N-Myc and STAT Interactor is an Endometriosis Suppressor
Abstract
:1. Introduction
2. Results
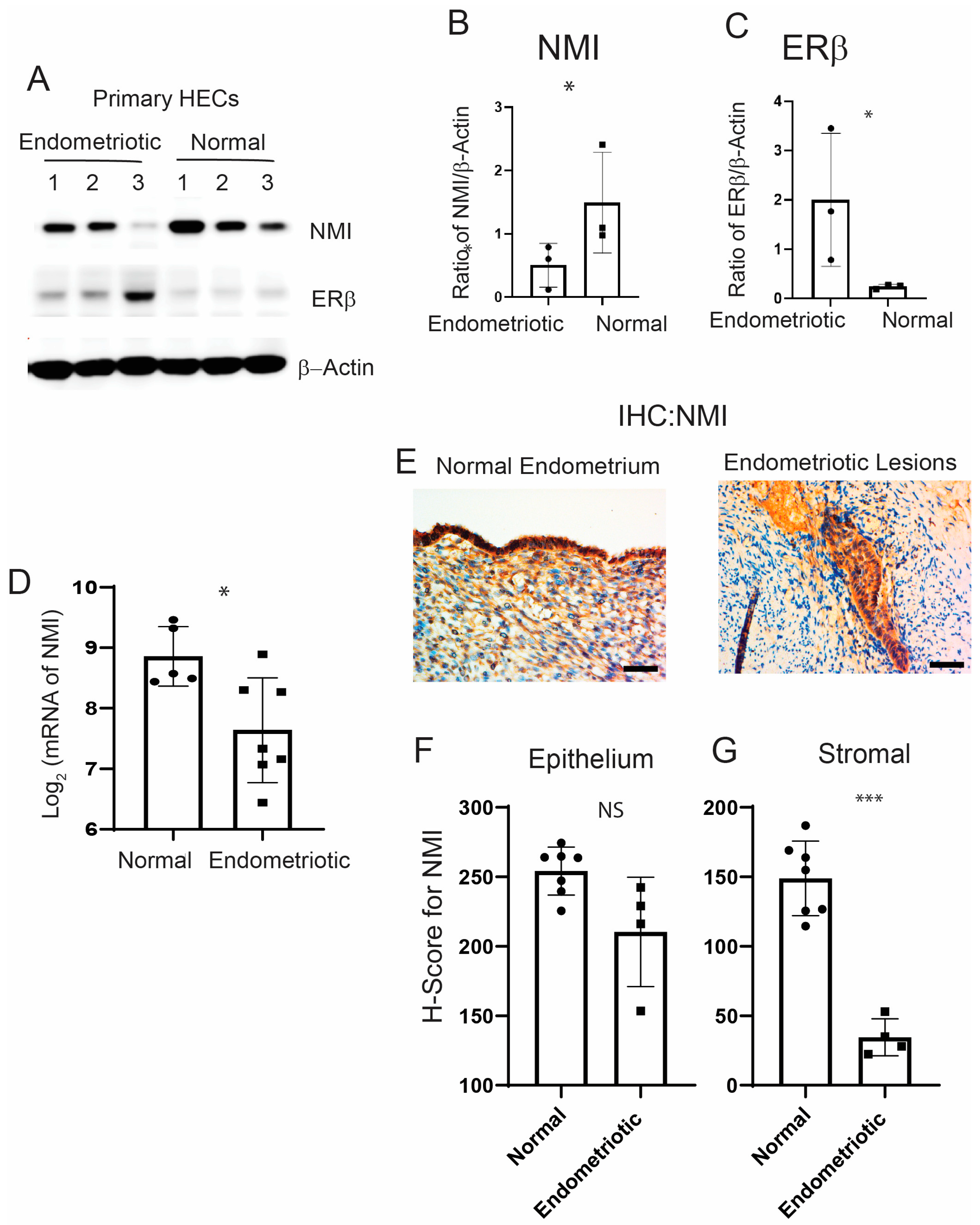
2.1. NMI Levels Are Reduced in Stromal Cells of Human Endometriotic Lesions
2.2. The ERβ/HDAC8 Axis Leads to the Reduction in NMI Expression in Endometrial Stromal Cells
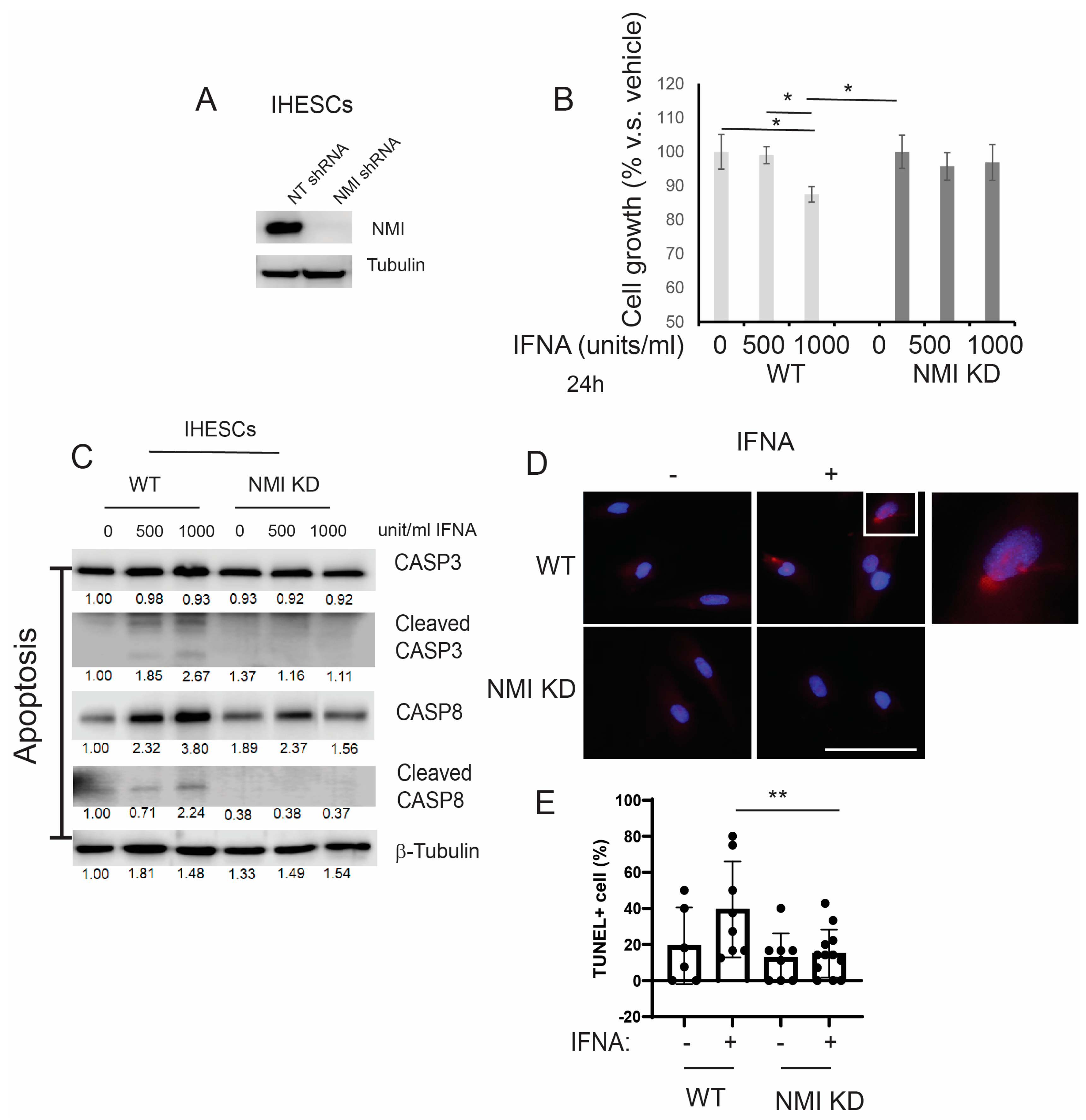
2.3. Reducing NMI Levels Induced an Anti-Apoptotic Phenotype in Human Endometrial Stromal Cells Following IFNA Treatment
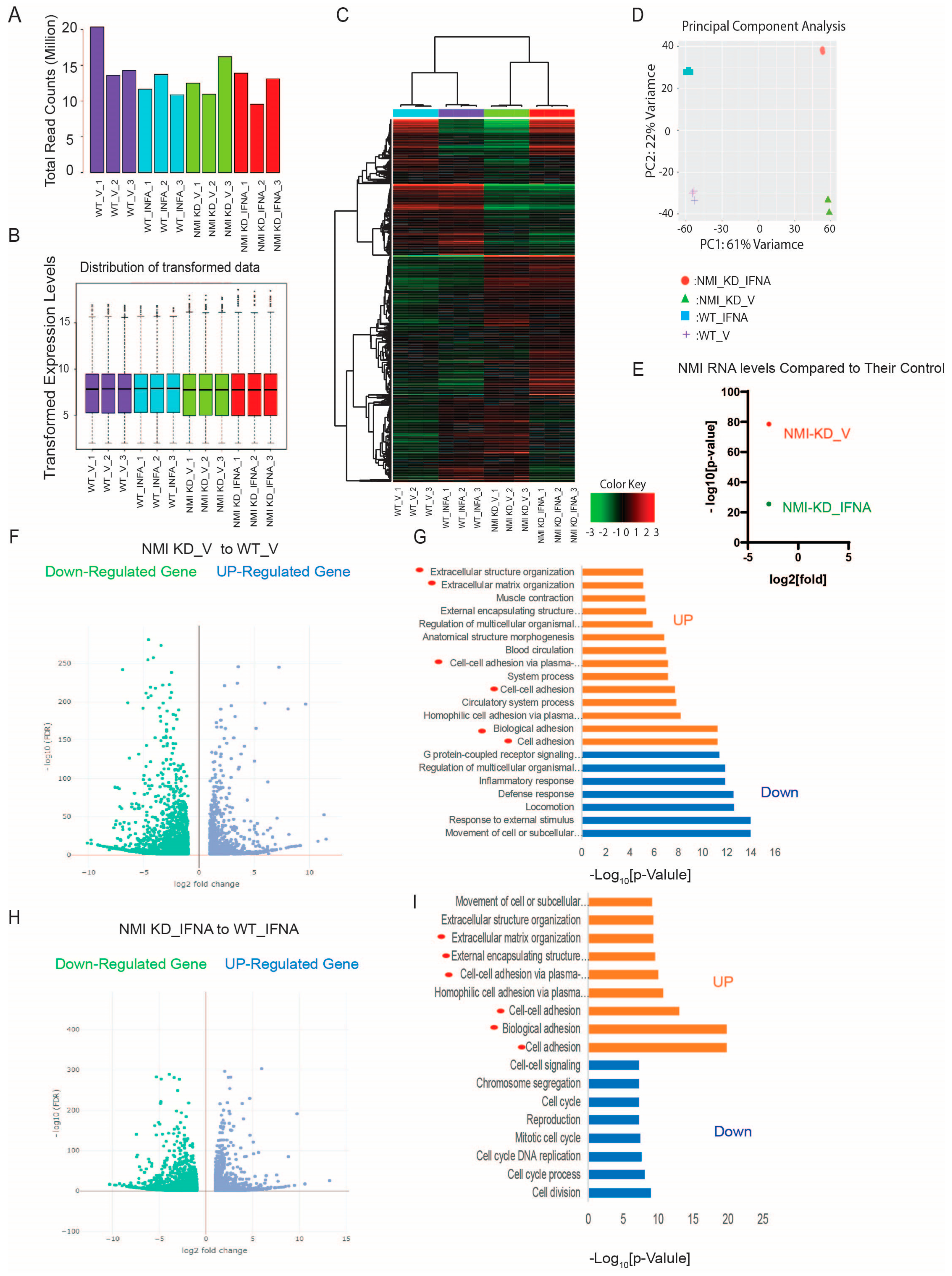
2.4. NMI KD Augmented the Expression of Genes Involved in Extracellular Matrix Signaling and Cell Adhesion in Human Endometrial Stromal Cells
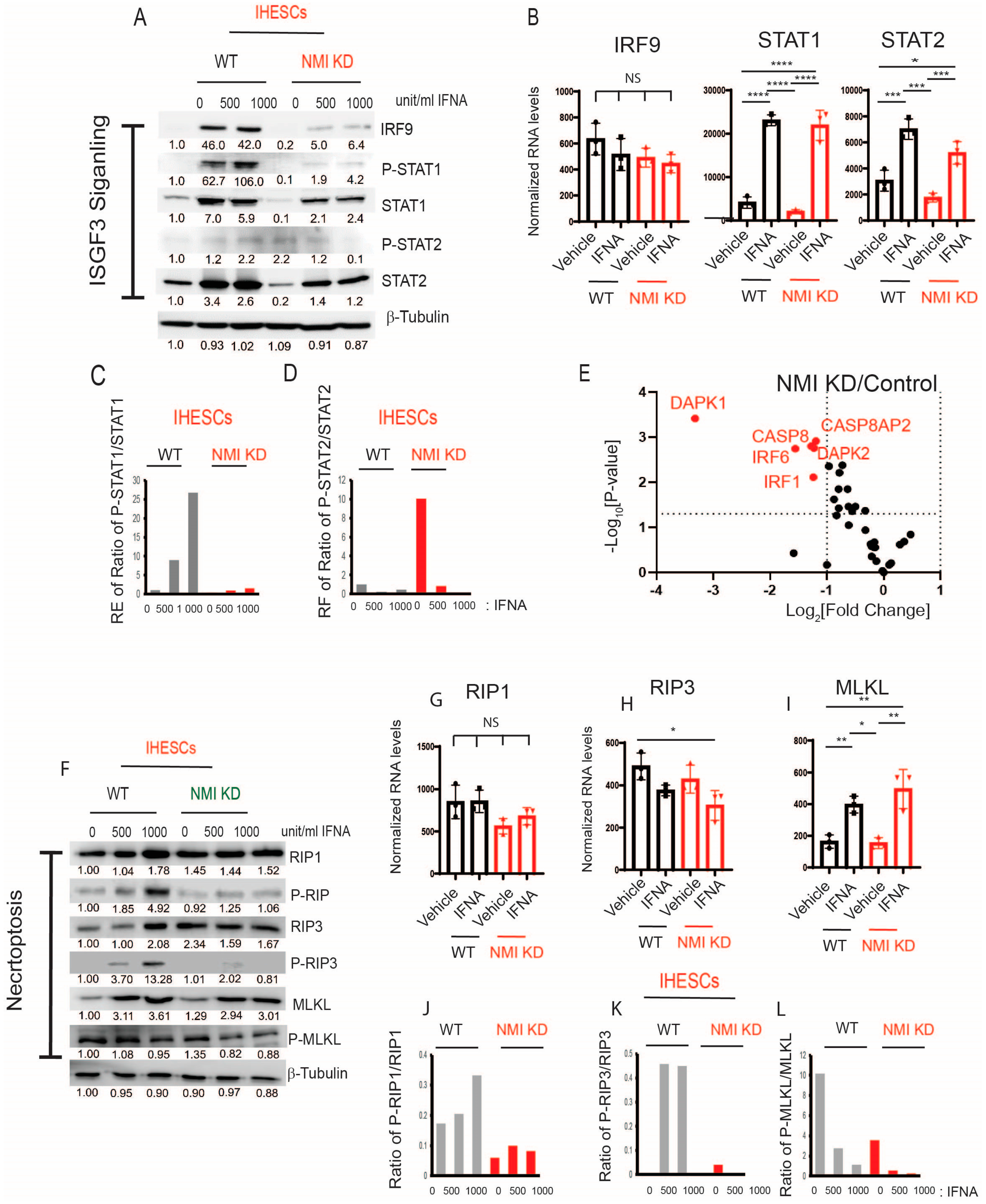
2.5. NMI KD Inhibited the Apoptosis and Necroptosis Mediated by Interferon-Stimulated Genes (ISGs) in IHESCs upon Stimulation with IFNA, Affecting the IFNA-Regulated Canonical Pathway
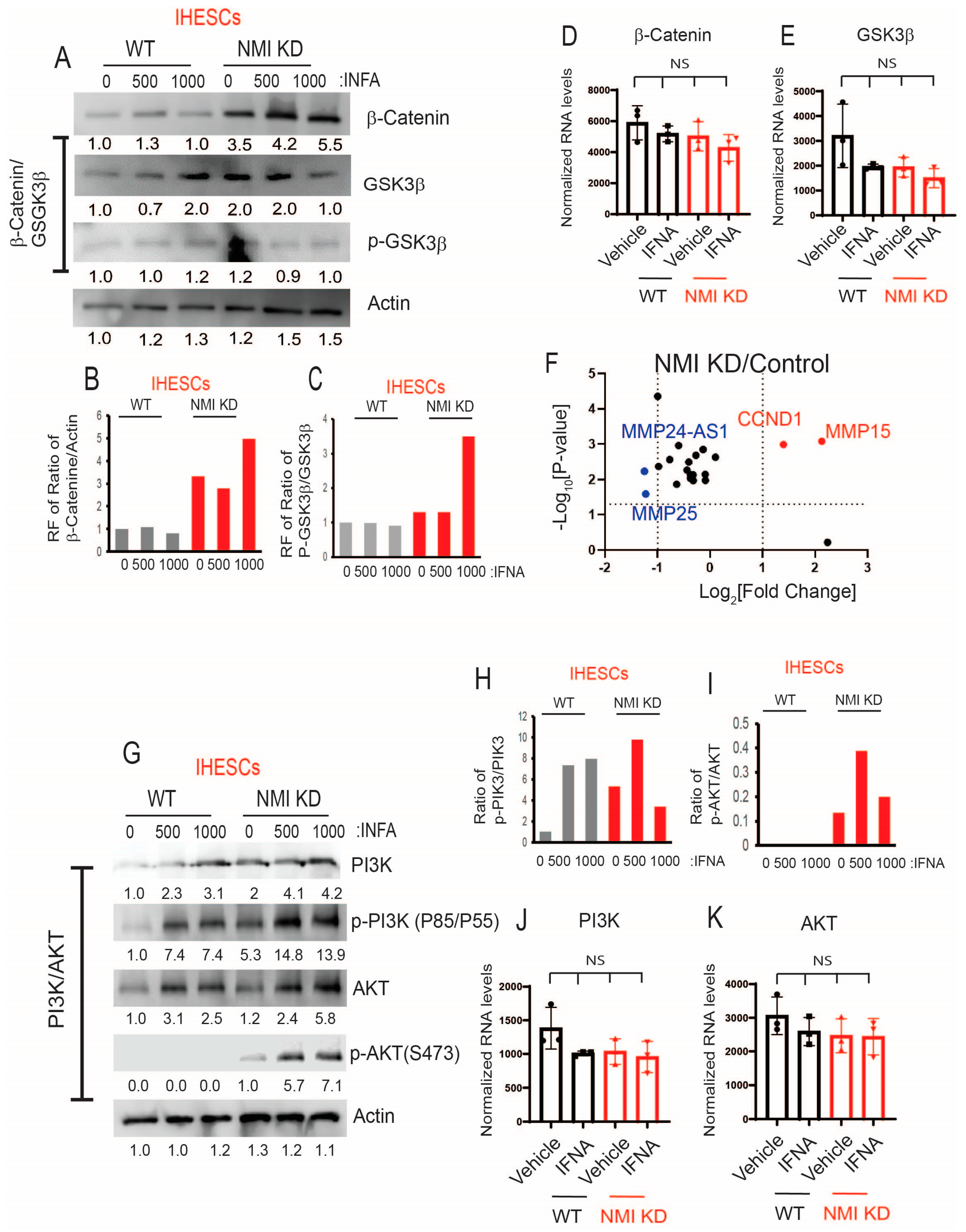
2.6. NMI Knockdown Enhanced IFNA-Induced Activation of Non-Canonical Pathways in Human Endometrial Stromal Cells
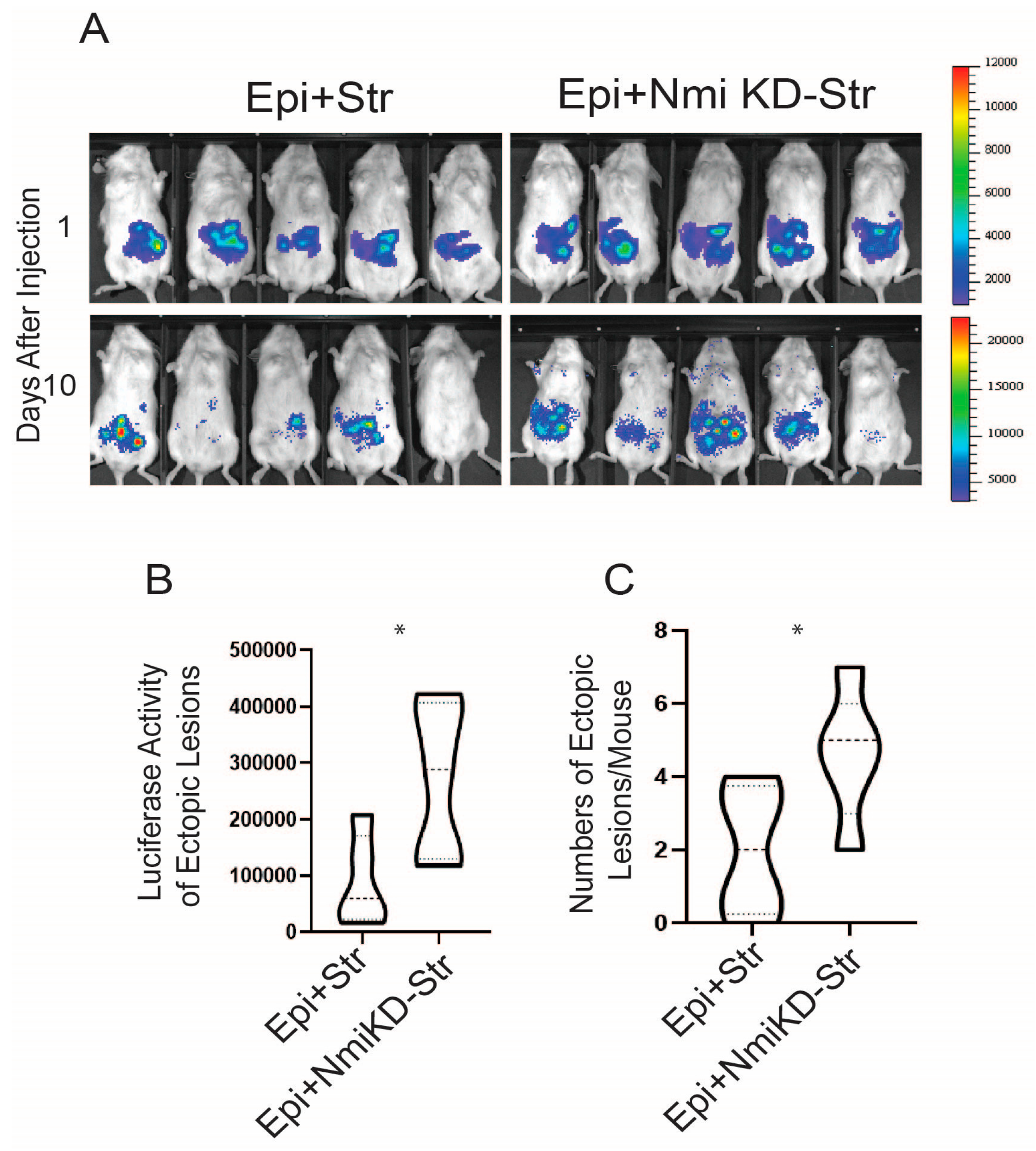
2.7. NMI KD Endometrial Stromal Cells Enhanced the Growth of Ectopic Lesions in Mice with Endometriosis
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Reagents
4.3. Cell Culture
4.4. Generation of Stable NMI Knockdown (KD) Cell Lines
4.5. HDAC8 and NCOR2 Knockdown in IHESCs
4.6. Growth Assay of IHECs NMI KD vs. Control
4.7. Terminal Deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) Assay
4.8. Reduction in NMI Levels by ERβ in HeLa Cells
4.9. Development of Human Endometriotic Lesions in NOD-SCID Female Mice
4.10. Quantifying Bioluminescence Data
4.11. Quantitative PCR (qPCR)
4.12. RNA Sequencing Analysis
4.13. Western Blotting
4.14. Formalin-Fixed Paraffin-Embedded (FFPE) for Human Endometriotic Lesions and Normal Endometrium
4.15. Immunohistochemistry of NMI in Human Endometriotic Lesions
4.16. Gene Expression Omnibus (GEO)
4.17. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| ADAM19 | ADAM metallopeptidase domain 19 |
| AKT | AKT Serine/Threonine Kinase |
| BSA | Bovine serum albumin |
| CDH10 | Cadherin 10 |
| CDH15 | Cadherin 15 |
| ChIP | Chromation Immuno Precipitation |
| COL4A1 | Collagen Type IV Alpha 1 Chain |
| COX-2 | Cyclooxygenase 2 |
| Dkk1 | Dickkopf 1 |
| DMEM | Dulbecco’s minimum essential medium |
| E2 | Estradiol-17β |
| ELFN1 | Extracellular Leucine Rich Repeat And Fibronectin Type III Domain Contain ing 1 |
| ERβ | Estrogen Receptor beta |
| FBS | Fetal bovine serum |
| FFPE | Formalin-Fixed Paraffin-Embedded |
| GSK3β | Glycogen Synthase Kinase 3 Beta |
| HAPLN3 | Hyaluronan And Proteoglycan Link Protein 3 |
| IFNa | Interferon alpha |
| IFNAR2 | Interferon Alpha And Beta Receptor Subunit 2 |
