Response of Soil Microbiota, Enzymes, and Plants to the Fungicide Azoxystrobin
Abstract
:1. Introduction
2. Results
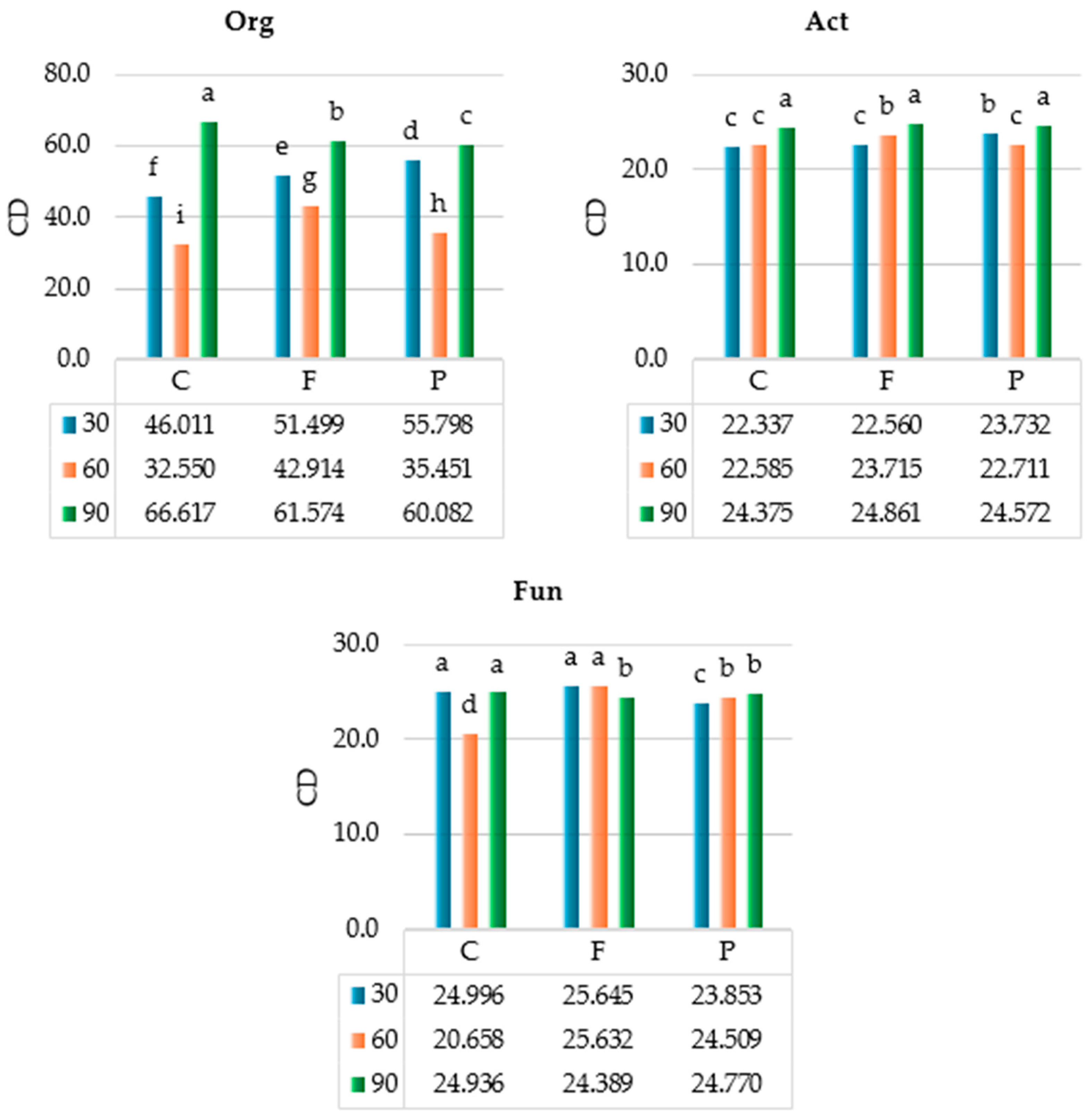
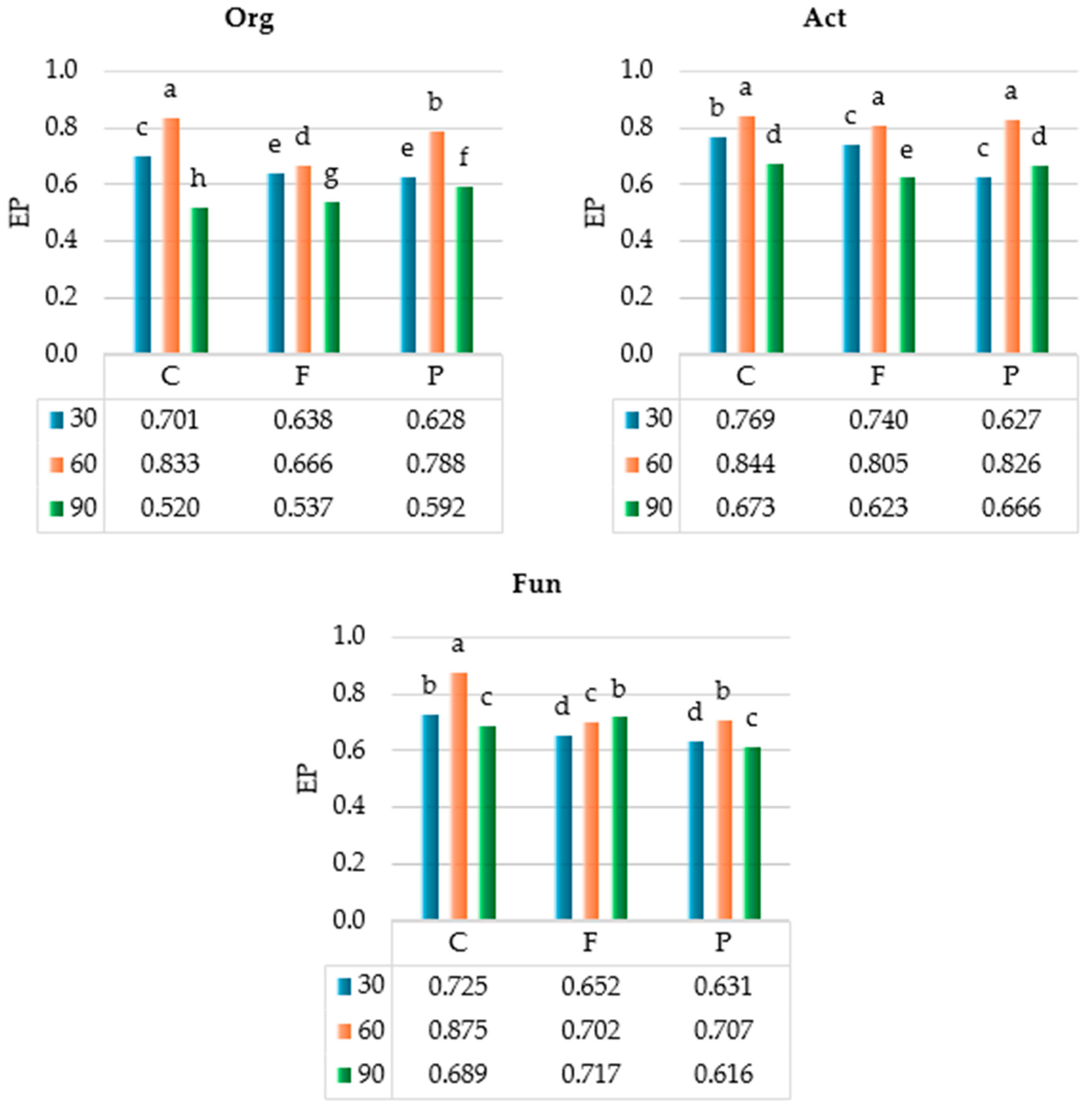
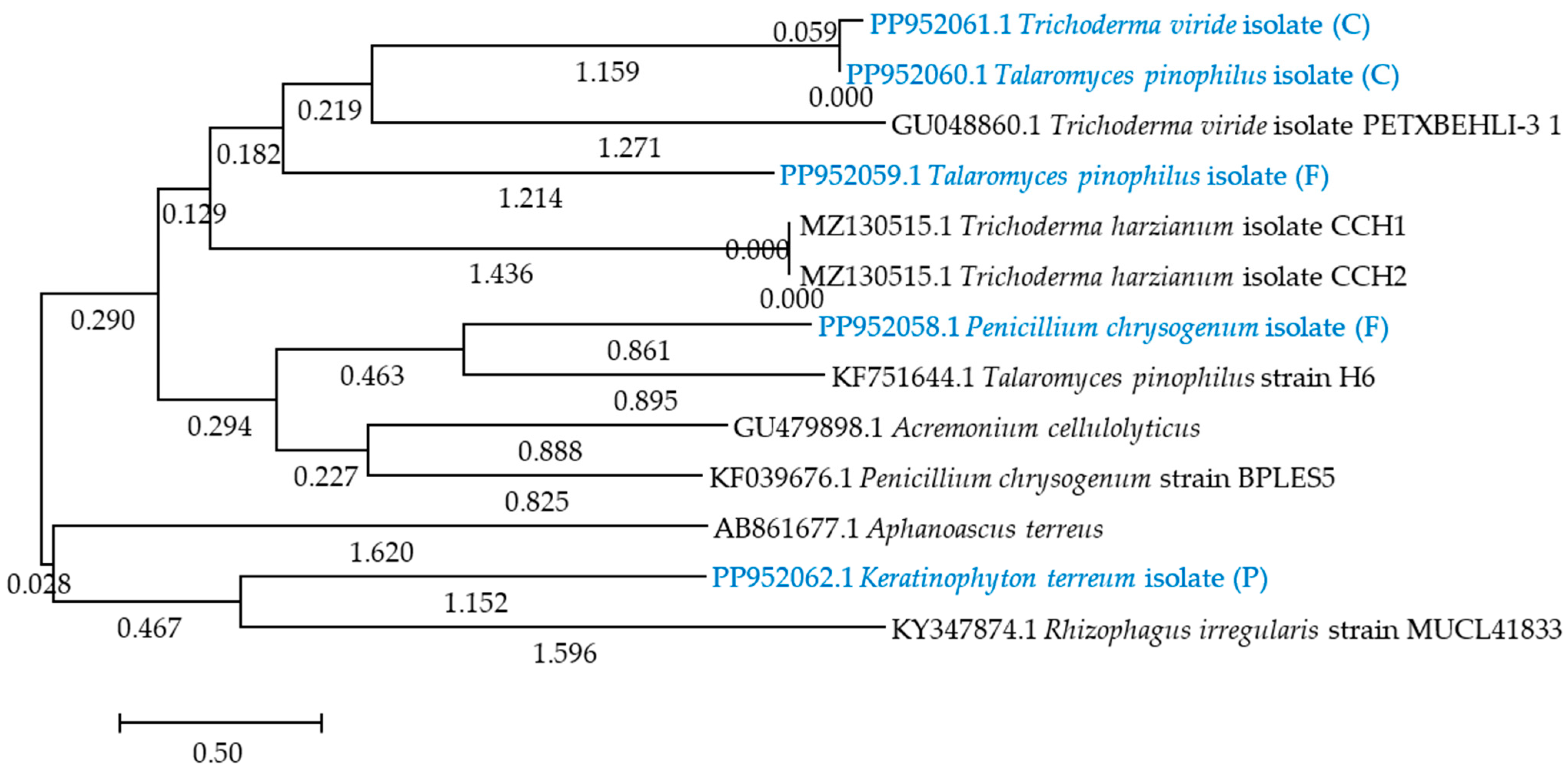
2.1. Response of Soil Microbiota to Azoxystrobin
2.2. Response of Soil Enzymes to Azoxystrobin
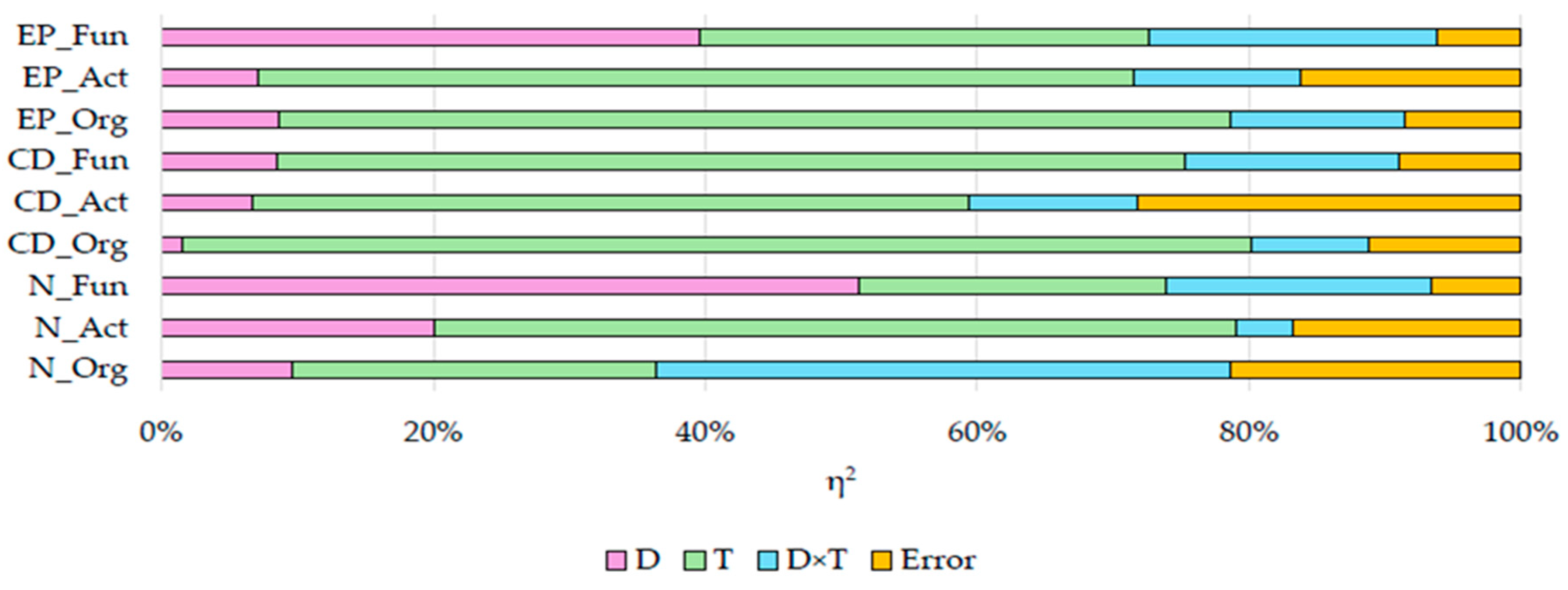
2.3. Pearson’s Simple Correlation Coefficients between Microbiological and Biochemical Soil Parameters
