | <0.0001 *** | 0.4246 | 0.3150 to 0.5722 |
| all the other haplotypes | 841 (0.937) | 1635 (0.862) | ||||
| H3: A T T C C C C C C T C C A C A 16 10 | 81 (0.090) | 384 (0.203) | 55.43, 1 | <0.0001 *** | 0.3904 | 0.3027 to 0.5034 |
| all the other haplotypes | 817 (0.910) | 1512 (0.797) | ||||
| H4: G T G C C C C C C T C C A C A 14 12 | 129 (0.144) | 405 (0.214) | 19.29, 1 | <0.0001 *** | 0.6176 | 0.4974 to 0.7668 |
| all the other haplotypes | 769 (0.856) | 1491 (0.786) | ||||
| H5: G T G C C C C T C T C C A C A 14 12 | 129 (0.144) | 86 (0.045) | 82.89, 1 | <0.0001 *** | 3.531 | 2.653 to 4.698 |
| all the other haplotypes | 769 (0.856) | 1810 (0.955) |
| Variation | Base Shift | Function | H2 | H3 | H4 | H5 |
|---|---|---|---|---|---|---|
| rs25531 | SNP A/G | Loss (G allele) | + | + | - | - |
| rs1042173 | SNP T/G | Gain (G allele) | + | - | + | + |
| rs25532 | SNP C/T | Loss (T allele) | + | + | + | - |
| 5-HTTLPR | VNTR 43 bp | Loss (S allele) | + | + | - | - |
| STin2 | VNTR 17 bp | Loss (9 and 10 repeats) | + | - | + | + |
| Study Sample (n = 1447) | |
|---|---|
| Age in years | 45.37 ± 10.05 |
| Ethnic Origin (%) | |
| Caucasian | 93.5 |
| African | 2.7 |
| Hispanics | 2.6 |
| Asian | 1.2 |
| Marital Status (%) | |
| Single | 37.1 |
| Married | 33.7 |
| Separated/Divorced | 27.4 |
| Widowed | 1.8 |
| Qualifications (%) | |
| Primary School | 1.2 |
| Middle School | 41.7 |
| High School | 45.3 |
| University Degree | 11.8 |
| Employment Status (%) | |
| Workers | 60.3 |
| Unemployed | 30.5 |
| Retired | 9.2 |
| Smokers (%) | 75 |
| Daily cigarettes’ numbers | 18.5 ± 2.5 |
| Family History of Alcoholism (%) | 83.9 |
| From both parents | 12.4 |
| From father | 30.6 |
| Alcohol Related Variables | |
| Age of onset of at-risk drinking | 24.8 ± 2.7 |
| Years of at-risk drinking | 14.4 ± 2.8 |
| Alcohol units’ intake per die 30 days before Day Hospital admission | 15.9 ± 2.3 |
| Alcohol preference | |
| Wine (%) | 49.8 |
| Beer (%) | 35.6 |
| Spirit (%) | 14.6 |
| Variation | Forward Primer | Reverse Primer |
|---|---|---|
| rs25531 | CAACCTCCCAGCAACTCCCTGTA | ATGCTGGGGGGGCTGCAG |
| rs2020933 | TTTTCTTCTGAACTGGGGCTTTTGC | CATCCATATTGGAACGGTCACTGC |
| rs1042173 | GCGTAGGAGAGAACAGGGATGC | TGGGCCCAAAATATTGGACTAGAG |
| rs140700 | TAGTGGGCTCAGAGGTAGTTCTCCTG | CTGCCAATTGGGTTTCAAGTAGAAG |
| rs199909202 | TAGTGGGCTCAGAGGTAGTTCTCCTG | TCTGCCAATTGGGTTTCAAGTAGAAG |
| rs755973197 | TGTGTGGTGGTCATGGCAGTC | TCCCAGGCTCAAGCAATCTTCC |
| rs28914834 | AGTCCCCCAGCCCCACTTTC | AGGTGCCCATCACCACACC |
| rs25532 | CTGCACCCCTCGCAGTATCC | GGCTGAGCGTCTAGAGGGACTG |
| rs16965628 | CCCCAAGCACTGATTGAGAGCAG | ATCACCACCATACATCCGCAACC |
| rs28914832 | AGATGGAAGCCCCACCCTTCC | CCTCACCGTGCTGTCCAAGC |
