Alzheimer’s Disease Neuropathological Change in Aged Non-Primate Mammals
Abstract
:1. Introduction: Human Brain Aging and Cognitive Impairment
2. ADNC in Non-Primate Mammals
2.1. Pinnipeds
2.2. Bears
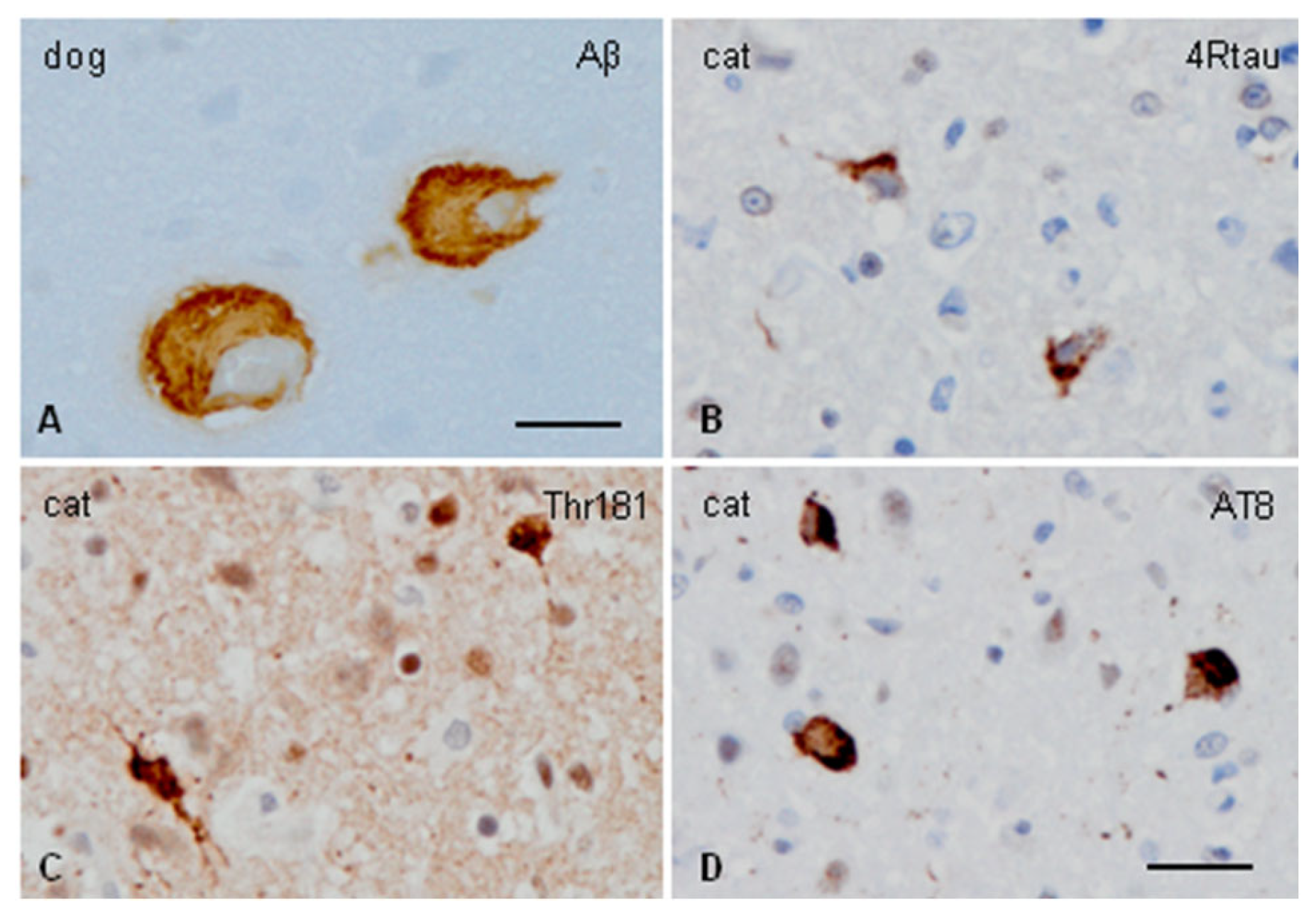
2.3. Dogs (Canis lupus familiaris)
2.4. Domestic and Wild Cats
2.5. Wolverine
2.6. Cetacea
2.7. Cattle
2.8. Sheep
2.9. Equids
2.10. Guinea Pigs (Cavia porcellus)
2.11. Degus
2.12. Rabbits
2.13. Tree Shrews
3. Summary of ADNC and Abnormal Behavior in Aged Mammals
4. Comparative Brain Aging ADNC in Mammals and Humans
Funding
Conflicts of Interest
Appendix A. Suggested Sampling and Staining for Age-Related Neurodegenerative Diseases in Veterinary Neuropathology
References
- Heuer, E.; Rosen, R.F.; Cintron, A.; Walker, L.C. Nonhuman primate models of Alzheimer-like cerebral proteopathy. Curr. Pharm. Des. 2012, 18, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.C.; Jucker, M. The exceptional vulnerability of humans to Alzheimer’s disease. Trends Mol. Med. 2017, 23, 534–545. [Google Scholar] [CrossRef]
- Freire-Cobo, C.; Edler, M.K.; Varghese, M.; Munger, E.; Laffey, J.; Raia, S.; Raia, S.; In, S.S.; Wicinski, B.; Medalla, M.; et al. Comparative neuropathology in aging primates: A perspective. Am. J. Primatol. 2021, 83, e23299. [Google Scholar] [CrossRef]
- Ferrer, I. The unique neuropathological vulnerability of the human brain to aging. Ageing Res. Rev. 2023, 87, 101916. [Google Scholar] [CrossRef]
- Glenner, G.G.; Wong, C.W. Alzheimer’s disease: Initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 1984, 120, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Glenner, G.G.; Wong, C.W.; Quaranta, V.; Eanes, E.D. The amyloid deposits in Alzheimer’s disease: Their nature and pathogenesis. Appl. Pathol. 1984, 12, 357–369. [Google Scholar] [PubMed]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef] [PubMed]
- Iwatsubo, T.; Odaka, A.; Suzuki, N.; Mizusawa, H.; Nukina, N.; Ihara, Y. Visualization of Ab42(43) and Ab40 in senile plaques with end-specific Aβ monoclonals: Evidence that an initially deposited species is Aβ42(43). Neuron 1994, 13, 45–53. [Google Scholar] [CrossRef]
- Gremer, L.; Schölzel, D.; Schenk, C.; Reinartz, E.; Labahn, J.; Ravelli, R.B.G.; Tusche, M.; Lopez-Iglesias, C.; Hoyer, W.; Heise, H.; et al. Fibril structure of amyloid-beta(1-42) by cryo-electron microscopy. Science 2017, 358, 116–119. [Google Scholar] [CrossRef]
- Ono, K.; Watanabe-Nakayama, T. Aggregation and structure of amyloid beta-protein. Neurochem. Int. 2021, 151, 105208. [Google Scholar] [CrossRef]
- Yang, Y.; Arseni, D.; Zhang, W.; Huang, M.; Lövestam, S.; Schweighauser, M.; Kotecha, A.; Murzin, A.G.; Peak-Chew, S.Y.; Macdonald, J.; et al. Cryo-EM structures of amyloid-beta 42 filaments from human brains. Science 2022, 375, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Goate, A.; Chartier-Harlin, M.C.; Mullan, M.; Brown, J.; Crawford, F.; Fidani, L.; Giuffra, L.; Haynes, A.; Irving, N.; James, L.; et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature 1991, 349, 704–706. [Google Scholar] [CrossRef] [PubMed]
- Chartier-Harlin, M.C.; Crawford, F.; Houlden, H.; Warren, A.; Hughes, D.; Fidani, L.; Goate, A.; Rossor, M.; Roques, P.; Hardy, J.; et al. Early-onset Alzheimer’s disease caused by mutations at codon 717 of the β-amyloid precursor protein gene. Nature 1991, 353, 844–846. [Google Scholar] [CrossRef] [PubMed]
- Murrell, J.; Farlow, M.; Ghetti, B.; Benson, M.D. A mutation in the amyloid precursor protein associated with hereditary Alzheimer’s disease. Science 1991, 254, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Levy-Lahad, E.; Wasco, W.; Poorkaj, P.; Romano, D.M.; Oshima, J.; Pettingell, W.H.; Yu, C.E.; Jondro, P.D.; Schmidt, S.D.; Wang, K.; et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 1995, 269, 973–977. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, R.; Rogaev, E.I.; Liang, Y.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Chi, H.; Lin, C.; Li, G.; Holman, K.; et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995, 375, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Rogaev, E.I.; Sherrington, R.