Study on the Mechanisms of Glrα3 in Pain Sensitization of Endometriosis
Abstract
:1. Introduction
2. Results
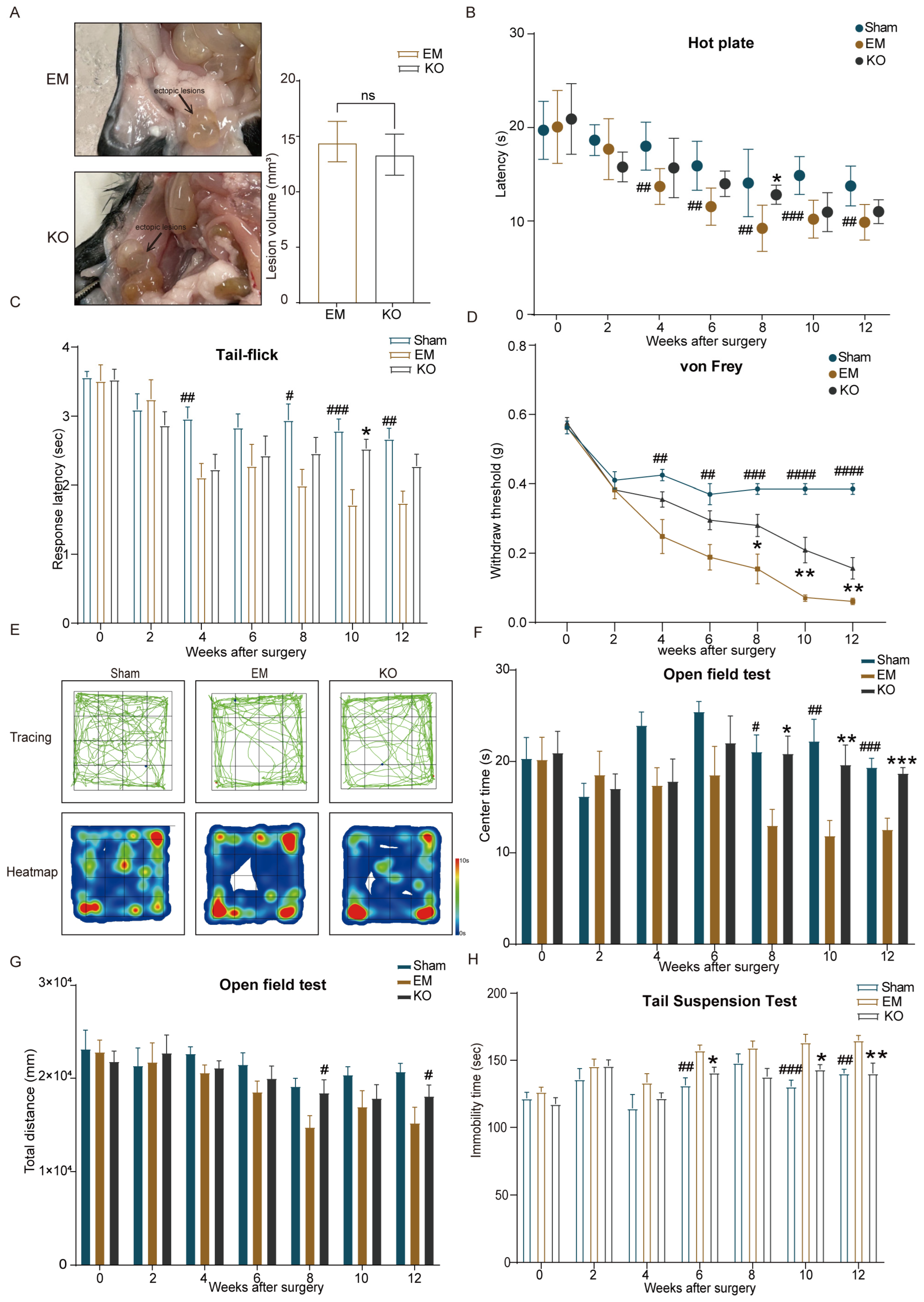
2.1. Endometriosis Induces Hypersensitivity to Pain and Anxiety–Depressive Behaviors
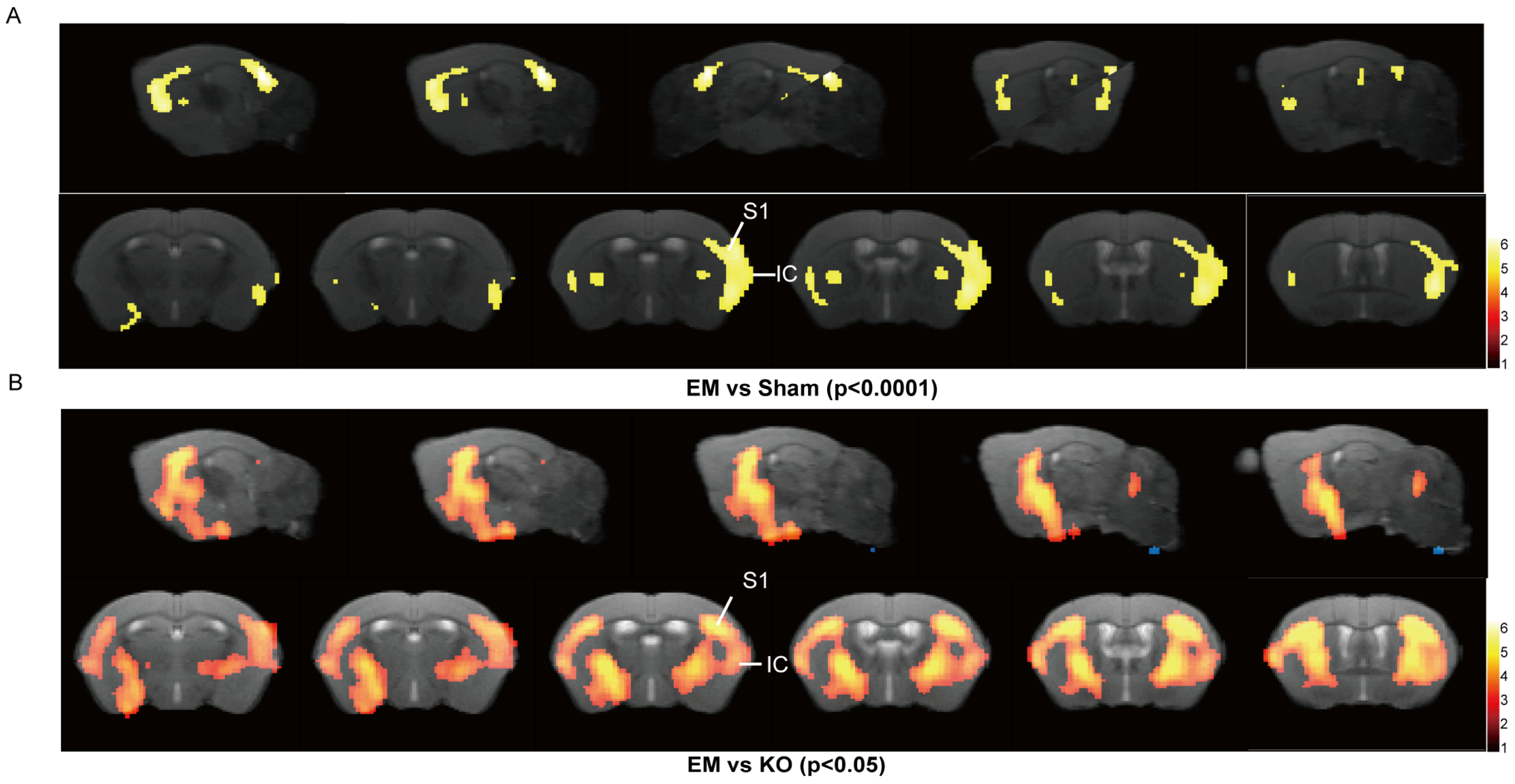
2.2. Upregulation of Glrα3 in the S1 and IC Brain Regions of Mice with Endometriosis
2.3. Inhibition of Glrα3 Alleviates Pain Hypersensitivity and Reduces Anxiety–Depressive Behaviors
2.4. Inhibition of Glrα3 Ameliorates Reductions in Gray Matter Volume in the S1 and IC Regions
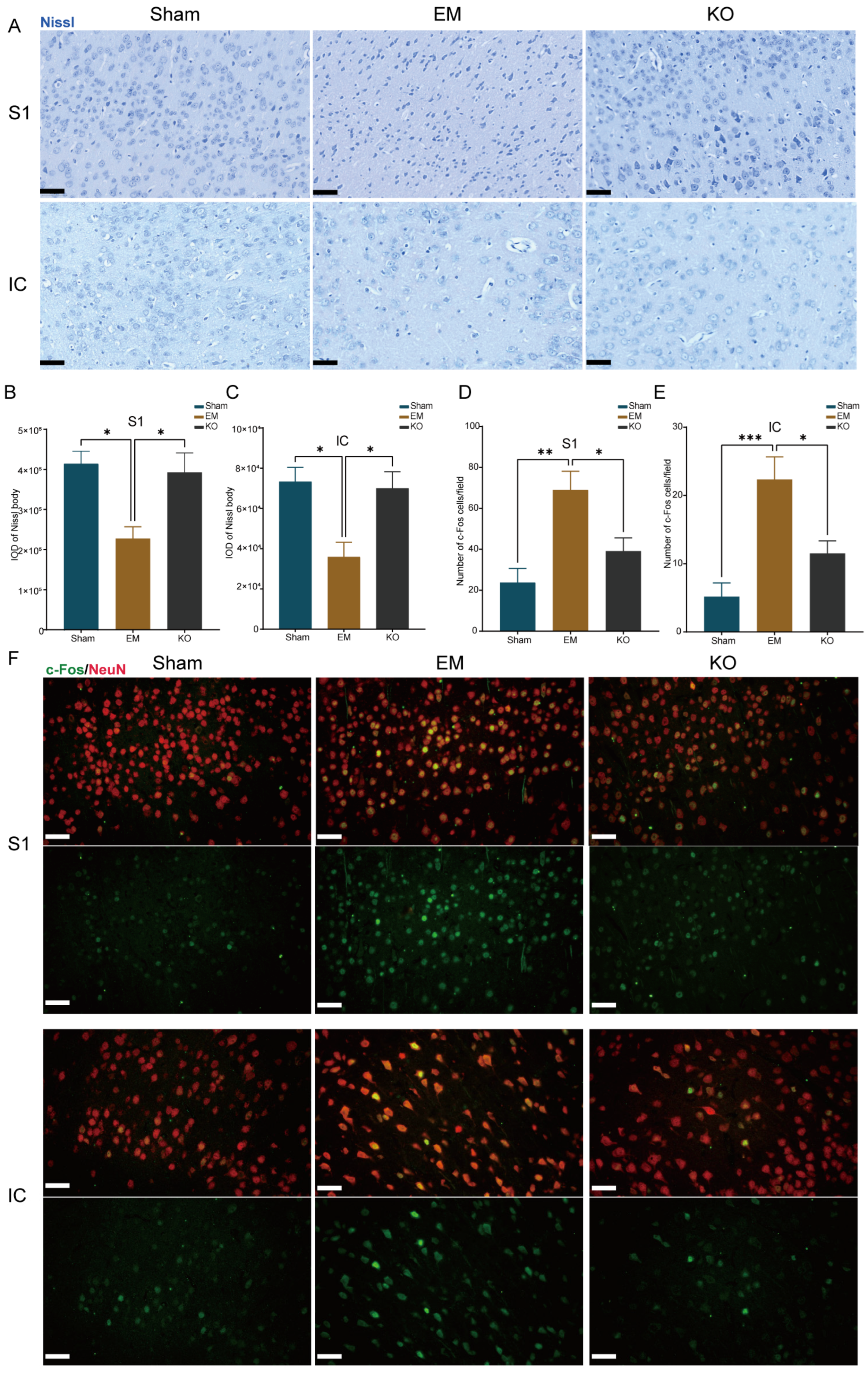
2.5. Inhibition of Glrα3 Alleviates Neuronal Alterations in the IC and S1 Regions
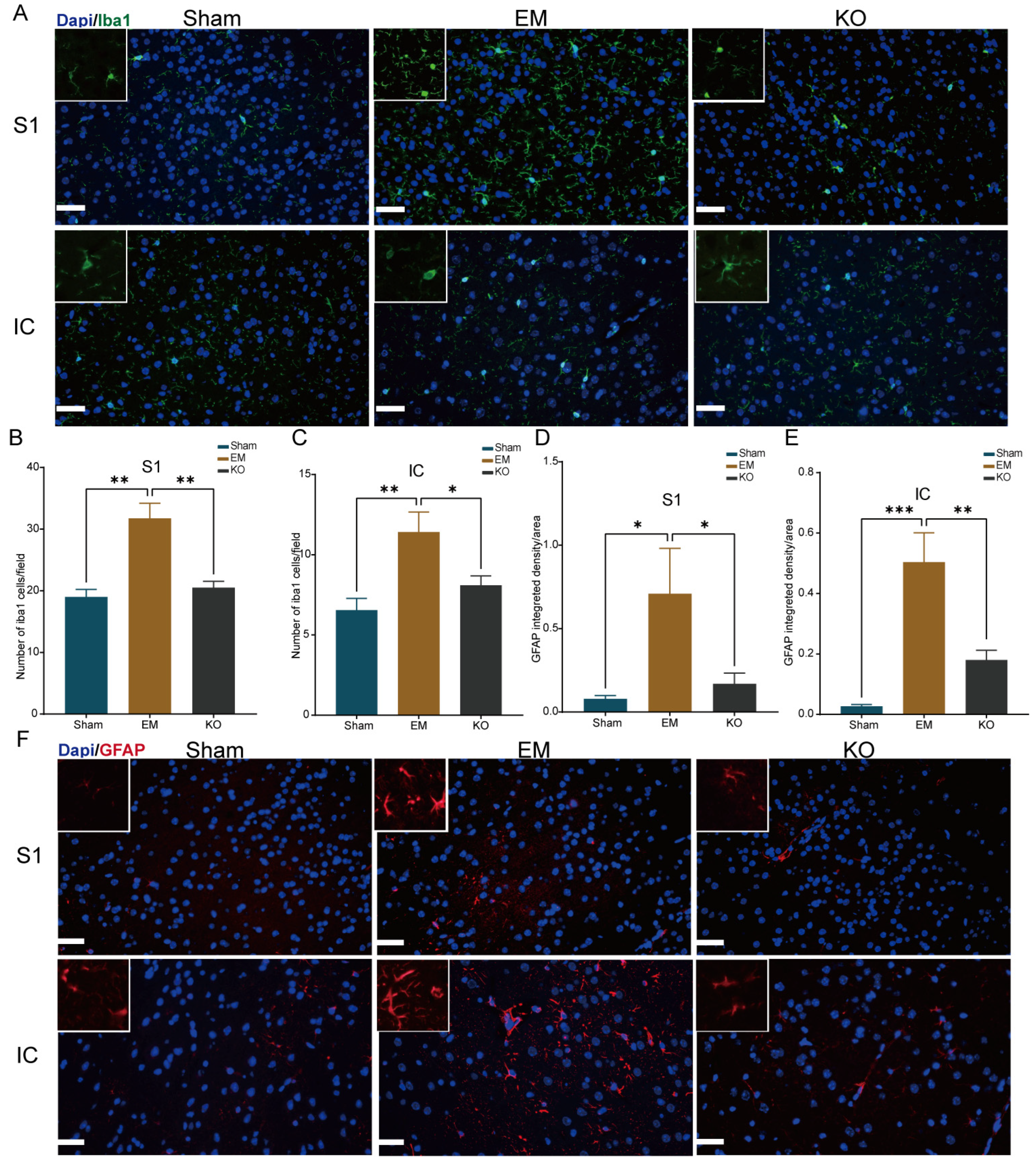
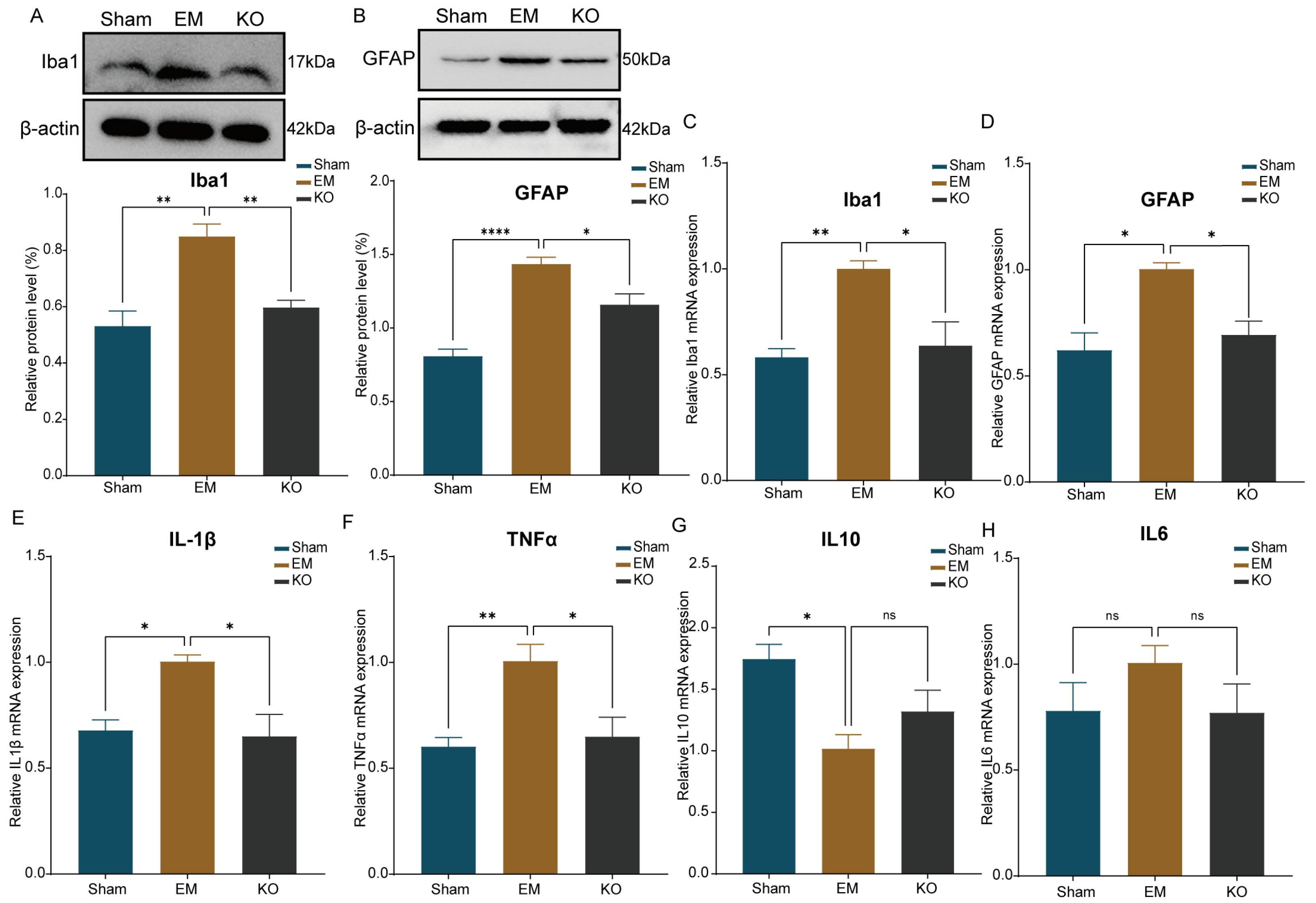
2.6. Inhibition of Glrα3 Reduces Activation of Glial Cells in the IC and S1 Regions
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Methods
4.2.1. Animal Model of Endometriosis
4.2.2. Behavioral Testing
4.2.3. MRI Image Acquisition and Processing
4.2.4. Western Blot
4.2.5. Immunofluorescence Staining
4.2.6. Nissl Staining
4.2.7. qPCR
4.2.8. Experimental Design
4.2.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamvu, G.; Carrillo, J.; Ouyang, C.; Rapkin, A. Chronic Pelvic Pain in Women: A Review. JAMA 2021, 325, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed]
- McNamara, H.C.; Frawley, H.C.; Donoghue, J.F.; Readman, E.; Healey, M.; Ellett, L.; Reddington, C.; Hicks, L.J.; Harlow, K.; Rogers, P.A.W.; et al. Peripheral, Central, and Cross Sensitization in Endometriosis-Associated Pain and Comorbid Pain Syndromes. Front. Reprod. Health 2021, 3, 729642. [Google Scholar] [CrossRef] [PubMed]
- Maddern, J.; Grundy, L.; Castro, J.; Brierley, S.M. Pain in Endometriosis. Front. Cell. Neurosci. 2020, 14, 590823. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, H.; Wu, J.; Liu, D.; Yao, S. Villainous role of estrogen in macrophage-nerve interaction in endometriosis. Reprod. Biol. Endocrinol. 2018, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Qiu, D.; Wang, W.; Mei, Y.; Tang, H.; Yuan, Z.; Zhang, P.; Zhang, Y.; Yu, X.; Yang, C.; Wang, Q.; et al. Brain structure and cortical activity changes of new daily persistent headache: Multimodal evidence from MEG/sMRI. J. Headache Pain 2023, 24, 45. