Specific Compounds Derived from Traditional Chinese Medicine Ameliorate Lipid-Induced Contractile Dysfunction in Cardiomyocytes
Abstract
:1. Introduction
2. Results
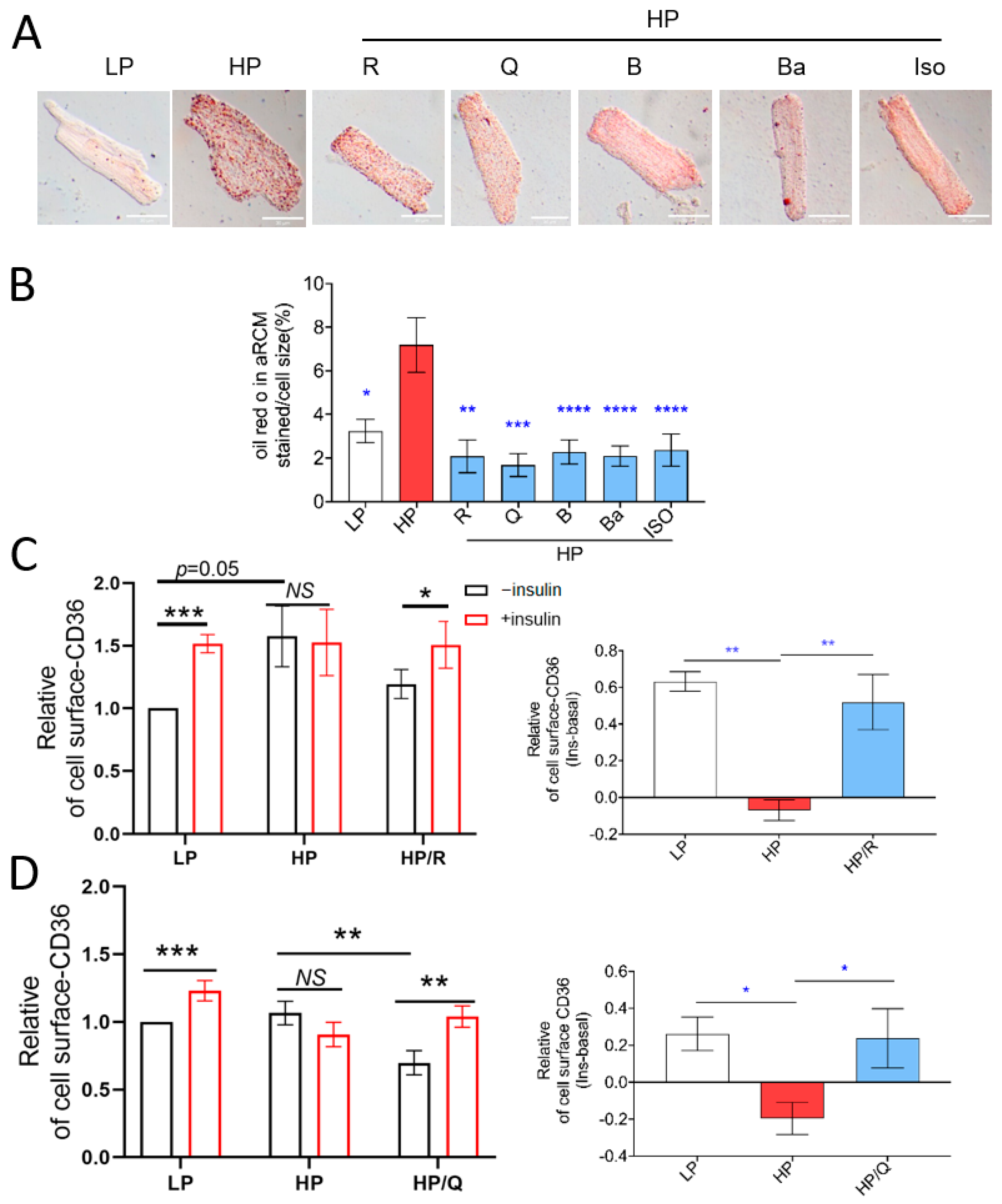
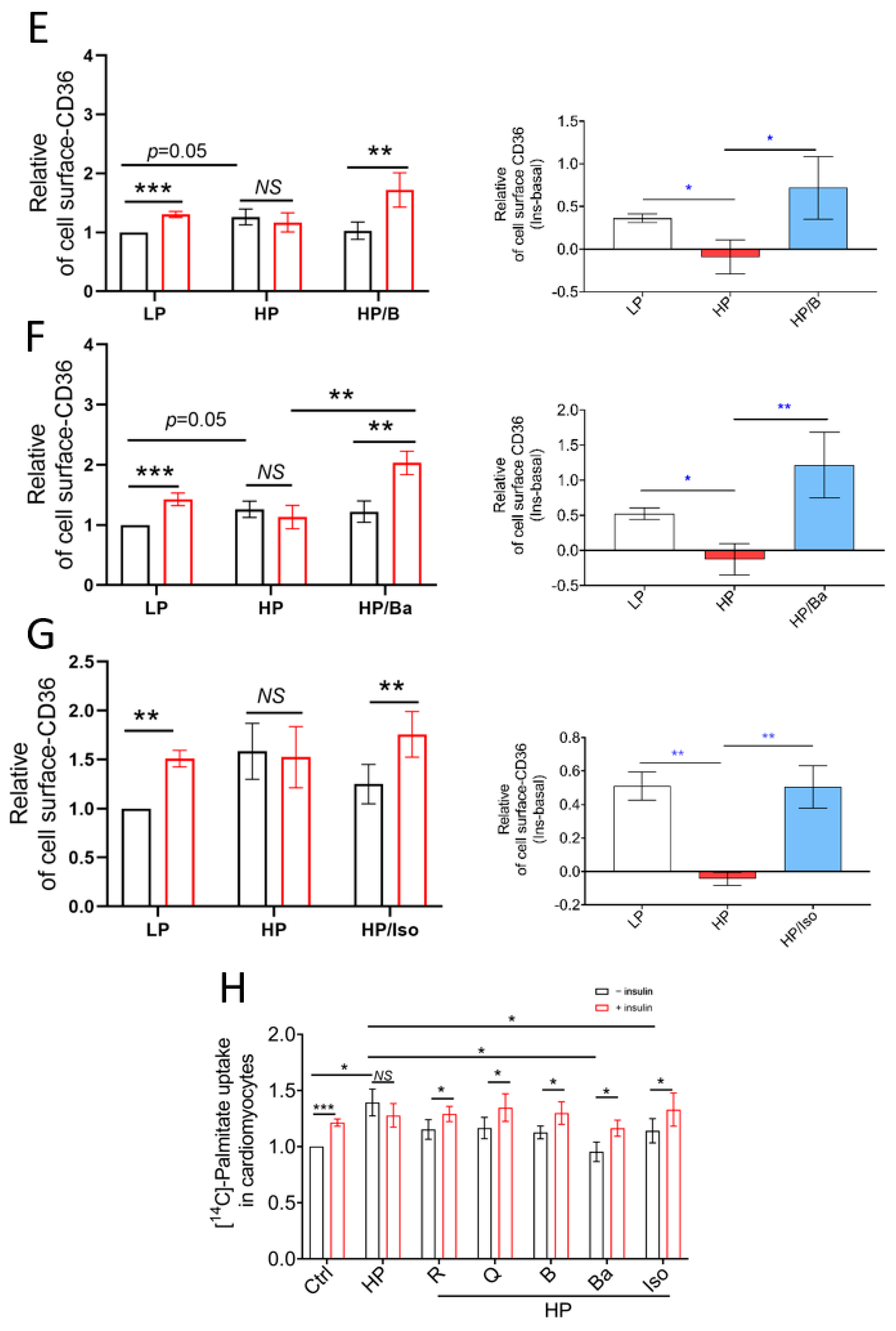
2.1. TCM Compounds Can Prevent Lipid Accumulation and Restore Intracellular Storage of CD36 Transporter Vesicles in Lipid-Overloaded Adult Rat Cardiomyocytes
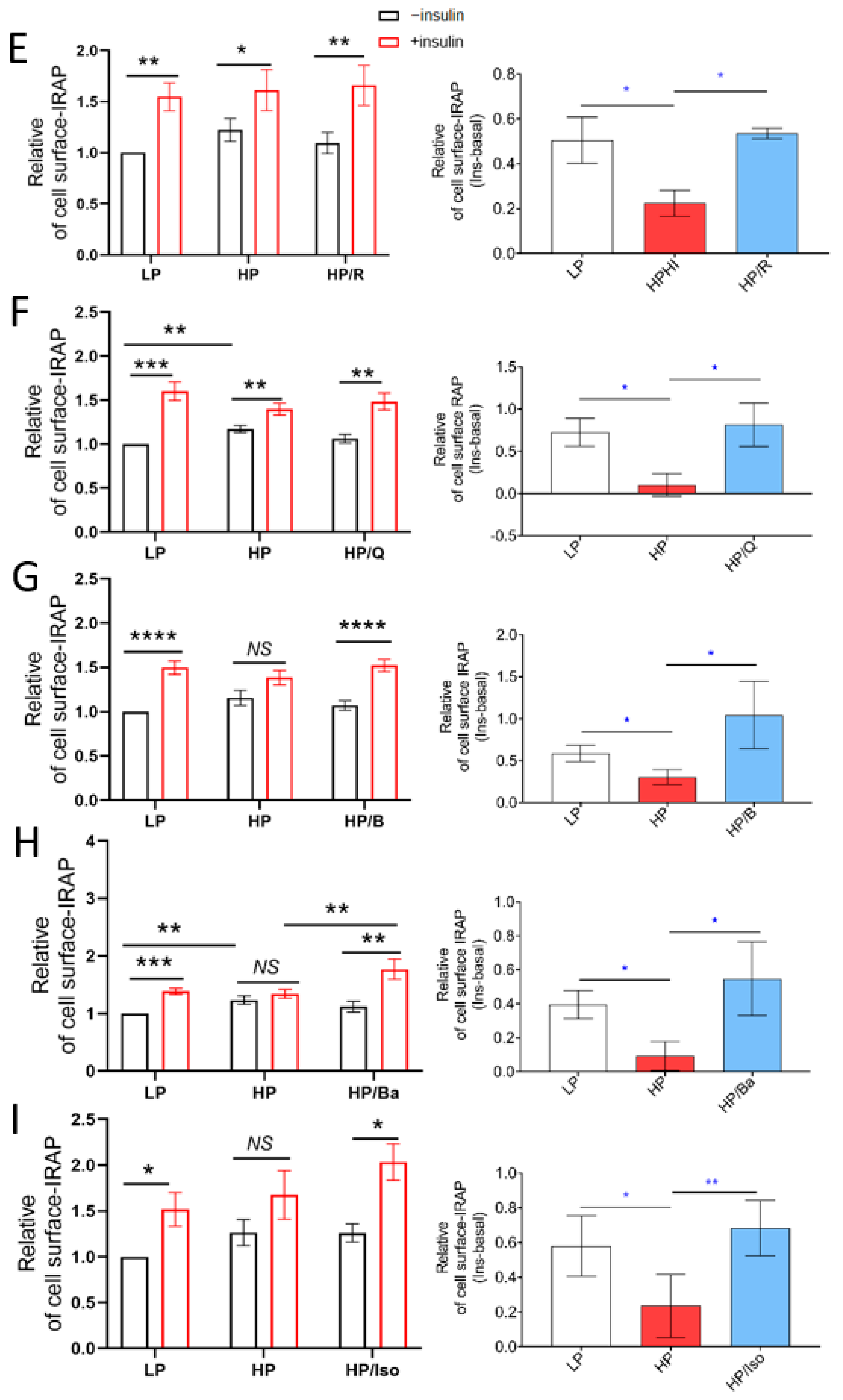
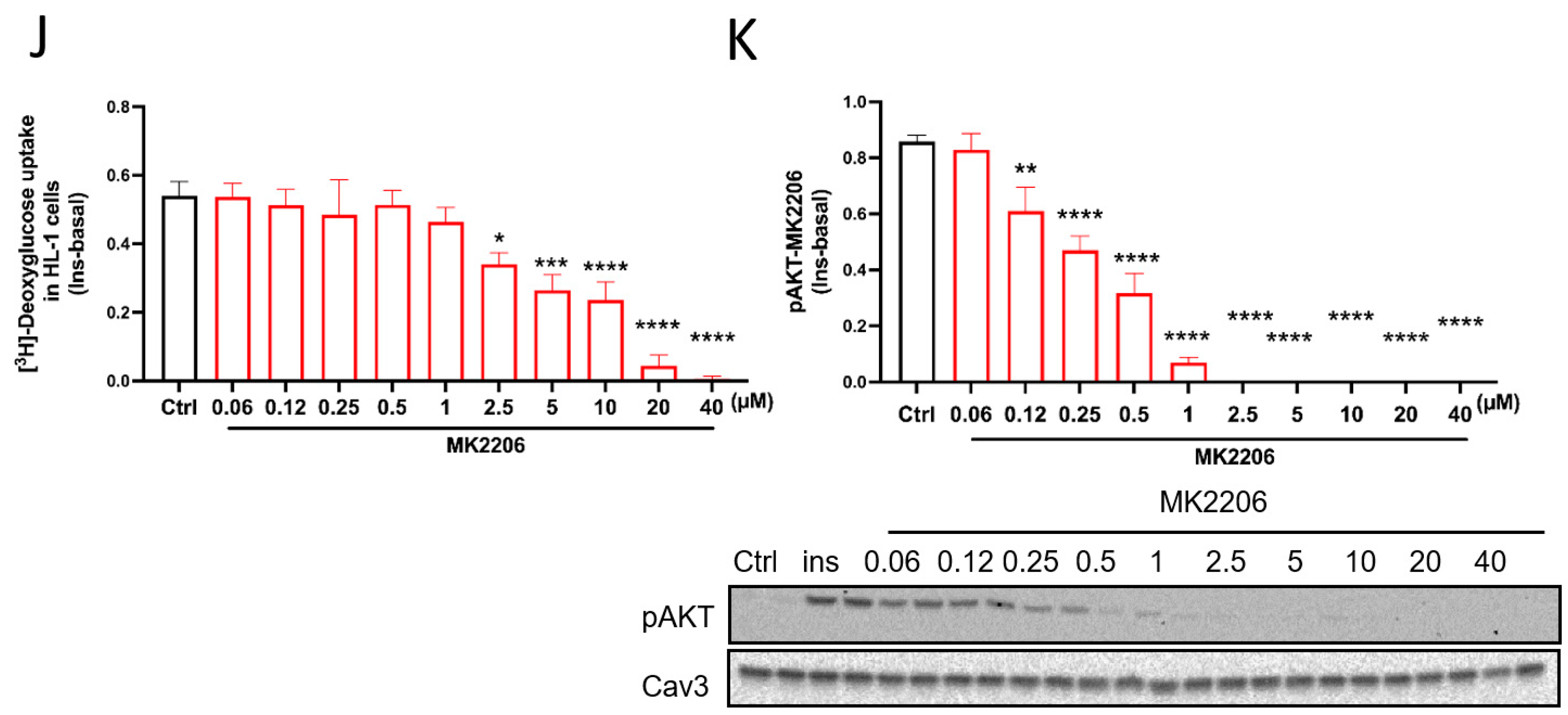
2.2. TCM Compounds Partially Prevent Decreased Insulin Signaling in Lipid-Overexposed Cardiomyocytes, but Completely Preserve Insulin-Stimulated Glucose Uptake in Lipid-Overexposed Cardiomyocytes
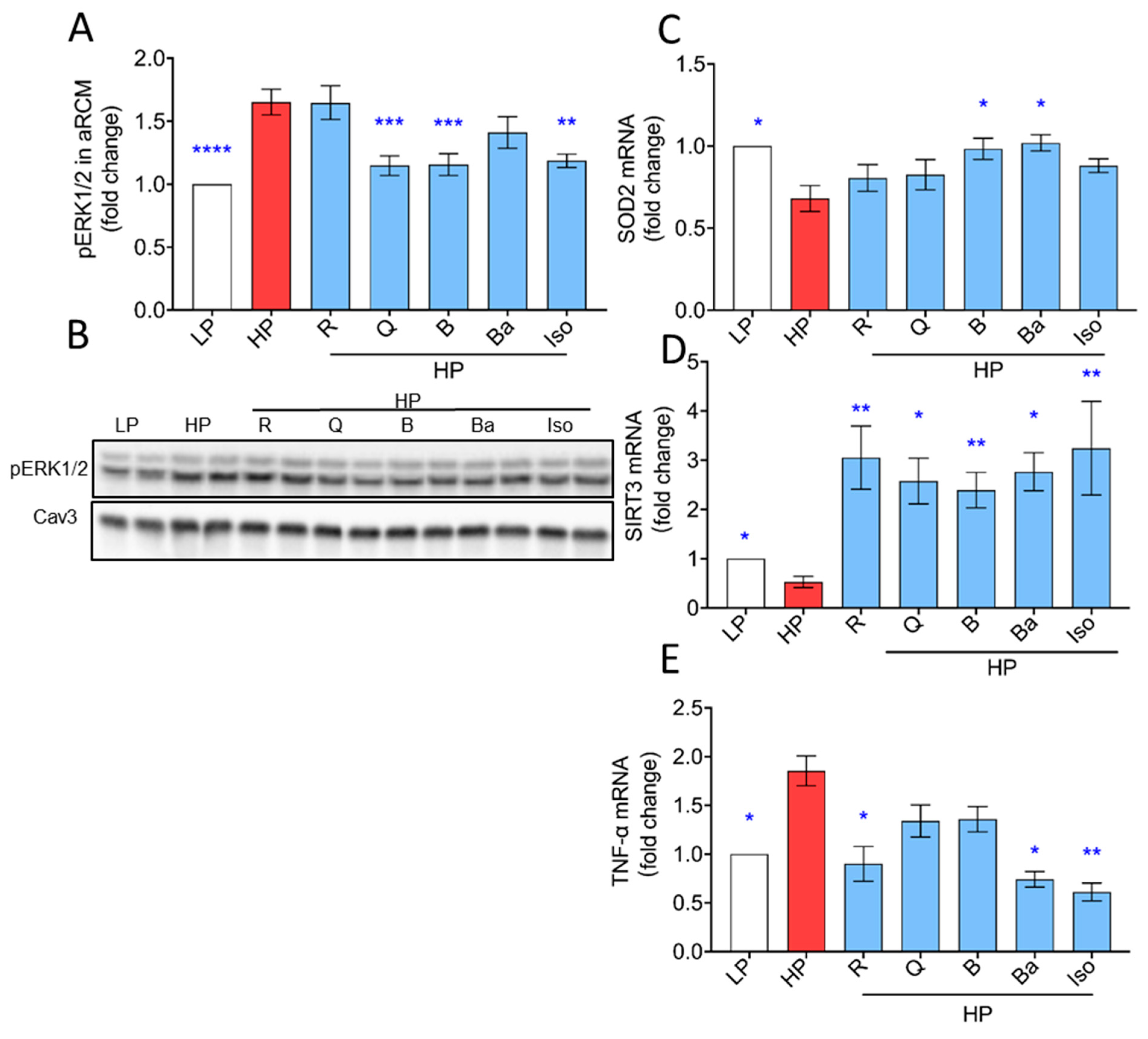
2.3. TCM Compounds Can Increase Antioxidant Levels and Prevent Increased Expression of Inflammatory Markers in Lipid-Overexposed Cardiomyocytes
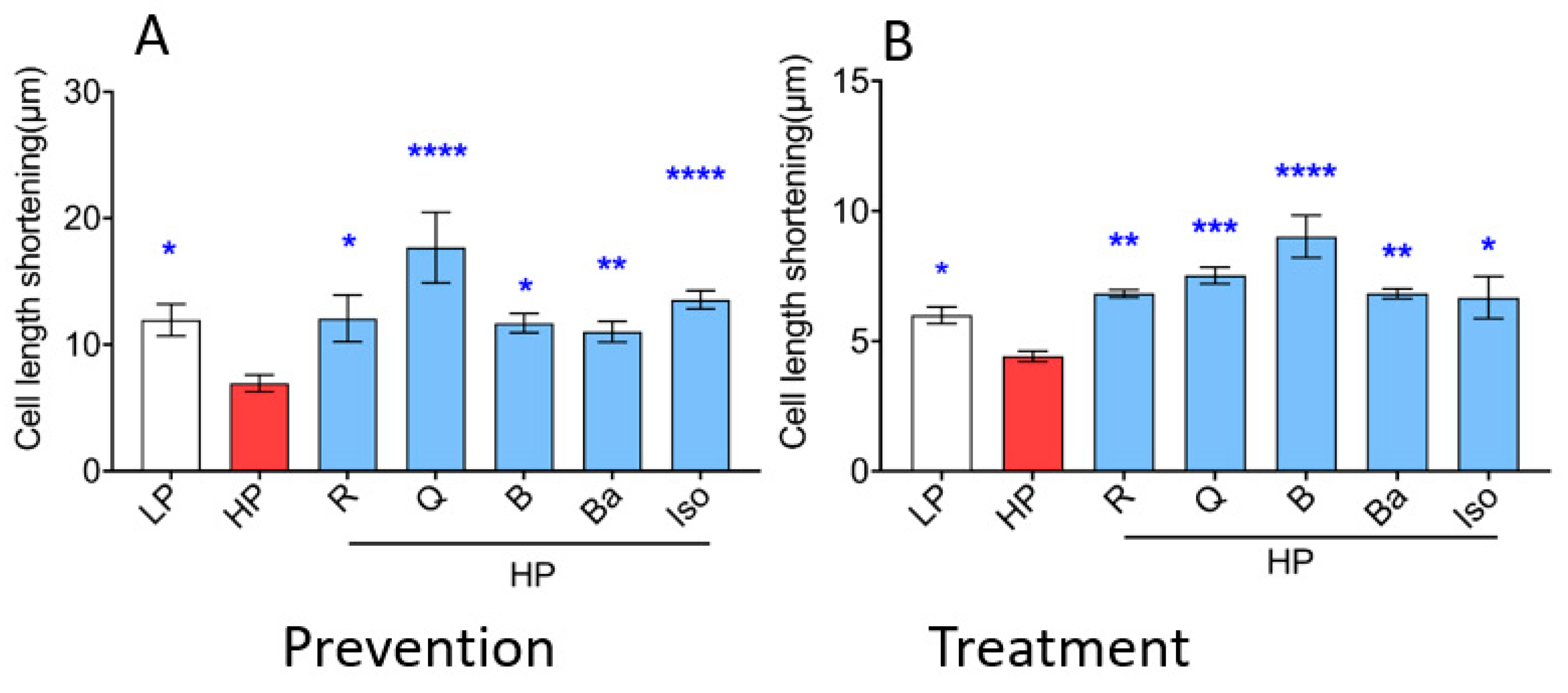
2.4. TCM Compounds Not Only Prevent but Also Ameliorate Cardiac Contractile Dysfunction in Lipid-Overexposed Cardiomyocytes
3. Discussion
3.1. Linkage between Prevention of Lipid Accumulation by the TCM Compounds and Preservation of Insulin-Stimulated Glucose Uptake
3.2. Mismatch between Preservation of Insulin-Stimulated Glucose Uptake by the TCM Compounds and Impairment of the Canonical Insulin Signaling Pathway
3.3. Effects of TCM Compounds on Anti-OxidativeAnti-Inflammatory Processes
3.4. Limitations
4. Materials and Methods
4.1. Compounds
4.2. Antibodies
4.3. Culturing of HL-1 Cardiomyocytes
4.4. Isolation and Culturing of Primary Rat Cardiomyocytes
4.5. Sulforhodamine B (SRB) Colorimetric Assay for Cytotoxicity Screening
4.6. Lipid Droplet Staining: Oil Red O Staining
4.7. Surface-Protein Biotinylation for Detecting GLUT4 and CD36 Translocation
4.8. Determination of Insulin Sensitivity
4.9. Measurement of Glucose Uptake
4.10. Real-Time RT-PCR
4.11. Measurement of Cardiomyocyte Contraction Dynamics
4.12. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TCM | Traditional Chinese Medicine |
| LCM | lipotoxic cardiomyopathy |
| aRCM | adult rat cardiomyocytes |
| R | Resveratrol |
| Q | Quercetin |
| B | Berberine |
| Ba | Baicalein |
| Iso | Isorhamnetin |
| HP | high palmitate |
| LP | low palmitate |
| IRAP | insulin-regulated aminopeptidase |
| LCFA | long-chain fatty acids |
| ORO | Oil red O |
| SRB | Sulforhodamine B |
References
