MMP-14 Exhibits Greater Expression, Content and Activity Compared to MMP-15 in Human Renal Carcinoma
Abstract
1. Introduction
2. Results
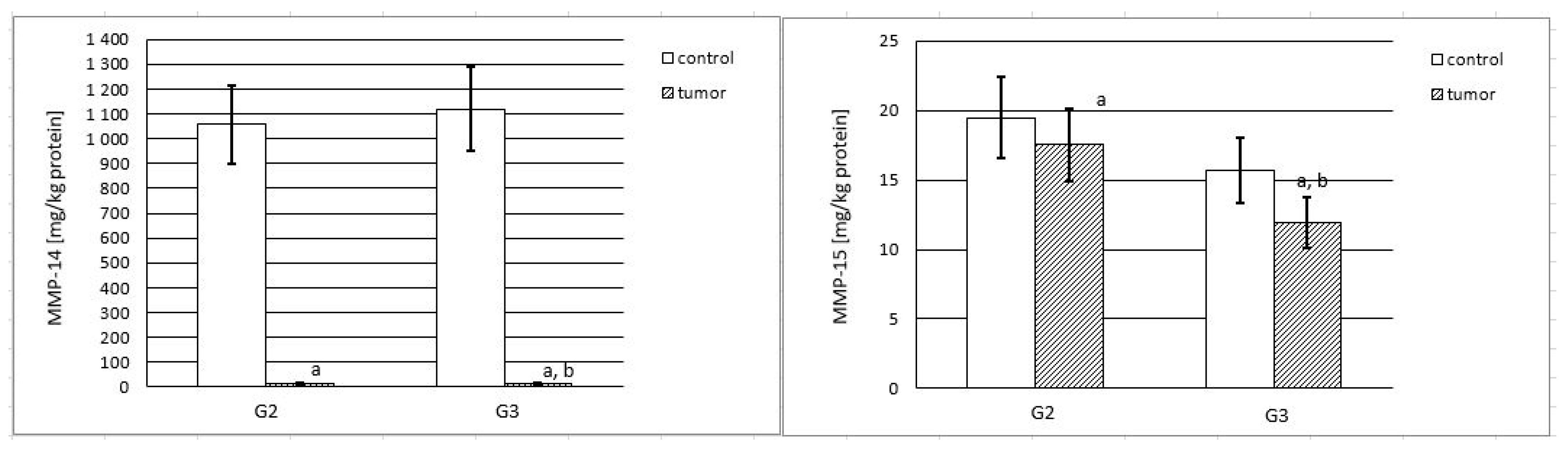
2.1. MMP-14 and MMP-15 Content in Tissue Samples
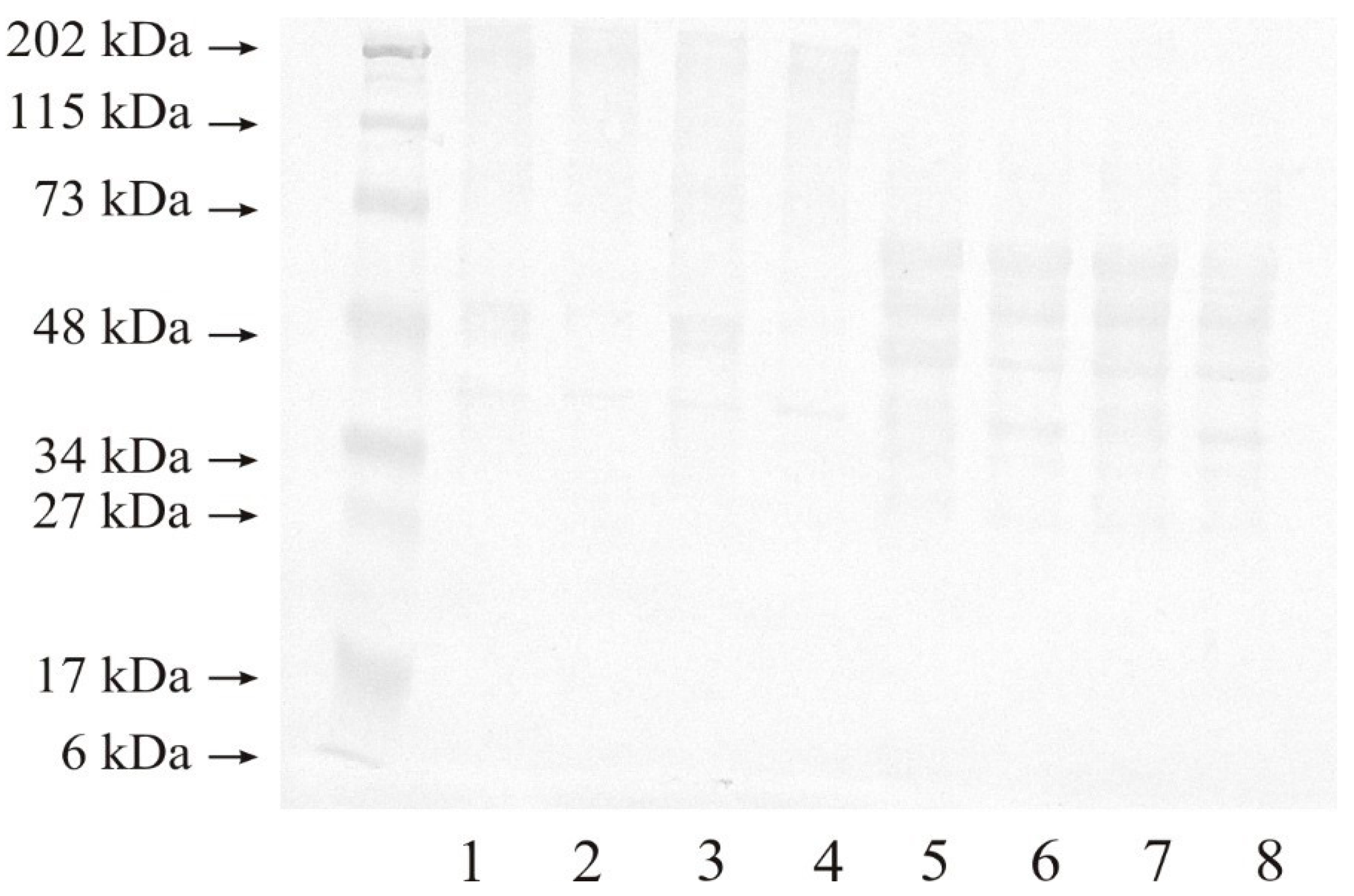
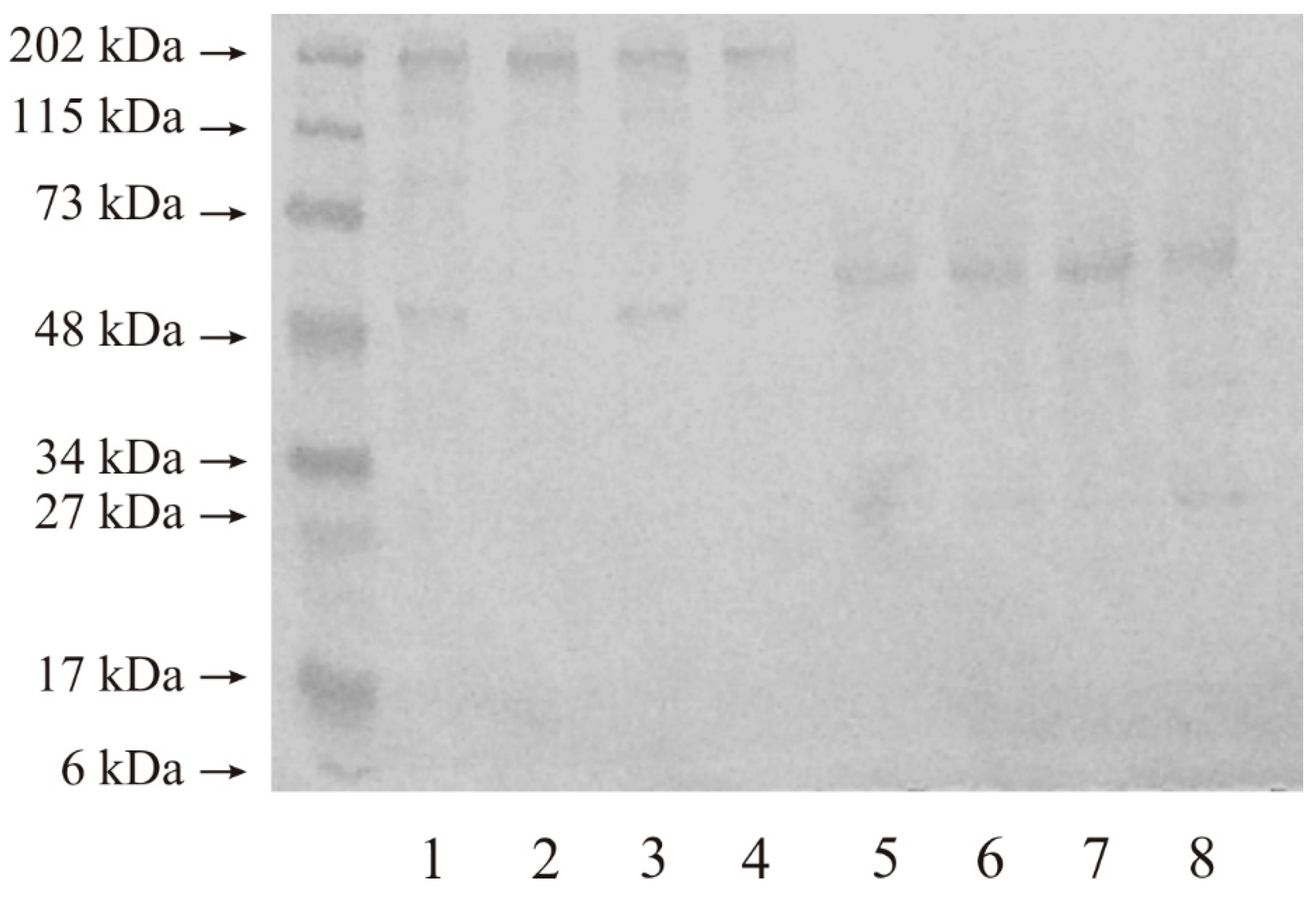
2.2. Western Blot Analysis of MMP-14 and MMP-15
2.2.1. Expression of MMP-14
2.2.2. Expression of MMP-15
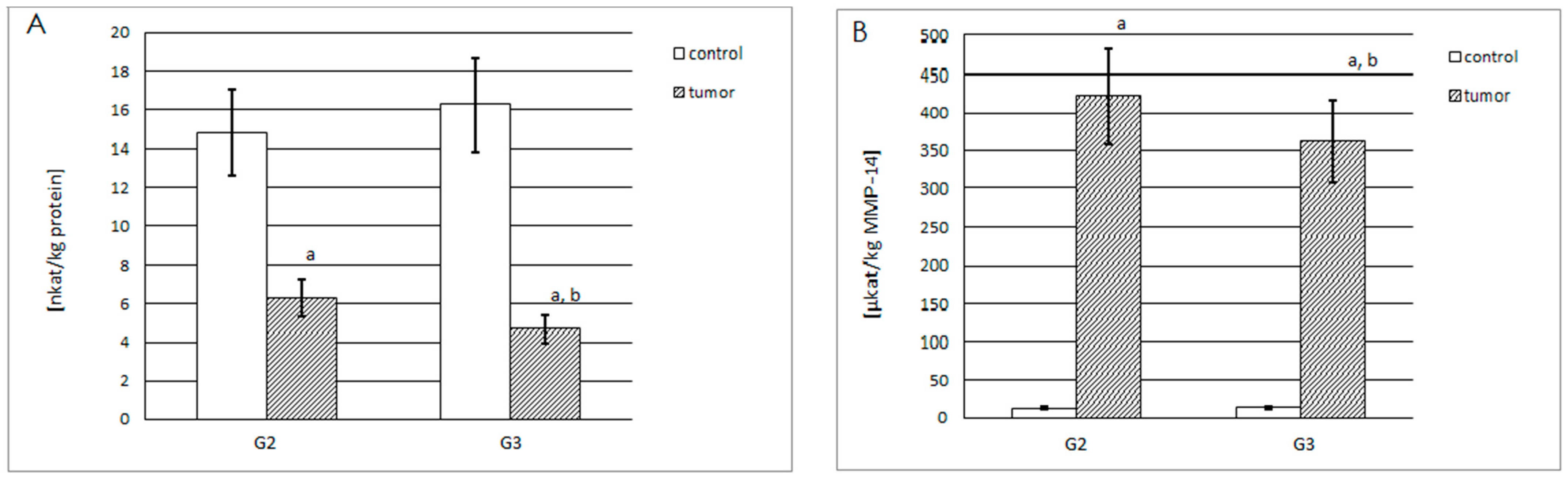
2.3. Activities of MMP-14 and MMP-15
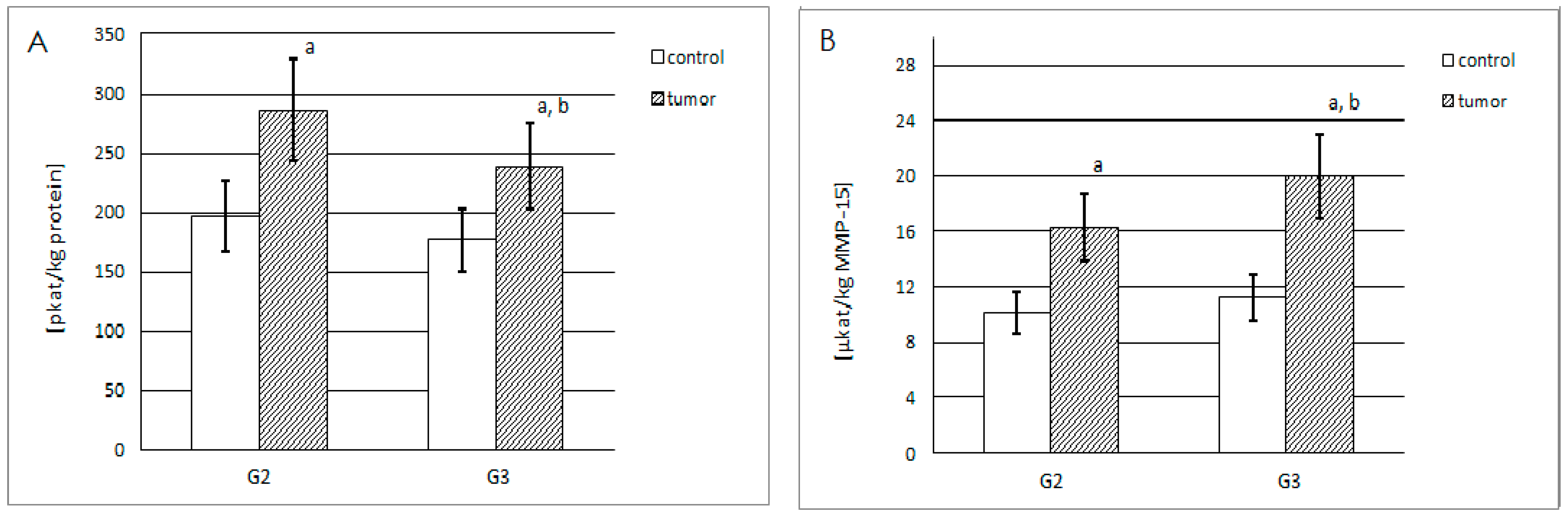
2.3.1. Actual and Specific Activity of MMP-14
2.3.2. Actual and Specific Activity of MMP-15
3. Discussion
4. Materials and Methods
4.1. Tissue Material
4.2. Specimen Preparation
4.3. MMP Contents
4.4. MMP Western Blot
4.5. Actual and Specific Activities of MMPs
4.6. Protein Determination
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Netter, F. Atlas of Human Anatomy, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Bochenek, A.; Reicher, M. Anatomia Człowieka Tom II; PZWL: Warsaw, Poland, 2004; pp. 476–537. [Google Scholar]
