The Possible Effects of Galectin-3 on Mechanisms of Renal and Hepatocellular Injury Induced by Intravascular Hemolysis
Abstract
1. Introduction
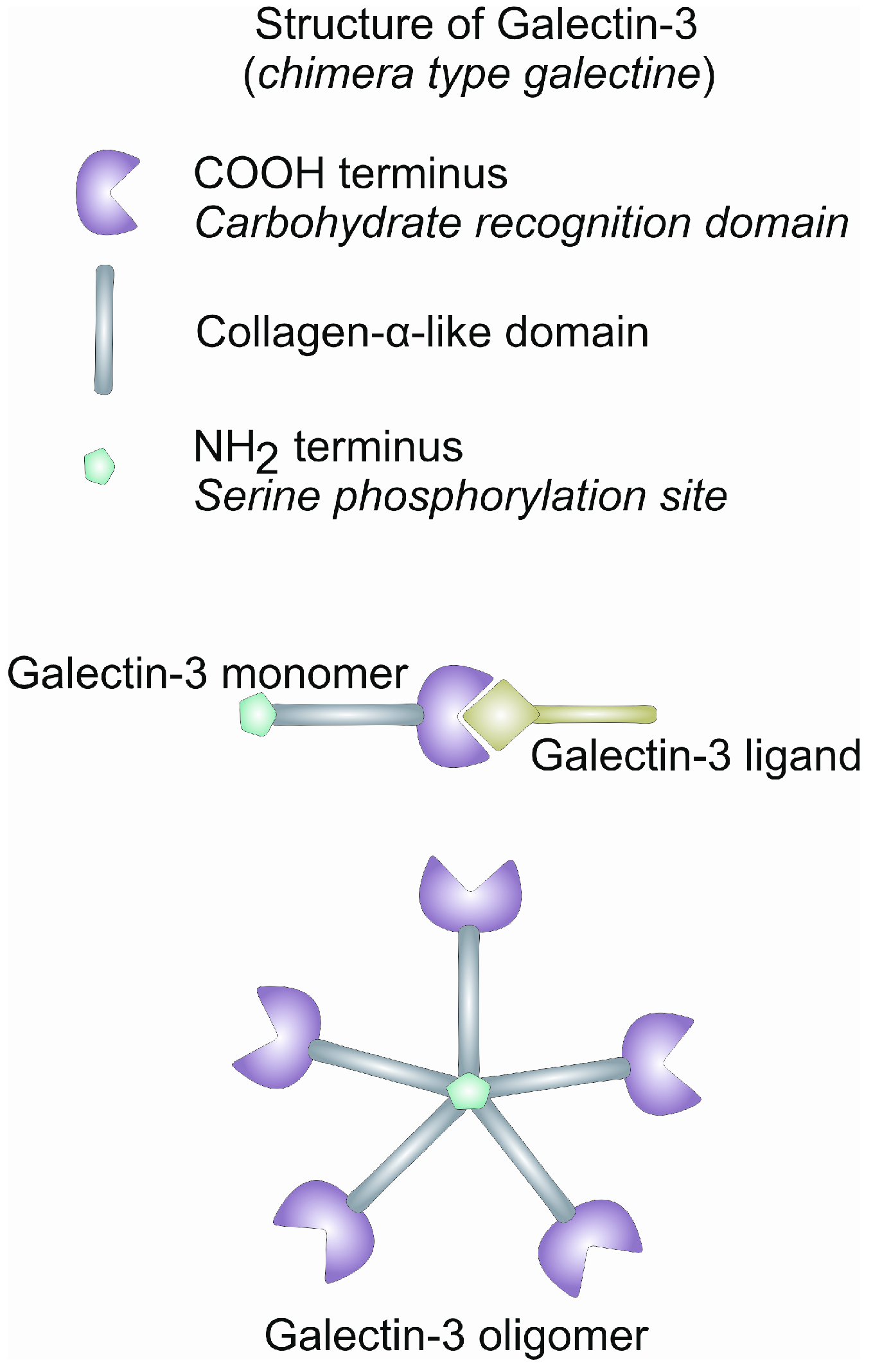
2. Gal-3: Structure and Function
3. Gal-3 and the Innate Immunity
4. Gal-3 and Tissue Fibrosis
5. Harmful Effects of the Products of Lysed Erythrocytes
6. The Role of Gal-3 in Renal Injury Induced by Intravascular Hemolysis
7. The Role of Gal-3 in Liver Injury Induced by Intravascular Hemolysis
8. Potential Therapeutic Effects of Gal-3 Inhibition
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| galectin-3 | Gal-3 |
| autoimmune hemolytic anemia | AIHA |
| sickle cell disease | SCD |
| paroxysmal nocturnal hemoglobinuria | PNH |
| heme oxygenase-1 | HO-1 |
| damage-associated molecular pattern | DAMP |
| carbon recognition domain | CRD |
| carbohydrate binding proteins | CBPs |
| NLR family pyrin domain-containing 3 | NLRP3 |
| dextran sulfate sodium | DSS |
| toll-like receptor 4 | TLR4 |
| myeloid differentiation primary response 88 | MyD88 |
| nuclear factor kappa B | NF-κB |
| nonalcoholic steatohepatitis | NASH |
| modified citrus pectin | MCP |
| α-smooth muscle actin | α-SMA |
| low-density lipoprotein | LDL |
| high-mobility group box 1 | HMGB1 |
| reactive oxygen species | ROS |
| tumor necrosis factor alpha | TNF-α |
| chemokine (C-C motif) ligand 2 | CCL2 |
| phenylhydrazine | PHZ |
| peroxisome proliferator activated receptor γ | PPARγ |
| hepatocellular carcinoma | HCC |
References
- Muller-Eberhard, U. Hemopexin. N. Engl. J. Med. 1970, 283, 1090–1094. [Google Scholar] [CrossRef]
- Schaer, D.J.; Vinchi, F.; Ingoglia, G.; Tolosano, E.; Buehler, P.W. Haptoglobin, Hemopexin, and Related Defense Pathways-Basic Science, Clinical Perspectives, and Drug Development. Front. Physiol. 2014, 5, 415. [Google Scholar] [CrossRef]
- Wagener, F.A.D.T.G.; Eggert, A.; Boerman, O.C.; Oyen, W.J.G.; Verhofstad, A.; Abraham, N.G.; Adema, G.; van Kooyk, Y.; de Witte, T.; Figdor, C.G. Heme Is a Potent Inducer of Inflammation in Mice and Is Counteracted by Heme Oxygenase. Blood 2001, 98, 1802–1811. [Google Scholar] [CrossRef]
- Nath, K.A.; Haggard, J.J.; Croatt, A.J.; Grande, J.P.; Poss, K.D.; Alam, J. The Indispensability of Heme Oxygenase-1 in Protecting against Acute Heme Protein-Induced Toxicity in Vivo. Am. J. Pathol. 2000, 156, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Van Avondt, K.; Nur, E.; Zeerleder, S. Mechanisms of Haemolysis-Induced Kidney Injury. Nat. Rev. Nephrol. 2019, 15, 671–692. [Google Scholar] [CrossRef]
- Zhong, H.; Yazdanbakhsh, K. Hemolysis and Immune Regulation. Curr. Opin. Hematol. 2018, 25, 177–182. [Google Scholar] [CrossRef]
- Bozza, M.T.; Jeney, V. Pro-Inflammatory Actions of Heme and Other Hemoglobin-Derived DAMPs. Front. Immunol. 2020, 11, 1323. [Google Scholar] [CrossRef] [PubMed]
- Theurl, I.; Hilgendorf, I.; Nairz, M.; Tymoszuk, P.; Haschka, D.; Asshoff, M.; He, S.; Gerhardt, L.M.S.; Holderried, T.A.W.; Seifert, M.; et al. On-Demand Erythrocyte Disposal and Iron Recycling Requires Transient Macrophages in the Liver. Nat. Med. 2016, 22, 945–951. [Google Scholar] [CrossRef]
- Yang, R.Y.; Rabinovich, G.A.; Liu, F.T. Galectins: Structure, Function and Therapeutic Potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G. Galectins as Pattern Recognition Receptors: Structure, Function, and Evolution. Adv. Exp. Med. Biol. 2012, 946, 21–36. [Google Scholar] [CrossRef]
- Sato, S.; St-Pierre, C.; Bhaumik, P.; Nieminen, J. Galectins in Innate Immunity: Dual Functions of Host Soluble Beta-Galactoside-Binding Lectins as Damage-Associated Molecular Patterns (DAMPs) and as Receptors for Pathogen-Associated Molecular Patterns (PAMPs). Immunol. Rev. 2009, 230, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Bouffette, S.; Botez, I.; De Ceuninck, F. Targeting Galectin-3 in Inflammatory and Fibrotic Diseases. Trends Pharmacol. Sci. 2023, 44, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Stowell, S.R. The Role of Galectins in Immunity and Infection. Nat. Rev. Immunol. 2023, 23, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Saccon, F.; Gatto, M.; Ghirardello, A.; Iaccarino, L.; Punzi, L.; Doria, A. Role of Galectin-3 in Autoimmune and Non-Autoimmune Nephropathies. Autoimmun. Rev. 2017, 16, 34–47. [Google Scholar] [CrossRef]
- Sciacchitano, S.; Lavra, L.; Morgante, A.; Ulivieri, A.; Magi, F.; De Francesco, G.P.; Bellotti, C.; Salehi, L.B.; Ricci, A. Galectin-3: One Molecule for an Alphabet of Diseases, from A to Z. Int. J. Mol. Sci. 2018, 19, 379. [Google Scholar] [CrossRef]
- Manganas, K.; Delicou, S.; Xydaki, A.; Kourakli, A.; Evliati, L.; Vlachaki, E.; Klironomos, E.; Diamantidis, M.; Lafiatis, I.; Kattamis, A.; et al. Predisposing Factors for Advanced Liver Fibrosis in Patients with Sickle Cell Disease. Br. J. Haematol. 2023, 202, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Cummings, R.D.; Liu, F.-T. Galectins. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Freeze, H.H., Stanley, P., Bertozzi, C.R., Hart, G.W., Etzler, M.E., Eds.; Cold Spring Harbor: New York, NY, USA, 2009; ISBN 9780879697709. [Google Scholar]
