The Many Faces of Hypusinated eIF5A: Cell Context-Specific Effects of the Hypusine Circuit and Implications for Human Health
Abstract
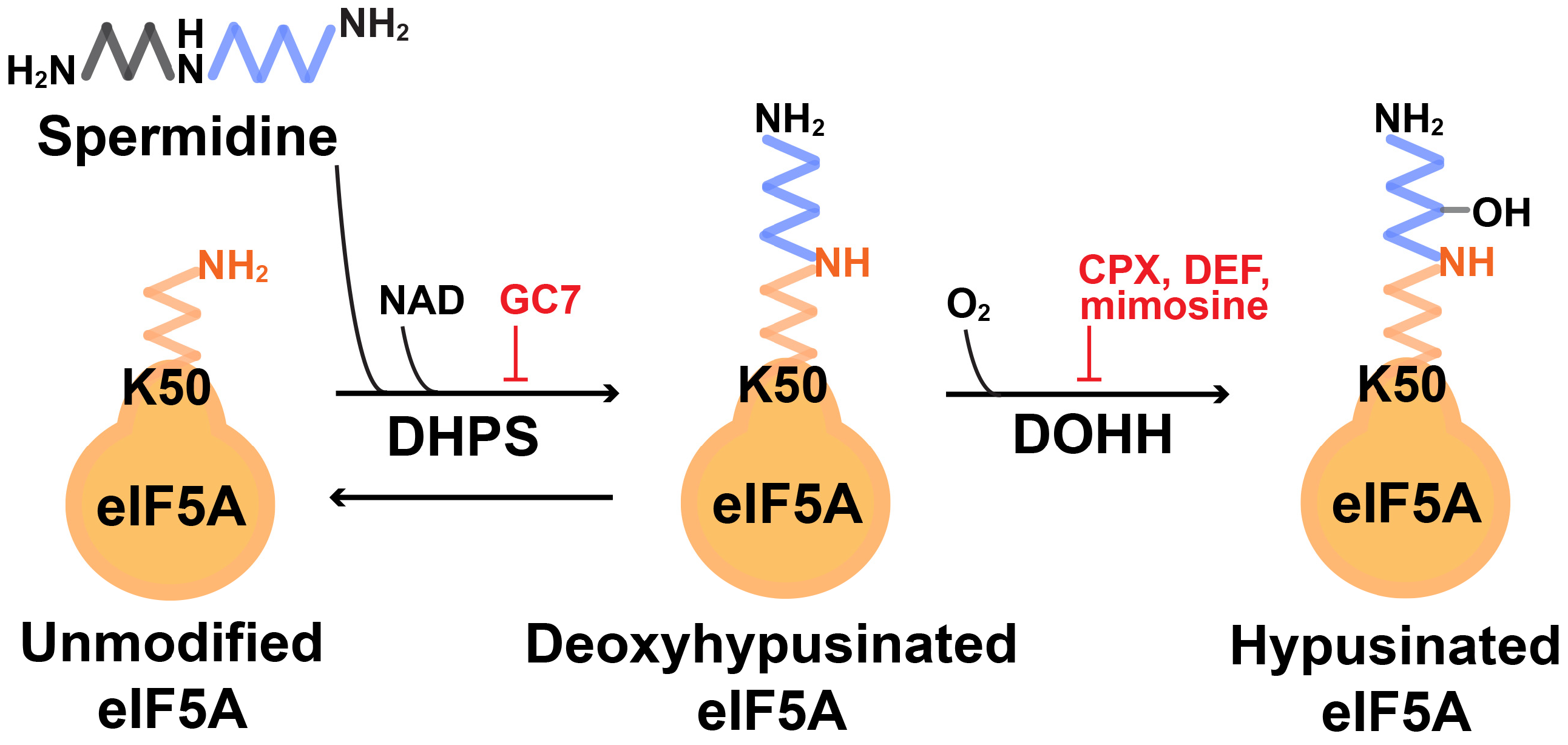
:1. Introduction
2. Tissue/Cell Specific Roles of eIF5AHyp
2.1. Gastrointestinal Tissues
2.1.1. Intestinal Epithelium Cells (IECs)
2.1.2. Pancreas and β Cells
2.1.3. Pancreatic Ductal Adenocarcinoma (PDAC) Pathogenesis
2.2. Breast Cancer
2.3. Immune Cells
2.3.1. B Cells and B Cell Aging
2.3.2. B Cell Malignancies
2.3.3. T Cells
2.3.4. Macrophages
2.3.5. Hematopoietic Stem and Progenitor Cells (HSPCs)
2.4. Other Noteworthy Topics
2.4.1. Roles of DOHH
2.4.2. Free Pools of Hypusine
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, M.H.; Cooper, H.L.; Folk, J.E. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc. Natl. Acad. Sci. USA 1981, 78, 2869–2873. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Wolff, E.C. Hypusine, a polyamine-derived amino acid critical for eukaryotic translation. J. Biol. Chem. 2018, 293, 18710–18718. [Google Scholar] [CrossRef] [PubMed]
- Clement, P.M.; Henderson, C.A.; Jenkins, Z.A.; Smit-McBride, Z.; Wolff, E.C.; Hershey, J.W.; Park, M.H.; Johansson, H.E. Identification and characterization of eukaryotic initiation factor 5A-2. Eur. J. Biochem. 2003, 270, 4254–4263. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, Z.A.; Haag, P.G.; Johansson, H.E. Human eIF5A2 on chromosome 3q25-q27 is a phylogenetically conserved vertebrate variant of eukaryotic translation initiation factor 5A with tissue-specific expression. Genomics 2001, 71, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Benne, R.; Brown-Luedi, M.L.; Hershey, J.W. Purification and characterization of protein synthesis initiation factors eIF-1, eIF-4C, eIF-4D, and eIF-5 from rabbit reticulocytes. J. Biol. Chem. 1978, 253, 3070–3077. [Google Scholar] [CrossRef] [PubMed]
- Schuller, A.P.; Wu, C.C.; Dever, T.E.; Buskirk, A.R.; Green, R. eIF5A Functions Globally in Translation Elongation and Termination. Mol. Cell 2017, 66, 194–205.e5. [Google Scholar] [CrossRef]
- Saini, P.; Eyler, D.E.; Green, R.; Dever, T.E. Hypusine-containing protein eIF5A promotes translation elongation. Nature 2009, 459, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Doerfel, L.K.; Wohlgemuth, I.; Kothe, C.; Peske, F.; Urlaub, H.; Rodnina, M.V. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science 2013, 339, 85–88. [Google Scholar] [CrossRef]
- Gutierrez, E.; Shin, B.S.; Woolstenhulme, C.J.; Kim, J.R.; Saini, P.; Buskirk, A.R.; Dever, T.E. eIF5A promotes translation of polyproline motifs. Mol. Cell 2013, 51, 35–45. [Google Scholar] [CrossRef]
- Ude, S.; Lassak, J.; Starosta, A.L.; Kraxenberger, T.; Wilson, D.N.; Jung, K. Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science 2013, 339, 82–85. [Google Scholar] [CrossRef]