- Lobstein, T.; Jackson-Leach, R.; Powis, J.; Brinsden, H.; Gray, M. World Obesity Atlas 2023; World Obesity Federation: London, UK, 2023; pp. 5–25. [Google Scholar]
- Doenst, T.; Nguyen, T.D.; Abel, E.D. Cardiac metabolism in heart failure: Implications beyond ATP production. Circ. Res. 2013, 113, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Albanese, G.; Di Martino, A.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Sasso, F.C. The Diabetic Cardiomyopathy: The Contributing Pathophysiological Mechanisms. Front. Med. 2021, 8, 695792. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Ravazzola, M.; Park, B.H.; Bashmakov, Y.K.; Orci, L.; Unger, R.H. Metabolic mechanisms of failure of intraportally transplanted pancreatic beta-cells in rats: Role of lipotoxicity and prevention by leptin. Diabetes 2007, 56, 2295–2301. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Ussher, J.R.; Folmes, C.D.L.; Jaswal, J.S.; Stanley, W.C. Myocardial fatty acid metabolism in health and disease. Physiol. Rev. 2010, 90, 207–258. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.; Mishra, M. Role of Oxidative Stress in Diabetic Cardiomyopathy. Antioxidants 2022, 11, 784. [Google Scholar] [CrossRef] [PubMed]
- Walls, S.M.; Cammarato, A.; Chatfield, D.A.; Ocorr, K.; Harris, G.L.; Bodmer, R. Ceramide-Protein Interactions Modulate Ceramide-Associated Lipotoxic Cardiomyopathy. Cell Rep. 2018, 22, 2702–2715. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Sambandam, N.; Han, X.; Gross, R.W.; Courtois, M.; Kovacs, A.; Febbraio, M.; Finck, B.N.; Kelly, D.P. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ. Res. 2007, 100, 1208–1217. [Google Scholar] [CrossRef]
- Shao, D.; Kolwicz, S.C., Jr.; Wang, P.; Roe, N.D.; Villet, O.; Nishi, K.; Hsu, Y.W.A.; Flint, G.V.; Caudal, A.; Wang, W.; et al. Increasing Fatty Acid Oxidation Prevents High-Fat Diet-Induced Cardiomyopathy Through Regulating Parkin-Mediated Mitophagy. Circulation 2020, 142, 983–997. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.; Nabben, M. CD36 (SR-B2) as a Target to Treat Lipid Overload-Induced Cardiac Dysfunction. J. Lipid Atheroscler. 2020, 9, 66–78. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Heather, L.C.; Luiken, J. CD36 as a gatekeeper of myocardial lipid metabolism and therapeutic target for metabolic disease. Physiol. Rev. 2024, 104, 727–764. [Google Scholar] [CrossRef]
