Epigenetic Regulation of the Renin–Angiotensin–Aldosterone System in Hypertension
Abstract
1. Introduction
2. Epigenetic Regulation of Gene Expression
2.1. DNA Methylation
2.2. Histone Modifications
2.3. Micro RNAs (miRNAs)
3. Epigenetic Regulation of the AGT Gene
3.1. Salt-Sensitive Hypertension (SSH)
3.2. Primary Aldosteronism
4. Epigenetic Regulation of the Angiotensin-Converting Enzyme (ACE)
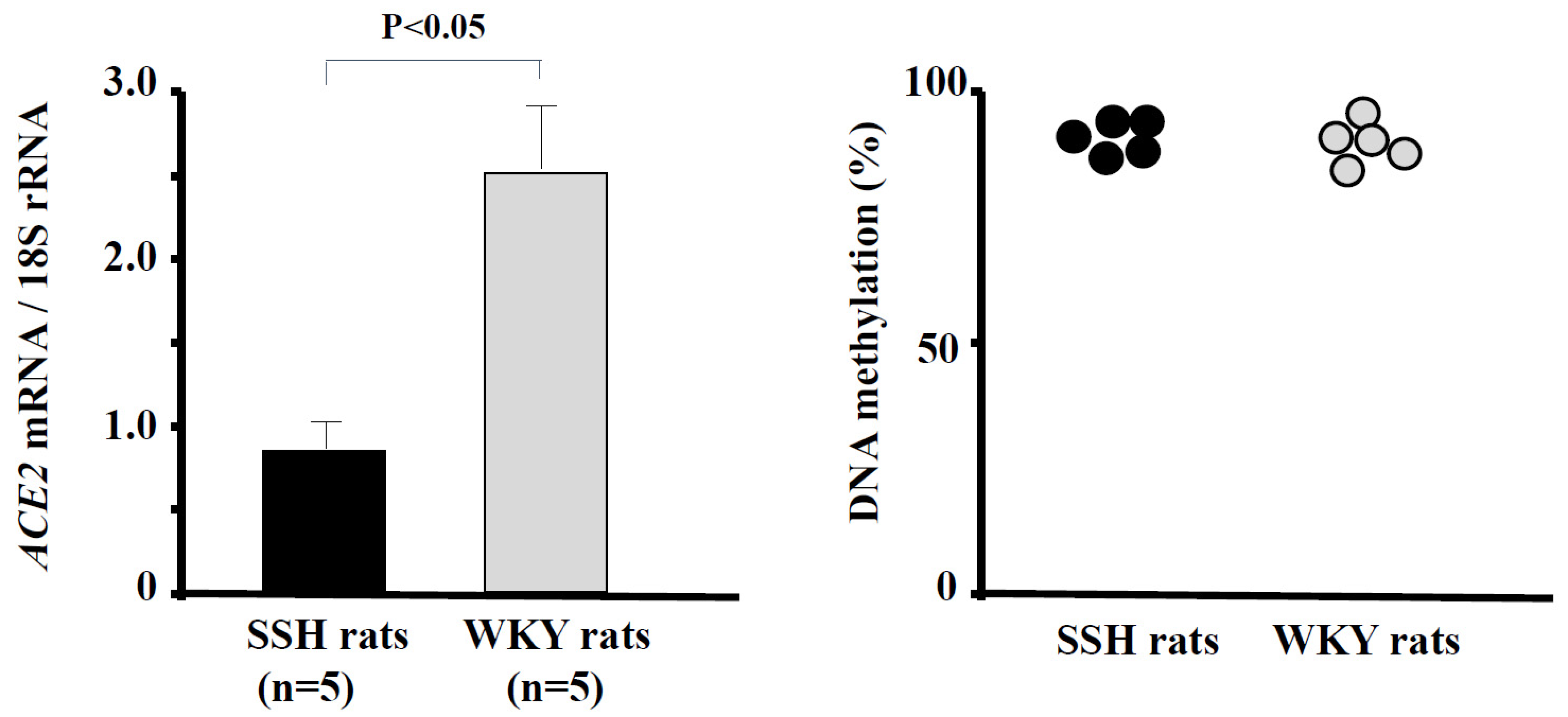
5. Epigenetic Regulation of ACE2
6. Epigenetic Regulation of AT1R
7. Epigenetic Regulation of CYP11B2
7.1. Epigenetics and Aldosterone-Producing Adenoma (APA)
7.2. Epigenetic Regulation of Mineralocorticoid-Related Genes in SSH
7.3. Epigenetic Control of Mineralocorticoid Receptors
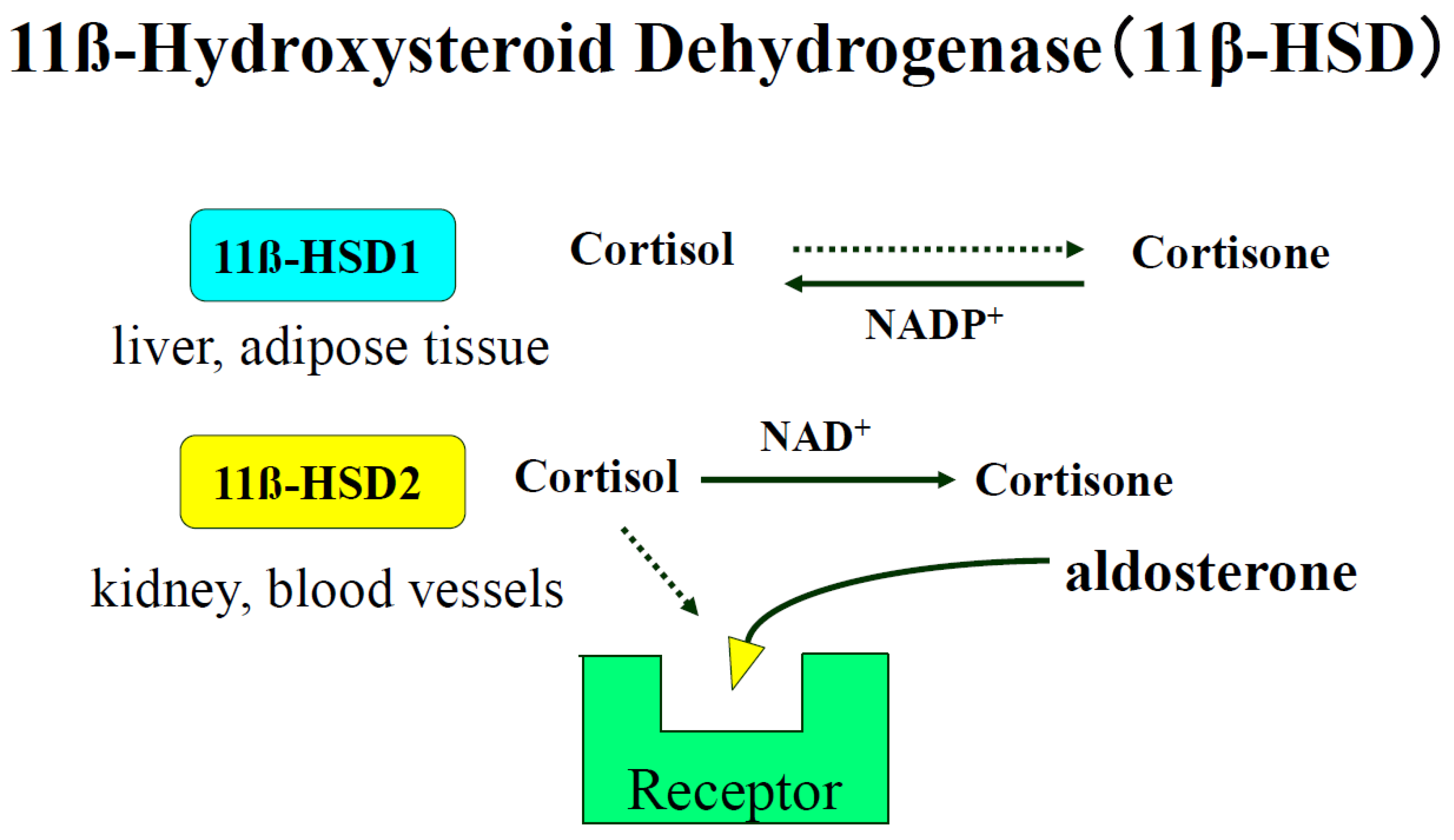
7.4. Epigenetic Control of 11ß-Hydroxysteroid Dehydrogenase Type 2
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACE | angiotensin-converting enzyme |
| AGT | angiotensinogen |
| APA | aldosterone-producing adenoma |
| AT1R | angiotensin II type 1 receptor |
| CEBP | CCAAT enhancer-binding protein |
| DNA | deoxyribonucleic acid |
| HATs | histone acetyl transferases |
| HCM | hypertrophic cardiomyopathy |
| HDACs | histone deacetylases |
| mRNA | messenger ribonucleic acid |
| miRNA | microRNA |
| MBD | methyl-CpG-binding domain |
| MR | mineralocorticoid receptor |
| MRA | mineralocorticoid receptor antagonist |
| PA | primary aldosteronism |
| RAAS | renin–angiotensin–aldosterone system |
| RNA | ribonucleic acid |
| SHR | spontaneously hypertensive rat |
| SSH | salt-sensitive hypertension |
| TFs | transcription factors |
| UTR | untranslated region |
References
- Paz Ocaranza, M.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Azushima, K.; Morisawa, N.; Tamura, K.; Nishiyama, A. Recent research advances in renin-angiotensin-aldosterone system receptors. Curr. Hypertens. Rep. 2020, 22, 22. [Google Scholar] [CrossRef] [PubMed]
- Ames, M.K.; Atkins, C.E.; Pitt, B.J. The renin-angiotensin-aldosterone system and its suppression. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Cruz-López, E.O.; Ye, D.; Wu, C.; Lu, H.S.; Uijl, E.; Mirabito Colafella, K.M.; Danser, A.H.J. Angiotensinogen suppression: A new tool to treat cardiovascular and renal disease. Hypertension 2022, 79, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Yasue, S.; Masuzaki, H.; Okada, S.; Ishii, T.; Kozuka, C.; Tanaka, T.; Fujikura, J.; Ebihara, K.; Hosoda, K.; Katsurada, A.; et al. Adipose tissue-specific regulation of angiotensinogen in obese humans and mice: Impact of nutritional status and adipocyte hypertrophy. Am. J. Hypertens. 2010, 23, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Cruz-López, E.O.; Uijl, E.; Danser, A.H.J. Perivascular adipose tissue in vascular function: Does locally synthesized angiotensinogen play a role? J. Cardiovasc. Pharmacol. 2021, 78 (Suppl. S6), S53–S62. [Google Scholar] [CrossRef]
- Khurana, V.; Goswami, B. Angiotensin converting enzyme (ACE). Clin. Chim. Acta 2022, 524, 113–122. [Google Scholar] [CrossRef]
- Le, D.; Brown, L.; Malik, K.; Murakami, S. Two opposing functions of angiotensin-converting enzyme (ACE) that links hypertension, dementia, and aging. Int. J. Mol. Sci. 2021, 22, 13178. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.R.; Khalil, R.A. Regulation of vascular angiotensin II type 1 and type 2 receptor and angiotensin-(1–7)/MasR signaling in normal and hypertensive pregnancy. Biochem. Pharmacol. 2024, 220, 115963. [Google Scholar] [CrossRef]
- Bierstadt, S.; Casaro, E.B.; Range, É.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Lawal, I.O.; Kgatle, M.M.; Mokoala, K.; Farate, A.; Sathekge, M.M. Cardiovascular disturbances in COVID-19: An updated review of the pathophysiology and clinical evidence of cardiovascular damage induced by SARS-CoV-2. BMC Cardiovasc. Disord. 2022, 22, 93. [Google Scholar] [CrossRef]
- Agarwal, R.; Kolkhof, P.; Bakris, G.; Bauersachs, J.; Haller, H.; Wada, T.; Zannad, F. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur. Heart J. 2021, 42, 152–161. [Google Scholar] [CrossRef]
- Barrera-Chimal, J.; Lima-Posada, I.; Bakris, G.L.; Jaisser, F. Mineralocorticoid receptor antagonists in diabetic kidney diseasemechanistic and therapeutic effects. Nat. Rev. Nephrol. 2022, 18, 56–70. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Uehara, T.; Azushima, K.; Wakui, H.; Tamura, K. Updates for cardio-kidney protective effects by angiotensin receptor-neprilysin inhibitor: Requirement for additional evidence of kidney protection. J. Am. Heart Assoc. 2023, 12, e029565. [Google Scholar] [CrossRef]
- Parksook, W.W.; Williamn, G.H. Aldosterone and cardiovascular diseases. Cardiovasc. Res. 2023, 119, 28–44. [Google Scholar] [CrossRef]