- Thijssen, V.L. Galectins in Endothelial Cell Biology and Angiogenesis: The Basics. Biomolecules 2021, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, J.; Kasai, K. The Family of Metazoan Metal-Independent Beta-Galactoside-Binding Lectins: Structure, Function and Molecular Evolution. Glycobiology 1993, 3, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Ochieng, J.; Leite-Browning, M.L.; Warfield, P. Regulation of Cellular Adhesion to Extracellular Matrix Proteins by Galectin-3. Biochem. Biophys. Res. Commun. 1998, 246, 788–791. [Google Scholar] [CrossRef]
- Ochieng, J.; Fridman, R.; Nangia-Makker, P.; Kleiner, D.E.; Liotta, L.A.; Stetler-Stevenson, W.G.; Raz, A. Galectin-3 Is a Novel Substrate for Human Matrix Metalloproteinases-2 and -9. Biochemistry 1994, 33, 14109–14114. [Google Scholar] [CrossRef]
- Ippel, H.; Miller, M.C.; Vértesy, S.; Zheng, Y.; Cañada, F.J.; Suylen, D.; Umemoto, K.; Romanò, C.; Hackeng, T.; Tai, G.; et al. Intra- and Intermolecular Interactions of Human Galectin-3: Assessment by Full-Assignment-Based NMR. Glycobiology 2016, 26, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Mehul, B.; Bawumia, S.; Colin Hughes, R. Cross-Linking of Galectin 3, a Galactose-Binding Protein of Mammalian Cells, by Tissue-Type Transglutaminase. FEBS Lett. 1995, 360, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.P.; Hughes, R.C. Determinants in the N-Terminal Domains of Galectin-3 for Secretion by a Novel Pathway Circumventing the Endoplasmic Reticulum-Golgi Complex. Eur. J. Biochem. 1999, 264, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a Glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.R.; Shankar, J.; Dennis, J.W. The Galectin Lattice at a Glance. J. Cell Sci. 2015, 128, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Akahani, S.; Nangia-Makker, P.; Inohara, H.; Kim, H.R.; Raz, A. Galectin-3: A Novel Antiapoptotic Molecule with a Functional BH1 (NWGR) Domain of Bcl-2 Family. Cancer Res. 1997, 57, 5272–5276. [Google Scholar] [PubMed]
- Park, J.; Park, J.W.; Voss, P.G.; Grabski, S.; Wang, J.L.; Patterson, R.J. Association of Galectin-1 and Galectin-3 with Gemin4 in Complexes Containing the SMN Protein. Nucleic Acids Res. 2001, 29, 3595–3602. [Google Scholar] [CrossRef] [PubMed]
- Elad-Sfadia, G.; Haklai, R.; Balan, E.; Kloog, Y. Galectin-3 Augments K-Ras Activation and Triggers a Ras Signal That Attenuates ERK but Not Phosphoinositide 3-Kinase Activity. J. Biol. Chem. 2004, 279, 34922–34930. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Takenaka, Y.; Tsutsumi, S.; Hogan, V.; Kikuchi, A.; Raz, A. Galectin-3, a Novel Binding Partner of β-Catenin. Cancer Res. 2004, 64, 6363–6367. [Google Scholar] [CrossRef]
- Sato, S.; Hughes, R.C. Binding Specificity of a Baby Hamster Kidney Lectin for H Type I and II Chains, Polylactosamine Glycans, and Appropriately Glycosylated Forms of Laminin and Fibronectin. J. Biol. Chem. 1992, 267, 6983–6990. [Google Scholar] [CrossRef]
- Talaga, M.L.; Fan, N.; Fueri, A.L.; Brown, R.K.; Bandyopadhyay, P.; Dam, T.K. Multitasking Human Lectin Galectin-3 Interacts with Sulfated Glycosaminoglycans and Chondroitin Sulfate Proteoglycans. Biochemistry 2016, 55, 4541–4551. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.; Cherayil, B.J.; Isselbacher, K.J.; Pillai, S. Mac-2-Binding Glycoproteins: Putative Ligands for a Cytosolic β-Galactoside Lectin. J. Biol. Chem. 1991, 266, 18731–18736. [Google Scholar] [CrossRef] [PubMed]
- Probstmeier, R.; Montag, D.; Schachner, M. Galectin-3, a β-Galactoside-Binding Animal Lectin, Binds to Neural Recognition Molecules. J. Neurochem. 1995, 64, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Hsu, D.K.; Zuberi, R.I.; Kuwabara, I.; Chi, E.Y.; Henderson, W.R.J. Expression and Function of Galectin-3, a beta-Galactoside-Binding Lectin, in Human Monocytes and Macrophages. Am. J. Pathol. 1995, 147, 1016–1028. [Google Scholar] [PubMed]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a Novel Biomarker for Disease Diagnosis and a Target for Therapy (Review). Int. J. Mol. Med. 2018, 41, 599–614. [Google Scholar] [CrossRef]
- Van den Brûle, F.A.; Fernandez, P.L.; Buicu, C.; Liu, F.T.; Jackers, P.; Lambotte, R.; Castronovo, V. Differential Expression of Galectin-1 and Galectin-3 during First Trimester Human Embryogenesis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1997, 209, 399–405. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, H.-W.; Gu Kang, H.; La, S.-H.; Choi, I.J.; Ro, J.Y.; Bresalier, R.S.; Song, J.; Chun, K.-H. Ablation of Galectin-3 Induces P27(KIP1)-Dependent Premature Senescence without Oncogenic Stress. Cell Death Differ. 2014, 21, 1769–1779. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Kuo, P.L. The Role of Galectin-3 in the Kidneys. Int. J. Mol. Sci. 2016, 17, 565. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kobayashi, S.; Hemmi, N.; Ikee, R.; Hyodo, N.; Saigusa, T.; Namikoshi, T.; Yamada, M.; Suzuki, S.; Miura, S. Galectin-3-Positive Cell Infiltration in Human Diabetic Nephropathy. Nephrol. Dial. Transpl. 2004, 19, 602–607. [Google Scholar] [CrossRef]
- Hsu, D.K.; Dowling, C.A.; Jeng, K.C.G.; Chen, J.T.; Yang, R.Y.; Liu, F.T. Galectin-3 Expression Is Induced in Cirrhotic Liver and Hepatocellular Carcinoma. Int. J. Cancer 1999, 81, 519–526. [Google Scholar] [CrossRef]
- Fermin Lee, A.; Chen, H.-Y.; Wan, L.; Wu, S.-Y.; Yu, J.-S.; Huang, A.C.; Miaw, S.-C.; Hsu, D.K.; Wu-Hsieh, B.A.; Liu, F.-T. Galectin-3 Modulates Th17 Responses by Regulating Dendritic Cell Cytokines. Am. J. Pathol. 2013, 183, 1209–1222. [Google Scholar] [CrossRef]