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Liang, Y.; Chi, H.; Lin, C.; Holman, K.; Tsuda, T.; et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature 1995, 376, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L.; Tanzi, R.E. Genetics of Alzheimer’s disease. In The Molecular Pathology of Dementias and Movement Disorders, 2nd ed.; Dickson, D.W., Weller, R.O., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 51–61. ISBN 978-1-405-19693-2. [Google Scholar]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The amyloid-β oligomer hypothesis: Beginning of the third decade. J. Alzheimers Dis. 2018, 64, S567–S610. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.; St George-Hyslop, P.H.; Pericak-Vance, M.A.; Joo, S.H. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993, 43, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Harold, D.; Abraham, R.; Hollingworth, P.; Sims, R.; Gerrish, A.; Hamshere, M.L.; Pahwa, J.S.; Moskvina, V.; Dowzell, K.; Williams, A.; et al. Genome-wide association studies identifies variants CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.C.; Heath, S.; Even, G.; Campion, D.; Sleegers, K.; Hiltunen, M.; Combarros, O.; Zelenika, D.; Bullido, M.J.; Tavernier, B.; et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 2009, 41, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Fitzpatrick, A.L.; Ikram, M.A.; DeStefano, A.L.; Gudnason, V.; Boada, M.; Bis, J.C.; Smith, A.V.; Carassquillo, M.M.; Lambert, J.C.; et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 2010, 303, 1832–1840. [Google Scholar] [CrossRef]
- Jun, G.; Naj, A.C.; Beecham, G.W.; Wang, L.S.; Buros, J.; Gallins, P.J.; Buxbaum, J.D.; Ertekin-Taner, N.; Fallin, M.D.; Friedland, R.; et al. Meta-analysis confirms CR1, CLU, and PICALM as Alzheimer disease risk loci and reveals interactions with APOE genotypes. Arch. Neurol. 2010, 67, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.; Holmans, P.A.; Hamshere, M.L.; Harold, D.; Moskvina, V.; Ivanov, D.; Pocklington, A.; Abraham, R.; Hollingworth, P.; Sims, R.; et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PLoS ONE 2010, 5, e13950. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; DeStafano, A.L.; Bis, J.C.; Beecham, G.W.; Grenier-Boley, B.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Naj, A.C.; Jun, G.; Reitz, C.; Kunkle, B.W.; Perry, W.; Park, Y.S.; Beecham, G.W.; Rajbhandary, R.A.; Hamilton-Nelson, K.L.; Wang, L.S.; et al. Effects of multiple genetic loci on age at onset in late-onset Alzheimer disease: A genome-wide association study. JAMA Neurol. 2014, 71, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Sims, R.; van der Lee, S.J.; Naj, A.C.; Bellenguez, C.; Badarinarayan, N.; Jakobsdottir, J.; Kunkle, B.W.; Boland, A.; Raybould, R.; Bis, J.C.; et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 2017, 49, 1373–1384. [Google Scholar] [CrossRef]
- Jansen, I.E.; Savage, J.E.; Watanabe, K.; Bryois, J.; Williams, D.M.; Steinberg, S.; Sealock, J.; Karlsson, I.K.; Hägg, S.; Athanasiu, L.; et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 2019, 51, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.J.; Fulton-Howard, B.; Goate, A. Interpretation of risk loci from genome-wide association studies of Alzheimer’s disease. Lancet Neurol. 2020, 19, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, H.M.; Narang, H.K.; Terry, R.D. Neurofibrillary tangles of paired helical filaments. J. Neurol. Sci. 1976, 27, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Delacourte, A.; Defossez, A. Alzheimer’s disease: Tau proteins, the promoting factors of microtubule assembly, are major components of paired helical filaments. J. Neurol. Sci. 1986, 76, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Kosik, K.S.; Joachim, C.L.; Selkoe, D.J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1986, 83, 4044–4048. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.G.; Mirra, S.S.; Pollock, N.J.; Binder, L.I. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau). Proc. Natl. Acad. Sci. USA 1986, 83, 4040–4043. [Google Scholar] [CrossRef]
- Grundke-Iqbal, I.; Iqbal, K.; Quinlan, M.; Tung, Y.C.; Zaidi, M.S.; Wisniewski, H.M. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J. Biol. Chem. 1986, 261, 6084–6089. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Wischik, C.M.; Crowther, R.A.; Walker, J.E.; Klug, A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: Identification as microtubule-associated protein tau. Proc. Natl. Acad. Sci. USA 1988, 85, 4051–4055. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G.; Cairns, N.J.; Crowther, R.A. Tau proteins in Alzheimer paired helical filaments: Abnormal phosphorylation of all six brain isoforms. Neuron 1992, 8, 159–168. [Google Scholar] [CrossRef]