- Ohno, Y.; Sone, M.; Inagaki, N.; Yamasaki, T.; Ogawa, O.; Takeda, Y.; Kurihara, I.; Itoh, H.; Umakoshi, H.; Tsuiki, M.; et al. Prevalence of cardiovascular disease and its risk factors in primary aldosteronism: A multicenter study in Japan. Hypertension 2018, 71, 530–537. [Google Scholar] [CrossRef]
- Zennaro, M.C.; Boulkroun, S.; Fernandes-Rosa, F.L. Pathogenesis and treatment of primary aldosteronism. Nat. Rev. Endocrinol. 2020, 16, 578–589. [Google Scholar] [CrossRef]
- Takeda, Y.; Zhu, A.; Yoneda, T.; Usukura, M.; Takata, H.; Yamagishi, M. Effects of aldosterone and angiotensin II receptor blockade on cardiac angiotensinogen and angiotensin-converting enzyme 2 expression in Dahl salt-sensitive hypertensive rats. Am. J. Hypertens. 2007, 20, 1119–1124. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Tostes, R.C.; Paradis, P.; Schiffrin, E.L. Aldosterone, inflammation, immune system, and hypertension. Am. J. Hypertens. 2021, 34, 15–27. [Google Scholar] [CrossRef]
- Dzau, V.J. Multiple pathways of angiotensin production in the blood vessel wall: Evidence, possibilities and hypotheses. J. Hypertens. 1989, 7, 933–936. [Google Scholar] [CrossRef]
- Briones, A.M.; Nguyen Dinh Cat, A.; Callera, G.E.; Yogi, A.; Burger, D.; He, Y.; Corrêa, J.W.; Gagnon, A.M.; Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P.; et al. Adipocytes produce aldosterone through calcineurin-dependent signaling pathways: Implications in diabetes mellitus-associated obesity and vascular dysfunction. Hypertension 2012, 59, 1069–1078. [Google Scholar] [CrossRef]
- Takeda, Y. Role of cardiovascular aldosterone in hypertension. Curr. Med. Chem. Cardiovasc. Hematol. Agents 2005, 3, 261–266. [Google Scholar] [CrossRef]
- Xu, C. Extra-adrenal aldosterone: A mini review focusing on the physiology and pathophysiology of intrarenal aldosterone. Endocrine 2023, 83, 285–301. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, J.Y.; Cho, Y.; Koh, S.B.; Kim, N.; Choi, J.R. Genetically, dietary sodium intake is causally associated with salt-sensitive hypertension risk in a community-based cohort study: A mendelian randomization approach. Curr. Hypertens. Rep. 2020, 22, 45. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Uzu, T.; Fujii, T.; Nishimura, M.; Kuroda, S.; Nakamura, S.; Inenaga, T.; Kimura, G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 1997, 350, 1734–1737. [Google Scholar] [CrossRef]
- Weinberger, M.H.; Fineberg, N.S.; Fineberg, S.E.; Weinberger, M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001, 37, 429–432. [Google Scholar] [CrossRef]
- Stoll, S.; Wang, C.; Qiu, H. DNA methylation and histone modification in hypertension. Int. J. Mol. Sci. 2018, 19, 1174. [Google Scholar] [CrossRef]
- Demura, M.; Demura, Y.; Takeda, Y.; Saijoh, K. Dynamic regulation of the angiotensinogen gene by DNA methylation, which is influenced by various stimuli experienced in daily life. Hypertens. Res. 2015, 38, 519–527. [Google Scholar] [CrossRef]
- Klimczak-Tomaniak, D.; Haponiuk-Skwarlińska, J.; Kuch, M.; Pączek, L. Crosstalk between microRNA and oxidative stress in heart failure: A systematic review. Int. J. Mol. Sci. 2022, 23, 15013. [Google Scholar] [CrossRef]
- Wang, F.; Demura, M.; Cheng, Y.; Zhu, A.; Karashima, S.; Yoneda, T.; Demura, Y.; Maeda, Y.; Namiki, M.; Ono, K.; et al. Dynamic CCAAT/enhancer binding protein-associated changes of DNA methylation in the angiotensinogen gene. Hypertension 2014, 63, 281–288. [Google Scholar] [CrossRef]
- Guarner-Lans, V.; Ramírez-Higuera, A.; Rubio-Ruiz, M.E.; Castrejón-Téllez, V.; Soto, M.E.; Pérez-Torres, I. Early programming of adult systemic essential hypertension. Int. J. Mol. Sci. 2020, 21, 1203. [Google Scholar] [CrossRef] [PubMed]
- Field, A.; Adelman, K. Evaluating enhancer function and transcription. Annu. Rev. Biochem. 2020, 89, 213–234. [Google Scholar] [CrossRef]
- Huang, Y.; Ting, P.Y.; Yao, T.M.; Homma, T.; Brooks, D.; Rangel, I.K.; Adler, G.K.; Romero, J.R.; Williams, J.S.; Pojoga, L.H.; et al. Histone demethylase LSD1 deficiency and biological sex: Impact on blood pressure and aldosterone production. J. Endocrinol. 2019, 240, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M.; Giacca, M. Small non-coding RNA therapeutics for cardiovascular disease. Eur. Heart J. 2022, 43, 4548–4561. [Google Scholar] [CrossRef]
- Mahtal, N.; Lenoir, O.; Tinel, C.; Anglicheau, D.; Tharaux, P.L. MicroRNAs in kidney injury and disease. Nat. Rev. Nephrol. 2022, 18, 643–662. [Google Scholar] [CrossRef]