- Jiang, H.-R.; Al Rasebi, Z.; Mensah-Brown, E.; Shahin, A.; Xu, D.; Goodyear, C.S.; Fukada, S.Y.; Liu, F.-T.; Liew, F.Y.; Lukic, M.L. Galectin-3 Deficiency Reduces the Severity of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2009, 182, 1167–1173. [Google Scholar] [CrossRef]
- Simovic Markovic, B.; Nikolic, A.; Gazdic, M.; Bojic, S.; Vucicevic, L.; Kosic, M.; Mitrovic, S.; Milosavljevic, M.; Besra, G.; Trajkovic, V.; et al. Galectin-3 Plays an Important Pro-Inflammatory Role in the Induction Phase of Acute Colitis by Promoting Activation of NLRP3 Inflammasome and Production of IL-1β in Macrophages. J. Crohns. Colitis 2016, 10, 593–606. [Google Scholar] [CrossRef]
- Pan, X.; Wang, H.; Zheng, Z.; Huang, X.; Yang, L.; Liu, J.; Wang, K.; Zhang, Y. Pectic Polysaccharide from Smilax china L. Ameliorated Ulcerative Colitis by Inhibiting the Galectin-3/NLRP3 Inflammasome Pathway. Carbohydr. Polym. 2022, 277, 118864. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, C.; Meng, J.; Li, N.; Xu, Z.; Liu, X.; Hou, S. Galectin-3 Regulates Microglial Activation and Promotes Inflammation through TLR4/MyD88/NF-KB in Experimental Autoimmune Uveitis. Clin. Immunol. 2022, 236, 108939. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, K.; Endo, J.; Kataoka, M.; Katsumata, Y.; Yoshida, N.; Yamamoto, T.; Isobe, S.; Moriyama, H.; Goto, S.; Kitakata, H.; et al. IL (Interleukin)-10-STAT3-Galectin-3 Axis Is Essential for Osteopontin-Producing Reparative Macrophage Polarization after Myocardial Infarction. Circulation 2018, 138, 2021–2035. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, S.; Wei, L.; Liu, M.; Liu, H.; Liu, T.; Zhou, Y.; Jia, Q.; Wang, D.; Yang, Z.; et al. Twist1 Regulates Macrophage Plasticity to Promote Renal Fibrosis through Galectin-3. Cell. Mol. Life Sci. 2022, 79, 137. [Google Scholar] [CrossRef] [PubMed]
- Siew, J.J.; Chen, H.M.; Chen, H.Y.; Chen, H.L.; Chen, C.M.; Soong, B.W.; Wu, Y.R.; Chang, C.P.; Chan, Y.C.; Lin, C.H.; et al. Galectin-3 Is Required for the Microglia-Mediated Brain Inflammation in a Model of Huntington’s Disease. Nat. Commun. 2019, 10, 3473. [Google Scholar] [CrossRef]
- Li, H.; Cao, Z.; Wang, L.; Li, J.; Cheng, X.; Tang, Y.; Xing, M.; Yao, P. Chronic High-Fat Diet Induces Galectin-3 and TLR4 to Activate NLRP3 Inflammasome in NASH. J. Nutr. Biochem. 2023, 112, 109217. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, N.-N.; Wang, D.; Meng, W.-H.; Chen, H.-S. Modified Citrus Pectin Alleviates Cerebral Ischemia/Reperfusion Injury by Inhibiting NLRP3 Inflammasome Activation via TLR4/NF-ĸB Signaling Pathway in Microglia. J. Inflamm. Res. 2022, 15, 3369–3385. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.C.; Raiten, J.; Li, Y. Understanding Fibrosis: Mechanisms, Clinical Implications, Current Therapies, and Prospects for Future Interventions. Biomed. Eng. Adv. 2024, 7, 100118. [Google Scholar] [CrossRef]
- Friedman, S.L.; Sheppard, D.; Duffield, J.S.; Violette, S. Therapy for Fibrotic Diseases: Nearing the Starting Line. Sci. Transl. Med. 2013, 5, 167sr1. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, A.C.; Gibbons, M.A.; Farnworth, S.L.; Leffler, H.; Nilsson, U.J.; Delaine, T.; Simpson, A.J.; Forbes, S.J.; Hirani, N.; Gauldie, J.; et al. Regulation of Transforming Growth Factor-Β1-Driven Lung Fibrosis by Galectin-3. Am. J. Respir. Crit. Care Med. 2012, 185, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Henderson, N.; Mackinnon, A.; Rooney, C.; Sethi, T. Galectin-3: A Central Regulator of Chronic Inflammation and Tissue Fibrosis. ACS Symp. Ser. 2012, 1115, 377–390. [Google Scholar] [CrossRef]
- Calvier, L.; Miana, M.; Reboul, P.; Cachofeiro, V.; Martinez-Martinez, E.; de Boer, R.A.; Poirier, F.; Lacolley, P.; Zannad, F.; Rossignol, P.; et al. Galectin-3 Mediates Aldosterone-Induced Vascular Fibrosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Filer, A.; Bik, M.; Parsonage, G.N.; Fitton, J.; Trebilcock, E.; Howlett, K.; Cook, M.; Raza, K.; Simmons, D.L.; Thomas, A.M.C.; et al. Galectin 3 Induces a Distinctive Pattern of Cytokine and Chemokine Production in Rheumatoid Synovial Fibroblasts via Selective Signaling Pathways. Arthritis Rheum. 2009, 60, 1604–1614. [Google Scholar] [CrossRef]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Poirier, F.; Russo, F.P.; Iredale, J.P.; Haslett, C.; Simpson, K.J.; Sethi, T. Galectin-3 Regulates Myofibroblast Activation and Hepatic Fibrosis. Proc. Natl. Acad. Sci. USA 2006, 103, 5060–5065. [Google Scholar] [CrossRef]
- Iacobini, C.; Oddi, G.; Menini, S.; Amadio, L.; Ricci, C.; Di Pippo, C.; Sorcini, M.; Pricci, F.; Pugliese, F.; Pugliese, G. Development of Age-Dependent Glomerular Lesions in Galectin-3/AGE-Receptor-3 Knockout Mice. Am. J. Physiol. Ren. Physiol. 2005, 289, F611–F621. [Google Scholar] [CrossRef]
- Nishi, Y.; Sano, H.; Kawashima, T.; Okada, T.; Kuroda, T.; Kikkawa, K.; Kawashima, S.; Tanabe, M.; Goto, T.; Matsuzawa, Y.; et al. Role of Galectin-3 in Human Pulmonary Fibrosis. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2007, 56, 57–65. [Google Scholar] [CrossRef]
- Henderson, N.C.; Mackinnon, A.C.; Farnworth, S.L.; Kipari, T.; Haslett, C.; Iredale, J.P.; Liu, F.-T.; Hughes, J.; Sethi, T. Galectin-3 Expression and Secretion Links Macrophages to the Promotion of Renal Fibrosis. Am. J. Pathol. 2008, 172, 288–298. [Google Scholar] [CrossRef]