| IHC | Immunohistochemistry |
| IHEECs | Immortalized human endometrial epithelial cells |
| IHEECs | ERΒ immortalized human endometrial epithelial cells overexpressing ERβ |
| IHESCs | Immortalized human endometrial stromal cells |
| IRF9 | Interferon Regulatory Factor 9 |
| ISGF3 | IFN-stimulated gene factor 3 |
| ISRE | IFN-stimulated responsive element |
| ITGB3 | Integrin Subunit Beta 3 |
| IVIS | In Vivo Imaging System |
| JAK1 | Janus Kinase 1 |
| JUP | Junction Plakoglobin |
| KD | Knockdown |
| MFAP5 | Microfibril Associated Protein 5 |
| MLKL | Mixed lineage kinase domain-like |
| MMP | Matrix Metallopeptidase |
| MPZL2 | Myelin Protein Zero Like 2 |
| NF-kB | Nuclear Factor Kappa B |
| NMI | N-Myc and STAT Interactor |
| NMI KD-IHEECs | NMI knockdown immortalized human endometrial epithelial cells |
| NMI KD-IHESCs | NMI knockdown immortalized human endometrial stromal cells |
| NOD-SCID | Nonobese diabetic-severe combined immunodeficiency |
| NT | Non-target |
| PCDH1 | Protocadherin 1 |
| PCDHA1 | Protocadherin Alpha 1 |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphoinositide 3-kinases |
| PML | Promyelocytic leukemia |
| p-MLKL | phospho-MLKL |
| p-RIP | phospho-RIP |
| p-STAT | phospho-STAT |
| RIP | Receptor-Interacting Serine/Threonine-Protein |
| SMAD | SMAD Family Member |
| SORBS1 | Sorbin And SH3 Domain Containing 1 |
| SPARC | Secreted Protein Acidic And Cysteine Rich |
| SPOCK1 | SPARC (Osteonectin), Cwcv And Kazal Like Domains Proteoglycan 1 |
| STAT | Signal Transducer And Activator Of Transcription |
| TBST | Tris-buffered saline |
| TGF-b | Transforming Growth Factor Beta 1 |
| VCAN | Versican |
| WT | Wild Type |
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef]
- Lyons, S.D.; Chew, S.S.; Thomson, A.J.; Lenart, M.; Camaris, C.; Vancaillie, T.G.; Abbott, J.A. Clinical and quality-of-life outcomes after fertility-sparing laparoscopic surgery with bowel resection for severe endometriosis. J. Minim. Invasive Gynecol. 2006, 13, 436–441. [Google Scholar] [CrossRef]
- Zakhari, A.; Delpero, E.; McKeown, S.; Tomlinson, G.; Bougie, O.; Murji, A. Endometriosis recurrence following post-operative hormonal suppression: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 96–107. [Google Scholar] [CrossRef]
- Tosti, C.; Biscione, A.; Morgante, G.; Bifulco, G.; Luisi, S.; Petraglia, F. Hormonal therapy for endometriosis: From molecular research to bedside. Eur. J. Obs. Gynecol. Reprod. Biol. 2017, 209, 61–66. [Google Scholar] [CrossRef]
- Sourial, S.; Tempest, N.; Hapangama, D.K. Theories on the pathogenesis of endometriosis. Int. J. Reprod. Med. 2014, 2014, 179515. [Google Scholar] [CrossRef]