2.4. Response of Plants to Azoxystrobin
3. Discussion
3.1. Response of Soil Microbiota to Azoxystrobin
3.2. Response of Soil Enzymes to Azoxystrobin
3.3. Response of Plants to Azoxystrobin
4. Materials and Methods
4.1. Soil Materials
4.2. Azoxystrobin
4.3. Establishment of the Experiment and Procedure for Conducting the Experiment
4.4. Conducting Microbiological Analysis of Soil
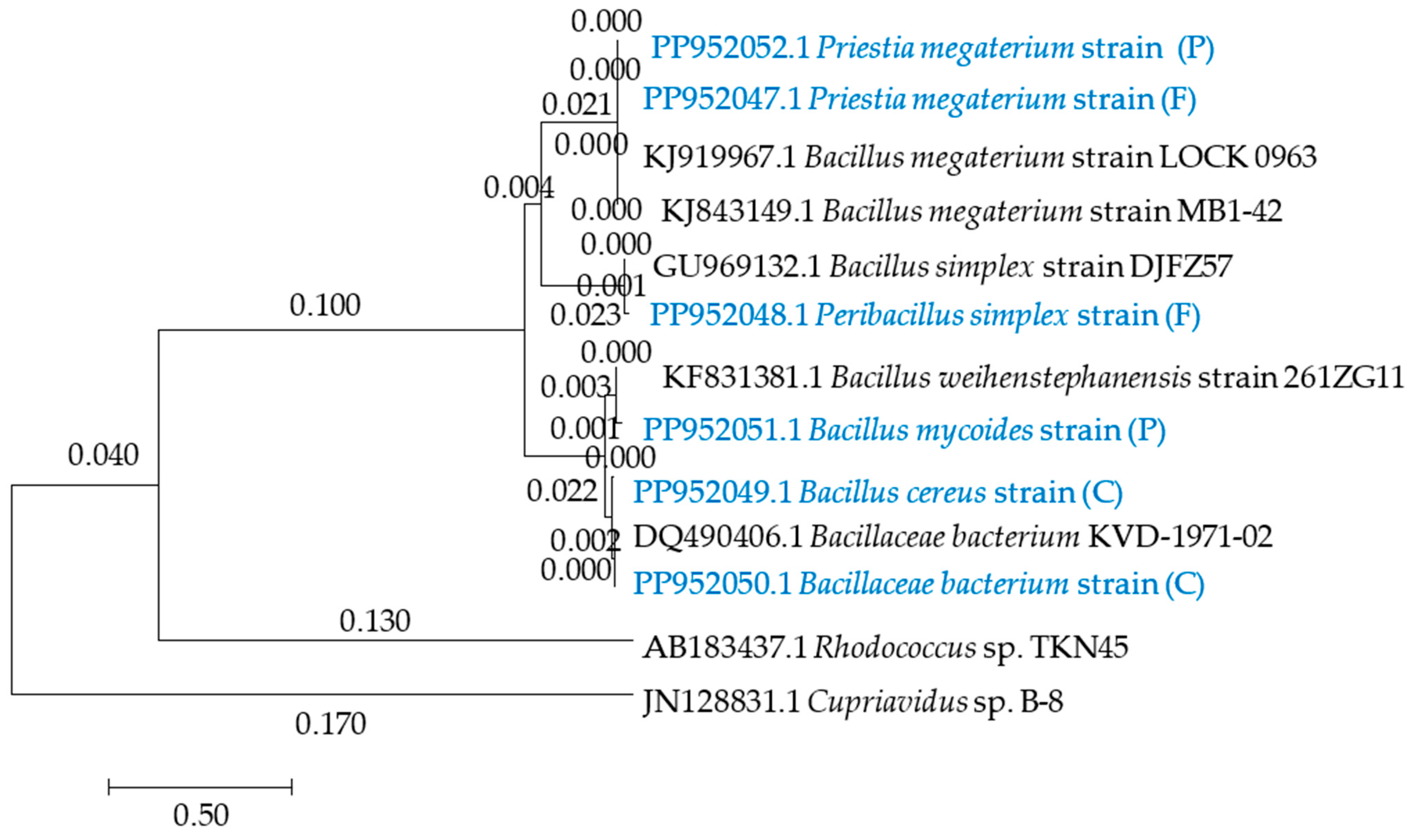
4.5. Isolation of Microorganisms from Soil and Their Identification
4.6. Conducting Enzymatic Analysis of Soil
4.7. The Effect of Azoxystrobin on Seed Germination and Plant Root Elongation
4.8. Calculation of Results
- ▪
- Colony development index (CD) of microorganisms [79]: CD values range from 0 to 100. CD values close to 100 indicate rapid growth of the microorganism population in a short period.