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Jia, S.; Guo, D.; Chen, S.; Zhang, W.; Cheng, A.; Xie, W.; Sun, G.; Leng, J.; Lang, J. Central Sensitization-Related Changes in Brain Function Activity in a Rat Endometriosis-Associated Pain Model. J. Pain Res. 2020, 13, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Terumitsu, M.; Takado, Y.; Fukuda, K.I.; Kato, E.; Tanaka, S. Neurometabolite Levels and Relevance to Central Sensitization in Chronic Orofacial Pain Patients: A Magnetic Resonance Spectroscopy Study. J. Pain Res. 2022, 15, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Zeilhofer, H.U.; Werynska, K.; Gingras, J.; Yévenes, G.E. Glycine Receptors in Spinal Nociceptive Control—An Update. Biomolecules 2021, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Werynska, K.; Gingras, J.; Benke, D.; Scheurer, L.; Neumann, E.; Zeilhofer, H.U. A Glra3 phosphodeficient mouse mutant establishes the critical role of protein kinase A-dependent phosphorylation and inhibition of glycine receptors in spinal inflammatory hyperalgesia. Pain 2021, 162, 2436–2445. [Google Scholar] [CrossRef]
- Harvey, R.J.; Depner, U.B.; Wässle, H.; Ahmadi, S.; Heindl, C.; Reinold, H.; Smart, T.G.; Harvey, K.; Schütz, B.; Abo-Salem, O.M.; et al. GlyR alpha3: An essential target for spinal PGE2-mediated inflammatory pain sensitization. Science 2004, 304, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Mamillapalli, R.; Ding, S.; Chang, H.; Liu, Z.W.; Gao, X.B.; Taylor, H.S. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice. Biol. Reprod. 2018, 99, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Xiao, L.; Fan, W.; Zou, J.; Yang, H.; Liu, B.; Ye, Y.; Wen, D.; Liao, L. Astrocyte Activation, but not Microglia, Is Associated with the Experimental Mouse Model of Schizophrenia Induced by Chronic Ketamine. J. Mol. Neurosci. 2022, 72, 1902–1915. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.A. Using c-fos as a neural marker of pain. Brain Res. Bull. 1998, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nature reviews. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.A.; Ibrahim, W.W.; Mohamed, A.F.; Abdelkader, N.F. Microglia polarization in nociplastic pain: Mechanisms and perspectives. Inflammopharmacology 2023, 31, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Dodds, K.N.; Beckett, E.A.H.; Evans, S.F.; Hutchinson, M.R. Spinal Glial Adaptations Occur in a Minimally Invasive Mouse Model of Endometriosis: Potential Implications for Lesion Etiology and Persistent Pelvic Pain. Reprod. Sci. 2019, 26, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Maulitz, L.; Stickeler, E.; Stickel, S.; Habel, U.; Tchaikovski, S.N.; Chechko, N. Endometriosis, psychiatric comorbidities and neuroimaging: Estimating the odds of an endometriosis brain. Front. Neuroendocrinol. 2022, 65, 100988. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, L.A.; Mechawar, N. Implication of cerebral astrocytes in major depression: A review of fine neuroanatomical evidence in humans. GLIA 2021, 69, 2077–2099. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Gao, Z.; Hu, H. Microglia in depression: Current perspectives. Sci. China Life Sci. 2021, 64, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Valet, M.; Gündel, H.; Sprenger, T.; Sorg, C.; Mühlau, M.; Zimmer, C.; Henningsen, P.; Tölle, T.R. Patients with pain disorder show gray-matter loss in pain-processing structures: A voxel-based morphometric study. Psychosom. Med. 2009, 71, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.L.B.; Pingueiro-Okada, E.M.; Luiz, K.G.; Pedroso, G.L.; Matsumoto, M.A.N.; de Menezes, L.M.; Küchler, E.C.; Nascimento, G.C.; Stuani, M.B.S. Orthodontic pain: C-Fos expression in rat brain nuclei after rapid maxillary expansion. J. World Fed. Orthod. 2023, 12, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Takeda, I.; Yoshihara, K.; Cheung, D.L.; Kobayashi, T.; Agetsuma, M.; Tsuda, M.; Eto, K.; Koizumi, S.; Wake, H.; Moorhouse, A.J.; et al. Controlled activation of cortical astrocytes modulates neuropathic pain-like behaviour. Nat. Commun. 2022, 13, 4100. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Jung, Y.W.; Shin, W.; Park, M.; Lee, G.W.; Jeong, S.; An, S.; Kim, K.; Ko, Y.B.; Lee, K.H.; et al. Endometriosis-Related Chronic Pelvic Pain. Biomedicines 2023, 11, 2868. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Missmer, S.A. Pathophysiology, diagnosis, and management of endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.M.; Gattrell, W.T.; Gude, K.; Singh, S.S. Reevaluating response and failure of medical treatment of endometriosis: A systematic review. Fertil. Steril. 2017, 108, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.M.; Mechsner, S. Pathogenesis of Endometriosis: The Origin of Pain and Subfertility. Cells 2021, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Harvey, V.L.; Caley, A.; Müller, U.C.; Harvey, R.J.; Dickenson, A.H. A Selective Role for alpha3 Subunit Glycine Receptors in Inflammatory Pain. Front. Mol. Neurosci. 2009, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Targowska-Duda, K.M.; Peters, D.; Marcus, J.L.; Zribi, G.; Toll, L.; Ozawa, A. Functional and anatomical analyses of active spinal circuits in a mouse model of chronic pain. Pain 2024, 165, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Niddam, D.M.; Wang, S.J.; Tsai, S.Y. Pain sensitivity and the primary sensorimotor cortices: A multimodal neuroimaging study. Pain 2021, 162, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Ferdek, M.A.; Oosterman, J.M.; Adamczyk, A.K.; van Aken, M.; Woudsma, K.J.; Peeters, B.; Nap, A.; Wyczesany, M.; van Rijn, C.M. Effective Connectivity of Beta Oscillations in Endometriosis-Related Chronic Pain during rest and Pain-Related Mental Imagery. J. Pain 2019, 20, 1446–1458. [Google Scholar] [CrossRef] [PubMed]
- Torrecillas-Martínez, L.; Catena, A.; O’Valle, F.; Padial-Molina, M.; Galindo-Moreno, P. Does experienced pain affects local brain volumes? Insights from a clinical acute pain model. Int. J. Clin. Health Psychol. 2019, 19, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Tracey, I.; Bushnell, M.C. How Neuroimaging Studies Have Challenged Us to Rethink: Is Chronic Pain a Disease? J. Pain 2009, 10, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Melum, T.A.; Vangberg, T.R.; Johnsen, L.H.; Steingrímsdóttir, Ó.A.; Stubhaug, A.; Mathiesen, E.B.; Nielsen, C. Gray matter volume and pain tolerance in a general