- Huter, P.; Arenz, S.; Bock, L.V.; Graf, M.; Frister, J.O.; Heuer, A.; Peil, L.; Starosta, A.L.; Wohlgemuth, I.; Peske, F.; et al. Structural Basis for Polyproline-Mediated Ribosome Stalling and Rescue by the Translation Elongation Factor EF-P. Mol. Cell 2017, 68, 515–527.e6. [Google Scholar] [CrossRef]
- Pelechano, V.; Alepuz, P. eIF5A facilitates translation termination globally and promotes the elongation of many non polyproline-specific tripeptide sequences. Nucleic Acids Res. 2017, 45, 7326–7338. [Google Scholar] [CrossRef] [PubMed]
- Coni, S.; Serrao, S.M.; Yurtsever, Z.N.; Di Magno, L.; Bordone, R.; Bertani, C.; Licursi, V.; Ianniello, Z.; Infante, P.; Moretti, M.; et al. Blockade of EIF5A hypusination limits colorectal cancer growth by inhibiting MYC elongation. Cell Death Dis. 2020, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Lee, S.B.; Park, J.H.; Park, M.H. Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids 2012, 42, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Sievert, H.; Pallmann, N.; Miller, K.K.; Hermans-Borgmeyer, I.; Venz, S.; Sendoel, A.; Preukschas, M.; Schweizer, M.; Boettcher, S.; Janiesch, P.C.; et al. A novel mouse model for inhibition of DOHH-mediated hypusine modification reveals a crucial function in embryonic development, proliferation and oncogenic transformation. Dis. Models Mech. 2014, 7, 963–976. [Google Scholar] [CrossRef] [PubMed]
- Pallmann, N.; Braig, M.; Sievert, H.; Preukschas, M.; Hermans-Borgmeyer, I.; Schweizer, M.; Nagel, C.H.; Neumann, M.; Wild, P.; Haralambieva, E.; et al. Biological Relevance and Therapeutic Potential of the Hypusine Modification System. J. Biol. Chem. 2015, 290, 18343–18360. [Google Scholar] [CrossRef]
- Faundes, V.; Jennings, M.D.; Crilly, S.; Legraie, S.; Withers, S.E.; Cuvertino, S.; Davies, S.J.; Douglas, A.G.L.; Fry, A.E.; Harrison, V.; et al. Impaired eIF5A function causes a Mendelian disorder that is partially rescued in model systems by spermidine. Nat. Commun. 2021, 12, 833. [Google Scholar] [CrossRef]
- Ganapathi, M.; Padgett, L.R.; Yamada, K.; Devinsky, O.; Willaert, R.; Person, R.; Au, P.B.; Tagoe, J.; McDonald, M.; Karlowicz, D.; et al. Recessive Rare Variants in Deoxyhypusine Synthase, an Enzyme Involved in the Synthesis of Hypusine, Are Associated with a Neurodevelopmental Disorder. Am. J. Hum. Genet. 2019, 104, 287–298. [Google Scholar] [CrossRef]
- Ziegler, A.; Steindl, K.; Hanner, A.S.; Kar, R.K.; Prouteau, C.; Boland, A.; Deleuze, J.F.; Coubes, C.; Bezieau, S.; Kury, S.; et al. Bi-allelic variants in DOHH, catalyzing the last step of hypusine biosynthesis, are associated with a neurodevelopmental disorder. Am. J. Hum. Genet. 2022, 109, 1549–1558. [Google Scholar] [CrossRef]