- Sharma, N.M.; Nandi, S.S.; Zheng, H.; Mishra, P.K.; Patel, K.P. A novel role for miR-133a in centrally mediated activation of the renin-angiotensin system in congestive heart failure. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H968–H979. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, X.; Zhao, T.; Zhang, X.; Qu, P.; Zhao, H. AGT, targeted by miR-149-5p, promotes IL-6-induced inflammatory responses of chondrocytes in osteoarthritis via activating JAK2/STAT3 pathway. Clin. Exp. Rheumatol. 2020, 38, 1088–1095. [Google Scholar]
- Liu, T.T.; Hao, Q.; Zhang, Y.; Li, Z.H.; Cui, Z.H.; Yang, W. Effects of microRNA-133b on retinal vascular endothelial cell proliferation and apoptosis through angiotensinogen-mediated angiotensin II- extracellular signal-regulated kinase 1/2 signalling pathway in rats with diabetic retinopathy. Acta Ophthalmol. 2018, 96, e626–e635. [Google Scholar] [CrossRef]
- Chen, X.K.; Ouyang, L.J.; Yin, Z.Q.; Xia, Y.Y.; Chen, X.R.; Shi, H.; Xiong, Y.; Pi, L.H. Effects of microRNA-29a on retinopathy of prematurity by targeting AGT in a mouse model. Am. J. Transl. Res. 2017, 9, 791–801. [Google Scholar]
- Novák, J.; Maceková, S.; Héžová, R.; Máchal, J.; Zlámal, F.; Hlinomaz, O.; Rezek, M.; Souček, M.; Vašků, A.; Slabý, O.; et al. Polymorphism rs7079 in miR-31/-584 binding site in angiotensinogen gene associates with earlier onset of coronary artery disease in Central European population. Genes 2022, 13, 1981. [Google Scholar] [CrossRef]
- Takeda, Y.; Demura, M.; Yoneda, T.; Takeda, Y. DNA methylation of the angiotensinogen gene, agt, and the aldosterone synthase gene, CYP11B2 in cardiovascular diseases. Int. J. Mol. Sci. 2021, 22, 4587. [Google Scholar] [CrossRef] [PubMed]
- Mopidevi, B.; Kaw, M.K.; Puri, N.; Ponnala, M.; Jain, S.; Rana, A.; Keetha, N.R.; Khuder, S.A.; Fiering, S.N.; Kumar, A. Variable transcriptional regulation of the human aldosterone synthase gene causes salt-dependent high blood pressure in transgenic mice. Circ. Cardiovasc. Genet. 2015, 8, 30–39. [Google Scholar] [CrossRef]
- Pellieux, C.; Montessuit, C.; Papageorgiou, I.; Pedrazzini, T.; Lerch, R. Differential effects of high-fat diet on myocardial lipid metabolism in failing and nonfailing hearts with angiotensin II-mediated cardiac remodeling in mice. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H1795–H1805. [Google Scholar] [CrossRef]
- Dogra, P.; Bancos, I.; Young, W.F., Jr. Primary aldosteronism: A pragmatic approach to diagnosis and management. Mayo Clin. Proc. 2023, 98, 1207–1215. [Google Scholar] [CrossRef]
- Hanslik, G.; Wallaschofski, H.; Dietz, A.; Riester, A.; Reincke, M.; Allolio, B.; Lang, K.; Quack, I.; Rump, L.C.; Willenberg, H.S.; et al. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldsoteronism of the German Conn’s registry. Eur. J. Endocrinol. 2015, 173, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Akehi, Y.; Yanase, T.; Motonaga, R.; Umakoshi, H.; Tsuiki, M.; Takeda, Y.; Yoneda, T.; Kurihara, I.; Itoh, H.; Katabami, T.; et al. High prevalence of diabetes in patients with primary aldosteronism (PA) associated with subclinical hypercortisolism and prediabetes more prevalent in bilateral than unilateral PA: A large, multicenter cohort study in Japan. Diabetes Care. 2019, 42, 938–945. [Google Scholar] [CrossRef]
- Garg, R.; Adler, G.K. Role of mineralocorticoid receptor in insulin resistance. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 168–175. [Google Scholar] [CrossRef]
- Adler, G.K.; Murray, G.R.; Turcu, A.F.; Nian, H.; Yu, C.; Solorzano, C.C.; Manning, R.; Dungeng Peng, D.; Luther, J.M. Primary aldosteronism decreases insulin secretion and increases insulin clearance in humans. Hypertension 2020, 75, 1251–1259. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, H.; Zhang, J.; Xie, C.; Fan, C.; Zhang, H.; Wu, P.; Wei, Q.; Tan, W.; Xu, L.; et al. Inflammation and fibrosis in peripheral adipose tissue of patients with aldosterone-producing adenoma. Endocrinology 2018, 159, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Kalupahana, N.S.; Massiera, F.; Quignard-Boulange, A.; Ailhaud, G.; Voy, B.H.; Wasserman, D.H.; Moustaid-Moussa, N. Overproduction of angiotensinogen from adipose tissue induces adipose inflammation, glucose intolerance, and insulin resistance. Obesity 2012, 20, 48–56. [Google Scholar] [CrossRef]
- Bader, M.; Steckelings, U.M.; Alenina, N.; Santos, R.A.S.; Ferrario, C.M. Alternative renin-angiotensin system. Hypertension 2024, 81, 964–976. [Google Scholar] [CrossRef] [PubMed]
- Jarajapu, Y.P.R. Targeting angiotensin-converting enzyme-2/angiotensin-(1–7)/mas receptor axis in the vascular progenitor cells for cardiovascular diseases. Mol. Pharmacol. 2021, 99, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Mudersbach, T.; Siuda, D.; Kohlstedt, K.; Fleming, I. Epigenetic control of the angiotensin-converting enzyme in endothelial cells during inflammation. PLoS ONE 2019, 14, e0216218. [Google Scholar] [CrossRef]