population: The Tromsø study. Pain 2023, 164, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, R.R.; Gupta, A.; Rapkin, A.; Kilpatrick, L.A.; Hamadani, K.; Pazmany, E.; Van Oudenhove, L.; Stains, J.; Aerts, L.; Enzlin, P.; et al. Altered gray matter volume in sensorimotor and thalamic regions associated with pain in localized provoked vulvodynia: A voxel-based morphometry study. Pain 2019, 160, 1529–1540. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Zhang, F.; Cheng, M.-L.; Wu, J.; Xie, M.; Yu, L.-Z.; Liu, L.; Xiong, J.; Zhu, H.-L. Glycogen synthase kinase-3β inhibition decreases inflammation and relieves cancer induced bone pain via reducing Drp1-mediated mitochondrial damage. J. Cell. Mol. Med. 2022, 26, 3965–3976. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, Z.; Li, S.; He, J.; Cao, S.; Xiao, Z. PRG-1 relieves pain and depressive-like behaviors in rats of bone cancer pain by regulation of dendritic spine in hippocampus. Int. J. Biol. Sci. 2021, 17, 4005–4020. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, C.; Pan, Y.; Yang, Y.; Li, W.; Ni, H.; Liu, B.; Nie, H.; Xu, R.; Wei, H.; et al. CXCL13 contributes to chronic pain of a mouse model of CRPS-I via CXCR5-mediated NF-κB activation and pro-inflammatory cytokine production in spinal cord dorsal horn. J. Neuroinflamm. 2023, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Siouti, E.; Andreakos, E. The many facets of macrophages in rheumatoid arthritis. Biochem. Pharmacol. 2019, 165, 152–169. [Google Scholar] [CrossRef] [PubMed]
- Watkins, L.R.; Milligan, E.D.; Maier, S.F. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001, 24, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef] [PubMed]
- Ou, M.; Chen, Y.; Liu, J.; Zhang, D.; Yang, Y.; Shen, J.; Miao, C.; Tang, S.J.; Liu, X.; Mulkey, D.K.; et al. Spinal astrocytic MeCP2 regulates Kir4.1 for the maintenance of chronic hyperalgesia in neuropathic pain. Prog. Neurobiol. 2023, 224, 102436. [Google Scholar] [CrossRef] [PubMed]
- Jeljeli, M.; Riccio, L.G.C.; Chouzenoux, S.; Moresi, F.; Toullec, L.; Doridot, L.; Nicco, C.; Bourdon, M.; Marcellin, L.; Santulli, P.; et al. Macrophage Immune Memory Controls Endometriosis in Mice and Humans. Cell Rep. 2020, 33, 108325. [Google Scholar] [CrossRef]