- ▪
- The ecophysiological diversity index (EP) of microorganisms [80] takes values from 0 to 1, measuring the stability and homogeneity of microorganisms over time. EP values close to 1 indicate steady growth of microorganisms in the environment.
- ▪
- Changes (Cha) of microbial abundance, enzyme activity, seed germination, and root elongation in soil caused by azoxystrobin: A positive Cha value indicates stimulation of the analyzed parameters under the influence of azoxystrobin, while a negative value indicates inhibition.
- ▪
- The resilience index (RL) of azoxystrobin-treated soil is determined by microbial abundance and enzyme activity [81]. RL values range from −1 to 1. A RL value close to −1 indicates that the soil is not returning to equilibrium. A RL value close to 1 indicates that the soil is returning to equilibrium. A RL value close to 0 indicates that the soil is out of or slightly out of equilibrium.
4.9. Statistical Analyses of Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, A.A.; Desoky, E.M.; Rady, M.M. Application of azoxystrobin fungicide improves drought tolerance in tomato, via enhancing physio-biochemical and anatomical feature. Int. Lett. Nat. Sci. 2019, 76, 34–49. [Google Scholar] [CrossRef]
- Heleno, F.F.; Rodrigues, A.A.; Queiroz, M.E.; Neves, A.A.; Oliveira, A.F.; Libardi, V.M. Determination of fungicides in bell pepper using solid-liquid extraction with low temperature partitioning. Microchem. J. 2019, 148, 79–84. [Google Scholar] [CrossRef]
- Han, L.; Liu, Y.; Fang, K.; Zhang, X.; Liu, T.; Wang, F.; Wang, X. Azoxystrobin dissipation and its effect on soil microbial community structure and function in the presence of chlorothalonil, chlortetracycline and ciprofloxacin. Environ. Pollut. 2020, 257, 113578. [Google Scholar] [CrossRef]
- Regueiro, J.; Olguín, N.; Simal-Gándara, J.; Suñol, C. Toxicity evaluation of new agricultural fungicides in primary cultured cortical neurons. Environ. Res. 2015, 140, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, E.; Zubrod, J.P.; Konschak, M.; Sulzer, L.; Schnurr, J.; Schreiner, V.C.; Schulz, R.; Bundschuh, M. As above, so below? Effects of fungicides on microbial organic matter decomposition are stronger in the hyporheic than in the benthic zone. Limnol. Oceanogr. 2022, 67, 39–52. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Q.; Lavoie, M.; Zhu, Y.; Ye, Y.; Yang, J.; Paerl, H.W.; Qian, H.; Zhu, Y.G. The fungicide azoxystrobin promotes freshwater cyanobacterial dominance through altering competition. Microbiome 2019, 7, 128. [Google Scholar] [CrossRef]
- Lu, C.; Hou, K.; Zhou, T.; Wang, X.; Zhang, J.; Cheng, C.; Du, Z.; Li, B.; Wang, J.; Wang, J.; et al. Characterization of the responses of soil micro-organisms to azoxystrobin and the residue dynamics of azoxystrobin in wheat–corn rotation fields over two years. Chemosphere 2023, 318, 137918. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, Y.; Zhan, H.; Bhatt, P.; Chen, S. An overview of strobilurin fungicide degradation: Current status and future perspective. Front. Microbiol. 2020, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Edwards, P.G.; Murphy, T.M.; Lydy, M.J. Fate and transport of agriculturally applied fungicidal compounds, azoxystrobin and propiconazole. Chemosphere 2016, 146, 450–457. [Google Scholar] [CrossRef]
- Sun, M.; Wang, H.; Shi, C.; Li, J.; Cai, L.; Xiang, L.; Liu, T.; Goodwin, P.H.; Chen, X.; Wang, L. Effect of azoxystrobin on tobacco leaf microbial composition and diversity. Front. Plant Sci. 2023, 13, 1101039. [Google Scholar] [CrossRef]
- Yao, J.; Cui, B.; Zhao, X.; Wang, Y.; Zeng, Z.; Sun, C.; Yang, D.; Liu, G.; Gao, J.; Cui, H. Preparation, characterization, and evaluation of azoxystrobin nanosuspension produced by wet media milling. Appl. Nanosci. 2018, 8, 297–307. [Google Scholar] [CrossRef]
- Li, D.; Liu, M.; Yang, Y.; Shi, H.; Zhou, J.; He, D. Strong lethality and teratogenicity of strobilurins on Xenopus tropicalis embryos: Basing on ten agricultural fungicides. Environ. Pollut. 2016, 208, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Pearson, B.L.; Simon, J.M.; McCoy, E.S.; Salazar, G.; Fragola, G.; Zylka, M.J. Identification of chemicals that mimic transcriptional changes associated with autism, brain aging and neurodegeneration. Nat. Commun. 2016, 7, 11173. [Google Scholar] [CrossRef] [PubMed]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, J.J.; Rasmussen, J.R.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An overlooked pesticide class? Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Mahmood, A.; Ito, T.; Kataoka, R. Non-target impact of dinotefuran and azoxystrobin on soil bacterial community and nitrification. Bull. Environ. Contam. Toxicol. 2021, 106, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- White, B.E.; Hovenden, M.J.; Barmuta, L.A. Multifunctional redundancy: Impossible or undetected? Ecol. Evol. 2023, 13, e10409. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity 827 and functionality. Nat. Commun. 2020, 11, 3072. [Google Scholar] [CrossRef] [PubMed]