- Najafipour, R.; Mohammadi, D.; Momeni, A.; Moghbelinejad, S.J. Effect of B12 and folate deficiency in hypomethylation of Angiotensin I converting enzyme 2 gene and severity of disease among the acute respiratory distress syndrome patients. Clin. Lab. Anal. 2023, 37, e24846. [Google Scholar] [CrossRef]
- Ceconi, C.; Francolini, G.; Olivares, A.; Comini, L.; Bachetti, T.; Ferrari, R. Angiotensin-converting enzyme (ACE) inhibitors have different selectivity for bradykinin binding sites of human somatic ACE. Eur. J. Pharmacol. 2007, 577, 1–6. [Google Scholar] [CrossRef]
- Ryznar, R.J.; Phibbs, L.; Van Winkle, L.J. Epigenetic modifications at the center of the Barker hypothesis and their transgenerational implications. Int. J. Environ. Res. Public Health 2021, 18, 12728. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Goyal, D.; Leitzke, A.; Gheorghe, C.P.; Longo, L.D. Brain renin-angiotensin system: Fetal epigenetic programming by maternal protein restriction during pregnancy. Reprod. Sci. 2010, 17, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Rivière, G.; Lienhard, D.; Andrieu, T.; Vieau, D.; Frey, B.M.; Frey, F.J. Epigenetic regulation of somatic angiotensin-converting enzyme by DNA methylation and histone acetylation. Epigenetics 2011, 6, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Cho, H.M.; Lee, D.Y.; Kim, K.C.; Han, H.S.; Kim, I.K. Tissue-specific upregulation of angiotensin-converting enzyme 1 in spontaneously hypertensive rats through histone code modifications. Hypertension 2012, 59, 621–626. [Google Scholar] [CrossRef]
- Hu, B.; Song, J.T.; Qu, H.Y.; Bi, C.L.; Huang, X.Z.; Liu, X.X.; Zhang, M. Mechanical stretch suppresses microRNA-145 expression by activating extracellular signal-regulated kinase 1/2 and upregulating angiotensin-converting enzyme to alter vascular smooth muscle cell phenotype. PLoS ONE 2014, 9, e96338. [Google Scholar] [CrossRef]
- Kohlstedt, K.; Trouvain, C.; Boettger, T.; Shi, L.; Fisslthaler, B.; Fleming, I. AMP-activated protein kinase regulates endothelial cell angiotensin-converting enzyme expression via p53 and the post-transcriptional regulation of microRNA-143/145. Circ. Res. 2013, 112, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.J.; Nalivaeva, N.N. Angiotensin-converting enzyme 2 (ACE2): Two decades of revelations and re-evaluation. Peptides 2022, 151, 170766. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.; Berdasco, C.; Lazartigues, E. Brain angiotensin converting enzyme-2 in central cardiovascular regulation. Clin. Sci. 2020, 134, 2535–2547. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Leitzke, A.; Goyal, D.; Gheorghe, C.P.; Longo, L.D. Antenatal maternal hypoxic stress: Adaptations in fetal lung renin-angiotensin system. Reprod. Sci. 2011, 18, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Demuea, M.; Yoneda, T.; Takeda, Y. Epigenesis of blood pressure regulating hormones. In Proceeding of the 45th Annual Scientific Meeting of Japanese Society of Hypertension, Osaka, Japan, 15–17 September 2023; p. 184. [Google Scholar]
- Sen, R.; Garbati, M.; Bryant, K.; Lu, Y. Epigenetic mechanisms influencing COVID-19. Genome 2021, 64, 372–385. [Google Scholar] [CrossRef]
- Lima, R.S.; Rocha, L.P.C.; Moreira, P.R. Genetic and epigenetic control of ACE2 expression and its possible role in COVID-19. Cell Biochem. Funct. 2021, 39, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Chlamydas, S.; Papavassiliou, A.G.; Piperi, C. Epigenetic mechanisms regulating COVID-19 infection. Epigenetics 2021, 16, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.G.G.; Oliveira, A.; Singh, Y.; Jimenez, L.; Gonçalves, A.N.A.; Ogava, R.L.T.; Creighton, R.; Schatzmann Peron, J.P.; Nakaya, H.I. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. J. Infect. Dis. 2020, 222, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, F.; Rutigliano, G.; Sestito, S.; Bandini, L.; Storti, B.; Bizzarri, R.; Zucchi, R. ACE2 in the era of SARS-CoV-2: Controversies and novel perspectives. Front. Mol. Biosci. 2020, 7, 588618. [Google Scholar] [CrossRef]
- Elemam, N.M.; Hasswan, H.; Aljaibeji, H.; Sulaiman, N. Circulating soluble ace2 and upstream microRNA expressions in serum of type 2 diabetes mellitus patients. Int. J. Mol. Sci. 2021, 22, 5263. [Google Scholar] [CrossRef]