- de Castro-Jorge, L.A.; de Carvalho, R.V.H.; Klein, T.M.; Hiroki, C.H.; Lopes, A.H.; Guimarães, R.M.; Fumagalli, M.J.; Floriano, V.G.; Agostinho, M.R.; Slhessarenko, R.D.; et al. The NLRP3 inflammasome is involved with the pathogenesis of Mayaro virus. PLoS Pathog. 2019, 15, e1007934. [Google Scholar] [CrossRef]
- Grim, T.W.; Schmid, C.L.; Stahl, E.L.; Pantouli, F.; Ho, J.H.; Acevedo-Canabal, A.; Kennedy, N.M.; Cameron, M.D.; Bannister, T.D.; Bohn, L.M. A G protein signaling-biased agonist at the μ-opioid receptor reverses morphine tolerance while preventing morphine withdrawal. Neuropsychopharmacology 2020, 45, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, S.G.; Naidu, P.S.; Cravatt, B.F.; Dudley, D.T.; Lichtman, A.H. Fatty acid amide hydrolase blockade attenuates the development of collagen-induced arthritis and related thermal hyperalgesia in mice. Pharmacol. Biochem. Behav. 2011, 99, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Barcelon, E.E.; Cho, W.H.; Jun, S.B.; Lee, S.J. Brain Microglial Activation in Chronic Pain-Associated Affective Disorder. Front. Neurosci. 2019, 13, 213. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Meng, X.; Zhou, P.; Yin, Z.; Xie, Q.; Zou, H.; Shen, N.; Ye, Z.; Tang, Y. Interferon-α exacerbates neuropsychiatric phenotypes in lupus-prone mice. Arthritis Res. Ther. 2019, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Wu, D.; Liang, S.; Liu, H.; Sun, X.; Li, P.; Huang, Q.; Zhang, T.; Feng, T.; Ye, S.; et al. A stereotaxic MRI template set of mouse brain with fine sub-anatomical delineations: Application to MEMRI studies of 5XFAD mice. Magn. Reson. Imaging 2019, 57, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Guo, E.; Xiao, R.; Wu, Y.; Lu, F.; Liu, C.; Yang, B.; Li, X.; Fu, Y.; Wang, Z.; Li, Y.; et al. WEE1 inhibition induces anti-tumor immunity by activating ERV and the dsRNA pathway. J. Exp. Med. 2022, 219, e20210789. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Shen, X.; Xu, X.; Liu, H.; Li, F.; Lu, S.; Gao, Z.; Zhang, J.; Wu, Q.; Yang, D.; et al. Astrocytic YAP Promotes the Formation of Glia Scars and Neural Regeneration after Spinal Cord Injury. J. Neurosci. 