- Mpofu, E.; Alias, A.; Tomita, K.; Suzuki-Minakuchi, C.; Tomita, K.; Chakraborty, J.; Malon, M.; Ogura, Y.; Takikawa, H.; Okada, K.; et al. Azoxystrobin amine: A novel azoxystrobin degradation product from Bacillus licheniformis strain TAB7. Chemosphere 2021, 273, 129663. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Baquerizo, M.; Oliverio, A.M.; Brewer, T.E.; Benavent-González, A.; Eldridge, D.J.; Bardgett, R.D.; Maestre, F.T.; Singh, B.K.; Fierer, N. A global atlas of the dominant bacteria found in soil. Science 2018, 359, 320–325. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, D.; Ding, J.; Zheng, F.; Zhou, S.; Lu, T.; Zhu, Y.-G.; Qian, H. The fungicide azoxystrobin perturbs the gut microbiota community and enriches antibiotic resistance genes in Enchytraeus crypticus. Environ. Int. 2019, 131, 104965. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Y.; Sun, R.; Zhang, J.; Hale, L.; Dumack, K.; Geisen, S.; Deng, Y.; Zhu, B.; Li, Y.; et al. Resource-dependent biodiversity and potential 833 multi-trophic interactions determine belowground functional trait stability. Microbiome 2023, 11, 95. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils–A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of agrochemicals on soil microbiota and management: A review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Cycoń, M.; Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for the remediation of pesticide-polluted soil: A review. Chemosphere 2017, 172, 52–71. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singh, G.; Barnwal, R.P.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Rawat, M.; Malyan, S.K.; Singh, R.; Tyagi, V.K.; Singh, K.; Kashyap, S.; Kumar, S.; Sharma, M.; Panday, B.K.; et al. Global distribution of pesticides in freshwater resources and their remediation approaches. Environ. Res. 2023, 225, 115605. [Google Scholar] [CrossRef]
- Verdenelli, R.A.; Dominchin, M.F.; Barbero, F.M.; Pérez-Brandán, C.; Aoki, A.; Gil, S.V.; Meriles, J.M. Effect of two broad-spectrum fungicides on the microbial communities of a soil subjected to different degrees of water erosion. Appl. Soil Ecol. 2023, 190, 104984. [Google Scholar] [CrossRef]
- Da Rocha, A.G.; Pitombo, L.M.; Bresolin, J.D.; da Silva, W.T.L.; Espindola, E.L.G.; de Menezes Oliveira, V.B. Single and com-bined toxicity of the pesticides abamectin and difenoconazole on soil microbial activity and Enchytraeus crypticus population. SN Appl. Sci. 2020, 2, 1390. [Google Scholar] [CrossRef]
- Chamberlain, L.A.; Aguayo, T.; Zerega, N.J.C.; Dybzinski, R.; Egerton-Warburton, L.M. Rapid improvement in soil health following the conversion of abandoned farm fields to annual or perennial agroecosystems. Front. Sustain. Food Syst. 2022, 6, 1010298. [Google Scholar] [CrossRef]
- Roman, D.L.; Voiculescu, D.I.; Filip, M.; Ostafe, V.; Isvoran, A. Effects of triazole fungicides on soil microbiota and on the activities of enzymes found in soil: A review. Agriculture 2021, 11, 893. [Google Scholar] [CrossRef]
- Filimon, M.N.; Voia, S.O.; Vlădoiu, D.L.; Isvoran, A.; Ostafe, V. Temperature dependent effect of difenoconazole on enzymatic activity from the soil. J. Serbian Chem. Soc. 2015, 80, 1127–1137. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Y.; Zhang, X.; Zhu, Y. Persistence of myclobutanil and its impact on soil microbial biomass C and dehydrogenase enzyme activity in tea orchard soils. Eurasian J. Soil Sci. 2017, 6, 106–113. [Google Scholar] [CrossRef]
- Satapute, P.; Kamble, M.V.; Adhikari, S.S.; Jogaiah, S. Influence of triazole pesticides on tillage soil microbial populations and metabolic changes. Sci. Total Environ. 2019, 651, 2334–2344. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.M.; Verma, V.K.; Rawat, B.S.; Kaur, B.; Babu, N.; Sharma, A.; Dewali, S.; Yadav, M.; Kumari, R.; Singh, S.; et al. Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Front. Microbiol. 2022, 13, 962619. [Google Scholar] [CrossRef]
- Yengkokpam, P.; Mazumder, P.B. Phytotoxicity of malathion (PM) and tatafen (PTF) towards Solanum melongena L. cv. Longai: A case study. Plant Phys. Rep. 2020, 25, 149–156. [Google Scholar] [CrossRef]
- Bhatt, P.; Huang, Y.; Zhang, W.; Sharma, A.; Chen, S. Enhanced cypermethrin degradation kinetics and metabolic pathway in Bacillus thuringiensis strain SG4. Microorganisms 2020, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Huang, Y.; Rene, E.R.; Kumar, A.J.; Chen, S. Mechanism of allethrin biodegradation by a newly isolated Sphingomonas trueperi strain CW3 from wastewater sludge. Bioresour. Technol. 2020, 305, 123074. [Google Scholar] [CrossRef] [PubMed]
- Conde-Avila, V.; Ortega-Martínez, L.D.; Loera, O.; El Kassis, E.G.; Dávila, J.G.; Valenzuela, C.M.; Armendáriz, B.P. Pesticides degradation by immobilised microorganisms. Int. J. Environ. Anal. Chem. 2021, 101, 2975–3005. [Google Scholar] [CrossRef]
- Alexandrino, D.A.; Mucha, A.P.; Almeida, C.M.R.; Carvalho, M.F. Microbial degradation of two highly persistent fluorinated fungicides-epoxiconazole and fludioxonil. J. Hazard. Mater. 2020, 394, 122545. [Google Scholar] [CrossRef]
- Dennis, P.G.; Kukulies, T.; Forstner, C.; Orton, T.G.; Pattison, A.B. The effects of glyphosate, glufosinate, 620 paraquat and paraquat-diquat on soil microbial activity and bacterial, archaeal and nematode 621 diversity. Sci. Rep. 2018, 8, 2119. [Google Scholar] [CrossRef]
- Storck, V.; Nikolaki, S.; Perruchon, C.; Chabanis, C.; Sacchi, A.; Pertile, G.; Baguelin, C.; Karas, P.A.; Spor, A.; Devers-Lamrani, M.; et al. Lab to field assessment of the ecotoxicological impact of chlorpyrifos, isoproturon, or tebuconazole on the diversity and composition of the soil bacterial community. Front. Microbiol. 2018, 9, 1412. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, X.; Zhu, L.; Du, Z.; Zhang, C.; Wang, J.; Wang, J.; Lv, D. Responses of soil microorganisms and enzymatic activities to azoxystrobin in Cambisol. Pol. J. Environ. Stud. 2018, 27, 2775–2783. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; He, J.; Yan, X.; Hong, Q.; Chen, K.; He, Q.; Zhang, L.; Liu, X.; Chuang, S.; Li, S.; et al. Microbial catabolism of chemical herbicides: 647 Microbial resources, metabolic pathways and catabolic genes. Pestic. Biochem. Physiol. 2017, 143, 272–297. [Google Scholar] [CrossRef]