- Sampson, J.A. Heterotopic or misplaced endometrial tissue. Am. J. Obstet. Gynecol. 1925, 10, 649–664. [Google Scholar] [CrossRef]
- Halme, J.; Hammond, M.G.; Hulka, J.F.; Raj, S.G.; Talbert, L.M. Retrograde menstruation in healthy women and in patients with endometriosis. Obs. Gynecol. 1984, 64, 151–154. [Google Scholar]
- Bulun, S.E.; Cheng, Y.H.; Pavone, M.E.; Xue, Q.; Attar, E.; Trukhacheva, E.; Tokunaga, H.; Utsunomiya, H.; Yin, P.; Luo, X.; et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin. Reprod. Med. 2010, 28, 36–43. [Google Scholar] [CrossRef]
- Bulun, S.E. Endometriosis. N. Engl. J. Med. 2009, 360, 268–279. [Google Scholar] [CrossRef]
- Monnin, N.; Fattet, A.J.; Koscinski, I. Endometriosis: Update of Pathophysiology, (Epi) Genetic and Environmental Involvement. Biomedicines 2023, 11, 978. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Lin, Z.; Cheng, Y.H.; Huang, C.C.; Marsh, E.; Yin, P.; Milad, M.P.; Confino, E.; Reierstad, S.; Innes, J.; et al. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol. Reprod. 2007, 77, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Monsivais, D.; Dyson, M.T.; Yin, P.; Coon, J.S.; Navarro, A.; Feng, G.; Malpani, S.S.; Ono, M.; Ercan, C.M.; Wei, J.J. vERbeta- and prostaglandin E2-regulated pathways integrate cell proliferation via Ras-like and estrogen-regulated growth inhibitor in endometriosis. Mol. Endocrinol. 2014, 28, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Jung, S.Y.; Wu, S.P.; Hawkins, S.M.; Park, M.J.; Kyo, S.; Qin, J.; Lydon, J.P.; Tsai, S.Y.; Tsai, M.J.; et al. Estrogen Receptor beta Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell 2015, 163, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Lee, J.E.; Cho, Y.J.; Park, M.J.; O’Malley, B.W. Genomic Function of Estrogen Receptor beta in Endometriosis. Endocrinology 2019, 160, 2495–2516. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Han, S.J. Interferon Signaling in the Endometrium and in Endometriosis. Biomolecules 2022, 12, 1554. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yao, X.; Fu, Y.; Wang, Y. Interferon-alpha and its effects on cancer cell apoptosis. Oncol. Lett. 2022, 24, 235. [Google Scholar] [CrossRef]
- Shi, W.Y.; Cao, C.; Liu, L. Interferon alpha Induces the Apoptosis of Cervical Cancer HeLa Cells by Activating both the Intrinsic Mitochondrial Pathway and Endoplasmic Reticulum Stress-Induced Pathway. Int. J. Mol. Sci. 2016, 17, 1832. [Google Scholar] [CrossRef] [PubMed]
- Herzer, K.; Hofmann, T.G.; Teufel, A.; Schimanski, C.C.; Moehler, M.; Kanzler, S.; Schulze-Bergkamen, H.; Galle, P.R. IFN-alpha-induced apoptosis in hepatocellular carcinoma involves promyelocytic leukemia protein and TRAIL independently of p53. Cancer Res. 2009, 69, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Mazewski, C.; Perez, R.E.; Fish, E.N.; Platanias, L.C. Type I Interferon (IFN)-Regulated Activation of Canonical and Non-Canonical Signaling Pathways. Front. Immunol. 2020, 11, 606456. [Google Scholar] [CrossRef]