- Hejenkowska, E.D.; Mitash, N.; Donovan, J.E.; Chandra, A.; Bertrand, C.; De Santi, C.; Greene, C.M.; Mu, F.; Swiatecka-Urban, A. TGF-β1 inhibition of ace2 mediated by miRNA uncovers novel mechanism of SARS-CoV-2 pathogenesis. J. Innate Immun. 2023, 15, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Wang, B.; Zhang, X.F.; Ma, Y.P.; Liu, J.D.; Wang, X.Z. Contribution of renin-angiotensin system to exercise-induced attenuation of aortic remodeling and improvement of endothelial function in spontaneously hypertensive rats. Cardiovasc. Pathol. 2014, 23, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Huang, Y.L.; Shih, Y.Y.; Wu, H.Y.; Peng, C.T.; Lo, W.Y. MicroRNA-146a decreases high glucose/thrombin-induced endothelial inflammation by inhibiting NAPDH oxidase 4 expression. Mediators Inflamm. 2014, 2014, 379537. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Xu, R.; Yu, H.M.; Chang, Q.; Zhong, J.C. The ace2/apelin signaling, microRNAs, and hypertension. Int. J. Hypertens. 2015, 2015, 896861. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Fan, X.; Zhao, M.; Wu, M.; Li, H.; Ji, B.; Zhu, X.; Li, L.; Ding, H.; Sun, M.; et al. DNA methylation-reprogrammed ang II (angiotensin II) type 1 receptor-early growth response gene 1-protein kinase C ε axis underlies vascular hypercontractility in antenatal hypoxic offspring. Hypertension 2021, 77, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.I.; Papadopoulou, A.; Aw, T.C. MicroRNA-155 mediates endogenous angiotensin II type 1 receptor regulation: Implications for innovative type 2 diabetes mellitus management. World J. Diabetes 2023, 14, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Kawakami-Mori, F.; Nishimoto, M.; Reheman, L.; Kawarazaki, W.; Ayuzawa, N.; Ueda, K.; Hirohama, D.; Kohno, D.; Oba, S.; Shimosawa, T.; et al. DNA methylation of hypothalamic angiotensin receptor in prenatal programmed hypertension. JCI Insight 2018, 3, e95625. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Zhou, J.J.; Shao, J.Y.; Chen, S.R.; Pan, H.L. DNA demethylation in the hypothalamus promotes transcription of Agtr1a and Slc12a2 and hypertension development. J. Biol. Chem. 2023, 300, 105597. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Li, S.; Zhang, Y.; Chen, Y.; Zhou, Y.; Shi, L. Maternal exercise upregulates the DNA methylation of Agtr1a to enhance vascular function in offspring of hypertensive rats. Hypertens. Res. 2023, 46, 654–666. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, C.C.; Chen, W.D.; Shen, W.L.; Ruan, C.C.; Zhu, L.M.; Zhu, D.L.; Gao, P.J. MicroRNA-155 regulates angiotensin II type 1 receptor expression and phenotypic differentiation in vascular adventitial fibroblasts. Biochem. Biophys. Res. Commun. 2010, 400, 483–488. [Google Scholar] [CrossRef]
- Takeda, Y. Effects of eplerenone, a selective mineralocorticoid receptor antagonist, on clinical and experimental salt-sensitive hypertension. Hypertens. Res. 2009, 32, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Booz, G.W.; Sigmund, C.D.; Coffman, T.M.; Kawai, T.; Rizzo, V.; Scalia, R.; Eguchi, S. Angiotensin II signal transduction: An update on mechanisms of physiology and pathophysiology. Physiol. Rev. 2018, 98, 1627–1738. [Google Scholar] [CrossRef] [PubMed]
- DuPont, J.J.; McCurley, A.; Davel, A.P.; McCarthy, J.; Bender, S.B.; Hong, K.; Yang, Y.; Yoo, J.K.; Aronovitz, M.; Baur, W.E.; et al. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight 2016, 1, e88942. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.F.; Sun, Y.L.; Hamet, P.; Inagami, T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001, 11, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, Q.; Sun, S.; Qiu, Y.; Li, J.; Liu, W.; Yuan, G.; Ma, H. Renal transplantation increases angiotensin II receptor-mediated vascular contractility associated with changes of epigenetic mechanisms. Int. J. Mol. Med. 2018, 41, 2375–2388. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, S.; Azushima, K.; Yamaji, T.; Suzuki, T.; Abe, E.; Tanaka, S.; Hirota, K.; Tsukamoto, S.; Morita, R.; Kobayashi, R.; et al. Angiotensin II type 1 receptor-associated protein deletion combined with angiotensin II stimulation accelerates the development of diabetic kidney disease in mice on a C57BL/6 strain. Hypertens. Res. 2024, 47, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Yamashita, A.; Abe, E.; Yamaji, T.; Azushima, K.; Tanaka, S.; Taguchi, S.; Tsukamoto, S.; Wakui, H.; Tamura, K. miR-125a-5p/miR-125b-5p contributes to pathological activation of angiotensin II-AT1R in mouse distal convoluted tubule cells by the suppression of Atrap. J. Biol. Chem. 2023, 299, 105478. [Google Scholar] [CrossRef]