- Buée, L.; Bussière, T.; Buée-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau protein isoforms, phosphorylation and role in neurodegenerative disorders. Brain Res. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef]
- Mandelkow, E.M.; Mandelkow, E. Biochemistry and Cell Biology of Tau Protein in Neurofibrillary Degeneration. Cold Spring Harb. Perspect. Med. 2012, 2, a006247. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Goedert, M. Tau pathology and neurodegeneration. Lancet Neurol. 2013, 12, 609–6022. [Google Scholar] [CrossRef]
- Arendt, T.; Stieler, J.T.; Holzer, M. Tau and tauopathies. Brain Res. Bull. 2016, 26, 238–292. [Google Scholar] [CrossRef]
- Wang, Y.; Mandelkow, E. Tau in physiology and pathology. Nat. Rev. Neurosci. 2016, 17, 5–21. [Google Scholar] [CrossRef]
- Goedert, M.; Spillantini, M.G. Ordered assembly of tau protein and neurodegeneration. Adv. Exp. Med. Biol. 2019, 1184, 3–21. [Google Scholar] [CrossRef]
- Wegmann, S.; Biernat, J.; Mandelkow, E. A current view on tau protein phosphorylation. Curr. Opin. Neurobiol. 2021, 69, 131–138. [Google Scholar] [CrossRef]
- Hernandez, F.; Ferrer, I.; Pérez, M.; Zabala, J.C.; Del Rio, J.A.; Avila, J. Tau aggregation. Neuroscience 2023, 518, 64–69. [Google Scholar] [CrossRef]
- Fitzpatrick, A.W.P.; Falcon, B.; He, S.; Murzin, A.G.; Murshudov, G.; Garringer, H.J.; Crowther, R.A.; Ghetti, B.; Goedert, M.; Scheres, S.H.W. Cryo-EM structures of tau filaments from Alzheimer’s disease. Nature 2017, 547, 185–190. [Google Scholar] [CrossRef]
- Falcon, B.; Zhang, W.; Schweighauser, M.; Murzin, A.G.; Vidal, R.; Garringer, H.J.; Ghetti, B.; Scheres, S.H.W.; Goedert, M. Tau filaments from multiple cases of sporadic and inherited Alzheimer’s disease adopt a common fold. Acta Neuropathol. 2018, 136, 699–708. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Staging of Alzheimer’s disease-related neurofibrillary tangles. Neurobiol. Aging 1995, 16, 271–278. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol. Aging 1997, 18, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011, 121, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 2015, 138, 2814–2833. [Google Scholar] [CrossRef]
- Arnsten, A.F.T.; Datta, D.; Del Tredici, K.; Braak, H. Hypothesis: Tau pathology is an initiating factor in sporadic Alzheimer’s disease. Alzheimers Dement. 2021, 17, 115–124. [Google Scholar] [CrossRef]
- Thal, R.D.; Rub, U.; Orantes, M.; Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the development of Alzheimer’s disease. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef]
- Ferrer, I. Defining Alzheimer as a common age-related neurodegenerative process not inevitably leading to dementia. Prog. Neurobiol. 2012, 97, 38–51. [Google Scholar] [CrossRef]
- Ferrer, I. Alzheimer’s disease is an inherent, natural part of human brain aging: An integrated perspective. Free Neuropathol. 2022, 3, 17. [Google Scholar] [CrossRef]
- Knopman, D. Clinical aspects of Alzheimer’s disease. In Neurodegeneration, the Molecular Pathology of Dementia and Movement Disorders; Dickson, D.W., Weller, R.O., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 39–50. ISBN 9781405196932. [Google Scholar]
- Nelson, P.T.; Alafuzoff, I.; Bigio, E.H.; Bouras, C.; Braak, H.; Cairns, N.J.; Castellani, R.J.; Crain, B.J.; Davies, P.; Del Tredici, K.; et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J. Neuropathol. Exp. Neurol. 2012, 71, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Hyman, B.T.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Carrillo, M.C.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; et al. National Institute on Aging-Alzheimer’s Association disease guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012, 8, 1–13. [Google Scholar] [CrossRef]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: A practical approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Cummings, J.L.; Dekosky, S.T.; Barberger-Gateau, P.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Revising the definition of Alzheimer’s disease: A new lexicon. Lancet Neurol. 2010, 9, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; Dekosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.D.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice guideline update summary: Mild cognitive impairment. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Crary, J.F.; Trojanowski, J.Q.; Schneider, J.A.; Abisambra, J.F.; Abner, E.L.; Alafuzoff, I.; Arnold, S.E.; Attems, J.; Beach, T.G.; Bigio, E.H.; et al. Primary age-related tauopathy (PART): A common pathology associated with human aging. Acta Neuropathol. 2014, 128, 755–766. [Google Scholar] [CrossRef]