- Coort, S.L.M. Regulation of FAT/CD 36-Mediated Long-Chain Fatty Acid Uptake by Cardiac Muscle: Implications for Diabetes and Its Treatment. Ph.D. Thesis, Maastricht University, Maastricht, The Netherlands, 2005; p. 9. [Google Scholar]
- Liu, Y.; Steinbusch, L.K.; Nabben, M.; Kapsokalyvas, D.; van Zandvoort, M.; Schönleitner, P.; Antoons, G.; Simons, P.J.; Coumans, W.A.; Geomini, A.; et al. Palmitate-Induced Vacuolar-Type H (+)-ATPase Inhibition Feeds Forward Into Insulin Resistance and Contractile Dysfunction. Diabetes 2017, 66, 1521–1534. [Google Scholar] [CrossRef]
- Luiken, J.J.; Coort, S.L.M.; Koonen, D.P.Y.; van der Horst, D.J.; Bonen, A.; Zorzano, A.; Glatz, J.F.C. Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pfluegers Arch. 2004, 448, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhao, W.; Wang, D.; Ma, X. Chinese Medicine in the Battle Against Obesity and Metabolic Diseases. Front. Physiol. 2018, 9, 850. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, H.; Vijg, J. New Insights into Bioactive Compounds of Traditional Chinese Medicines for Insulin Resistance Based on Signaling Pathways. Chem. Biodivers. 2019, 16, e1900176. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Zhao, Y.; Liu, Y.; Liu, Y.; Chen, K.; Lyu, S. Roles and Mechanisms of Herbal Medicine for Diabetic Cardiomyopathy: Current Status and Perspective. Oxidative Med. Cell. Longev. 2017, 2017, 8214541. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, F.; Sun, W.; Gao, L.; Kim, K.S.; Kim, K.T.; Cai, L.; Zhang, Z.; Zheng, Y. Extracts of Magnolia Species-Induced Prevention of Diabetic Complications: A Brief Review. Int. J. Mol. Sci. 2016, 17, 1629. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Zhang, H.H.; Zheng, J.; Hu, X.; Kong, W.; Hu, D.; Wang, S.X.; Zhang, P. Resveratrol attenuates high-fat diet-induced insulin resistance by influencing skeletal muscle lipid transport and subsarcolemmal mitochondrial beta-oxidation. Metabolism 2011, 60, 1598–1609. [Google Scholar] [CrossRef]
- Hong, H.J.; Kang, W.; Kim, D.G.; Lee, D.H.; Lee, Y.; Han, C.-H. Effects of resveratrol on the insulin signaling pathway of obese mice. J. Vet. Sci. 2014, 15, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Song, Z.; Zhang, H.; Li, S.; Wang, C.; Zhang, L.; Wang, T. The therapeutic effects of resveratrol on hepatic steatosis in high-fat diet-induced obese mice by improving oxidative stress, inflammation and lipid-related gene transcriptional expression. Med. Mol. Morphol. 2019, 52, 187–197. [Google Scholar] [CrossRef]