- Sherpa, M.D.; Sonkawade, S.D.; Jonnala, V.; Pokharel, S.; Khazaeli, M.; Yatsynovich, Y.; Kalot, M.A.; Weil, B.R.; Canty, J.M.J.; Sharma, U.C. Galectin-3 Is Associated with Cardiac Fibrosis and an Increased Risk of Sudden Death. Cells 2023, 12, 1218. [Google Scholar] [CrossRef]
- Xu, G.-R.; Zhang, C.; Yang, H.-X.; Sun, J.-H.; Zhang, Y.; Yao, T.-T.; Li, Y.; Ruan, L.; An, R.; Li, A.-Y. Modified Citrus Pectin Ameliorates Myocardial Fibrosis and Inflammation via Suppressing Galectin-3 and TLR4/MyD88/NF-ΚB Signaling Pathway. Biomed. Pharmacother. 2020, 126, 110071. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wu, J.; Gu, H.; Deng, X.; Xu, W.; Feng, S.; Wang, S.; Song, Y.; Pang, Z.; Deng, X.; et al. Galectin-3-Centered Paracrine Network Mediates Cardiac Inflammation and Fibrosis upon β-Adrenergic Insult. Sci. China Life Sci. 2023, 66, 1067–1078. [Google Scholar] [CrossRef]
- Bender, M.A.; Carlberg, K. Sickle Cell Disease. In GeneReviews; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Ackers, G.K.; Halvorson, H.R. The Linkage between Oxygenation and Subunit Dissociation in Human Hemoglobin. Proc. Natl. Acad. Sci. USA 1974, 71, 4312–4316. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Møller, H.J.; Moestrup, S.K. Hemoglobin and heme scavenger receptors. Antioxid. Redox Signal. 2010, 12, 261–273. [Google Scholar] [CrossRef]
- Balla, G.; Vercellotti, G.M.; Muller-Eberhard, U.; Eaton, J.; Jacob, H.S. Exposure of Endothelial Cells to Free Heme Potentiates Damage Mediated by Granulocytes and Toxic Oxygen Species. Lab. Investig. 1991, 64, 648–655. [Google Scholar]
- Butt, O.I.; Buehler, P.W.; D’Agnillo, F. Differential Induction of Renal Heme Oxygenase and Ferritin in Ascorbate and Nonascorbate Producing Species Transfused with Modified Cell-Free Hemoglobin. Antioxid. Redox Signal. 2010, 12, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Doherty, D.H.; Doyle, M.P.; Curry, S.R.; Vali, R.J.; Fattor, T.J.; Olson, J.S.; Lemon, D.D. Rate of Reaction with Nitric Oxide Determines the Hypertensive Effect of Cell-Free Hemoglobin. Nat. Biotechnol. 1998, 16, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Minneci, P.C.; Deans, K.J.; Zhi, H.; Yuen, P.S.T.; Star, R.A.; Banks, S.M.; Schechter, A.N.; Natanson, C.; Gladwin, M.T.; Solomon, S.B. Hemolysis-Associated Endothelial Dysfunction Mediated by Accelerated NO Inactivation by Decompartmentalized Oxyhemoglobin. J. Clin. Investig. 2005, 115, 3409–3417. [Google Scholar] [CrossRef]
- Haymann, J.-P.; Stankovic, K.; Levy, P.; Avellino, V.; Tharaux, P.-L.; Letavernier, E.; Grateau, G.; Baud, L.; Girot, R.; Lionnet, F. Glomerular Hyperfiltration in Adult Sickle Cell Anemia: A Frequent Hemolysis Associated Feature. Clin. J. Am. Soc. Nephrol. 2010, 5, 756–761. [Google Scholar] [CrossRef]
- Wesson, D.E. The Initiation and Progression of Sickle Cell Nephropathy. Kidney Int. 2002, 61, 2277–2286. [Google Scholar] [CrossRef]
- Guerrero-Hue, M.; Rubio-Navarro, A.; Sevillano, Á.; Yuste, C.; Gutiérrez, E.; Palomino-Antolín, A.; Román, E.; Praga, M.; Egido, J.; Moreno, J.A. Adverse Effects of the Renal Accumulation of Haem Proteins. Novel Therapeutic Approaches. Nefrologia 2018, 38, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Tonegawa, S. Reduced Stress Defense in Heme Oxygenase 1-Deficient Cells. Proc. Natl. Acad. Sci. USA 1997, 94, 10925–10930. [Google Scholar] [CrossRef]
- Maines, M.D. The Heme Oxygenase System: A Regulator of Second Messenger Gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef]
- Belcher, J.D.; Chen, C.; Nguyen, J.; Milbauer, L.; Abdulla, F.; Alayash, A.I.; Smith, A.; Nath, K.A.; Hebbel, R.P.; Vercellotti, G.M. Heme Triggers TLR4 Signaling Leading to Endothelial Cell Activation and Vaso-Occlusion in Murine Sickle Cell Disease. Blood 2014, 123, 377–390. [Google Scholar] [CrossRef]
- Balla, G.; Jacob, H.S.; Eaton, J.W.; Belcher, J.D.; Vercellotti, G.M. Hemin: A Possible Physiological Mediator of Low Density Lipoprotein Oxidation and Endothelial Injury. Arterioscler. Thromb. A J. Vasc. Biol. 1991, 11, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bandyopadhyay, U. Free Heme Toxicity and Its Detoxification Systems in Human. Toxicol. Lett. 2005, 157, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Tyrrell, R.M. The Heme Synthesis and Degradation Pathways: Role in Oxidant Sensitivity. Heme Oxygenase Has Both pro- and Antioxidant Properties. Free Radic. Biol. Med. 2000, 28, 289–309. [Google Scholar] [CrossRef]
- Tolosano, E.; Fagoonee, S.; Morello, N.; Vinchi, F.; Fiorito, V. Heme Scavenging and the Other Facets of Hemopexin. Antioxid. Redox Signal. 2010, 12, 305–320. [Google Scholar] [CrossRef]
- Fortes, G.B.; Alves, L.S.; de Oliveira, R.; Dutra, F.F.; Rodrigues, D.; Fernandez, P.L.; Souto-Padron, T.; De Rosa, M.J.; Kelliher, M.; Golenbock, D.; et al. Heme Induces Programmed Necrosis on Macrophages through Autocrine TNF and ROS Production. Blood 2012, 119, 2368–2375. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, R.T.; Fernandez, P.L.; Mourao-Sa, D.S.; Porto, B.N.; Dutra, F.F.; Alves, L.S.; Oliveira, M.F.; Oliveira, P.L.; Graça-Souza, A.V.; Bozza, M.T. Characterization of Heme as Activator of Toll-like Receptor 4. J. Biol. Chem. 2007, 282, 20221–20229. [Google Scholar] [CrossRef] [PubMed]