- Górski, J. Fizjologiczne Podstawy Wysiłku Fizycznego; PZWL: Warsaw, Poland, 2008; pp. 368–387. [Google Scholar]
- Boor, P.; Sebekova, K.; Ostendorf, T.; Floege, J. Treatment targets in renal fibrosis. Nephrol. Dial. Transpl. 2007, 22, 3391–3407. [Google Scholar] [CrossRef]
- Catania, J.M.; Chen, G.; Parrish, A.R. Role of matrix metalloproteinases in renal pathophysiologies. Am. J. Physiol. Renal. Physiol. 2007, 292, F905–F911. [Google Scholar] [CrossRef]
- Pardo, A.; Selman, M. Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc. Am. Thorac. Soc. 2006, 3, 383–388. [Google Scholar] [CrossRef]
- Castro-Castro, A.; Marchesin, V.; Monteiro, P.; Lodillinsky, C.; Rossé, C.; Chavrier, P. Cellular and molecular mechanisms of MT1-MMP-dependent cancer cell Invasion. Annu. Rev. Cell Dev. Biol. 2016, 32, 555–576. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Watkins, G.A.; Fung Jones, E.; Scott Shell, M.; VanBrocklin, H.F.; Pan, M.-H.; Hanrahan, S.M.; Feng, J.J.; He, J.; Sounni, N.E.; Dill, K.A.; et al. Development of an optimized activatable MMP-14 targeted SPECT imaging probe. Bioorg. Med. Chem. 2009, 17, 653–659. [Google Scholar] [CrossRef]
