Enhanced Assessment of Cross-Reactive Antigenic Determinants within the Spike Protein
Abstract
:1. Introduction
2. Results
2.1. Mapping of IgG Epitopes within SARS-CoV-2 Spike Protein
2.2. Cross-Reactivity with Anti-DENV Antibodies
2.3. Bioinformatic Analysis
2.4. Pre-Pandemic DENV Sera Display Antibody-Dependent Enhancement In Vitro
2.5. Antibody Binding to Peptides from Spike Protein
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. B-Linear Epitope Mapping
4.3. Peptide Synthesis
4.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.5. In Silico Analysis
4.6. Cells, Viruses, and Reagents
4.7. Infections and Virus Titration
4.8. Molecular Detection of Viral RNA Levels
4.9. LDH Measurement
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hadj Hassine, I. Covid-19 vaccines and variants of concern: A review. Rev. Med. Virol. 2022, 32, e2313. [Google Scholar] [CrossRef]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.S.; Winkler, M.S.; et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 2022, 185, 447–456.e11. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; Lam, E.C.; St Denis, K.; Nitido, A.D.; Garcia, Z.H.; Hauser, B.M.; Feldman, J.; Pavlovic, M.N.; Gregory, D.J.; Poznansky, M.C.; et al. Multiple SARS-CoV-2 variants escape neutralization by vaccine-induced humoral immunity. Cell 2021, 184, 2372–2383. [Google Scholar] [CrossRef]
- Ladner, J.T.; Henson, S.N.; Boyle, A.S.; Engelbrektson, A.L.; Fink, Z.W.; Rahee, F.; D’ambrozio, J.; Schaecher, K.E.; Stone, M.; Dong, W.; et al. Epitope-resolved profiling of the SARS-CoV-2 antibody response identifies cross-reactivity with endemic human coronaviruses. Cell Rep. Med. 2021, 2, 100189. [Google Scholar] [CrossRef]
- Devaux, C.A.; Fantini, J. Unravelling antigenic cross-reactions toward the world of coronaviruses: Extent of the stability of shared epitopes and SARS-CoV-2 anti-spike cross-neutralizing antibodies. Pathogens 2023, 12, 713. [Google Scholar] [CrossRef]
- Polyiam, K.; Phoolcharoen, W.; Butkhot, N.; Srisaowakarn, C.; Thitithanyanont, A.; Auewarakul, P.; Hoonsuwan, T.; Ruengjitchatchawalya, M.; Mekvichitsaeng, P.; Roshorm, Y.M. Immunodominant linear B cell epitopes in the spike and membrane proteins of SARS-CoV-2 identified by immunoinformatics prediction and immunoassay. Sci. Rep. 2021, 11, 20383. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chao, C.H.; Lai, Y.C.; Hsieh, K.H.; Wang, J.R.; Wan, S.W.; Huang, H.J.; Chuang, Y.C.; Chuang, W.J.; Yeh, T.M. Antibodies against the SARS-CoV-2 S1-RBD cross-react with dengue virus and hinder dengue pathogenesis. Front. Immunol. 2022, 13, 941923. [Google Scholar] [CrossRef]
- Murray, S.M.; Ansari, A.M.; Frater, J.; Klenerman, P.; Dunachie, S.; Barnes, E.; Ogbe, A. The impact of pre-existing cross-reactive immunity on SARS-CoV-2 infection and vaccine responses. Nat. Rev. Immunol. 2022, 23, 304–316. [Google Scholar] [CrossRef]
- Takada, A.; Kawaoka, Y. Antibody-dependent enhancement of viral infection: Molecular mechanisms and in vivo implications. Rev. Med. Virol. 2003, 13, 387–398. [Google Scholar] [CrossRef]
- Tirado, S.M.C.; Yoon, K.J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003, 16, 69–86. [Google Scholar] [CrossRef]
- Thomas, S.; Smatti, M.K.; Ouhtit, A.; Cyprian, F.S.; Almaslamani, M.A.; Thani, A.A.; Yassine, H.M.; Thomas, S. Antibody-Dependent Enhancement (ADE) and the role of complement system in disease pathogenesis. Mol. Immunol. 2022, 152, 172–182. [Google Scholar] [CrossRef]
- Gorlani, A.; Forthal, D.N. Antibody-dependent enhancement and the risk of HIV infection. Curr. HIV Res. 2013, 11, 421–426. [Google Scholar] [CrossRef]
- Sullivan, N.; Sun, Y.; Binley, J.; Lee, J.; Barbas, C.F., 3rd; Parren, P.W.; Burton, D.R.; Sodroski, J. Determinants of human immunodeficiency virus type 1 envelope glycoprotein activation by soluble CD4 and monoclonal antibodies. J. Virol. 1998, 72, 6332–6338. [Google Scholar] [CrossRef]