- Yao, Z.; Gu, Y.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Xia, Y.; Bao, X.; Shi, H.; Sun, S.; et al. Estimated daily quercetin intake and association with the prevalence of type 2 diabetes mellitus in Chinese adults. Eur. J. Nutr. 2019, 58, 819–830. [Google Scholar] [CrossRef]
- Liu, L.; Gao, C.; Yao, P.; Gong, Z. Quercetin Alleviates High-Fat Diet-Induced Oxidized Low-Density Lipoprotein Accumulation in the Liver: Implication for Autophagy Regulation. Biomed. Res. Int. 2015, 2015, 607531. [Google Scholar] [CrossRef] [PubMed]
- Arias, N.; Macarulla, M.T.; Aguirre, L.; Martínez-Castaño, M.G.; Portillo, M.P. Quercetin can reduce insulin resistance without decreasing adipose tissue and skeletal muscle fat accumulation. Genes Nutr. 2014, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Dong, F.; Li, S.; Chu, M.; Zhou, H.; Lu, Z.; Huang, W. Berberine-induced inhibition of adipocyte enhancer-binding protein 1 attenuates oxidized low-density lipoprotein accumulation and foam cell formation in phorbol 12-myristate 13-acetate-induced macrophages. Eur. J. Pharmacol. 2012, 690, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, Y.; Gong, Z.; Sheng, X.; Li, Z.; Zhang, W.; Qin, Y. Berberine inhibits 3T3-L1 adipocyte differentiation through the PPARgamma pathway. Biochem. Biophys. Res. Commun. 2006, 348, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Yang, N.; Kong, B.; Cao, B.; Feng, D.; Yu, X.; Ge, C.; Huang, J.; Shen, J.; Wang, P.; et al. Orally Administered Berberine Modulates Hepatic Lipid Metabolism by Altering Microbial Bile Acid Metabolism and the Intestinal FXR Signaling Pathway. Mol. Pharmacol. 2017, 91, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, W.; Zhang, J.; Xie, M.; Wang, X. Baicalein improves glucose metabolism in insulin resistant HepG2 cells. Eur. J. Pharmacol. 2019, 854, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Min, W.; Wu, M.; Fang, P.; Yu, M.; Shi, M.; Zhang, Z.; Bo, P. Effect of Baicalein on GLUT4 Translocation in Adipocytes of Diet-Induced Obese Mice. Cell. Physiol. Biochem. 2018, 50, 426–436. [Google Scholar] [CrossRef]