- Thiour-Mauprivez, C.; Martin-Laurent, F.; Calvayrac, C.; Barthelmebs, L. Effects of herbicide on non-target microorganisms: Towards a new class of biomarkers? Sci. Total Environ. 2019, 684, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Święciło, A.; Krzepiłko, A.; Michałek, S. Evaluation of azoxystrobin toxicity to saprophytic fungi and radish in the early stages of growth. Ecol. Chem. Eng. A 2018, 25, 81–92. [Google Scholar] [CrossRef]
- Andreolli, M.; Lampis, S.; Tosi, L.; Marano, V.; Zapparoli, G. Fungicide sensitivity of grapevine bacteria with plant growth-promoting traits and antagonistic activity as non-target microorganisms. World J. Microbiol. Biotechnol. 2023, 39, 121. [Google Scholar] [CrossRef] [PubMed]
- Baćmaga, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Effects of tebuconazole application on soil microbiota and enzymes. Molecules 2022, 27, 7501. [Google Scholar] [CrossRef]
- Sułowicz, S.; Cycoń, M.; Piotrowska-Seget, Z. Non-target impact of fungicide tetraconazole on microbial communities in soils with different agricultural management. Ecotoxicology 2016, 25, 1047–1060. [Google Scholar] [CrossRef] [PubMed]
- Podbielska, M.; Kus-Liśkiewicz, M.; Jagusztyn, B.; Szpyrka, E. Effect of microorganisms on degradation of fluopyram and tebuconazole in laboratory and field studies. Environ. Sci. Pollut. Res. 2023, 30, 47727–47741. [Google Scholar] [CrossRef]
- Cycoń, M.; Markowicz, A.; Piotrowska-Seget, Z. Structural and functional diversity of bacterial community in soil treated with the herbicide napropamide estimated by the DGGE, CLPP and r/K-strategy approaches. Appl. Soil Ecol. 2013, 72, 242–250. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. Biostimulation as a process aiding tebuconazole degradation in soil. J. Soils Sediments 2019, 19, 3728–3741. [Google Scholar] [CrossRef]
- Lipińska, A.; Wyszkowska, J.; Kucharski, J. Microbiological and biochemical activity in soil contaminated with pyrene subjected to bioaugmentation. Water Air Soil Pollut. 2021, 232, 45. [Google Scholar] [CrossRef]
- Chen, X.; He, S.; Liang, Z.; Li, Q.X.; Yan, H.; Hu, J.; Liu, X. Biodegradation of pyraclostrobin by two microbial communities from Hawaiian soils and metabolic mechanism. J. Hazard. Mater. 2018, 354, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Luong, T.T.; Nguyen, T.H.T.; Nguyen, T.D.; Pham, T.H.T.; Ho, T.T.; Nguyen, N.L. Degradation of triazole fungicides by plant growth-promoting bacteria from contaminated agricultural soil. J. Microbiol. Biotechnol. 2024, 34, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Clinton, B.; Warden, A.C.; Haboury, S.; Easton, C.J.; Kotsonis, S.; Taylor, M.C.; Oakeshott, J.G.; Russell, R.J.; Scott, C. Bacterial degradation of strobilurin fungicides: A role for a promiscuous methyl esterase activity of the subtilisin proteases? Biocatal. Biotransform. 2011, 29, 119–129. [Google Scholar] [CrossRef]
- Howell, C.C.; Semple, K.T.; Bending, G.D. Isolation and characterisation of azoxystrobin degrading bacteria from soil. Chemosphere 2014, 95, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Hocinat, A.; Boudemagh, A. Biodegradation of commercial ortiva fungicide by isolated actinomycetes from the activated sludge, Desalin. Water Treat. 2016, 57, 6091–6097. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, W.; Pang, S.; Lin, Z.; Zhang, Y.; Huang, Y.; Bhatt, P.; Chen, S. Kinetics and new mechanism of azoxystrobin biodegradation by an Ochrobactrum anthropi strain SH14. Microorganisms 2020, 8, 625. [Google Scholar] [CrossRef] [PubMed]
- Kenarova, A.; Boteva, S. Fungicides in agriculture and their side effects on soil enzyme activities: A review. Bulg. J. Agric. Sci. 2023, 29, 33–42. [Google Scholar]
- Wang, X.; Lu, Z.; Miller, H.; Liu, J.; Hou, Z.; Liang, S.; Zhao, H.; Borch, T. Fungicide azoxystrobin induced changes on the soil microbiome. Appl. Soil Ecol. 2020, 145, 103343. [Google Scholar] [CrossRef]
- Boteva, S.B.; Kenarova, A.E.; Petkova, M.R.; Georgieva, S.S.; Chanev, C.D.; Radeva, G.S. Soil enzyme activities after application of fungicide QuadrisR at increasing concentration rates. Plant Soil Environ. 2022, 68, 382–392. [Google Scholar] [CrossRef]
- Roman, D.L.; Matica, M.A.; Ciorsac, A.; Boros, B.V.; Isvoran, A. The effects of the fungicide myclobutanil on soil enzyme activity. Agriculture 2023, 13, 1956. [Google Scholar] [CrossRef]
- Amaro, A.C.E.; Ramos, A.R.P.; Macedo, A.C.; Ono, E.O.; Rodrigues, J.D. Effects of the fungicides azoxystrobin, pyraclostrobin and boscalid on the physiology of Japanese cucumber. Sci. Hortic. 2018, 228, 66–75. [Google Scholar] [CrossRef]
- Chiu-Yueh, L.A.N.; Kuan-Hung, L.I.N.; Huang, W.D.; Chang-Chang, C.H.E.N. Physiological effects of the fungicide azoxystrobin on wheat seedlings under extreme heat. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 683–690. [Google Scholar] [CrossRef]
- Emam, S.S.; Abd-Eldaim, F.A. Effect of difenoconazole and azoxystrobin on wheat and radish seeds germination and tomato seedling growth. Egypt. Acad. J. Biol. Sci. 2022, 14, 139–148. [Google Scholar] [CrossRef]
- Macar, O.; Kalefetoğlu Macar, T.; Yalçın, E.; Çavuşoğlu, K. Acute multiple toxic effects of trifloxystrobin fungicide on Allium cepa L. Sci. Rep. 2022, 12, 15216. [Google Scholar] [CrossRef]
- IUSS Working Group World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Reference Base for Soil Resources, Update 2015; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2014. [Google Scholar]
- Borowik, A.; Wyszkowska, J.; Wyszkowski, M. Resistance of aerobic microorganisms and soil enzyme response to soil contamination with Ekodiesel Ultra fuel. Environ. Sci. Pollut. Res. 2017, 24, 24346–24363. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Development. The Register of Authorized Plant Protection Products, 2024—Update 21 March 2024. Available online: https://www.gov.pl (accessed on 31 March 2024).