- Dutra, F.F.; Alves, L.S.; Rodrigues, D.; Fernandez, P.L.; de Oliveira, R.B.; Golenbock, D.T.; Zamboni, D.S.; Bozza, M.T. Hemolysis-Induced Lethality Involves Inflammasome Activation by Heme. Proc. Natl. Acad. Sci. USA 2014, 111, E4110–E4118. [Google Scholar] [CrossRef] [PubMed]
- Kanakiriya, S.K.R.; Croatt, A.J.; Haggard, J.J.; Ingelfinger, J.R.; Tang, S.; Alam, J.; Nath, K.A.; Sharan, K.R.; Croatt, A.J.; Jill, J.; et al. Heme: A Novel Inducer of MCP-1 through HO-Dependent and HO-Independent Mechanisms. 2024, 284, 546–554.
- Merle, N.S.; Grunenwald, A.; Figueres, M.L.; Chauvet, S.; Daugan, M.; Knockaert, S.; Robe-Rybkine, T.; Noe, R.; May, O.; Frimat, M.; et al. Characterization of Renal Injury and Inflammation in an Experimental Model of Intravascular Hemolysis. Front. Immunol. 2018, 9, 179. [Google Scholar] [CrossRef] [PubMed]
- Porto, B.N.; Alves, L.S.; Fernández, P.L.; Dutra, T.P.; Figueiredo, R.T.; Graça-Souza, A.V.; Bozza, M.T. Heme Induces Neutrophil Migration and Reactive Oxygen Species Generation through Signaling Pathways Characteristic of Chemotactic Receptors. J. Biol. Chem. 2007, 282, 24430–24436. [Google Scholar] [CrossRef] [PubMed]
- Graça-Souza, A.V.; Arruda, M.A.B.; de Freitas, M.S.; Barja-Fidalgo, C.; Oliveira, P.L. Neutrophil Activation by Heme: Implications for Inflammatory Processes. Blood 2002, 99, 4160–4165. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhang, D.; Fuchs, T.A.; Manwani, D.; Wagner, D.D.; Frenette, P.S. Heme-Induced Neutrophil Extracellular Traps Contribute to the Pathogenesis of Sickle Cell Disease. Blood 2014, 123, 3818–3827. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Sammy, F.; Yang, H.; Thundivalappil, S.; Hellman, J.; Tracey, K.J.; Warren, H.S. Identification of Hemopexin as an Anti-Inflammatory Factor That Inhibits Synergy of Hemoglobin with HMGB1 in Sterile and Infectious Inflammation. J. Immunol. 2012, 189, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A.; Croatt, A.J.; Haggard, J.J.; Grande, J.P. Renal Response to Repetitive Exposure to Heme Proteins: Chronic Injury Induced by an Acute Insult. Kidney Int. 2000, 57, 2423–2433. [Google Scholar] [CrossRef][Green Version]
- Tracz, M.J.; Alam, J.; Nath, K.A. Physiology and Pathophysiology of Heme: Implications for Kidney Disease. J. Am. Soc. Nephrol. 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Qian, Q.; Nath, K.A.; Wu, Y.; Daoud, T.M.; Sethi, S. Hemolysis and Acute Kidney Failure. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2010, 56, 780–784. [Google Scholar] [CrossRef]
- Gonzalez-Michaca, L.; Farrugia, G.; Croatt, A.J.; Alam, J.; Nath, K.A. Heme: A Determinant of Life and Death in Renal Tubular Epithelial Cells. Am. J. Physiol. Ren. Physiol. 2004, 286, F370–F377. [Google Scholar] [CrossRef] [PubMed]
- Adedoyin, O.; Boddu, R.; Traylor, A.; Lever, J.M.; Bolisetty, S.; George, J.F.; Agarwal, A. Heme Oxygenase-1 Mitigates Ferroptosis in Renal Proximal Tubule Cells. Am. J. Physiol. Ren. Physiol. 2018, 314, F702–F714. [Google Scholar] [CrossRef] [PubMed]
- Sponsel, H.T.; Alfrey, A.C.; Hammond, W.S.; Durr, J.A.; Ray, C.; Anderson, R.J. Effect of Iron on Renal Tubular Epithelial Cells. Kidney Int. 1996, 50, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Fu, W.; Yao, J.; Ji, Z.; Wang, Y.; Zhou, Z.; Yan, J.; Li, W. Heme Induces IL-1β Secretion through Activating NLRP3 in Kidney Inflammation. Cell Biochem. Biophys. 2014, 69, 495–502. [Google Scholar] [CrossRef]
- Erdei, J.; Tóth, A.; Balogh, E.; Nyakundi, B.B.; Bányai, E.; Ryffel, B.; Paragh, G.; Cordero, M.D.; Jeney, V. Induction of NLRP3 Inflammasome Activation by Heme in Human Endothelial Cells. Oxid. Med. Cell. Longev. 2018, 2018, 4310816. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Kwak, Y.H.; Sammy, F.; He, P.; Thundivalappil, S.; Sun, G.; Chao, W.; Warren, H.S. Synergistic Inflammation Is Induced by Blood Degradation Products with Microbial Toll-like Receptor Agonists and Is Blocked by Hemopexin. J. Infect. Dis. 2010, 202, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Pulskens, W.P.; Teske, G.J.; Butter, L.M.; Roelofs, J.J.; van der Poll, T.; Florquin, S.; Leemans, J.C. Toll-like Receptor-4 Coordinates the Innate Immune Response of the Kidney to Renal Ischemia/Reperfusion Injury. PLoS ONE 2008, 3, e3596. [Google Scholar] [CrossRef]
- Vázquez-Carballo, C.; Herencia, C.; Guerrero-Hue, M.; García-Caballero, C.; Rayego-Mateos, S.; Morgado-Pascual, J.L.; Opazo-Rios, L.; González-Guerrero, C.; Vallejo-Mudarra, M.; Cortegano, I.; et al. Role of Toll-like Receptor 4 in Intravascular Hemolysis-Mediated Injury. J. Pathol. 2022, 258, 236–249. [Google Scholar] [CrossRef]
- Merle, N.S.; Grunenwald, A.; Rajaratnam, H.; Gnemmi, V.; Frimat, M.; Figueres, M.-L.; Knockaert, S.; Bouzekri, S.; Charue, D.; Noe, R.; et al. Intravascular Hemolysis Activates Complement via Cell-Free Heme and Heme-Loaded Microvesicles. JCI Insight 2018, 3, e96910. [Google Scholar] [CrossRef]
- Faubel, S.; Lewis, E.C.; Reznikov, L.; Ljubanovic, D.; Hoke, T.S.; Somerset, H.; Oh, D.-J.; Lu, L.; Klein, C.L.; Dinarello, C.A.; et al. Cisplatin-Induced Acute Renal Failure Is Associated with an Increase in the Cytokines Interleukin (IL)-1beta, IL-18, IL-6, and Neutrophil Infiltration in the Kidney. J. Pharmacol. Exp. Ther. 2007, 322, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.P.T.; Pinheiro, C.S.; Luna-Gomes, T.; Alves, L.R.; Maya-Monteiro, C.M.; Porto, B.N.; Barja-Fidalgo, C.; Benjamim, C.F.; Peters-Golden, M.; Bandeira-Melo, C.; et al. Leukotriene B4 Mediates Neutrophil Migration Induced by Heme. J. Immunol. 2011, 186, 6562–6567. [Google Scholar] [CrossRef] [PubMed]