- Levasseur, E.M.; Yamada, K.; Pineros, A.R.; Wu, W.; Syed, F.; Orr, K.S.; Anderson-Baucum, E.; Mastracci, T.L.; Maier, B.; Mosley, A.L.; et al. Hypusine biosynthesis in beta cells links polyamine metabolism to facultative cellular proliferation to maintain glucose homeostasis. Sci. Signal 2019, 12, eaax0715. [Google Scholar] [CrossRef]
- Schultz, C.R.; Sheldon, R.D.; Xie, H.; Demireva, E.Y.; Uhl, K.L.; Agnew, D.W.; Geerts, D.; Bachmann, A.S. New K50R mutant mouse models reveal impaired hypusination of eif5a2 with alterations in cell metabolite landscape. Biol. Open 2023, 12, bio059647. [Google Scholar] [CrossRef]
- Gobert, A.P.; Smith, T.M.; Latour, Y.L.; Asim, M.; Barry, D.P.; Allaman, M.M.; Williams, K.J.; McNamara, K.M.; Delgado, A.G.; Short, S.P.; et al. Hypusination Maintains Intestinal Homeostasis and Prevents Colitis and Carcinogenesis by Enhancing Aldehyde Detoxification. Gastroenterology 2023, 165, 656–669.e8. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Chen, K.Y. Hypusine is required for a sequence-specific interaction of eukaryotic initiation factor 5A with postsystematic evolution of ligands by exponential enrichment RNA. J. Biol. Chem. 2001, 276, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, K.; Wright, T.; Strnadel, J.; Kaushal, S.; Metildi, C.; Lowy, A.M.; Bouvet, M.; Kelber, J.A.; Klemke, R.L. A hypusine-eIF5A-PEAK1 switch regulates the pathogenesis of pancreatic cancer. Cancer Res. 2014, 74, 6671–6681. [Google Scholar] [CrossRef]
- Fujimura, K.; Choi, S.; Wyse, M.; Strnadel, J.; Wright, T.; Klemke, R. Eukaryotic Translation Initiation Factor 5A (EIF5A) Regulates Pancreatic Cancer Metastasis by Modulating RhoA and Rho-associated Kinase (ROCK) Protein Expression Levels. J. Biol. Chem. 2015, 290, 29907–29919. [Google Scholar] [CrossRef] [PubMed]
- Lubas, M.; Harder, L.M.; Kumsta, C.; Tiessen, I.; Hansen, M.; Andersen, J.S.; Lund, A.H.; Frankel, L.B. eIF5A is required for autophagy by mediating ATG3 translation. EMBO Rep. 2018, 19, e46072. [Google Scholar] [CrossRef]
- Zhang, H.; Alsaleh, G.; Feltham, J.; Sun, Y.; Napolitano, G.; Riffelmacher, T.; Charles, P.; Frau, L.; Hublitz, P.; Yu, Z.; et al. Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol. Cell 2019, 76, 110–125.e9. [Google Scholar] [CrossRef]
- Nakanishi, S.; Li, J.; Berglund, A.E.; Kim, Y.; Zhang, Y.; Zhang, L.; Yang, C.; Song, J.; Mirmira, R.G.; Cleveland, J.L. The Polyamine-Hypusine Circuit Controls an Oncogenic Translational Program Essential for Malignant Conversion in MYC-Driven Lymphoma. Blood Cancer Discov. 2023, 4, 294–317. [Google Scholar] [CrossRef]
- Tan, T.C.J.; Kelly, V.; Zou, X.; Wright, D.; Ly, T.; Zamoyska, R. Translation factor eIF5a is essential for IFNgamma production and cell cycle regulation in primary CD8+ T lymphocytes. Nat. Commun. 2022, 13, 7796. [Google Scholar] [CrossRef]