- Guillon, C.; Schutten, M.; Boers, P.H.; Gruters, R.A.; Osterhaus, A.D. Antibody-mediated enhancement of human immunodeficiency virus type 1 infectivity is determined by the structure of gp120 and depends on modulation of the gp120-CCR5 interaction. J. Virol. 2002, 76, 2827–2834. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengue. Lancet 2007, 370, 1644–1652. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Z.; Li, S.; Xu, W.; Zhang, Q.; Silva, I.T.; Li, C.; Wu, Y.; Jiang, Q.; Liu, Z.; et al. Enhancement versus neutralization by SARS-CoV-2 antibodies from a convalescent donor associate with distinct epitopes on the RBD. Cell Rep. 2021, 34, 108699. [Google Scholar] [CrossRef]
- Okuya, K.; Hattori, T.; Saito, T.; Takadate, Y.; Sasaki, M.; Furuyama, W.; Marzi, A.; Ohiro, Y.; Konno, S.; Hattori, T.; et al. Multiple routes of antibody-dependent enhancement of SARS-CoV-2 infection. Microbiol. Spectr. 2022, 10, e0155321. [Google Scholar] [CrossRef]
- Goncalvez, A.P.; Engle, R.E.; St. Claire, M.; Purcell, R.H.; Lai, C.J. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. USA 2007, 104, 9422–9427. [Google Scholar] [CrossRef]
- Pierson, T.C.; Xu, Q.; Nelson, S.; Oliphant, T.; Nybakken, G.E.; Fremont, D.H.; Diamond, M.S. The stoichiometry of antibody-mediated neutralization and enhancement of West Nile virus infection. Cell Host Microbe 2007, 1, 135–145. [Google Scholar] [CrossRef]
- Taylor, A.; Foo, S.S.; Bruzzone, R.; Dinh, L.V.; King, N.J.; Mahalingam, S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015, 268, 340–364. [Google Scholar] [CrossRef]
- Mu, S.; Song, S.; Hao, Y.; Luo, F.; Wu, R.; Wang, Y.; Han, X.; Li, T.; Hu, C.; Li, S.; et al. Neutralizing antibodies from the rare convalescent donors elicited antibody-dependent enhancement of SARS-CoV-2 variants infection. Front. Med. 2022, 9, 952697. [Google Scholar] [CrossRef]
- Arvin, A.M.; Fink, K.; Schmid, M.A.; Cathcart, A.; Spreafico, R.; Havenar-Daughton, C.; Lanzavecchia, A.; Corti, D.; Virgin, H.W. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature 2020, 584, 353–363. [Google Scholar] [CrossRef]
- Cloutier, M.; Nandi, M.; Ihsan, A.U.; Chamard, H.A.; Ilangumaran, S.; Ramanathan, S. ADE and hyperinflammation in SARS-CoV2 infection—Comparison with dengue hemorrhagic fever and feline infectious peritonitis. Cytokine 2020, 136, 155256. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, F. Evolving understanding of antibody-dependent enhancement (ADE) of SARS-CoV-2. Front. Immunol. 2022, 13, 1008285. [Google Scholar] [CrossRef]
- Pinto, D.; Park, Y.J.; Beltramello, M.; Walls, A.C.; Tortorici, M.A.; Bianchi, S.; Jaconi, S.; Culap, K.; Zatta, F.; De Marco, A.; et al. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature 2020, 583, 290–295. [Google Scholar] [CrossRef]
- Bates, T.A.; Weinstein, J.B.; Farley, S.; Leier, H.C.; Messer, W.B.; Tafesse, F.G. Cross-reactivity of SARS-CoV structural protein antibodies against SARS-CoV-2. Cell Rep. 2021, 34, 108737. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, J.; Jiao, S.; Gu, C.; Xu, W.; Chen, B.; Wang, R.; Chen, H.; Xie, Y.; Wang, A.; et al. Characterization of MW06, a human monoclonal antibody with cross-neutralization activity against both SARS-CoV-2 and SARS-CoV. MAbs 2021, 13, 1953683. [Google Scholar] [CrossRef]
- Watanabe, Y.; Hosokawa, N.; Yoshida, M.; Miura, T.; Kawano, M. Identification of closed linear epitopes in S1-RBD and S2-HR1/2 of SARS-CoV-2 spike protein able to induce neutralizing Abs. Vaccines 2023, 11, 287. [Google Scholar] [CrossRef]