- Takeda, Y.; Demura, M.; Wang, F.; Karashima, S.; Yoneda, T.; Kometani, M.; Hashimoto, A.; Aono, D.; Horike, S.I.; Meguro-Horike, M.; et al. Epigenetic regulation of aldosterone synthase gene by sodium and angiotensin II. J. Am. Heart Assoc. 2018, 7, e008281. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; MacKenzie, S.M.; Alvarez-Madrazo, S.; Diver, L.A.; Lin, J.; Stewart, P.M.; Fraser, R.; Connell, J.M.; Davies, E. MicroRNA-24 is a novel regulator of aldosterone and cortisol production in the human adrenal cortex. Hypertension 2013, 62, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.; Diver, L.A.; Alvarez-Madrazo, S.; Livie, C.; Ejaz, A.; Fraser, R.; Connell, J.M.; MacKenzie, S.M.; Davies, E. Regulation of corticosteroidogenic genes by microRNAs. Int. J. Endocrinol. 2017, 2017, 2021903. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Liu, Q.; Xie, T.; Huang, R.; Kang, H.; Lai, C.; Zhu, J. MiR-193a-3p functions as a tumour suppressor in human aldosterone-producing adrenocortical adenoma by down-regulating CYP11B2. Int. J. Exp. Pathol. 2018, 99, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Ball, J.P.; Mathis, K.W.; Hall, M.E.; Ryan, M.J.; Rothenberg, M.E.; Yanes Cardozo, L.L.; Romero, D.G. MicroRNA-21 ablation exacerbates aldosterone-mediated cardiac injury, remodeling, and dysfunction. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E1154–E1167. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, K.; Ogishima, T.; Sugiura, Y.; Suematsu, M.; Mukai, K. Pathology and gene mutations of aldosterone-producing lesions. Endocr. J. 2023, 70, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Howard, B.; Wang, Y.; Xekouki, P.; Faucz, F.R.; Jain, M.; Zhang, L.; Meltzer, P.G.; Stratakis, C.A.; Kebebew, E. Integrated analysis of genome-wide methylation and gene expression shows epigenetic regulation of CYP11B2 in aldosteronomas. J. Clin. Endocrinol. Metab. 2014, 99, E536–E543. [Google Scholar] [CrossRef] [PubMed]
- Di Dalmazi, G.; Morandi, L.; Rubin, B.; Pilon, C.; Asioli, S.; Vicennati, V.; De Leo, A.; Ambrosi, F.; Santini, D.; Pagotto, U.; et al. DNA methylation of steroidogenic enzymes in benign adrenocortical tumors: New insights in aldosterone-producing adenomas. J. Clin. Endocrinol. Metab. 2020, 105, dgaa585. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Demura, M.; Wang, F.; Karashima, S.; Yoneda, T.; Kometani, M.; Aomo, D.; Hashimoto, A.; Horike, S.; Meguro-Horike, M.; et al. Effect of potassium on DNA methylation of aldosterone synthase gene. J. Hypertens. 2021, 39, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Kometani, M.; Yoneda, T.; Demura, M.; Aono, D.; Gondoh, Y.; Karashima, S.; Nishimoto, K.; Yasuda, M.; Horike, S.; Takeda, Y. Genetic and epigenetic analyses of aldosterone-producing adenoma with hypercortisolemia. Steroids 2019, 151, 108470. [Google Scholar] [CrossRef] [PubMed]
- Ayuzawa, N.; Fujita, T. The mineralocorticoid receptor in salt-sensitive hypertension and renal injury. J. Am. Soc. Nephrol. 2021, 32, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T. Recent advances in hypertension: Epigenetic mechanism involved in development of salt-sensitive hypertension. Hypertension 2023, 80, 711–718. [Google Scholar] [CrossRef]
- Cao, N.; Lan, C.; Chen, C.; Xu, Z.; Luo, H.; Zheng, S.; Gong, X.; Ren, H.; Li, Z.; Qu, S.; et al. Prenatal lipopolysaccharides exposure induces transgenerational inheritance of hypertension. Circulation 2022, 146, 1082–1095. [Google Scholar] [CrossRef]
- Takeda, Y.; Demura, M.; Kometani, M.; Karashima, S.; Yoneda, T.; Takeda, Y. Molecular and epigenetic control of aldosterone synthase, CYP11B2 and 11-hydroxylase, CYP11B1. Int. J. Mol. Sci. 2023, 24, 5782. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Yoneda, T.; Demura, M.; Furukawa, K.; Miyamori, I.; Mabuchi, H. Effects of high sodium intake on cardiovascular aldosterone synthesis in stroke-prone spontaneously hypertensive rats. J. Hypertens. 2001, 19, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Garnier, A.; Bendall, J.K.; Fuchs, S.; Escoubet, B.; Rochais, F.; Hoerter, J.; Nehme, J.; Ambroisine, M.L.; De Angelis, N.; Morineau, G.; et al. Cardiac specific increase in aldosterone production induces coronary dysfunction in aldosterone synthase-transgenic mice. Circulation 2004, 110, 1819–1825. [Google Scholar] [CrossRef] [PubMed]
- Alesutan, I.; Voelkl, J.; Feger, M.; Kratschmar, D.V.; Castor, T.; Mia, S.; Sacherer, M.; Viereck, R.; Borst, O.; Leibrock, C.; et al. Involvement of vascular aldosterone synthase in phosphate-induced osteogenic transformation of vascular smooth muscle cells. Sci. Rep. 2017, 7, 2059. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Yoneda, T.; Demura, M.; Usukura, M.; Mabuchi, H. Calcineurin inhibition attenuates mineralocorticoid-induced cardiac hypertrophy. Circulation 2002, 105, 677–679. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshimura, M.; Nakamura, S.; Ito, T.; Nakayama, M.; Harada, E.; Mizuno, Y.; Sakamoto, T.; Yamamuro, M.; Saito, Y.; Nakao, K.; et al. Expression of aldosterone synthase gene in failing human heart: Quantitative analysis using modified real-time polymerase chain reaction. J. Clin. Endocrinol. Metab. 2002, 87, 3936–3940. [Google Scholar] [CrossRef] [PubMed]