- Van Avondt, K.; Schimmel, M.; Bulder, I.; van Bruggen, R.; Biemond, B.J.; Luken, B.M.; Zeerleder, S.S. Free Iron in Sera of Patients with Sickle Cell Disease Contributes to the Release of Neutrophil Extracellular Traps. Blood 2016, 128, 161. [Google Scholar] [CrossRef]
- Stillman, B.N.; Hsu, D.K.; Pang, M.; Brewer, C.F.; Johnson, P.; Liu, F.T.; Baum, L.G. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J. Immunol. 2006, 176, 778–789. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Fu, C.; Cong, Z.; Peng, L.; Peng, Z.; Chen, T.; Wang, W.; Jiang, H.; Wei, Q.; Qin, C. Intracellular Gal-3 promotes caspase-independent cell death of HIV-1-infected macrophages. FEBS J. 2017, 284, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Al-Salam, S.; Jagadeesh, G.S.; Sudhadevi, M.; Tageldeen, H.; Yasin, J. Galectin-3 Possesses Anti-Necroptotic and Anti-Apoptotic Effects in Cisplatin-Induced Acute Tubular Necrosis. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2021, 55, 344–363. [Google Scholar]
- Li, M.; Tian, M.; Jiang, X.; Liu, Y.; Wang, Y.; Li, Y. Inhibition of galectin-3 ameliorates high-glucose-induced oxidative stress and inflammation in ARPE-19 cells. Cutan. Ocul. Toxicol. 2022, 41, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Seropian, I.M.; Fontana Estevez, F.S.; Villaverde, A.; Cacciagiú, L.; Bustos, R.; Touceda, V.; Penas, F.; Selser, C.; Morales, C.; Miksztowicz, V.; et al. Galectin-3 contributes to acute cardiac dysfunction and toxicity by increasing oxidative stress and fibrosis in doxorubicin-treated mice. Int. J. Cardiol. 2023, 393, 131386. [Google Scholar] [CrossRef] [PubMed]
- Al-Salam, S.; Kandhan, K.; Sudhadevi, M.; Yasin, J.; Tariq, S. Early Doxorubicin Myocardial Injury: Inflammatory, Oxidative Stress, and Apoptotic Role of Galectin-3. Int. J. Mol. Sci. 2022, 23, 12479. [Google Scholar] [CrossRef]
- Colomb, F.; Wang, W.; Simpson, D.; Zafar, M.; Beynon, R.; Rhodes, J.M.; Yu, L.G. Galectin-3 interacts with the cell-surface glycoprotein CD146 (MCAM, MUC18) and induces secretion of metastasis-promoting cytokines from vascular endothelial cells. J. Biol. Chem. 2017, 292, 8381–8389. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.H.; Chen, H.L.; Yao, C.I.; Weng, I.C.; Li, C.S.; Huang, C.C.; Chen, N.J.; Lin, C.H.; Liu, F.T. Galectin-3 promotes noncanonical inflammasome activation through intracellular binding to lipopolysaccharide glycans. Proc. Natl. Acad. Sci. USA 2021, 118, e2026246118. [Google Scholar] [CrossRef]
- Tian, J.; Yang, G.; Chen, H.Y.; Hsu, D.K.; Tomilov, A.; Olson, K.A.; Dehnad, A.; Fish, S.R.; Cortopassi, G.; Zhao, B.; et al. Galectin-3 regulates inflammasome activation in cholestatic liver injury. FASEB J. 2016, 30, 4202–4213. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Tu, D.; Liu, X.; Niu, S.; Suo, Y.; Liu, T.; Li, G.; Liu, C. Role of NLRP3-Inflammasome/Caspase-1/Galectin-3 Pathway on Atrial Remodeling in Diabetic Rabbits. J. Cardiovasc. Transl. Res. 2020, 13, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wu, X.; Yang, J.; Yu, X.; Liu, B.; Yan, Z. Hippocampal Galectin-3 knockdown alleviates lipopolysaccharide-induced neurotoxicity and cognitive deficits by inhibiting TLR4/NF-κB signaling in aged mice. Eur. J. Pharmacol. 2022, 936, 175360. [Google Scholar] [CrossRef]
- Li, X.D.; Du, S.X.; Xie, P.; Zheng, G.Z.; Han, J.M. Galectin-3 deficiency protects lipopolysaccharide-induced chondrocytes injury via regulation of TLR4 and PPAR-γ-mediated NF-κB signaling pathway. J. Cell. Biochem. 2019, 120, 10195–10204. [Google Scholar] [CrossRef]
- Nishiyama, J.; Kobayashi, S.; Ishida, A.; Nakabayashi, I.; Tajima, O.; Miura, S.; Katayama, M.; Nogami, H. Up-Regulation of Galectin-3 in Acute Renal Failure of the Rat. Am. J. Pathol. 2000, 157, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, M.; Coutrot, M.; Michel, T.; Boutin, L.; Genest, M.; Poirier, F.; Launay, J.-M.; Kane, B.; Kinugasa, S.; Prakoura, N.; et al. Acute Kidney Injury Induces Remote Cardiac Damage and Dysfunction Through the Galectin-3 Pathway. JACC Basic Transl. Sci. 2019, 4, 717–732. [Google Scholar] [CrossRef]
- Li, H.; Yang, S.; Li, J.; Feng, J. Galectin 3 Inhibition Attenuates Renal Injury Progression in Cisplatin-Induced Nephrotoxicity. Biosci. Rep. 2018, 38, BSR20181803. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Markovic, B.S.; Jankovic, M.G.; Djokovic, B.; Jovicic, N.; Harrell, C.R.; Fellabaum, C.; Djonov, V.; Arsenijevic, N.; Lukic, M.L. Galectin 3 Protects from Cisplatin-Induced Acute Kidney Injury by Promoting TLR-2-Dependent Activation of IDO1/Kynurenine Pathway in Renal DCs. Theranostics 2019, 9, 5976–6001. [Google Scholar] [CrossRef]
- Frenay, A.R.S.; Yu, L.; van der Velde, A.R.; Vreeswijk-Baudoin, I.; López-Andrés, N.; van Goor, H.; Silljé, H.H.; Ruifrok, W.P.; de Boer, R.A. Pharmacological Inhibition of Galectin-3 Protects against Hypertensive Nephropathy. Am. J. Physiol. Ren. Physiol. 2015, 308, F500–F509. [Google Scholar] [CrossRef]