- Sakai, M.; Ohnishi, K.; Masuda, M.; Ohminami, H.; Yamanaka-Okumura, H.; Hara, T.; Taketani, Y. Isorhamnetin, a 3′-methoxylated flavonol, enhances the lysosomal proteolysis in J774.1 murine macrophages in a TFEB-independent manner. Biosci. Biotechnol. Biochem. 2020, 84, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Guan, Y.-Y.; Zhang, Z.-L.; Rahman, K.; Wang, S.-J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Alqudah, A.; Qnais, E.Y.; Wedyan, M.A.; Altaber, S.; Bseiso, Y.; Oqal, M.; AbuDalo, R.; Alrosan, K.; Alrosan, A.Z.; Melhim, S.B.; et al. Isorhamnetin Reduces Glucose Level, Inflammation, and Oxidative Stress in High-Fat Diet/Streptozotocin Diabetic Mice Model. Molecules 2023, 28, 502. [Google Scholar] [CrossRef]
- Lee, J.; Jung, E.; Lee, J.; Kim, S.; Huh, S.; Kim, Y.; Kim, Y.; Byun, S.Y.; Kim, Y.S.; Park, D. Isorhamnetin Represses Adipogenesis in 3T3-L1 Cells. Obesity 2009, 17, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Deng, J.; Liu, X.; Li, Q.; Zhang, J.; Bai, H.; Zhang, J. Echinacoside reverses myocardial remodeling and improves heart function via regulating SIRT1/FOXO3a/MnSOD axis in HF rats induced by isoproterenol. J. Cell. Mol. Med. 2021, 25, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ichikawa, T.; Li, J.; Si, Q.; Yang, H.; Chen, X.; Goldblatt, C.S.; Meyer, C.J.; Li, X.; Cai, L.; et al. Diabetic downregulation of Nrf2 activity via ERK contributes to oxidative stress-induced insulin resistance in cardiac cells in vitro and in vivo. Diabetes 2011, 60, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Sun, J.; Tong, Q.; Lin, Q.; Qian, L.; Park, Y.; Zheng, Y. The Role of ERK1/2 in the Development of Diabetic Cardiomyopathy. Int. J. Mol. Sci. 2016, 17, 2001. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Lin, Y.; Lei, Q.; Guan, K.-L.; Zhao, S.; Xiong, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011, 12, 534–541. [Google Scholar] [CrossRef]
- Wenzl, F.A.; Ambrosini, S.; Mohammed, S.A.; Kraler, S.; Lüscher, T.F.; Costantino, S.; Paneni, F. Inflammation in Metabolic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 742178. [Google Scholar] [CrossRef] [PubMed]