- Puleston, D.J.; Buck, M.D.; Klein Geltink, R.I.; Kyle, R.L.; Caputa, G.; O’Sullivan, D.; Cameron, A.M.; Castoldi, A.; Musa, Y.; Kabat, A.M.; et al. Polyamines and eIF5A Hypusination Modulate Mitochondrial Respiration and Macrophage Activation. Cell Metab. 2019, 30, 352–363.e8. [Google Scholar] [CrossRef]
- Gobert, A.P.; Finley, J.L.; Latour, Y.L.; Asim, M.; Smith, T.M.; Verriere, T.G.; Barry, D.P.; Allaman, M.M.; Delagado, A.G.; Rose, K.L.; et al. Hypusination Orchestrates the Antimicrobial Response of Macrophages. Cell Rep. 2020, 33, 108510. [Google Scholar] [CrossRef]
- Anderson-Baucum, E.; Pineros, A.R.; Kulkarni, A.; Webb-Robertson, B.J.; Maier, B.; Anderson, R.M.; Wu, W.; Tersey, S.A.; Mastracci, T.L.; Casimiro, I.; et al. Deoxyhypusine synthase promotes a pro-inflammatory macrophage phenotype. Cell Metab. 2021, 33, 1883–1893.e7. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Menendez, P.; Phadke, I.; Olive, M.E.; Joly, A.; Papoin, J.; Yan, H.; Galtier, J.; Platon, J.; Kang, S.W.S.; McGraw, K.L.; et al. Arginine metabolism regulates human erythroid differentiation through hypusination of eIF5A. Blood 2023, 141, 2520–2536. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Buttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Puleston, D.J.; Zhang, H.; Powell, T.J.; Lipina, E.; Sims, S.; Panse, I.; Watson, A.S.; Cerundolo, V.; Townsend, A.R.; Klenerman, P.; et al. Autophagy is a critical regulator of memory CD8+ T cell formation. eLife 2014, 3, e03706. [Google Scholar] [CrossRef] [PubMed]
- Ennishi, D.; Hsi, E.D.; Steidl, C.; Scott, D.W. Toward a New Molecular Taxonomy of Diffuse Large B-cell Lymphoma. Cancer Discov. 2020, 10, 1267–1281. [Google Scholar] [CrossRef]
- Roschewski, M.; Staudt, L.M.; Wilson, W.H. Diffuse large B-cell lymphoma-treatment approaches in the molecular era. Nat. Rev. Clin. Oncol. 2014, 11, 12–23. [Google Scholar] [CrossRef]
- Olszewski, A.J.; Kurt, H.; Evens, A.M. Defining and treating high-grade B-cell lymphoma, NOS. Blood 2022, 140, 943–954. [Google Scholar] [CrossRef]
- Savage, K.J.; Johnson, N.A.; Ben-Neriah, S.; Connors, J.M.; Sehn, L.H.; Farinha, P.; Horsman, D.E.; Gascoyne, R.D. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood 2009, 114, 3533–3537. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef]