- Calvier, L.; Martinez-Martinez, E.; Miana, M.; Cachofeiro, V.; Rousseau, E.; Sádaba, J.R.; Zannad, F.; Rossignol, P.; López-Andrés, N. The Impact of Galectin-3 Inhibition on Aldosterone-Induced Cardiac and Renal Injuries. JACC Heart Fail. 2015, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, G.; Pricci, F.; Leto, G.; Amadio, L.; Iacobini, C.; Romeo, G.; Lenti, L.; Sale, P.; Gradini, R.; Liu, F.T.; et al. The Diabetic Milieu Modulates the Advanced Glycation End Product-Receptor Complex in the Mesangium by Inducing or Upregulating Galectin-3 Expression. Diabetes 2000, 49, 1249–1257. [Google Scholar] [CrossRef][Green Version]
- Berry, P.A.; Cross, T.J.S.; Thein, S.L.; Portmann, B.C.; Wendon, J.A.; Karani, J.B.; Heneghan, M.A.; Bomford, A. Hepatic Dysfunction in Sickle Cell Disease: A New System of Classification Based on Global Assessment. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2007, 5, 1469–1476; quiz 1369. [Google Scholar] [CrossRef]
- Englert, F.A.; Seidel, R.A.; Galler, K.; Gouveia, Z.; Soares, M.P.; Neugebauer, U.; Clemens, M.G.; Sponholz, C.; Heinemann, S.H.; Pohnert, G.; et al. Labile Heme Impairs Hepatic Microcirculation and Promotes Hepatic Injury. Arch. Biochem. Biophys. 2019, 672, 108075. [Google Scholar] [CrossRef] [PubMed]
- Theocharidou, E.; Suddle, A.R. The Liver in Sickle Cell Disease. Clin. Liver Dis. 2019, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Nyakundi, B.B.; Tóth, A.; Balogh, E.; Nagy, B.; Erdei, J.; Ryffel, B.; Paragh, G.; Cordero, M.D.; Jeney, V. Oxidized Hemoglobin Forms Contribute to NLRP3 Inflammasome-Driven IL-1β Production upon Intravascular Hemolysis. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 464–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, K.; Jin, N.; Tang, B.; Zhang, W. Macrophage in Liver Fibrosis: Identities and Mechanisms. Int. Immunopharmacol. 2023, 120, 110357. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Hughes, R.C. Regulation of Secretion and Surface Expression of Mac-2, a Galactoside-Binding Protein of Macrophages. J. Biol. Chem. 1994, 269, 4424–4430. [Google Scholar] [CrossRef]
- Ezhilarasan, D. Unraveling the Pathophysiologic Role of Galectin-3 in Chronically Injured Liver. J. Cell. Physiol. 2023, 238, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, K.; Tsuneyama, K.; Abdel Aziz, H.O.; Takahashi, H.; Murai, Y.; Cui, Z.-G.; Fujimoto, M.; Kato, I.; Hiraga, K.; Hsu, D.K.; et al. Disrupted Galectin-3 Causes Non-Alcoholic Fatty Liver Disease in Male Mice. J. Pathol. 2006, 210, 469–477. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Tsuneyama, K.; Nomoto, K.; Fujimoto, M.; Salunga, T.L.; Nakajima, T.; Miwa, S.; Murai, Y.; Hayashi, S.; Kato, I.; et al. Nonalcoholic Steatohepatitis and Hepatocellular Carcinoma in Galectin-3 Knockout Mice. Hepatol. Res. 2008, 38, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Jeftic, I.; Jovicic, N.; Pantic, J.; Arsenijevic, N.; Lukic, M.L.; Pejnovic, N. Galectin-3 Ablation Enhances Liver Steatosis, but Attenuates Inflammation and IL-33-Dependent Fibrosis in Obesogenic Mouse Model of Nonalcoholic Steatohepatitis. Mol. Med. 2015, 21, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yang, F.; Zhong, W.; Jiang, X.; Zhang, F.; Ji, X.; Xue, M.; Qiu, Y.; Yu, J.; Hu, X.; et al. Secretory Galectin-3 Promotes Hepatic Steatosis via Regulation of the PPARγ/CD36 Signaling Pathway. Cell. Signal. 2021, 84, 110043. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Marcos, L.V.; Martínez-Beamonte, R.; Macías-Herranz, M.; Arnal, C.; Barranquero, C.; Puente-Lanzarote, J.J.; Gascón, S.; Herrero-Continente, T.; Gonzalo-Romeo, G.; Alastrué-Vera, V.; et al. Hepatic Galectin-3 Is Associated with Lipid Droplet Area in Non-Alcoholic Steatohepatitis in a New Swine Model. Sci. Rep. 2022, 12, 1024. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, A.; Milovanovic, J.; Stojanovic, B.; Djordjevic, D.; Stanojevic, I.; Jankovic, N.; Vojvodic, D.; Arsenijevic, N.; Lukic, M.L.; Milovanovic, M. Gal-3 Deficiency Suppresses Novosphyngobium aromaticivorans Inflammasome Activation and IL-17 Driven Autoimmune Cholangitis in Mice. Front. Immunol. 2019, 10, 1309. [Google Scholar] [CrossRef] [PubMed]
- Traber, P.G.; Zomer, E. Therapy of Experimental NASH and Fibrosis with Galectin Inhibitors. PLoS ONE 2013, 8, e83481. [Google Scholar] [CrossRef] [PubMed]
- Setayesh, T.; Colquhoun, S.D.; Wan, Y.-J.Y. Overexpression of Galectin-1 and Galectin-3 in Hepatocellular Carcinoma. Liver Res. 2020, 4, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Nassar, E.S.; Elkalbashawy, Y.A.; Kamal, A.; Zakaria, N.H.E. Galectin-3 Is Not Useful for Hepatocellular Carcinoma Surveillance in Cirrhotic Patients but It May Be a Marker of Cirrhosis Development. Clin. Exp. Hepatol. 2021, 7, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Alvarez, E.; Limon-de la Rosa, N.; Vilatoba, M.; Pérez-Monter, C.; Hurtado-Gomez, S.; Martinez-Cabrera, C.; Argemi, J.; Alatorre-Arenas, E.; Yarza-Regalado, S.; Tejeda-Dominguez, F.; et al. Galectin-3 Is Overexpressed in Advanced Cirrhosis and Predicts Post-Liver Transplant Infectious Complications. Liver Int. Off. J. Int. Assoc. Study Liver 2022, 42, 2260–2273. [Google Scholar] [CrossRef]
- Yoeli, D.; Mack, C.L.; Luo, Y.; Chaidez, A.; De La Rosa, N.L.; Wang, Z.; Cervantes-Alvarez, E.; Huang, C.A.; Navarro-Alvarez, N. Galectin-3 in Biliary Atresia and Other Pediatric Cholestatic Liver Diseases. Hepatol. Res. 2024, 54, 392–402. [Google Scholar] [CrossRef]