- Ouwens, D.M.; Diamant, M.; Fodor, M.; Habets, D.D.J.; Pelsers, M.M.A.L.; El Hasnaoui, M.; Dang, Z.C.; Brom, C.E.v.D.; Vlasblom, R.; Rietdijk, A.; et al. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated CD36-mediated fatty acid uptake and esterification. Diabetologia 2007, 50, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Neumann, D.; Glatz, J.F.; Luiken, J.J. Molecular mechanism of lipid-induced cardiac insulin resistance and contractile dysfunction. Prostaglandins Leukot. Essent. Fat. Acids 2018, 136, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Han, Y.; Nabben, M.; Neumann, D.; Luiken, J.J.F.P.; Glatz, J.F.C. Endosomal v-ATPase as a Sensor Determining Myocardial Substrate Preference. Metabolites 2022, 12, 579. [Google Scholar] [CrossRef]
- Angin, Y.; Steinbusch, L.K.M.; Simons, P.J.; Greulich, S.; Hoebers, N.T.H.; Douma, K.; van Zandvoort, M.A.M.J.; Coumans, W.A.; Wijnen, W.; Diamant, M.; et al. CD36 inhibition prevents lipid accumulation and contractile dysfunction in rat cardiomyocytes. Biochem. J. 2012, 448, 43–53. [Google Scholar] [CrossRef]
- Wang, S.; Neumann, D.; Westenbrink, B.D.; Schianchi, F.; Wong, L.Y.; Sun, A.; Strzelecka, A.; Glatz, J.F.; Luiken, J.J.; Nabben, M. Ketone Body Exposure of Cardiomyocytes Impairs Insulin Sensitivity and Contractile Function through Vacuolar-Type H(+)-ATPase Disassembly-Rescue by Specific Amino Acid Supplementation. Int. J. Mol. Sci. 2022, 23, 12909. [Google Scholar] [CrossRef] [PubMed]
- Bostrom, P.; Andersson, L.; Rutberg, M.; Perman, J.; Lidberg, U.; Johansson, B.R.; Fernandez-Rodriguez, J.; Ericson, J.; Nilsson, T.; Borén, J.; et al. SNARE proteins mediate fusion between cytosolic lipid droplets and are implicated in insulin sensitivity. Nat. Cell Biol. 2007, 9, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- D’Oria, R.; Schipani, R.; Leonardini, A.; Natalicchio, A.; Perrini, S.; Cignarelli, A.; Laviola, L.; Giorgino, F. The Role of Oxidative Stress in Cardiac Disease: From Physiological Response to Injury Factor. Oxid. Med. Cell. Longev. 2020, 2020, 5732956. [Google Scholar] [CrossRef] [PubMed]
- Chatuphonprasert, W.; Lao-Ong, T.; Jarukamjorn, K. Improvement of superoxide dismutase and catalase in streptozotocin-nicotinamide-induced type 2-diabetes in mice by berberine and glibenclamide. Pharm. Biol. 2013, 52, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Zhang, R.; Piao, M.J.; Chae, S.; Kim, H.S.; Park, J.H.; Jung, K.S.; Hyun, J.W. Baicalein inhibits oxidative stress-induced cellular damage via antioxidant effects. Toxicol. Ind. Health 2012, 28, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Nath, K.; Banu, S. Modulatory effect of baicalein on gene expression and activity of antioxidant enzymes in streptozotocin-nicotinamide induced diabetic rats. Braz. J. Pharm. Sci. 2019, 55, e18201. [Google Scholar] [CrossRef]