- Pia, M.M.; Anna, L.V.; Ulla, B.K. Virus Infection and Type I Interferon in Endometriosis. In Endometriosis; Koel, C., Baidyanath, C., Eds.; IntechOpen: Rijeka, Croatia, 2012; p. Ch. 12. [Google Scholar]
- Kao, L.C.; Germeyer, A.; Tulac, S.; Lobo, S.; Yang, J.P.; Taylor, R.N.; Osteen, K.; Lessey, B.A.; Giudice, L.C. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology 2003, 144, 2870–2881. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Canis, M.; Pouly, J.L.; Botchorishvili, R.; Déchelotte, P.J.; Mage, G. Differential expression of genes in eutopic and ectopic endometrium from patients with ovarian endometriosis. Fertil. Steril. 2006, 86, 548–553. [Google Scholar] [CrossRef]
- Lebrun, S.J.; Shpall, R.L.; Naumovski, L. Interferon-induced upregulation and cytoplasmic localization of Myc-interacting protein Nmi. J. Interferon Cytokine Res. 1998, 18, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chai, K.; Chen, Y.; Lin, Y.; Zhang, S.; Li, X.; Qiao, W.; Tan, J. Interferon activates promoter of Nmi gene via interferon regulator factor-1. Mol. Cell Biochem. 2018, 441, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; John, S.; Berg, M.; Leonard, W.J. Functional association of Nmi with Stat5 and Stat1 in IL-2- and IFNgamma-mediated signaling. Cell 1999, 96, 121–130. [Google Scholar] [CrossRef]
- Fillmore, R.A.; Mitra, A.; Xi, Y.; Ju, J.; Scammell, J.; Shevde, L.A.; Samant, R.S. Nmi (N-Myc interactor) inhibits Wnt/beta-catenin signaling and retards tumor growth. Int. J. Cancer 2009, 125, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zou, K.; Feng, X.; Chen, M.; Li, C.; Tang, R.; Xuan, Y.; Luo, M.; Chen, W.; Qiu, H.; et al. Downregulation of NMI promotes tumor growth and predicts poor prognosis in human lung adenocarcinomas. Mol. Cancer 2017, 16, 158. [Google Scholar] [CrossRef]
- Meng, D.; Chen, Y.; Yun, D.; Zhao, Y.; Wang, J.; Xu, T.; Li, X.; Wang, Y.; Yuan, L.; Sun, R.; et al. High expression of N-myc (and STAT) interactor predicts poor prognosis and promotes tumor growth in human glioblastoma. Oncotarget 2015, 6, 4901–4919. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, K.; Li, X.; Sung, N.; Cho, Y.J.; Han, S.J.; Safe, S. Bis-Indole-Derived Nuclear Receptor 4A1 (NR4A1, Nur77) Ligands as Inhibitors of Endometriosis. Endocrinology 2020, 161, bqaa027. [Google Scholar] [CrossRef]