- Ibarrola, J.; Jaffe, I.Z. The mineralocorticoid receptor in the vasculature: Friend or foe? Annu. Rev. Physiol. 2024, 86, 49–70. [Google Scholar] [CrossRef]
- Brown, J.M. Adverse effects of aldosterone: Beyond blood pressure. J. Am. Heart Assoc. 2024, 13, e030142. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Miyamori, I.; Fujita, T.; Takeda, Y.; Takeda, R.; Yamamoto, H. Vascular aldosterone. biosynthesis and a link to angiotensin II-induced hypertrophy of vascular smooth muscle cells. J. Biol. Chem. 1994, 269, 24316–24320. [Google Scholar] [CrossRef]
- Mesquita, T.R.; Auguste, G.; Falcón, D.; Ruiz-Hurtado, G.; Salazar-Enciso, R.; Sabourin, J.; Lefebvre, F.; Viengchareun, S.; Kobeissy, H.; Lechène, P.; et al. Specific activation of the alternative cardiac promoter of cacna1c by the mineralocorticoid receptor. Circ. Res. 2018, 122, e49–e61. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, X.; Huang, Z.; Fan, X.; Tan, X.; Lu, C.; Yang, J. Aldosterone is a possible new stimulating factor for promoting vascular calcification. Front. Biosci. 2021, 26, 1052–1063. [Google Scholar] [CrossRef]
- Lee, H.A.; Song, M.J.; Seok, Y.M.; Kang, S.H.; Kim, S.Y.; Kim, I. Histone Deacetylase 3 and 4 Complex Stimulates the Transcriptional Activity of the Mineralocorticoid Receptor. PLoS ONE 2015, 10, e0136801. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, N.; Zhang, Y.; Li, J.; Zhang, E.; Xu, Z. Fat-diets in perinatal stages altered nr3c2-mediated Ca(2+) currents in mesenteric arteries of offspring rats. Mol. Nutr. Food Res. 2023, 67, e2200722. [Google Scholar] [CrossRef] [PubMed]
- Camarda, N.D.; Ibarrola, J.; Biwer, L.A.; Jaffe, I.Z. Mineralocorticoid receptors in vascular smooth muscle: Blood pressure and beyond. Hypertension 2024, 81, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Sõber, S.; Laan, M.; Annilo, T. MicroRNAs miR-124 and miR-135a are potential regulators of the mineralocorticoid receptor gene (NR3C2) expression. Biochem. Biophys. Res. Commun. 2010, 391, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.C.; Lilyanna, S.; Wang, P.; Vardy, L.A.; Jiang, X.; Armugam, A.; Jeyaseelan, K.; Richards, A.M. MicroRNA-31 promotes adverse cardiac remodeling and dysfunction in ischemic heart disease. J. Mol. Cell. Cardiol. 2017, 112, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Foinquinos, A.; Jung, M.; Janssen-Peters, H.; Biss, S.; Bauersachs, J.; Gupta, S.K.; Thum, T. MiRNA-181a is a novel regulator of aldosterone-mineralocorticoid receptor-mediated cardiac remodelling. Eur. J. Heart Fail. 2020, 22, 1366–1377. [Google Scholar] [CrossRef]
- Koyama, R.; Mannic, T.; Ito, J.; Amar, L.; Zennaro, M.C.; Rossier, M.F.; Maturana, A.D. MicroRNA-204 is necessary for aldosterone-stimulated T-type calcium channel expression in cardiomyocytes. Int. J. Mol. Sci. 2018, 19, 2941. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhang, L.; Cong, G.; Ren, L.; Hao, L. MicroRNA-34b/c inhibits aldosterone-induced vascular smooth muscle cell calcification via a SATB2/Runx2 pathway. Cell Tissue Res. 2016, 366, 733–746. [Google Scholar] [CrossRef]
- Hayakawa, K.; Kawasaki, M.; Hirai, T.; Yoshida, Y.; Tsushima, H.; Fujishiro, M.; Ikeda, K.; Morimoto, S.; Takamori, K.; Sekigawa, I. MicroRNA-766-3p contributes to anti-inflammatory responses through the indirect inhibition of NF-kappaB signaling. Int. J. Mol. Sci. 2019, 20, 809. [Google Scholar] [CrossRef]
- Zhu, A.; Yoneda, T.; Demura, M.; Karashima, S.; Usukura, M.; Yamagishi, M.; Takeda, Y. Effect of mineralocorticoid receptor blockade on the renal renin-angiotensin system in Dahl salt-sensitive hypertensive rats. J. Hypertens. 2009, 27, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Ozbaki-Yagan, N.; Liu, X.; Bodnar, A.J.; Ho, J.; Butterworth, M.B. Aldosterone-induced microRNAs act as feedback regulators of mineralocorticoid receptor signaling in kidney epithelia. FASEB J. 2020, 4, 11714–11728. [Google Scholar] [CrossRef]
- Sierra-Ramos, C.; Velazquez-Garcia, S.; Keskus, A.G.; Vastola-Mascolo, A.; Rodríguez-Rodríguez, A.E.; Luis-Lima, S.; Hernández, G.; Navarro-González, J.F.; Porrini, E.; Konu, O.; et al. Increased SGK1 activity potentiates mineralocorticoid/NaCl-induced kidney injury. Am. J. Physiol. Ren. Physiol. 2021, 320, F628–F643. [Google Scholar] [CrossRef]
- Park, E.-J.; Jung, H.J.; Choi, H.-J.; Cho, J.-I.; Park, H.-J.; Kwon, T.-H. miR-34c-5p and CaMKII are involved in aldosterone-induced fibrosis in kidney collecting duct cells. Am. J. Physiol. Ren. Physiol. 2018, 314, F329–F342. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Gao, J.; Wang, M.; Li, X.; Cui, Z.; Fu, G. Long noncoding RNA Tug1 promotes angiotensin II-induced renal fibrosis by binding to mineralocorticoid receptor and negatively regulating microR-29b-3p. Hypertension 2021, 78, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Xiong, Z.-C.; Zhang, S.-L.; Hao, Q.-Y.; Liu, Z.-Y.; Zhang, H.-F.; Wang, J.-F.; Gao, J.-W.; Liu, P.-M. Upregulated LncRNA H19 sponges miR-106a-5p and contributes to aldosterone-induced vascular calcification via activating the Runx2-dependent pathway. Arterioscler. Thromb. Vasc. Biol. 2023, 43, 1684–1699. [Google Scholar] [CrossRef]