- Jeong, S.M.; Kang, M.-J.; Choi, H.-N.; Kim, J.-H.; Kim, J.-I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 2012, 6, 201–207. [Google Scholar] [CrossRef]
- Chen, D.S.; Li, C.; Michalsen, A.; Kessler, C.; Huang, Y.; Meng, J.; Ke, B.; Wang, Y.; Zhang, J.; Qin, J. Modified Ling-Gui-Zhu-Gan decoction combined with short-term fasting improves therapeutic response in type 2 diabetic patients. Eur. J. Integr. Med. 2012, 4, E309–E314. [Google Scholar] [CrossRef]
- Wu, H.; Li, G.N.; Xie, J.; Li, R.; Chen, Q.H.; Chen, J.Z.; Wei, Z.H.; Kang, L.N.; Xu, B. Resveratrol ameliorates myocardial fibrosis by inhibiting ROS/ERK/TGF-beta/periostin pathway in STZ-induced diabetic mice. BMC Cardiovasc. Disord. 2016, 16, 5. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, H.; Peng, J.; He, J.; Tang, M.; Yan, S.; Rong, J.; Li, J.; Zheng, Z.; Wang, H.; et al. Resveratrol inhibits necroptosis by mediating the TNF-alpha/RIP1/RIP3/MLKL pathway in myocardial hypoxia/reoxygenation injury. Acta Biochim. Biophys. Sin. 2021, 53, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, C.; Zhuang, J.; Li, H.; Yao, Y.; Shao, C.; Wang, H. Resveratrol attenuates inflammation in the rat heart subjected to ischemia-reperfusion: Role of the TLR4/NF-kappaB signaling pathway. Mol. Med. Rep. 2015, 11, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, X.; Chu, Y.; Lu, S. Heart protective effects and mechanism of quercetin preconditioning on anti-myocardial ischemia reperfusion (IR) injuries in rats. Gene 2014, 545, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, M.; Hu, Y.; Zhao, Y.; Teng, F.; Lv, X.; Li, J.; Zhang, Y.; Hatch, G.M.; Chen, L. Increased Bioavailable Berberine Protects Against Myocardial Ischemia Reperfusion Injury Through Attenuation of NFkappaB and JNK Signaling Pathways. Int. Heart J. 2018, 59, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Sun, G.; Zhang, Y.; Li, X.; Wang, R. Involvement of the NF-kappaB signaling pathway in the renoprotective effects of isorhamnetin in a type 2 diabetic rat model. Biomed. Rep. 2016, 4, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, Y.; Ma, Z. Multi-target mechanism of Tripteryguim wilfordii Hook for treatment of ankylosing spondylitis