- Amar, S.; Smith, L.; Fields, G.B. Matrix metalloproteinase collagenolysis in health and disease. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1940–1951. [Google Scholar] [CrossRef]
- Yoshizaki, T.; Sato, H.; Furukawa, M. Recent advances in the regulation of matrix metalloproteinase 2 activation: From basic research to clinical implication (Review). Oncol. Rep. 2002, 9, 607–611. [Google Scholar] [CrossRef]
- Ingvarsen, S.; Madsen, D.H.; Hillig, T.; Lund, L.R.; Holmbeck, K.; Behrendt, N.; Engelholm, L.H. Dimerization of endogenous MT1-MMP is a regulatory step in the activation of the 72-kDa gelatinase MMP-2 on fibroblasts and fibrosarcoma cells. Biol. Chem. 2008, 389, 943–953. [Google Scholar] [CrossRef]
- Itoh, Y.; Ito, N.; Nagase, H.; Evans, R.D.; Bird, S.A.; Seiki, M. Cell surface collagenolysis requires homodimerization of the membrane-bound collagenase MT1-MMP. Mol. Biol. Cell. 2006, 17, 5390–5399. [Google Scholar] [CrossRef]
- Itoh, Y.; Ito, N.; Nagase, H.; Seiki, M. The second dimer interface of MT1-MMP, the transmembrane domain, is essential for ProMMP-2 activation on the cell surface. J. Biol. Chem. 2008, 283, 13053–13062. [Google Scholar] [CrossRef]
- Sabeh, F.; Ota, I.; Holmbeck, K.; Birkedal-Hansen, H.; Soloway, P.; Balbin, M.; Lopez-Otin, C.; Shapiro, S.; Inada, M.; Weiss, S.J. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 2004, 167, 769–781. [Google Scholar] [CrossRef]
- Szabova, L.; Chrysovergis, K.; Yamada, S.; Holmbeck, K. MT1-MMP is required for efficient tumor dissemination in experimental metastatic disease. Oncogene 2008, 27, 3274–3281. [Google Scholar] [CrossRef][Green Version]
- Andreucci, M.; Provenzano, M.; Faga, T.; Michael, A.; Patella, G.; Mastroroberto, P.; Serraino, G.F.; Bracale, U.M.; Ielapi, N.; Serra, R. Aortic Aneurysms, Chronic Kidney Disease and Metalloproteinases. Biomolecules 2021, 11, 194. [Google Scholar] [CrossRef]