- Hickman, R.A.; Flowers, X.E.; Wisniewski, T. Primary Age-Related Tauopathy (PART): Addressing the Spectrum of Neuronal Tauopathic Changes in the Aging Brain. Curr. Neurol. Neurosci. Rep. 2020, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Duyckaerts, C.; Braak, H.; Brion, J.P.; Buée, L.; Del Tredici, K.; Goedert, M.; Halliday, G.; Neumann, M.; Spillantini, M.G.; Tolnay, M.; et al. PART is part of Alzheimer disease. Acta Neuropathol. 2015, 129, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L.; Attems, J.; Thal, D.R. Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol. 2017, 134, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I. Hypothesis review: Alzheimer’s overture guidelines. Brain Pathol. 2023, 33, e13122. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I. Amyloid-β pathology is the common nominator proteinopathy of the primate brain aging. J. Alzheimer’s Dis. 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I. Review: Brain aging and Alzheimer’s disease, a perspective from non-human primates. Aging, 2024; in press. [Google Scholar]
- Takaichi, Y.; Chambers, J.K.; Takahashi, K.; Soeda, Y.; Koike, R.; Katsumata, E.; Kita, C.; Matsuda, F.; Haritani, M.; Takashima, A.; et al. Amyloid beta and tau pathology in brains of aged pinniped species (sea lion, seal, and walrus). Acta Neuropathol. Commun. 2021, 9, 10. [Google Scholar] [CrossRef]
- Takahashi, K.; Chambers, J.K.; Takaichi, Y.; Uchida, K. Different Aβ43 deposition patterns in the brains of aged dogs, sea lions, and cats. J. Vet. Med. Sci. 2022, 84, 1563–1573. [Google Scholar] [CrossRef]
- Cork, L.C.; Powers, R.E.; Selkoe, D.J.; Davies, P.; Geyer, J.J.; Price, D.L. Neurofibrillary tangles and senile plaques in aged bears. J. Neuropathol. Exp. Neurol. 1988, 47, 629–641. [Google Scholar] [CrossRef]
- Tekirian, T.L.; Saido, T.C.; Markesbery, W.R.; Russell, M.J.; Wekstein, D.R.; Patel, E.; Geddes, J.W. N-terminal heterogeneity of parenchymal and cerebrovascular Aβ deposits. J. Neuropathol. Exp. Neurol. 1998, 57, 76–94. [Google Scholar] [CrossRef]
- Lucot, K.L.; Bukhari, S.A.; Webber, E.D.; Bonham, T.A.; Darian-Smith, C.; Montine, T.J.; Green, S.L. Semi-quantitative assessment of Alzheimer’s-like pathology in two aged polar bears (Ursus maritimus). Comp. Med. 2022, 72, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Yoshino, T.; Yamaguchi, R.; Tateyama, S.; Kimoto, Y.; Nakayama, H.; Goto, N. Senile plaques and other senile changes in the brain of an aged American black bear. Vet. Pathol. 1995, 32, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Kuwamura, M.; Umemura, T.; Takada, K.; Ohama, E.; Itakura, C. Modified Bielschowsky and immunohistochemical studies on senile plaques in aged dogs. Neurosci. Lett. 1991, 129, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Kuwamura, M.; Awakura, T.; Umemura, T.; Takada, K.; Ohama, E.; Itakura, C. Topographic relationship between senile plaques and cerebrovascular amyloidosis in the brain of aged dogs. J. Vet. Med. Sci. 1992, 54, 137–144. [Google Scholar] [CrossRef]
- Uchida, K.; Tani, Y.; Uetsuka, K.; Nakayama, H.; Goto, N. Immunohistochemical studies on canine cerebral amyloid angiopathy and senile plaques. J. Vet. Med. Sci. 1992, 54, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Bobik, M.; White, R.G.; Hou, Y.; Benjamin, S.A.; Geddes, J.W. Age-specific onset of beta-amyloid in beagle brains. Neurobiol. Aging 1996, 17, 269–273. [Google Scholar] [CrossRef]
- Wisniewski, T.; Lalowski, M.; Bobik, M.; Russell, M.; Strosznajder, J.; Frangione, B. Amyloid beta 1-42 deposits do not lead to Alzheimer’s neuritic plaques in aged dogs. Biochem. J. 1996, 313, 575–580. [Google Scholar] [CrossRef]