based on network pharmacology and molecular docking. Ann. Med. 2021, 53, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yuan, Z.; Wu, H.; Li, W.; Li, L.; Huang, H. Network Pharmacology-Based Strategy Reveals the Effects of Hedysarum multijugum Maxim.Radix Salviae Compound on Oxidative Capacity and Cardiomyocyte Apoptosis in Rats with Diabetic Cardiomyopathy. Biomed. Res. Int. 2020, 2020, 8260703. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Deinum, E. Lipid Accumulation in Cardiometabolic Disease. Bachelor’s Thesis, Maastricht University, Maastricht, The Netherlands, 2023; p. 31. [Google Scholar]
- Wang, S.; Schianchi, F.; Neumann, D.; Wong, L.Y.; Sun, A.; van Nieuwenhoven, F.A.; Zeegers, M.P.; Strzelecka, A.; Col, U.; Glatz, J.F.; et al. Specific amino acid supplementation rescues the heart from lipid overload-induced insulin resistance and contractile dysfunction by targeting the endosomal mTOR-v-ATPase axis. Mol. Metab. 2021, 53, 101293. [Google Scholar] [CrossRef]
- Luiken, J.J.F.P.; Koonen, D.P.; Willems, J.; Zorzano, A.; Becker, C.; Fischer, Y.; Tandon, N.N.; Van Der Vusse, G.J.; Bonen, A.; Glatz, J.F. Insulin Stimulates Long-Chain Fatty Acid Utilization by Rat Cardiac Myocytes Through Cellular Redistribution of FAT/CD36. Diabetes 2002, 51, 3113–3119. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Neumann, D.; Kapsokalyvas, D.; Hoes, M.F.; Schianchi, F.; Glatz, J.F.C.; Nabben, M.; Luiken, J.J.F.P. Specific Compounds Derived from Traditional Chinese Medicine Ameliorate Lipid-Induced Contractile Dysfunction in Cardiomyocytes. Int. J. Mol. Sci. 2024, 25, 8131. https://doi.org/10.3390/ijms25158131
Wang F, Neumann D, Kapsokalyvas D, Hoes MF, Schianchi F, Glatz JFC, Nabben M, Luiken JJFP. Specific Compounds Derived from Traditional Chinese Medicine Ameliorate Lipid-Induced Contractile Dysfunction in Cardiomyocytes. International Journal of Molecular Sciences. 2024; 25(15):8131. https://doi.org/10.3390/ijms25158131
Chicago/Turabian StyleWang, Fang, Dietbert Neumann, Dimitris Kapsokalyvas, Martijn F. Hoes, Francesco Schianchi, Jan F. C. Glatz, Miranda Nabben, and Joost J. F. P. Luiken. 2024. "Specific Compounds Derived from Traditional Chinese Medicine Ameliorate Lipid-Induced Contractile Dysfunction in Cardiomyocytes" International Journal of Molecular Sciences 25, no. 15: 8131. https://doi.org/10.3390/ijms25158131