- Elvira-Matelot, E.; Zhou, X.; Farman, N.; Beaurain, G.; Henrion-Caude, A.; Hadchouel, J.; Jeunemaitre, X. Regulation of WNK1 expression by miR-192 and aldosterone. J. Am. Soc. Nephrol. 2010, 21, 1724–1731. [Google Scholar] [CrossRef]
- Subramanya, A.R.; Yang, C.L.; McCormick, J.A.; Ellison, D.H. WNK kinases regulate sodium chloride and potassium transport by the aldosterone-sensitive distal nephron. Kidney Int. 2006, 70, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.H.; Yue, P.; Zhang, C.; Wang, W.H. MicroRNA-194 (miR-194) regulates ROMK channel activity by targeting intersectin 1. Am. J. Physiol. Ren. Physiol. 2014, 306, F53–F60. [Google Scholar] [CrossRef][Green Version]
- Lin, D.H.; Yue, P.; Pan, C.; Sun, P.; Wang, W.H. MicroRNA 802 stimulates ROMK channels by suppressing caveolin-1. J. Am. Soc. Nephrol. 2011, 22, 1087–1098. [Google Scholar] [CrossRef]
- Edinger, R.S.; Coronnello, C.; Bodnar, A.J.; Labarca, M.; Bhalla, V.; LaFramboise, W.A.; Benos, P.V.; Ho, J.; Johnson, J.P.; Butterworth, M.B. Aldosterone regulates microRNAs in the cortical collecting duct to alter sodium transport. J. Am. Soc. Nephrol. 2014, 25, 2445–2457. [Google Scholar] [CrossRef] [PubMed]
- Funder, J.W. Apparent mineralocorticoid excess: Research as an art form. Endocrine 2020, 70, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Nishimoto, M.; Hirohama, D.; Ayuzawa, N.; Kawarazaki, W.; Watanabe, A.; Shimosawa, T.; Loffing, J.; Zhang, M.-Z.; Marumo, T.; et al. Renal dysfunction induced by kidney-specific gene deletion of Hsd11b2 as a primary cause of salt-dependent hypertension. Hypertension 2017, 70, 111–118. [Google Scholar] [CrossRef]
- Takeda, Y. Pathophysiological roles of vascular 11beta-hydroxysteroid dehydrogenase and aldosterone. J. Steroid Biochem. Mol. Biol. 2003, 85, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Alikhani-Koopaei, R.; Fouladkou, F.; Frey, F.J.; Frey, B.M. Epigenetic regulation of 11 beta-hydroxysteroid dehydrogenase type 2 expression. J. Clin. Investig. 2004, 114, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- White, P.C.; Mune, T.; Agarwal, A.K. 11 beta-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr. Rev. 1997, 18, 135–156. [Google Scholar] [PubMed][Green Version]
- Pizzolo, F.; Friso, S.; Morandini, F.; Antoniazzi, F.; Zaltron, C.; Udali, S.; Gandini, A.; Cavarzere, P.; Salvagno, G.; Giorgetti, A.; et al. Apparent mineralocorticoid excess by a novel mutation and epigenetic modulation by HSD11B2 promoter methylation. J. Clin. Endocrinol. Metab. 2015, 100, E1234–E1241. [Google Scholar] [CrossRef]
- Rezaei, M.; Andrieu, T.; Neuenschwander, S.; Bruggmann, R.; Mordasini, D.; Frey, F.J.; Vogt, B.; Frey, B.M. Regulation of 11beta-hydroxysteroid dehydrogenase type 2 by microRNA. Hypertension 2014, 64, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, M.; Barone, S.; Luo, H.; Zahedi, K. Pathogenesis of hypertension in metabolic syndrome: The role of fructose and salt. Int. J. Mol. Sci. 2023, 24, 4294. [Google Scholar] [CrossRef]
- Nouchi, Y.; Munetsuna, E.; Yamada, H.; Yamazaki, M.; Ando, Y.; Mizuno, G.; Ikeya, M.; Kageyama, I.; Wakasugi, T.; Teshigawara, A.; et al. Maternal high-fructose corn syrup intake impairs corticosterone clearance by reducing renal 11β-Hsd2 activity via miR-27a-mediated mechanism in rat offspring. Nutrients 2023, 15, 2122. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, Y.; Demura, M.; Yoneda, T.; Takeda, Y. Epigenetic Regulation of the Renin–Angiotensin–Aldosterone System in Hypertension. Int. J. Mol. Sci. 2024, 25, 8099. https://doi.org/10.3390/ijms25158099
Takeda Y, Demura M, Yoneda T, Takeda Y. Epigenetic Regulation of the Renin–Angiotensin–Aldosterone System in Hypertension. International Journal of Molecular Sciences. 2024; 25(15):8099. https://doi.org/10.3390/ijms25158099
Chicago/Turabian StyleTakeda, Yoshimichi, Masashi Demura, Takashi Yoneda, and Yoshiyu Takeda. 2024. "Epigenetic Regulation of the Renin–Angiotensin–Aldosterone System in Hypertension" International Journal of Molecular Sciences 25, no. 15: 8099. https://doi.org/10.3390/ijms25158099
APA StyleTakeda, Y., Demura, M., Yoneda, T., & Takeda, Y. (2024). Epigenetic Regulation of the Renin–Angiotensin–Aldosterone System in Hypertension. International Journal of Molecular Sciences, 25(15), 8099. https://doi.org/10.3390/ijms25158099