- Klinge, C.M.; Jernigan, S.C.; Mattingly, K.A.; Risinger, K.E.; Zhang, J. Estrogen response element-dependent regulation of transcriptional activation of estrogen receptors alpha and beta by coactivators and corepressors. J. Mol. Endocrinol. 2004, 33, 387–410. [Google Scholar] [CrossRef]
- Duong, V.; Licznar, A.; Margueron, R.; Boulle, N.; Busson, M.; Lacroix, M.; Katzenellenbogen, B.S.; Cavaillès, V.; Lazennec, G. ERα and ERβ expression and transcriptional activity are differentially regulated by HDAC inhibitors. Oncogene 2006, 25, 1799–1806. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, X.; Guo, S.W. Aberrant expression of histone deacetylase 8 in endometriosis and its potential as a therapeutic target. Reprod. Med. Biol. 2023, 22, e12531. [Google Scholar] [CrossRef]
- Sugihara, K.; Kobayashi, Y.; Suzuki, A.; Tamura, N.; Motamedchaboki, K.; Huang, C.T.; Akama, T.O.; Pecotte, J.; Frost, P.; Bauer, C.; et al. Development of pro-apoptotic peptides as potential therapy for peritoneal endometriosis. Nat. Commun. 2014, 5, 4478. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.I.; Hamza, A.M.; Eldabah, N.; Habiba, D.A. IFN-α and TNF-α serum levels and their association with disease severity in Egyptian children and adults with alopecia areata. Int. J. Dermatol. 2021, 60, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, J.; Yang, Y.; Liu, L.; Zhu, G.; Wang, X.; Wang, S.; Guo, W.; Yue, Q.; Zhao, T.; et al. Impact of Interferon-alpha1b (IFN-α1b) on Antitumor Immune Response: An Interpretation of the Promising Therapeutic Effect of IFN-alpha1b on Melanoma. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e922790. [Google Scholar] [CrossRef] [PubMed]
- Punt, C.J.; Eggermont, A.M. Adjuvant interferon-alpha for melanoma revisited: News from old and new studies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2001, 12, 1663–1666. [Google Scholar] [CrossRef]
- Sarhan, J.; Liu, B.C.; Muendlein, H.I.; Weindel, C.G.; Smirnova, I.; Tang, A.Y.; Ilyukha, V.; Sorokin, M.; Buzdin, A.; Fitzgerald, K.A.; et al. Constitutive interferon signaling maintains critical threshold of MLKL expression to license necroptosis. Cell Death Differ. 2019, 26, 332–347. [Google Scholar] [CrossRef] [PubMed]
- McComb, S.; Cessford, E.; Alturki, N.A.; Joseph, J.; Shutinoski, B.; Startek, J.B.; Gamero, A.M.; Mossman, K.L.; Sad, S. Type-I interferon signaling through ISGF3 complex is required for sustained Rip3 activation and necroptosis in macrophages. Proc. Natl. Acad. Sci. USA 2014, 111, E3206–E3213. [Google Scholar] [PubMed]
- Chawla-Sarkar, M.; Lindner, D.J.; Liu, Y.F.; Williams, B.R.; Sen, G.C.; Silverman, R.H.; Borden, E.C. Apoptosis and interferons: Role of interferon-stimulated genes as mediators of apoptosis. Apoptosis 2003, 8, 237–249. [Google Scholar] [CrossRef]
- Vanden Berghe, T.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef]