2020, 40, 2644–2662. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Gao, G.; Zhong, B.; Zhang, H.; Ding, S.; Sun, Z.; Zhang, Y.; Li, W. NLRP1 inflammasome involves in learning and memory impairments and neuronal damages during aging process in mice. Behav. Brain Funct. 2021, 17, 11. [Google Scholar] [CrossRef] [PubMed]



| Brain Region | Voxel | MNI | Peak T Value | Brain Region | Voxel | MNI | Peak T Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X (mm) | Y (mm) | Z (mm) | X (mm) | Y (mm) | Z (mm) | ||||||
| Somatosensory Cortex_left | 2461 | −2.7555 | 3.2743 | 0.9373 | 5.7204 | Caudate Putamen_left | 5837 | −2.2803 | 1.8555 | −0.0222 | 2.4598 |
| Temporal Cortex_left | 2384 | −4.0197 | 1.2495 | −7.9058 | 6.1893 | Caudate Putamen_right | 4971 | 1.9587 | −0.2203 | −0.6442 | 2.401 |
| Visual Cortex_left | 1984 | −2.2701 | 3.2313 | −5.7674 | 6.2919 | Somatosensory Cortex_right | 4437 | 2.3421 | −0.7132 | −0.1807 | 2.3139 |
| Auditory Cortex_left | 1765 | −4.0197 | 1.2495 | −7.9058 | 6.1893 | Somatosensory Cortex_left | 4243 | −2.2803 | 1.8713 | 0.2132 | 2.5247 |
| Ectorhinal Cortex_left | 1616 | −4.0107 | 3.1684 | −2.4721 | 6.1514 | Amygdala_right | 1719 | 1.7673 | 0.078 | −0.8753 | 2.112 |
| Caudate Putamen_left | 1456 | −2.5611 | 3.4787 | 0.9395 | 5.7985 | Insular Cortex_right | 1370 | 2.9181 | −1.297 | 0.5165 | 2.0951 |
| Amygdala_right | 1373 | 2.3541 | 1.3608 | −0.1547 | 5.9067 | Entorhinal Cortex_right | 1358 | 3.6177 | 2.8 | 1.5074 | 2.5537 |
| Insular Cortex_left | 1018 | −2.7549 | 3.378 | 0.9386 | 5.7204 | Ectorhinal Cortex_left | 984 | −3.6081 | 5.6384 | −7.2635 | 2.899 |
| Entorhinal Cortex_left | 741 | −3.1443 | 2.4705 | −4.9521 | 5.9972 | Piriform Cortex_right | 937 | 2.7249 | −1.3019 | 0.3992 | 2.1228 |
| Piriform Cortex_right | 736 | 2.3541 | 1.3608 | −0.1547 | 5.9067 | Temporal Cortex_left | 922 | −3.6081 | 5.6384 | −7.2635 | 2.22 |
| Hippocampus_right | 466 | 1.6809 | 1.7318 | −2.5008 | 5.5494 | Ectorhinal Cortex_right | 801 | 4.0899 | 0.9575 | 1.9538 | 1.9625 |
| Entorhinal Cortex_right | 429 | 3.4389 | 4.9995 | −2.8159 | 5.6935 | Entorhinal Cortex_left | 634 | −3.6081 | 5.6384 | −7.2635 | 2.567 |