- Turunen, S.P.; Tatti-Bugaeva, O.; Lehti, K. Membrane-type matrix metalloproteases as diverse effectors of cancer progression. Biochim. Biophys. Acta 2017, 1864 Pt A, 1974–1988. [Google Scholar] [CrossRef]
- Mylona, E.; Nomikos, A.; Magkou, C.; Kamberou, M.; Papassideri, I.; Keramopoulos, A.; Nakopoulou, L. The clinicopathological and prognostic significance of membrane type 1 matrix metalloproteinase (MT1-MMP) and MMP-9 according to their localization in invasive breast carcinoma. Histopathology 2007, 50, 338–347. [Google Scholar] [CrossRef]
- Shanbhogue, A.K.; Prasad, S.R.; Takahashi, N.; Vikram, R.; Sahani, D.V. Recent advances in cytogenetics and molecular biology of adult hepatocellular tumors: Implications for imaging and management. Radiology 2011, 258, 673–693. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, S.; Wei, Y.; Wang, C.; Zou, H.; Hu, J.; Jia, W.; Pang, L. Prognostic Significance of Matrix Metalloproteinase 14 in Patients with Cancer: A Systematic Review and Meta-Analysis. Clin. Lab. 2020, 66, 745. [Google Scholar] [CrossRef]
- Available online: http://www.uniprot.org/uniprot/P45452 (accessed on 23 July 2024).