| rs2228673 | AACGGCAGGGCCACTTTTCC | GGCCGTGGAGCACTTGAGGTAG |
| rs200850098 | CCCCTGCTGTGTTCCAGGTG | CCGTCGGTCCAATCACCTTCC |
| rs765035150 | GAGTCAATCCCGACGTGTCAATCC | ATCCACCTTCTTGCCCCAGGTC |
| rs6355 | GAGTCAATCCCGACGTGTCAATCC | ATCCACCTTCTTGCCCCAGGTC |
| rs28914833 | GAAGTTCTGTCCACGTGTGCTATTTTG | GGAGTAACAACCTCCCCTCCTTTG |
| 5-HTTLPR | CAACCTCCCAGCAACTCCCTGTA | GAGGGACTGAGCTGGACAACCAC |
| STin2 | GGGAGACCTGGGGCAAGAAG | TCAAGAGGACCTACAGCCCATCC |
| SNP | Sequence | Primer Length |
|---|---|---|
| rs25531 | AAAATCCCCCCTGCACCCCC | 20 (16 + 4) |
| rs2020933 | AAAGAAATCAGTTTTGTCCAGAAAAGTGAACC | 32 (26 + 6) |
| rs1042173 | GAAAAGAAAAAAAGGCCATATATTTTCTGAGTAGCATATA | 40 (26 + 14) |
| rs140700 | AGAAAAGAAAAAAAAAGGAAAAAGAAGACCTTGAGAAAGGAGGG* | 44 (21 + 23) |
| rs199909202 | AAAGAGAAAAAAAAAAAAAGAAGCCACCTTCCCTTATATCATCCTTT | 47 (26 + 21) |
| rs755973197 | AGAGAAAAAAGAAAAAAGGAAAAAAAGAAAAAAAAAAAAAGTTTTCCCCTCCAGAGATGCC | 61 (21 + 40) |
| rs28914834 | GGAAAAAAAAAAAGAAAAAAAAAAAAAAAAAGAAGAAAAAAAAAAAGCCATCAGCCCTCTGTTTCTC | 67 (21 + 46) |
| rs25532 | AACCCATGCACCCCCGG | 17 |
| rs16965628 | GCTAGGGTATGAAGTAGAAAGGCA | 24 |
| rs28914832 | AAGGAAAAGACGTGATTAACATCAGAAAGAAGATGA * | 36 (26 + 10) |
| rs2228673 | GAGAAAAAAAAAGAGGAAGGAAAAAAAAGTTGCCAGTGTTCCAGGAGTT * | 49 (22 + 27) |
| rs200850098 | AAAAAAAGAAAAAAAGAGAGAAAGAAAACTCAGATCTTCTTCTCTCTTGGTC | 52 (24 + 28) |
| rs765035150 | AAAGAAAAAAAAGAGAAAGAGAAGAAAAAGAATACTAACCAGCAGGATGGAGACG | 55 (23 + 32) |
| rs6355 | GAAGGAAAAGAAAGAAGAAAAAAAAAAAAAAAAAAAGATAGAGTGCCGTGTGTCATCT * | 58 (22 + 36) |
| rs28914833 | GAGAAAGAGGGGAAAGAAAAAGAAGAAAGAAAGGGAGGAAGAAAAATGACCACGGCGAGCACGA * | 64 (19 + 45) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraguti, G.; Francati, S.; Codazzo, C.; Blaconà, G.; Testino, G.; Angeloni, A.; Fiore, M.; Ceccanti, M.; Lucarelli, M. DNA Sequence Variations Affecting Serotonin Transporter Transcriptional Regulation and Activity: Do They Impact Alcohol Addiction? Int. J. Mol. Sci. 2024, 25, 8089. https://doi.org/10.3390/ijms25158089
Ferraguti G, Francati S, Codazzo C, Blaconà G, Testino G, Angeloni A, Fiore M, Ceccanti M, Lucarelli M. DNA Sequence Variations Affecting Serotonin Transporter Transcriptional Regulation and Activity: Do They Impact Alcohol Addiction? International Journal of Molecular Sciences. 2024; 25(15):8089. https://doi.org/10.3390/ijms25158089
Chicago/Turabian StyleFerraguti, Giampiero, Silvia Francati, Claudia Codazzo, Giovanna Blaconà, Giancarlo Testino, Antonio Angeloni, Marco Fiore, Mauro Ceccanti, and Marco Lucarelli. 2024. "DNA Sequence Variations Affecting Serotonin Transporter Transcriptional Regulation and Activity: Do They Impact Alcohol Addiction?" International Journal of Molecular Sciences 25, no. 15: 8089. https://doi.org/10.3390/ijms25158089