| Thalamus_right | 422 | 1.2045 | 2.8958 | −2.2501 | 5.6191 | Insular Cortex_left | 605 | −3.1641 | −1.078 | −6.8782 | 1.9983 |
| Insular Cortex_right | 393 | 3.3039 | −1.3672 | 1.1029 | 5.2575 | Olfactory bulb_right | 548 | 0.1059 | −3.3413 | −2.7967 | 2.1406 |
| Subiculum_left | 382 | −2.3667 | 3.2249 | −5.8849 | 6.2687 | globus pallidus_right | 575 | 1.8639 | 0.0923 | −0.6401 | 2.401 |
| Dentate gyrus_right | 369 | 1.4799 | 0.4736 | −1.2226 | 5.5979 | Temporal Cortex_right | 547 | 3.8127 | 2.9027 | −1.5493 | 1.9627 |
| Visual Cortex_right | 341 | 2.1519 | −0.1996 | −0.2915 | 5.6628 | globus pallidus_left | 541 | −1.7133 | −0.6709 | −5.1118 | 2.2825 |
| Piriform Cortex_left | 324 | −3.3201 | 5.3465 | −6.9149 | 5.4311 | Primary motor cortex_left | 265 | −1.9113 | −1.5054 | −5.2395 | 2.1345 |
| Caudate Putamen_right | 302 | 3.0417 | 3.368 | 0.5748 | 5.2151 | Thalamus_left | 153 | −1.1319 | −0.3214 | −4.4029 | 1.923 |
| Somatosensory Cortex_right | 278 | 3.3039 | −1.3672 | 1.1029 | 5.2575 | Thalamus_right | 146 | 1.2873 | 0.5645 | −1.4563 | 1.8657 |
| Subiculum_right | 235 | 2.0769 | 3.5193 | −0.4799 | 5.724 | Hippocampus_right | 108 | 2.9547 | 4.7443 | −3.6414 | 2.2463 |
| Cerebellum_right | 199 | 1.7907 | 4.1223 | −0.8246 | 1.9713 | Hypothalamus_right | 58 | 1.6011 | 4.7712 | −0.4633 | 2.2093 |
| Retrosplenial cortex_right | 178 | 2.0769 | 3.5193 | −0.4799 | 5.724 | Hippocampus_left | 32 | −1.8801 | 3.887 | −5.1719 | 1.792 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, P.; Liu, R.; Li, Y.; Wang, S.; Li, T. Study on the Mechanisms of Glrα3 in Pain Sensitization of Endometriosis. Int. J. Mol. Sci. 2024, 25, 8143. https://doi.org/10.3390/ijms25158143
Fan P, Liu R, Li Y, Wang S, Li T. Study on the Mechanisms of Glrα3 in Pain Sensitization of Endometriosis. International Journal of Molecular Sciences. 2024; 25(15):8143. https://doi.org/10.3390/ijms25158143
Chicago/Turabian StyleFan, Peiya, Rong Liu, Yan Li, Shixuan Wang, and Tian Li. 2024. "Study on the Mechanisms of Glrα3 in Pain Sensitization of Endometriosis" International Journal of Molecular Sciences 25, no. 15: 8143. https://doi.org/10.3390/ijms25158143