- Li, W.X. Canonical and non-canonical JAK-STAT signaling. Trends Cell Biol. 2008, 18, 545–551. [Google Scholar] [CrossRef]
- Marineau, A.; Khan, K.A.; Servant, M.J. Roles of GSK-3 and beta-Catenin in Antiviral Innate Immune Sensing of Nucleic Acids. Cells 2020, 9, 897. [Google Scholar] [CrossRef]
- Madanes, D.; Bilotas, M.A.; Bastón, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I.; Ricci, A.G. PI3K/AKT pathway is altered in the endometriosis patient’s endometrium and presents differences according to severity stage. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2020, 36, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Pazhohan, A.; Amidi, F.; Akbari-Asbagh, F.; Seyedrezazadeh, E.; Farzadi, L.; Khodarahmin, M.; Mehdinejadiani, S.; Sobhani, A. The Wnt/β-catenin signaling in endometriosis, the expression of total and active forms of β-catenin, total and inactive forms of glycogen synthase kinase-3β, WNT7a and DICKKOPF-1. Eur. J. Obs. Gynecol. Reprod. Biol. 2018, 220, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Santulli, P.; Marcellin, L.; Tosti, C.; Chouzenoux, S.; Cerles, O.; Borghese, B.; Batteux, F.; Chapron, C. MAP kinases and the inflammatory signaling cascade as targets for the treatment of endometriosis? Expert. Opin. Ther. Targets 2015, 19, 1465–1483. [Google Scholar] [CrossRef]
- Alam, S.; Zunic, A.; Venkat, S.; Feigin, M.E.; Atanassov, B.S. Regulation of Cyclin D1 Degradation by Ubiquitin-Specific Protease 27X Is Critical for Cancer Cell Proliferation and Tumor Growth. Mol. Cancer Res. MCR 2022, 20, 1751–1762. [Google Scholar] [CrossRef]
- Majali-Martinez, A.; Hoch, D.; Tam-Amersdorfer, C.; Pollheimer, J.; Glasner, A.; Ghaffari-Tabrizi-Wizsy, N.; Beristain, A.G.; Hiden, U.; Dieber-Rotheneder, M.; Desoye, G. Matrix metalloproteinase 15 plays a pivotal role in human first trimester cytotrophoblast invasion and is not altered by maternal obesity. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 10720–10730. [Google Scholar] [CrossRef]
- Suen, J.L.; Chang, Y.; Shiu, Y.S.; Hsu, C.Y.; Sharma, P.; Chiu, C.C.; Chen, Y.J.; Hour, T.C.; Tsai, E.M. IL-10 from plasmacytoid dendritic cells promotes angiogenesis in the early stage of endometriosis. J. Pathol. 2019, 249, 485–497. [Google Scholar] [CrossRef]
- Ali, S.; Mann-Nüttel, R.; Schulze, A.; Richter, L.; Alferink, J.; Scheu, S. Sources of Type I Interferons in Infectious Immunity: Plasmacytoid Dendritic Cells Not Always in the Driver’s Seat. Front. Immunol. 2019, 10, 778. [Google Scholar] [CrossRef]
- Newby, B.N.; Brusko, T.M.; Zou, B.; Atkinson, M.A.; Clare-Salzler, M.; Mathews, C.E. Type 1 Interferons Potentiate Human CD8(+) T-Cell Cytotoxicity Through a STAT4- and Granzyme B-Dependent Pathway. Diabetes 2017, 66, 3061–3071. [Google Scholar] [CrossRef]