- ISIS-Draw, MDL, Version 2.3. 2004. Available online: https://mdl-isis-draw.software.informer.com/2.3/ (accessed on 18 April 2024).
- Lewis, K.; Tzilivakis, J.G. Development of a data set of pesticide dissipation rates in/on various plant matrices for the Pesticide Properties Database (PPDB). Data 2017, 2, 28. [Google Scholar] [CrossRef]
- Kucharski, J.; Tomkiel, M.; Baćmaga, M.; Borowik, A.; Wyszkowska, J. Enzyme activity and microorganisms diversity in soil contaminated with the Boreal 58 WG herbicide. J. Environ. Sci. Health B 2016, 51, 446–454. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Boros-Lajszner, E.; Kucharski, J. The impact of soil contamination with lead on the biomass of maize intended for energy purposes, and the biochemical and physicochemical properties of the soil. Energies 2024, 17, 1156. [Google Scholar] [CrossRef]
- Baćmaga, M.; Wyszkowska, J.; Kucharski, J. The effect of the Falcon 460 EC fungicide on soil microbial communities, enzyme activities and plant growth. Ecotoxicology 2016, 25, 1575–1587. [Google Scholar] [CrossRef] [PubMed]
- Lipińska, A.; Wyszkowska, J.; Kucharski, J. Diversity of organotrophic bacteria, activity of dehydrogenases and urease as well as seed germination and root growth Lepidium sativum, Sorghum saccharatum and Sinapis alba under the influence of polycyclic aromatic hydrocarbons. Environ. Sci. Pollut. Res. 2015, 22, 18519–18530. [Google Scholar] [CrossRef] [PubMed]
- Starchel, R.; Wyszkowska, J.; Baćmaga, M. Bioaugmentation of soil contaminated with zinc. Water Air Soil Pollut. 2020, 231, 443. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. The usability of sorbents in restoring enzymatic activity in soils polluted with petroleum-derived products. Materials 2023, 16, 3738. [Google Scholar] [CrossRef] [PubMed]
- Borowik, A.; Wyszkowska, J.; Kucharski, J. Bacteria and soil enzymes supporting the valorization of forested soils. Materials 2022, 15, 3287. [Google Scholar] [CrossRef] [PubMed]
- Wyszkowska, J.; Boros-Lajszner, E.; Kucharski, J. Calorific value of Festuca rubra biomass in the phytostabilization of soil contaminated with nickel, cobalt and cadmium which disrupt the microbiological and biochemical properties of soil. Energies 2022, 15, 3445. [Google Scholar] [CrossRef]
- Lipińska, A.; Kucharski, J.; Wyszkowska, J. Activity of phosphatases in soil contaminated with PAHs. Water Air Soil Pollut. 2019, 230, 298. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica. Data Analysis Software System, Version 13. 2017. Available online: https://www.statistica.com (accessed on 13 April 2024).