- Borràs, D.; Ferrer, I.; Pumarola, M. Age-related changes in the brain of the dog. Vet. Pathol. 1999, 36, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, M.; Mascort, J.; Mahy, N.; Ferrer, I. Diffuse beta-amyloid plaques and hyperphosphorylated tau are unrelated processes in aged dogs with behavioral deficits. Acta Neuropathol. 2006, 112, 175–183. [Google Scholar] [CrossRef]
- Neus Bosch, M.; Pugliese, M.; Andrade, C.; Gimeno-Bayón, J.; Mahy, N.; Rodriguez, M.J. Amyloid-beta immunotherapy reduces amyloid plaques and astroglial reaction in aged domestic dogs. Neurodegener. Dis. 2015, 15, 24–37. [Google Scholar] [CrossRef]
- Czasch, S.; Paul, S.; Baumgärtner, W. A comparison of immunohistochemical and silver staining methods for the detection of diffuse plaques in the aged canine brain. Neurobiol. Aging 2006, 27, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Habiba, U.; Ozawa, M.; Chambers, J.K.; Uchida, K.; Descallar, J.; Nakayama, H.; Summers, B.A.; Morley, J.W.; Tayebi, M. Neuronal deposition of amyloid-beta oligomers and hyperphosphorylated tau is closely connected with cognitive dysfunction in aged dogs. J. Alzheimers Dis. Rep. 2021, 5, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.J.; Satou, T.; Head, E.; Milgram, N.W.; Cole, G.M.; Savage, M.J.; Podlisny, M.B.; Selkoe, D.J.; Siman, R.; Greenberg, B.D.; et al. Diffuse plaques contain C-terminal A beta 42 and not A beta 40: Evidence from cats and dogs. Neurobiol. Aging 1996, 17, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Tamaoka, A.; Sawamura, N.; Kiatipattanasakul, W.; Nakayama, H.; Shoji, S.; Yoshikawa, Y.; Doi, K. Deposition of amyloid beta protein (Aβ) subtypes [Aβ40 and Aβ42(43)] in canine senile plaques and cerebral amyloid angiopathy. Acta Neuropathol. 1997, 94, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, L.L.R.; Mesquita, L.P.; Wadt, D.; Bruhn, F.R.P.; Maiorka, P.C. Heterogenous deposition of β-amyloid in the brain of aged dogs. Neurobiol. Aging 2021, 99, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.L.; Le, K.X.; Cynis, H.; Ekpo, E.; Kleinschmidt, M.; Palmour, R.M.; Ervin, F.R.; Snigdha, S.; Cotman, C.W.; Saido, T.C.; et al. Pyroglutamate-3 amyloid-beta deposition in the brains of humans, non-human primates, canines, and Alzheimer disease-like transgenic mouse models. Am. J. Pathol. 2013, 183, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Frost, J.L.; Cotman, C.W.; Head, E.; Palmour, R.; Lemere, C.A.; Walter, J. Deposition of phosphorylated amyloid-beta in brains of aged nonhuman primates and canines. Brain Pathol. 2018, 28, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Satou, T.; Cummings, B.J.; Head, E.; Nielson, K.A.; Hahn, F.F.; Milgram, N.W.; Velazquez, P.; Cribbs, D.H.; Tenner, A.J.; Cotman, C.W.; et al. The progression of beta-amyloid deposition in the frontal cortex of the aged canine. Brain Res. 1997, 774, 35–43. [Google Scholar] [CrossRef] [PubMed]
- 101 Dewey, C.W.; Davies, E.S.; Xie, H.; Wakshlag, J.J. Canine cognitive dysfunction: Pathophysiology, diagnosis, and treatment. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 477–499. [Google Scholar] [CrossRef]
- Schütt, T.; Helboe, L.; Pedersen, L.Ø.; Waldemar, G.; Berendt, M.; Pedersen, J.T. Dogs with Cognitive dysfunction as a spontaneous model for early Alzheimer’s disease: A translational study of neuropathological and inflammatory markers. J. Alzheimers Dis. 2016, 52, 433–449. [Google Scholar] [CrossRef]
- Cummings, B.J.; Head, E.; Afagh, A.J.; Milgram, N.W.; Cotman, C.W. Beta-amyloid accumulation correlates with cognitive dysfunction in the aged canine. Neurobiol. Learn. Mem. 1996, 66, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Urfer, S.R.; Darvas, M.; Czeibert, K.; Sándor, S.; Promislow, D.E.L.; Creevy, K.E.; Kubinyi, E.; Kaeberlein, M. Canine Cognitive Dysfunction (CCD) scores correlate with amyloid beta 42 levels in dog brain tissue. Geroscience 2021, 43, 2379–2386. [Google Scholar] [CrossRef] [PubMed]
- Abey, A.; Davies, D.; Goldsbury, C.; Buckland, M.; Valenzuela, M.; Duncan, T. Distribution of tau hyperphosphorylation in canine dementia resembles early Alzheimer’s disease and other tauopathies. Brain Pathol. 2021, 31, 144–162. [Google Scholar] [CrossRef]