- Available online: https://www.wikigenes.org/e/gene/e/4322.html (accessed on 23 July 2024).
- Available online: http://www.genecards.org/cgi-bin/carddisp.pl (accessed on 23 July 2024).
- Kitagawa, Y.; Kunimi, K.; Uchibayashi, T.; Sato, H.; Namiki, M. Expression of messenger RNAs for membrane-type 1, 2, and 3 matrix metalloproteinases in human renal cell carcinomas. J. Urol. 1999, 162 Pt 1, 905–909. [Google Scholar] [CrossRef]
- Bosman, F.T.; Stamenkovic, I. Functional structure and composition of the extracellular matrix. J. Pathol. 2003, 200, 423–428. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.J.; Affolter, M.; Kussmann, M. A nutrigenomics view of protein intake: Macronutrient, bioactive peptides, and protein turnover. Prog. Mol. Biol. Transl. Sci. 2012, 108, 51–74. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.K.; Li, Y.L.; Lu, H.T.; Wang, K.L.; Xu, W.H. Expression of tissue levels of matrix metalloproteinases and tissue inhibitors of metalloproteinases in renal cell carcinoma. World J. Surg. Oncol. 2013, 11, 1. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Cannon, G.M., Jr.; Getzenburg, R.H. Urinary matrix metalloproteinases activity is not significantly altered in patients with renal cell carcinoma. Urology 2006, 67, 848–850. [Google Scholar] [CrossRef] [PubMed]
- Genovese, F.; Manresa, A.A.; Leeming, D.J.; Karsdal, M.A.; Boor, P. The extracellular matrix in the kidney: A source of novel non-invasive biomarkers of kidney fibrosis? Fibrogenesis Tissue Repair 2014, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Yang, K.E.; Hwang, J.W.; Kang, H.S.; Lee, S.Y.; Choi, S.; Shin, J.; Jang, I.S. An, as Indicators of Post-Mortem Interval in a Rat Model, with Use of Lateral Flow Technology. PLoS ONE 2016, 11, e0160557. [Google Scholar] [CrossRef]
- Shi, Z.D.; Ji, X.Y.; Qazi, H.; Tarbell, J.M. Interstitial flow promotes vascular fibroblast myofibroblast smooth muscle cell motility in 3-D collagen I via upregulation of MMP-1. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1225–H1234. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młynarczyk, G.; Tokarzewicz, A.; Gudowska-Sawczuk, M.; Mroczko, B.; Novák, V.; Novák, A.; Mitura, P.; Romanowicz, L. MMP-14 Exhibits Greater Expression, Content and Activity Compared to MMP-15 in Human Renal Carcinoma. Int. J. Mol. Sci. 2024, 25, 8107. https://doi.org/10.3390/ijms25158107
Młynarczyk G, Tokarzewicz A, Gudowska-Sawczuk M, Mroczko B, Novák V, Novák A, Mitura P, Romanowicz L. MMP-14 Exhibits Greater Expression, Content and Activity Compared to MMP-15 in Human Renal Carcinoma. International Journal of Molecular Sciences. 2024; 25(15):8107. https://doi.org/10.3390/ijms25158107
Chicago/Turabian StyleMłynarczyk, Grzegorz, Anna Tokarzewicz, Monika Gudowska-Sawczuk, Barbara Mroczko, Vojtěch Novák, Adam Novák, Przemysław Mitura, and Lech Romanowicz. 2024. "MMP-14 Exhibits Greater Expression, Content and Activity Compared to MMP-15 in Human Renal Carcinoma" International Journal of Molecular Sciences 25, no. 15: 8107. https://doi.org/10.3390/ijms25158107
APA StyleMłynarczyk, G., Tokarzewicz, A., Gudowska-Sawczuk, M., Mroczko, B., Novák, V., Novák, A., Mitura, P., & Romanowicz, L. (2024). MMP-14 Exhibits Greater Expression, Content and Activity Compared to MMP-15 in Human Renal Carcinoma. International Journal of Molecular Sciences, 25(15), 8107. https://doi.org/10.3390/ijms25158107