| Object | Org | Act | Fun | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Soil Incubation Time (Days) | |||||||||
| 30 | 60 | 90 | 30 | 60 | 90 | 30 | 60 | 90 | |
| C | 1.720 i | 3.378 b | 2.940 d | 0.832 h | 1.865 d | 2.059 c | 1.651 d | 4.798 a | 3.193 b |
| F | 2.133 g | 3.057 c | 3.772 a | 1.575 f | 2.142 b | 2.437 a | 1.418 e | 2.158 c | 2.197 c |
| P | 2.780 e | 2.095 h | 2.496 f | 1.115 g | 1.670 e | 1.819 d | 0.982 g | 1.208 f | 1.246 f |
| Average | 2.211 C | 2.843 B | 3.069 A | 1.174 C | 1.892 B | 2.105 A | 1.350 C | 2.721 A | 2.212 B |
| Object | Org | Act | Fun | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Soil Incubation Time (Days) | |||||||||
| 30 | 60 | 90 | 30 | 60 | 90 | 30 | 60 | 90 | |
| F | 24.012 c | −9.503 d | 28.299 b | 89.303 a | 14.852 d | 18.358 c | −14.113 a | −55.023 d | −31.193 b |
| P | 61.678 a | −37.981 f | −15.102 e | 34.014 b | −10.456 e | −11.656 f | −40.521 c | −74.823 f | −60.977 e |
| Average | 42.845 A | −23.742 C | 6.598 B | 61.685 A | 2.198 C | 3.351 B | −27.317 A | −64.923 C | −46.085 B |
| Object | Org | Act | Fun | |||
|---|---|---|---|---|---|---|
| Soil Incubation Time (Days) | ||||||
| 60 | 90 | 60 | 90 | 60 | 90 | |
| F | 0.131 b | −0.288 d | 0.451 a | 0.038 c | −0.779 d | −0.450 a |
| P | −0.089 c | 0.367 a | 0.123 b | −0.038 d | −0.689 c | −0.491 b |
| Average | 0.021 B | 0.040 A | 0.287 A | 0.000 B | −0.734 B | −0.471 A |
| Object | Deh (µmol TPF) | Cat (mol O2) | Pal (mmol PNP) | Pac (mmol PNP) | Ure (mmol N-NH4) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Incubation Time (Days) | |||||||||||||||
| 30 | 60 | 90 | 30 | 60 | 90 | 30 | 60 | 90 | 30 | 60 | 90 | 30 | 60 | 90 | |
| C | 28.050 a | 27.454 b | 19.159 e | 0.086 e | 0.516 b | 0.515 b | 2.261 f | 2.266 f | 0.334 b | 1.327 c | 1.048 e | 2.034 a | 2.129 a | 2.089 b | 1.721 c |
| F | 28.359 a | 28.139 a | 19.719 d | 0.102 d | 0.542 a | 0.521 a | 2.325 e | 2.455 d | 0.363 a | 1.301 c | 1.133 d | 2.014 a | 2.147 a | 2.127 b | 1.671 d |
| P | 27.034 b | 26.424 c | 19.073 f | 0.156 c | 0.549 a | 0.508 b | 2.002 g | 2.387 e | 0.316 c | 1.220 d | 1.126 e | 1.860 b | 2.111 a | 2.078 b | 1.396 e |
| Average | 28.361 A | 27.339 B | 19.317 C | 0.115 C | 0.536 A | 0.515 B | 2.196 B | 2.369 A | 0.338 C | 1.283 C | 1.102 B | 1.969 A | 2.129 A | 2.098 B | 1.596 C |
| Object | Deh | Cat | Pal | Pac | Ure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Incubation Time (Days) | |||||||||||||||
| 30 | 60 | 90 | 30 | 60 | 90 | 30 | 60 | 90 | 30 | 60 | 90 | 30 | 60 | 90 | |
| F | 1.102 c | 2.495 b | 2.923 a | 18.605 b | 1.342 d | 1.165 e | 2.831 d | 8.341 b | 8.683 a | −1.959 d | 8.112 a | −0.983 c | 0.845 b | 1.819 a | −2.905 e |
| P | −3.622 e | −3.752 f | −0.449 d | 81.395 a | 6.395 c | −1.359 f | −11.455 f | 5.340 c | −5.389 e | −8.063 e | 7.443 b | −8.554 f | −0.845 c | −1.100 d | −18.884 f |
| Average | −1.260 C | −0.628 B | 1.237 A | 50.000 A | 3.868 B | −0.097 C | −4.312 C | 6.840 A | 1.647 B | −5.011 C | 7.777 A | −4.768 B | 0.000 B | 0.359 A | −10.894 C |
| Object | Deh | Cat | Pal | Pac | Ure | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Soil Incubation Time (Days) | ||||||||||
| 60 | 90 | 60 | 90 | 60 | 90 | 60 | 90 | 60 | 90 | |
| F | −0.362 d | −0.269 c | −0.241 d | 0.467 b | −0.166 d | 0.370 c | −0.549 c | −0.499 b | −0.597 d | 0.854 a |
| P | 0.075 b | 0.195 a | 0.359 c | 0.843 a | 0.783 b | 0.876 a | −0.638 d | −0.423 a | −0.491 c | −0.267 b |
| Average | −0.144 B | −0.037 A | 0.059 B | 0.655 A | 0.309 B | 0.623 A | −0.594 B | −0.461 A | −0.544 B | 0.294 A |
| Object | Lepidium sativum L. | Sinapsis alba L. | Sorghum saccharatum L. | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Soil Incubation Time (Days) | |||||||||
| 30 | 60 | 90 | 30 | 60 | 90 | 30 | 60 | 90 | |
| Inhibition of seed germination | |||||||||
| F | −21.847 a | −41.681 c | −38.777 b | −55.130 c | −56.910 c | −22.919 a | −9.202 a | −19.756 b | −44.299 e |
| P | −40.442 c | −52.044 d | −58.432 e | −56.257 c | −63.924 d | −52.375 b | −39.323 c | −42.413 d | −54.227 f |
| Average | −31.145 | −46.863 | −48.605 | −55.693 | −60.417 | −37.647 | −24.262 | −31.084 | −49.263 |
| Inhibition of root elongation | |||||||||
| F | −23.390 a | −35.112 d | −25.607 b | −26.557 b | −43.438 c | −19.846 a | −20.378 a | −24.188 b | −41.184 d |
| P | −33.634 c | −47.244 e | −54.902 f | −49.520 e | −44.427 d | −50.917 f | −33.615 c | −46.562 e | −53.784 f |
| Average | −28.512 A | −41.178 C | −40.255 B | −38.039 B | −43.932 C | −35.381 A | −26.997 A | −35.375 B | −47.484 C |
| Soil | pH | HAC | EBC | CEC | BS % | Corg | Ntot | C:N | Total Exchangeable Cations | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K+ | Na+ | Ca2+ | Mg2+ | |||||||||
| mmol+ kg−1 | g kg−1 | mg kg−1 | ||||||||||
| Sandy loam | 7.0 | 6.4 | 165.9 | 172.3 | 96.28 | 14.30 | 0.98 | 14.6:1 | 186.0 | 20.0 | 2571.40 | 59.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baćmaga, M.; Wyszkowska, J.; Kucharski, J. Response of Soil Microbiota, Enzymes, and Plants to the Fungicide Azoxystrobin. Int. J. Mol. Sci. 2024, 25, 8104. https://doi.org/10.3390/ijms25158104
Baćmaga M, Wyszkowska J, Kucharski J. Response of Soil Microbiota, Enzymes, and Plants to the Fungicide Azoxystrobin. International Journal of Molecular Sciences. 2024; 25(15):8104. https://doi.org/10.3390/ijms25158104
Chicago/Turabian StyleBaćmaga, Małgorzata, Jadwiga Wyszkowska, and Jan Kucharski. 2024. "Response of Soil Microbiota, Enzymes, and Plants to the Fungicide Azoxystrobin" International Journal of Molecular Sciences 25, no. 15: 8104. https://doi.org/10.3390/ijms25158104