- Yu, C.H.; Song, G.S.; Yhee, J.Y.; Kim, J.H.; Im, K.S.; Nho, W.G.; Lee, J.H.; Sur, J.H. Histopathological and immunohistochemical comparison of the brain of human patients with Alzheimer’s disease and the brain of aged dogs with cognitive dysfunction. J. Comp. Pathol. 2011, 145, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Brellou, G.; Vlemmas, I.; Lekkas, S.; Papaioannou, N. Immunohistochemical investigation of amyloid β-protein (Aβ) in the brain of aged cats. Histol. Histopathol. 2005, 20, 725–731. [Google Scholar] [CrossRef]
- Head, E.; Moffat, K.; Das, P.; Sarsoza, F.; Poon, W.W.; Landsberg, G.; Cotman, C.W.; Murphy, M.P. Beta-amyloid deposition and tau phosphorylation in clinically characterized aged cats. Neurobiol. Aging 2005, 26, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Sordo, L.; Gunn-Moore, D.A. Cognitive dysfunction in cats: Update on neuropathological and behavioural changes plus clinical management. Vet. Rec. 2021, 188, e3. [Google Scholar] [CrossRef] [PubMed]
- Gunn-Moore, D.A.; McVee, J.; Bradshaw, J.M.; Pearson, G.R.; Head, E.; Gunn-Moore, F.J. Ageing changes in cat brains demonstrated by beta-amyloid and AT8-immunoreactive phosphorylated tau deposits. J. Feline Med. Surg. 2006, 8, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Sordo, L.; Martini, A.C.; Houston, E.F.; Head, E.; Gunn-Moore, D. Neuropathology of aging in cats and its similarities to human Alzheimer’s disease. Front. Aging 2021, 2, 684607. [Google Scholar] [CrossRef]
- Fiock, K.L.; Smith, J.D.; Crary, J.F.; Hefti, M.M. β-amyloid and tau pathology in the aging feline brain. J. Comp. Neurol. 2020, 528, 108–113. [Google Scholar] [CrossRef]
- Serizawa, S.; Chambers, J.K.; Une, Y. Beta amyloid deposition and neurofibrillary tangles spontaneously occur in the brains of captive cheetahs (Acinonyx jubatus). Vet. Pathol. 2012, 49, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.K.; Uchida, K.; Harada, T.; Tsuboi, M.; Sato, M.; Kubo, M.; Kawaguchi, H.; Miyoshi, N.; Tsujimoto, H.; Nakayama, H. Neurofibrillary tangles and the deposition of a β-amyloid peptide with a novel N-terminal epitope in the brains of Tsushima leopard cats. PLoS ONE 2012, 7, e46452. [Google Scholar] [CrossRef] [PubMed]
- Roertgen, K.E.; Parisi, J.E.; Clark, H.B.; Barnes, D.L.; O’Brien, T.D.; Johnson, K.H. Aβ-associated cerebral angiopathy and senile plaques with neurofibrillary tangles and cerebral hemorrhage in an aged wolverine (Gulo gulo). Neurobiol. Aging 1996, 17, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Vacher, M.C.; Durrant, C.S.; Rose, J.; Hall, A.J.; Spires-Jones, T.L.; Gunn-Moore, F.; Dagleish, M.P. Alzheimer’s disease-like neuropathology in three species of oceanic dolphin. Eur. J. Neurosci. 2023, 57, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Sacchini, S.; Díaz-Delgado, J.; Espinosa de Los Monteros, A.; Paz, Y.; Bernaldo de Quirós, Y.; Sierra, E.; Arbelo, M.; Herráez, P.; Fernández, A. Amyloid-beta peptide and phosphorylated tau in the frontopolar cerebral cortex and in the cerebellum of toothed whales: Aging versus hypoxia. Biol. Open 2020, 9, bio054734. [Google Scholar] [CrossRef] [PubMed]
- Stylianaki, I.; Komnenou, A.T.; Posantzis, D.; Nikolaou, K.; Papaioannou, N. Alzheimer’s disease-like pathological lesions in an aged bottlenose dolphin (Tursiops truncatus). Vet. Rec. Case Rep. 2019, 7, e000700. [Google Scholar] [CrossRef]
- Davis, D.A.; Mondo, K.; Stern, E.; Annor, A.K.; Murch, S.J.; Coyne, T.M.; Brand, L.E.; Niemeyer, M.E.; Sharp, S.; Bradley, W.G.; et al. Cyanobacterial neurotoxin BMAA and brain pathology in stranded dolphins. PLoS ONE 2019, 14, e0213346. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.A.; Garamszegi, S.P.; Banack, S.A.; Dooley, P.D.; Coyne, T.M.; McLean, D.W.; Rotstein, D.S.; Mash, D.C.; Cox, P.A. BMAA, methylmercury, and mechanisms of neurodegeneration in dolphins: A natural model of toxin exposure. Toxins 2021, 13, 697. [Google Scholar] [CrossRef] [PubMed]
- Garamszegi, S.P.; Brzostowicki, D.J.; Coyne, T.M.; Vontell, R.T.; Davis, D.A. TDP-43 and Alzheimer’s disease pathology in the brain of a harbor porpoise exposed to the Cyanobacterial toxin BMAA. Toxins 2024, 16, 42. [Google Scholar] [CrossRef]
- Vallino Costassa, E.; Fiorini, M.; Zanusso, G.; Peletto, S.; Acutis, P.; Baioni, E.; Maurella, C.; Tagliavini, F.; Catania, M.; Gallo, M.; et al. Characterization of amyloid-beta deposits in bovined brains. J. Alzheimers Dis. 2016, 51, 875–887. [Google Scholar] [CrossRef]