- Mackinnon, A.C.; Tonev, D.; Jacoby, B.; Pinzani, M.; Slack, R.J. Galectin-3: Therapeutic Targeting in Liver Disease. Expert Opin. Ther. Targets 2023, 27, 779–791. [Google Scholar] [CrossRef]
- Sotoudeheian, M. Galectin-3 and Severity of Liver Fibrosis in Metabolic Dysfunction-Associated Fatty Liver Disease. Protein Pept. Lett. 2024, 31, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Volarevic, V.; Milovanovic, M.; Ljujic, B.; Pejnovic, N.; Arsenijevic, N.; Nilsson, U.; Leffler, H.; Lukic, M.L. Galectin-3 Deficiency Prevents Concanavalin A-Induced Hepatitis in Mice. Hepatology 2012, 55, 1954–1964. [Google Scholar] [CrossRef] [PubMed]
- Zetterberg, F.R.; MacKinnon, A.; Brimert, T.; Gravelle, L.; Johnsson, R.E.; Kahl-Knutson, B.; Leffler, H.; Nilsson, U.J.; Pedersen, A.; Peterson, K.; et al. Discovery and Optimization of the First Highly Effective and Orally Available Galectin-3 Inhibitors for Treatment of Fibrotic Disease. J. Med. Chem. 2022, 65, 12626–12638. [Google Scholar] [CrossRef]
- Boutin, L.; Dépret, F.; Gayat, E.; Legrand, M.; Chadjichristos, C.E. Galectin-3 in Kidney Diseases: From an Old Protein to a New Therapeutic Target. Int. J. Mol. Sci. 2022, 23, 3124. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Martinez, E.; Ibarrola, J.; Calvier, L.; Fernandez-Celis, A.; Leroy, C.; Cachofeiro, V.; Rossignol, P.; Lopez-Andres, N. Galectin-3 Blockade Reduces Renal Fibrosis in Two Normotensive Experimental Models of Renal Damage. PLoS ONE 2016, 11, e0166272. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.S.; Liu, E.; Paniagua, S.M.; Sarma, A.A.; Zampierollo, G.; López, B.; Díez, J.; Wang, T.J.; Ho, J.E. Galectin-3 Inhibition with Modified Citrus Pectin in Hypertension. JACC Basic Transl. Sci. 2021, 6, 12–21. [Google Scholar] [CrossRef]

| Mechanisms of Renal Injury during Intravascular Hemolysis | How Gal-3 Affects the Mechanism |
|---|---|
| Heme and iron induce apoptotic death, necroptosis, and ferroptosis in renal cells [95,96]. | Extracellular Gal-3 induces apoptosis of T cells [107] and monocytes/macrophages [108]. Intracellular Gal-3 attenuates cisplatin-induced apoptosis and necroptosis of renal tubule cells [109]. |
| Heme and free iron induce oxidative stress and consequent inflammation and cell death [97]. | Inhibition of Gal-3 reduces oxidative stress, apoptosis, and gene expression of inflammatory molecules in the retinal pigment epithelium [110]. Gal-3 enhances oxidative stress and fibrosis in doxorubicin-induced cardiac disfunction [111]. Gal-3 attenuates oxidative stress and apoptotic cell death in doxorubicin myocardial injury [112]. |
| Heme increases the expression of adhesion molecules in endothelial cells in kidneys [86,87]. The activation of endothelia further promotes the influx of leukocytes into the vessel wall, leading to further propagation of renal inflammation. | Gal-3 interacts with different adhesive molecules expressed on endothelial cells including integrins, cadherins, endoglin, and the melanoma adhesion molecule (CD146) [18]. Interaction with CD146 activates the release of inflammatory cytokines from endothelial cells and enhances inflammatory processes [113]. |
| Heme induces the activation of NLRP3-dependent inflammasomes in macrophages, followed by the secretion of proinflammatory IL-1β and sterile injury [85]. | Gal-3 is a known enhancer of inflammasome activation and, thus, affects the development of different diseases [51,114,115,116]. |
| Heme binds to TLR4 expressed on the cells of innate immunity, inducing a cellular signal cascade that finally activates NF-κB, thus stimulating the expression of adhesion molecules and proinflammatory cytokines [84]. | Gal-3 stimulates TLR4/NF-κB signaling in immune cells and enhances inflammation [46,117,118]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grujcic, M.; Milovanovic, M.; Nedeljkovic, J.; Jovanovic, D.; Arsenijevic, D.; Solovjova, N.; Stankovic, V.; Tanaskovic, I.; Arsenijevic, A.; Milovanovic, J. The Possible Effects of Galectin-3 on Mechanisms of Renal and Hepatocellular Injury Induced by Intravascular Hemolysis. Int. J. Mol. Sci. 2024, 25, 8129. https://doi.org/10.3390/ijms25158129
Grujcic M, Milovanovic M, Nedeljkovic J, Jovanovic D, Arsenijevic D, Solovjova N, Stankovic V, Tanaskovic I, Arsenijevic A, Milovanovic J. The Possible Effects of Galectin-3 on Mechanisms of Renal and Hepatocellular Injury Induced by Intravascular Hemolysis. International Journal of Molecular Sciences. 2024; 25(15):8129. https://doi.org/10.3390/ijms25158129
Chicago/Turabian StyleGrujcic, Mirjana, Marija Milovanovic, Jelena Nedeljkovic, Danijela Jovanovic, Dragana Arsenijevic, Natalija Solovjova, Vesna Stankovic, Irena Tanaskovic, Aleksandar Arsenijevic, and Jelena Milovanovic. 2024. "The Possible Effects of Galectin-3 on Mechanisms of Renal and Hepatocellular Injury Induced by Intravascular Hemolysis" International Journal of Molecular Sciences 25, no. 15: 8129. https://doi.org/10.3390/ijms25158129
APA StyleGrujcic, M., Milovanovic, M., Nedeljkovic, J., Jovanovic, D., Arsenijevic, D., Solovjova, N., Stankovic, V., Tanaskovic, I., Arsenijevic, A., & Milovanovic, J. (2024). The Possible Effects of Galectin-3 on Mechanisms of Renal and Hepatocellular Injury Induced by Intravascular Hemolysis. International Journal of Molecular Sciences, 25(15), 8129. https://doi.org/10.3390/ijms25158129