- Kay, J.E.; Pegg, A.E. Effect of inhibition of spermidine formation on protein and nucleic acid synthesis during lymphocyte activation. FEBS Lett. 1973, 29, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Bowlin, T.L.; McKown, B.J.; Babcock, G.F.; Sunkara, P.S. Intracellular polyamine biosynthesis is required for interleukin 2 responsiveness during lymphocyte mitogenesis. Cell Immunol. 1987, 106, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Puleston, D.J.; Baixauli, F.; Sanin, D.E.; Edwards-Hicks, J.; Villa, M.; Kabat, A.M.; Kaminski, M.M.; Stanckzak, M.; Weiss, H.J.; Grzes, K.M.; et al. Polyamine metabolism is a central determinant of helper T cell lineage fidelity. Cell 2021, 184, 4186–4202.e20. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.; Mirmira, R.G.; Jaume, J.C. Eukaryotic translation initiation factor 5A inhibition alters physiopathology and immune responses in a “humanized” transgenic mouse model of type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E791–E798. [Google Scholar] [CrossRef]
- Imam, S.; Prathibha, R.; Dar, P.; Almotah, K.; Al-Khudhair, A.; Hasan, S.A.; Salim, N.; Jilani, T.N.; Mirmira, R.G.; Jaume, J.C. eIF5A inhibition influences T cell dynamics in the pancreatic microenvironment of the humanized mouse model of Type 1 Diabetes. Sci. Rep. 2019, 9, 1533. [Google Scholar] [CrossRef] [PubMed]
- Lemke, G. How macrophages deal with death. Nat. Rev. Immunol. 2019, 19, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Park, M.D.; Silvin, A.; Ginhoux, F.; Merad, M. Macrophages in health and disease. Cell 2022, 185, 4259–4279. [Google Scholar] [CrossRef]
- Hardbower, D.M.; Asim, M.; Luis, P.B.; Singh, K.; Barry, D.P.; Yang, C.; Steeves, M.A.; Cleveland, J.L.; Schneider, C.; Piazuelo, M.B.; et al. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc. Natl. Acad. Sci. USA 2017, 114, E751–E760. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kurihara, S.; Takahashi, D.; Ohashi, W.; Nakamura, Y.; Kimura, S.; Onuki, M.; Kume, A.; Sasazawa, Y.; Furusawa, Y.; et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat. Commun. 2021, 12, 2105. [Google Scholar] [CrossRef]
- Russo, S.; Kwiatkowski, M.; Govorukhina, N.; Bischoff, R.; Melgert, B.N. Meta-Inflammation and Metabolic Reprogramming of Macrophages in Diabetes and Obesity: The Importance of Metabolites. Front. Immunol. 2021, 12, 746151. [Google Scholar] [CrossRef]
- Yao, J.; Wu, D.; Qiu, Y. Adipose tissue macrophage in obesity-associated metabolic diseases. Front. Immunol. 2022, 13, 977485. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Aravind, L.; Wolff, E.C.; Kaevel, J.; Kim, Y.S.; Park, M.H. Molecular cloning, expression, and structural prediction of deoxyhypusine hydroxylase: A HEAT-repeat-containing metalloenzyme. Proc. Natl. Acad. Sci. USA 2006, 103, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, D.; Zhu, J.; Wang, M.; Zhang, Y.; Fu, Q.; Zhang, S.; Lin, H. Oxygen level regulates N-terminal translation elongation of selected proteins through deoxyhypusine hydroxylation. Cell Rep. 2022, 39, 110855. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.T.; Lok, E.; Swanson, K.D. Alternating Electric Fields Therapy for Malignant Gliomas: From Bench Observation to Clinical Reality. Prog. Neurol. Surg. 2018, 32, 180–195. [Google Scholar]