- Moreno-Gonzalez, I.; Edwards, G., 3rd; Morales, R.; Duran-Aniotz, C.; Escobedo, G., Jr.; Marquez, M.; Pumarola, M.; Soto, C. Aged cattle brain displays Alzheimer’s disease-like pathology and promotes brain amyloidosis in a transgenic animal model. Front. Aging Neurosci. 2022, 13, 815361. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Greenberg, S.G.; Saper, C.B. Neurofibrillary tangles in the cerebral cortex of sheep. Neurosci. Lett. 1994, 170, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.T.; Saper, C.B. Ultrastructure of neurofibrillary tangles in the cerebral cortex of sheep. Neurobiol. Aging 1995, 16, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.J.; Mckean, N.E.; Henty, K.; Portelius, E.; Blennow, K.; Rudiger, S.R.; Bawden, C.S.; Handley, R.R.; Verma, P.J.; Faull, R.L.M.; et al. Alzheimer’s disease markers in the aged sheep (Ovis aries). Neurobiol. Aging 2017, 58, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Malbon, A.J.; Sordo, L.; Wilson, L.A.; Gunn-Moore, D.; Paraschou, G.; Macintyre, N.; Schwarz, T.; McGorum, B.; Hahn, C. Alzheimer-like pathology in the parietal cortex and hippocampus of aged donkeys. Neurobiol. Aging 2022, 113, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Bates, K.; Vink, R.; Martins, R.; Harvey, A. Aging, cortical injury and Alzheimer’s disease-like pathology in the guinea pig brain. Neurobiol. Aging 2014, 35, 1345–1351. [Google Scholar] [CrossRef]
- Wahl, D.; Moreno, J.A.; Santangelo, K.S.; Zhang, Q.; Afzali, M.F.; Walsh, M.A.; Musci, R.V.; Cavalier, A.N.; Hamilton, K.L.; LaRocca, T.J.; et al. Nontransgenic guinea pig strains exhibit hallmarks of human brain aging and Alzheimer’s disease. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Reyes, A.E.; Chacón, M.A.; Cerpa, W.; Villalón, A.; Montiel, J.; Merabachvili, G.; Aldunate, R.; Bozinovic, F.; Aboitiz, F. Human-like rodent amyloid-beta-peptide determines Alzheimer pathology in aged wild-type Octodon degu. Neurobiol. Aging 2005, 26, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Munoz, P.; Palacios, A.G.; Castellano-Gonzalez, G.; Inestrosa, N.C.; Chung, R.S.; Sachdev, P.; Guillemin, G.J. Recent rodent models for Alzheimer’sdisease: Clinical implications and basic research. J. Neural Transm. 2012, 119, 173–195. [Google Scholar] [CrossRef]
- van Groen, T.; Kadish, I.; Popović, N.; Popović, M.; Caballero-Bleda, M.; Baño-Otálora, B.; Vivanco, P.; Rol, M.Á.; Madrid, J.A. Age-related brain pathology in Octodon degu: Blood vessel, white matter and Alzheimer-like pathology. Neurobiol. Aging 2011, 32, 1651–1661. [Google Scholar] [CrossRef]
- Cisternas, P.; Zolezzi, J.M.; Lindsay, C.; Rivera, D.S.; Martinez, A.; Bozinovic, F.; Inestrosa, N.C. New insights into the spontaneous human Alzheimer’s disease-like model Octodon degu: Unraveling amyloid-beta peptide aggregation and age-related amyloid pathology. Alzheimers Dis. 2018, 66, 1145–1163. [Google Scholar] [CrossRef]
- Steffen, J.; Krohn, M.; Paarmann, K.; Schwitlick, C.; Brüning, T.; Marreiros, R.; Müller-Schiffmann, A.; Korth, C.; Braun, K.; Pahnke, J. Revisiting rodent models: Octodon degu as Alzheimer’s disease model? Acta Neuropathol. Commun. 2016, 4, 91. [Google Scholar] [CrossRef] [PubMed]
- Bourdenx, M.; Dovero, S.; Thiolat, M.L.; Bezard, E.; Dehay, B. Lack of spontaneous age-related brain pathology in Octodon degu: A reappraisal of the model. Sci. Rep. 2017, 7, 45831. [Google Scholar] [CrossRef]
- Tan, Z.; Garduño, B.M.; Aburto, P.F.; Chen, L.; Ha, N.; Cogram, P.; Holmes, T.C.; Xu, X. Cognitively impaired aged Octodon degu recapitulate major neuropathological features of sporadic Alzheimer’s disease. Acta Neuropathol. Commun. 2022, 10, 182. [Google Scholar] [CrossRef]
- Sparks, D.L.; Schreurs, B.G. Trace amounts of copper in water induce β-amyloid plaques and learning deficits in a rabbit model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2003, 100, 11065–11069. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L. Cholesterol, copper, and accumulation of thioflavine S-reactive Alzheimer’s-like amyloid beta in rabbit brain. J. Mol. Neurosci. 2004, 24, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ghribi, O.; Larsen, B.; Schrag, M.; Herman, M.M. High cholesterol content in neurons increases BACE, beta-amyloid, and phosphorylated tau levels in rabbit hippocampus. Exp. Neurol. 2006, 200, 460–467. [Google Scholar] [CrossRef]