- Ito, M.; Hiramatsu, H.; Kobayashi, K.; Suzue, K.; Kawahata, M.; Hioki, K.; Ueyama, Y.; Koyanagi, Y.; Sugamura, K.; Tsuji, K.; et al. NOD/SCID/gamma(c)(null) mouse: An excellent recipient mouse model for engraftment of human cells. Blood 2002, 100, 3175–3182. [Google Scholar] [CrossRef]
- Spranger, S.; Frankenberger, B.; Schendel, D.J. NOD/scid IL-2Rg(null) mice: A preclinical model system to evaluate human dendritic cell-based vaccine strategies in vivo. J. Transl. Med. 2012, 10, 30. [Google Scholar] [CrossRef]
- Sun, F.; Yang, C.L.; Wang, F.X.; Rong, S.J.; Luo, J.H.; Lu, W.Y.; Yue, T.T.; Wang, C.Y.; Liu, S.W. Pancreatic draining lymph nodes (PLNs) serve as a pathogenic hub contributing to the development of type 1 diabetes. Cell Biosci. 2023, 13, 156. [Google Scholar] [CrossRef]
- Li, Q.; Xu, B.; Michie, S.A.; Rubins, K.H.; Schreriber, R.D.; McDevitt, H.O. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc. Natl. Acad. Sci. USA 2008, 105, 12439–12444. [Google Scholar] [CrossRef]
- Acien, P.; Quereda, F.; Campos, A.; Gomez-Torres, M.J.; Velasco, I.; Gutierrez, M. Use of intraperitoneal interferon alpha-2b therapy after conservative surgery for endometriosis and postoperative medical treatment with depot gonadotropin-releasing hormone analog: A randomized clinical trial. Fertil. Steril. 2002, 78, 705–711. [Google Scholar] [CrossRef]
- Dicitore, A.; Castiglioni, S.; Saronni, D.; Gentilini, D.; Borghi, M.O.; Stabile, S.; Vignali, M.; Di Blasio, A.M.; Persani, L.; Vitale, G. Effects of human recombinant type I IFNs (IFN-alpha2b and IFN-beta1a) on growth and migration of primary endometrial stromal cells from women with deeply infiltrating endometriosis: A preliminary study. Eur. J. Obs. Gynecol. Reprod. Biol. 2018, 230, 192–198. [Google Scholar] [CrossRef]
- Ingelmo, J.M.; Quereda, F.; Acien, P. Intraperitoneal and subcutaneous treatment of experimental endometriosis with recombinant human interferon-alpha-2b in a murine model. Fertil. Steril. 1999, 71, 907–911. [Google Scholar] [CrossRef]
- Pruitt, H.C.; Devine, D.J.; Samant, R.S. Roles of N-Myc and STAT interactor in cancer: From initiation to dissemination. Int. J. Cancer 2016, 139, 491–500. [Google Scholar] [CrossRef]
- He, T.; Qiao, Y.; Yang, Q.; Chen, J.; Chen, Y.; Chen, X.; Hao, Z.; Lin, M.; Shao, Z.; Wu, P.; et al. NMI: A potential biomarker for tumor prognosis and immunotherapy. Front. Pharmacol. 2022, 13, 1047463. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone deacetylases and mechanisms of regulation of gene expression. Crit. Rev. Oncog. 2015, 20, 35–47. [Google Scholar] [CrossRef]