- Preukschas, M.; Hagel, C.; Schulte, A.; Weber, K.; Lamszus, K.; Sievert, H.; Pallmann, N.; Bokemeyer, C.; Hauber, J.; Braig, M.; et al. Expression of eukaryotic initiation factor 5A and hypusine forming enzymes in glioblastoma patient samples: Implications for new targeted therapies. PLoS ONE 2012, 7, e43468. [Google Scholar] [CrossRef]
- Ofek, P.; Yeini, E.; Arad, G.; Danilevsky, A.; Pozzi, S.; Luna, C.B.; Dangoor, S.I.; Grossman, R.; Ram, Z.; Shomron, N.; et al. Deoxyhypusine hydroxylase: A novel therapeutic target differentially expressed in short-term vs long-term survivors of glioblastoma. Int. J. Cancer J. Int. Cancer 2023, 153, 654–668. [Google Scholar] [CrossRef]
- Shiba, T.; Mizote, H.; Kaneko, T.; Nakajima, T.; Kakimoto, Y. Hypusine, a new amino acid occurring in bovine brain. Isolation and structural determination. Biochim. Biophys. Acta 1971, 244, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Matsubayashi, T.; Kakimoto, Y.; Sano, I. Distribution of hypusine, N 6 -(4-amino-2-hydroxybutyl)-2,6-diaminohexanoic acid, in mammalian organs. Biochim. Biophys. Acta 1971, 252, 92–97. [Google Scholar] [CrossRef]
- Park, M.H.; Kar, R.K.; Banka, S.; Ziegler, A.; Chung, W.K. Post-translational formation of hypusine in eIF5A: Implications in human neurodevelopment. Amino Acids 2022, 54, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Tamborlin, L.; Pereira, K.D.; Guimaraes, D.; Silveira, L.R.; Luchessi, A.D. The first evidence of biological activity for free Hypusine, an enigmatic amino acid discovered in the ′70s. Amino Acids 2023, 55, 913–929. [Google Scholar] [CrossRef] [PubMed]
| Gene | Alleles | Target Regions | Group | References |
|---|---|---|---|---|
| Dhps | Dhps+/gt | Gt 1 in exon 2 | Park | Nishimura et al., 2012 [14] |
| Dhpsfl/fl | Exon 2–7 | Balabanov | Pallmann et al., 2015 [16] | |
| Dhpsfl/fl | Exon 2–7 | Mirmira | Levasseur et al., 2019 [20] | |
| DhpsN173S | N173S | Lutz | Donated to the Jackson lab (JAX) | |
| Dohh | Dohhfl/fl | Exon 2–4 | Balabanov | Sievert et al., 2014 [15] |
| Eif5a | Eif5a+/gt | Gt in intron1 | Park | Nishimura et al., 2012 [14] |
| Eif5a+/K50R | K50R | Bachmann | Shultz et al., 2023 [21] | |
| Eif5a2 | Eif5a2fl/fl | Exon 2–3 | Balabanov | Pallmann et al., 2015 [16] |
| Eif5a2+/K50R, Eif5a2K50R/K50R | K50R | Bachmann | Shultz et al., 2023 [21] |
| Tissue/Cell Type | Mouse Model or Cell/Condition * | Primary Method | Additional Method | Major Representative Targets | Cellular Function | References |
|---|---|---|---|---|---|---|