- Sparks, D.L. The early and ongoing experience with the cholesterol-fed rabbit as a model of Alzheimer’s disease: The old, the new and the pilot. J. Alzheimers Dis 2008, 15, 641–656. [Google Scholar] [CrossRef]
- Perez-Garmendia, R.; Hernandez-Zimbron, L.F.; Morales, M.A.; Luna-Muñoz, J.; Mena, R.; Nava-Catorce, M.; Acero, G.; Vasilevko, V.; Viramontes-Pintos, A.; Cribbs, D.H.; et al. Identification of N-terminally truncated pyroglutamate amyloid-β in cholesterol-enriched diet-fed rabbit and AD brain. J. Alzheimers Dis. 2014, 39, 441–455. [Google Scholar] [CrossRef]
- Li, H.; Xiang, B.L.; Li, X.; Li, C.; Li, Y.; Miao, Y.; Ma, G.L.; Ma, Y.H.; Chen, J.Q.; Zhang, Q.Y.; et al. Cognitive deficits and Alzheimer’s disease-like pathologies in the aged Chinese tree shrew. Mol. Neurobiol. 2024, 61, 1892–1906. [Google Scholar] [CrossRef]
- Yamashita, A.; Fuchs, E.; Taira, M.; Hayashi, M. Amyloid beta (Aβ) protein- and amyloid precursor protein (APP)-immunoreactive structures in the brains of aged tree shrews. Curr. Aging Sci. 2010, 3, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, A.; Fuchs, E.; Taira, M.; Yamamoto, T.; Hayashi, M. Somatostatin-immunoreactive senile plaque-like structures in the frontal cortex and nucleus accumbens of aged tree shrews and Japanese macaques. J. Med. Primatol. 2012, 41, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Ghetti, B.; Goedert, M. Classification of diseases with accumulation of tau protein. Neuropathol. Appl. Neurobiol. 2022, 48, e12792. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Ferrer, I. Diagnostic approach to the neuropathology of neurodegenerative diseases. In Greenfield’s Neuropathology Chapter 35, 10th ed.; Smith, C., Perry, A., Kovacs, G.G., Jacques, T., Eds.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2024; pp. 1192–1200. [Google Scholar]
- Ferrer, I.; López-González, I.; Carmona, M.; Arregui, L.; Dalfó, E.; Torrejón-Escribano, B.; Diehl, R.; Kovacs, G.G. Glial and neuronal tau pathology in tauopathies: Characterization of disease-specific phenotypes and tau pathology progression. J. Neuropathol. Exp. Neurol. 2014, 73, 81–97. [Google Scholar] [CrossRef] [PubMed]
| Carnivora | Carnifornia, Pinnipedia | pinnipeds | sea lions, seals, walruses |
| Carnifornia, Ursidae | bears | polar bear, Asian brown bear, American black bear | |
| Cannidae | dogs | ||
| Felidae, Felinae | cats | domestic cats, cheetahs, Tsushima leopard cats | |
| Mustelidae | wolverine | wolverine | |
| Artiodactyla | Cetacea | cetacea | Risso’s dolphins, long-finned pilot whales, white-beaked dolphins, harbor porpoises, bottlenose dolphin, Cuvier’s beaked whale, Blainville’s beaked whales, short-finned pilot whale, Atlantic spotted dolphins, bottlenose dolphin |
| Bovidae, Bovinae | cattle | cattle | |
| Bovidae, Caprinae | sheep | sheep | |
| Perissodactyla | Equidae | donkey | donkey |
| Rodentia | Cavidae | guinea-pig | guinea |
| Octodontidae | degus | degus | |
| Lagomorpha | rabbits | rabbit | |
| Euarchonta | Scadentia | three shrew | three shrew |
| Species | DP | NP | CAA | Hp-tau | NFT | Comments |
|---|---|---|---|---|---|---|
| pinnipeds | + | + | + | + | 0 | |
| bears | ++ | ++ | + | + | + | |
| dogs | + | 0 | + | + | 0 | |
| cats | ++ | 0 | + | + | 0 | NFT+: cheethas |
| wolverine | ++ | + | + | ++ | + | n = 1 |
| cetacea | + | + | nd | + | + | higher in animals exposed to BMAA |
| cattle | + | 0 | nd | 0 | 0 | |
| sheep | + | 0 | nd | + | + | |
| donkey | + | 0 | nd | + | 0 | |
| guinea-pig | + | 0 | 0 | 0 | 0 | |
| degus | + | 0 | 0 | + (?) | 0 | depending on environmental factors |
| rabbits | 0 | 0 | 0 | 0 | 0 | DP in cholesterol-fed + cooper |
| three shrew | + | 0 | 0 | + | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer, I. Alzheimer’s Disease Neuropathological Change in Aged Non-Primate Mammals. Int. J. Mol. Sci. 2024, 25, 8118. https://doi.org/10.3390/ijms25158118
Ferrer I. Alzheimer’s Disease Neuropathological Change in Aged Non-Primate Mammals. International Journal of Molecular Sciences. 2024; 25(15):8118. https://doi.org/10.3390/ijms25158118
Chicago/Turabian StyleFerrer, Isidro. 2024. "Alzheimer’s Disease Neuropathological Change in Aged Non-Primate Mammals" International Journal of Molecular Sciences 25, no. 15: 8118. https://doi.org/10.3390/ijms25158118