- Wu, Y.; Starzinski-Powitz, A.; Guo, S.W. Trichostatin A, a histone deacetylase inhibitor, attenuates invasiveness and reactivates E-cadherin expression in immortalized endometriotic cells. Reprod. Sci. 2007, 14, 374–382. [Google Scholar] [CrossRef]
- Lu, Y.; Nie, J.; Liu, X.; Zheng, Y.; Guo, S.W. Trichostatin A, a histone deacetylase inhibitor, reduces lesion growth and hyperalgesia in experimentally induced endometriosis in mice. Hum. Reprod. 2010, 25, 1014–1025. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, X.; Guo, S.W. Corroborating evidence for aberrant expression of histone deacetylase 8 in endometriosis. Reprod. Med. Biol. 2023, 22, e12527. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Ramos, J.; Luo, W.; Sirisawad, M.; Verner, E.; Buggy, J.J. A novel histone deacetylase 8 (HDAC8)-specific inhibitor PCI-34051 induces apoptosis in T-cell lymphomas. Leukemia 2008, 22, 1026–1034. [Google Scholar] [CrossRef]
- Yang, W.; Feng, Y.; Zhou, J.; Cheung, O.K.; Cao, J.; Wang, J.; Tang, W.; Tu, Y.; Xu, L.; Wu, F.; et al. A selective HDAC8 inhibitor potentiates antitumor immunity and efficacy of immune checkpoint blockade in hepatocellular carcinoma. Sci. Transl. Med. 2021, 13, eaaz6804. [Google Scholar] [CrossRef]
- Park, Y.; Cho, Y.J.; Sung, N.; Park, M.J.; Guan, X.; Gibbons, W.E.; O’Malley, B.W.; Han, S.J. Oleuropein suppresses endometriosis progression and improves the fertility of mice with endometriosis. J. Biomed. Sci. 2022, 29, 100. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.N.; Wang, K.L.; Li, N.; Pang, A.N.; Liu, L.H.; Li, B.; Hou, J.; Wang, S.; Nie, P. Interaction of Nmi and IFP35 Promotes Mutual Protein Stabilization and IRF3 and IRF7 Degradation to Suppress Type I IFN Production in Teleost Fish. J. Immunol. 2023, 210, 1494–1507. [Google Scholar] [CrossRef]
- Wu, G.; Huang, H.; Garcia Abreu, J.; He, X. Inhibition of GSK3 phosphorylation of beta-catenin via phosphorylated PPPSPXS motifs of Wnt coreceptor LRP6. PLoS ONE 2009, 4, e4926. [Google Scholar] [CrossRef]
- Community, T.G. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2022 update. Nucleic Acids Res. 2022, 50, W345–W351. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Guan, X.; Han, S.J. N-Myc and STAT Interactor is an Endometriosis Suppressor. Int. J. Mol. Sci. 2024, 25, 8145. https://doi.org/10.3390/ijms25158145
Park Y, Guan X, Han SJ. N-Myc and STAT Interactor is an Endometriosis Suppressor. International Journal of Molecular Sciences. 2024; 25(15):8145. https://doi.org/10.3390/ijms25158145
Chicago/Turabian StylePark, Yuri, Xiaoming Guan, and Sang Jun Han. 2024. "N-Myc and STAT Interactor is an Endometriosis Suppressor" International Journal of Molecular Sciences 25, no. 15: 8145. https://doi.org/10.3390/ijms25158145