| IECs 1 | Dhpsfl/fl;Vil1-Cre | Proteomics | IB 2 | GSTA4, GSTM3, GSTM2, GSTP1, GSTO1, GSTM1, AL1A7, AL1B1, ALDH2 | Aldehyde detoxification | Gobert et al., 2023 [22] |
| Pancreatic islet β cells | Dhpsfl/fl;MIP1-CreERT on HFD 3 | Proteomics | IB | Cyclin D2 | Proliferation | Levasseur et al., 2019 [20] |
| PDAC 4 | PANK1, 779E/knockdown of EIFA, EIF5A2, or both genes, GC7, or CPX treatment | IB | PEAK1 | Src kinase activity | Fujimura et al., 2014 [24] | |
| PDAC | 779E cell/knockdown of EIF5A | Proteomics | IB | RhoA, ROCK2, TRIM29, XRN1, ZO1 | Rho/ROCK signaling, cell motility | Fujimura et al., 2015 [25] |
| Breast cancer | MCF-7 cell/knockdown of EIF5A | Proteomics | IB | ATG3 | Autophagosome formation | Lubas et al., 2018 [26] |
| B cells | Primary B cells/GC7 treatment | Proteomics | IB | TFEB | Autophagy | Zhang et al., 2019 [27] |
| B-cell lymphoma | Eμ-Myc lymphoma/knockdown of Eif5a or Dhps | RP 5, proteomics | IB | POLD1, E2F, PIM3, SCD1, Cyclin D3 | Cell cycle, replication, proliferation | Nakanishi et al., 2023 [28] |
| T cells | OT-1 CD8+ T cells/knockout of Eif5a or GC7 treatment | Proteomics | FACS | CDK1, TBET, IRF4 | Cytokine production | Tan et al., 2022 [29] |
| Macrophages | BMDMs (+IL-4) 6/GC7 treatment | Proteomics | IB | SUCLG1, SDH, MCM, pyruvate dehydrogenases | TCA cycle, ETC | Puleston et al., 2019 [30] |
| Macrophages | Dhpsfl/fl;Lyz2-Cre BMDMs (+H. pylori) 7 | Proteomics | IB | NOS2, IRG1, SQSTM | Antibacterial response, autophagy | Gobert et al., 2020 [31] |
| Macrophages | Dhpsfl/fl; Lyz2-Cre BMDMs (+LPS + IFN-γ) 8 Dhpsfl/fl; Lyz2-Cre BMDMs (+IL-4) | Proteomics | IB, RIP 9, PP 10 | M1: IL17RA, SRK11/LKB1, TRIM13, PARP1, IκBα, CCL3, IL1b M2 11 | NF-κB signaling, proinflammatory signaling | Anderson-Baucum et al., 2021 [32] |
| HSPCs 12 | CD34+ cells (+EPO)/GC7 treatment | Proteomics | Mitochondrial proteins, including mitochondrial ribosomal proteins | Mitochondria function/OXPHOS | Gonzalez-Menendez et al., 2023 [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, S.; Cleveland, J.L. The Many Faces of Hypusinated eIF5A: Cell Context-Specific Effects of the Hypusine Circuit and Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 8171. https://doi.org/10.3390/ijms25158171
Nakanishi S, Cleveland JL. The Many Faces of Hypusinated eIF5A: Cell Context-Specific Effects of the Hypusine Circuit and Implications for Human Health. International Journal of Molecular Sciences. 2024; 25(15):8171. https://doi.org/10.3390/ijms25158171
Chicago/Turabian StyleNakanishi, Shima, and John L. Cleveland. 2024. "The Many Faces of Hypusinated eIF5A: Cell Context-Specific Effects of the Hypusine Circuit and Implications for Human Health" International Journal of Molecular Sciences 25, no. 15: 8171. https://doi.org/10.3390/ijms25158171