- Hicks, J.; Klumpp-Thomas, C.; Kalish, H.; Shunmugavel, A.; Mehalko, J.; Denson, J.P.; Snead, K.R.; Drew, M.; Corbett, K.S.; Graham, B.S.; et al. Serologic cross-reactivity of SARS-CoV-2 with endemic and seasonal betacoronaviruses. J. Clin. Immunol. 2021, 41, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Holenya, P.; Lange, P.J.; Reimer, U.; Woltersdorf, W.; Panterodt, T.; Glas, M.; Wasner, M.; Eckey, M.; Drosch, M.; Hollidt, J.M.; et al. Peptide microarray-based analysis of antibody responses to SARS-CoV-2 identifies unique epitopes with potential for diagnostic test development. Eur. J. Immunol. 2021, 51, 1839–1849. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, L.; Hao, Y.; Zhu, M.; Jiang, H.; Wang, S.; Li, D.; Shao, Y. Pre-existing anti-HCoV-OC43 immunity influences the durability and cross-reactivity of humoral response to SARS-CoV-2 vaccination. Front. Cell Infect. Microbiol. 2022, 12, 978440. [Google Scholar] [CrossRef]
- Ng, K.W.; Faulknerm, N.; Cornishm, G.H.; Rosam, A.; Harveym, R.; Hussainm, S.; Ulfertsm, R.; Earlm, C.; Wrobelm, A.G.; Benton, D.J.; et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.W.; Cheng, V.C.C.; Cai, J.P.; Chan, K.H.; Chen, L.L.; Wong, L.H.; Choi, C.Y.K.; Fong, C.H.Y.; Ng, A.C.K.; Lu, L.; et al. Seroprevalence of SARS-CoV-2 in Hong Kong and in residents evacuated from Hubei province, China: A multicohort study. Lancet Microbe 2020, 1, e111–e118. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Alahmari, A.A.; Almuzaini, Y.; Alamri, F.; Alsofayan, Y.M.; Aburas, A.; Al-Muhsen, S.; Van Kerkhove, M.; Yezli, S.; Ciottone, G.R.; et al. Potential cross-reactive immunity to COVID-19 infection in individuals with laboratory-confirmed MERS-CoV infection: A national retrospective cohort study from Saudi Arabia. Front. Immunol. 2021, 12, 727989. [Google Scholar] [CrossRef] [PubMed]
- Klompus, S.; Leviatan, S.; Vogl, T.; Mazor, R.D.; Kalka, I.N.; Stoler-Barak, L.; Nathan, N.; Peres, A.; Moss, L.; Godneva, A.; et al. Cross-reactive antibodies against human coronaviruses and the animal coronavirome suggest diagnostics for future zoonotic spillovers. Sci. Immunol. 2021, 6, eabe9950. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, C.; Cai, L.; Liao, C.; Yi, H.; Li, Q.; Hu, H.; Deng, Q.; Lu, Y.; Guo, Z.; et al. Pre-existing cross-reactive antibody responses do not significantly impact inactivated COVID-19 vaccine-induced neutralization. Front. Immunol. 2021, 12, 772511. [Google Scholar] [CrossRef] [PubMed]
- Majdoubi, A.; Michalski, C.; O’Connell, S.E.; Dada, S.; Narpala, S.; Gelinas, J.; Mehta, D.; Cheung, C.; Winkler, D.F.; Basappa, M.; et al. A majority of uninfected adults show preexisting antibody reactivity against SARS-CoV-2. JCI Insight 2021, 6, e146316. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Donina, S.; Zabrodskaya, Y.; Rudenko, L.; Isakova-Sivak, I. Cross-reactivity of SARS-CoV-2 nucleocapsid-binding antibodies and its implication for COVID-19 serology tests. Viruses 2022, 14, 2041. [Google Scholar] [CrossRef] [PubMed]
- Souris, M.; Tshilolo, L.; Parzy, D.; Ingoba, L.L.; Ntoumi, F.; Kamgaing, R.; Ndour, M.; Mbongi, D.; Phoba, B.; Tshilolo, M.A.; et al. Pre-pandemic cross-reactive immunity against SARS-CoV-2 among Central and West African populations. Viruses 2022, 14, 2259. [Google Scholar] [CrossRef] [PubMed]
- Vigan-Womas, I.; Spadoni, J.L.; Poiret, T.; Taïeb, F.; Randrianarisaona, F.; Faye, R.; Mbow, A.A.; Gaye, A.; Dia, N.; Loucoubar, C.; et al. Linear epitope mapping of the humoral response against SARS-CoV-2 in two independent African cohorts. Sci. Rep. 2023, 13, 782. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.A.; Kamath, K.; Bozekowski, J.; Baum-Jones, E.; Campbell, M.; Casanovas-Massana, A.; Daugherty, P.S.; Dela Cruz, C.S.; Dhal, A.; Farhadian, S.F.; et al. High-resolution epitope mapping and characterization of SARS-CoV-2 antibodies in large cohorts of subjects with COVID-19. Commun. Biol. 2021, 4, 1317. [Google Scholar] [CrossRef]
- Camerini, D.; Randall, A.Z.; Trappl-Kimmons, K.; Oberai, A.; Hung, C.; Edgar, J.; Shandling, A.; Huynh, V.; Teng, A.A.; Hermanson, G.; et al. Mapping SARS-CoV-2 antibody epitopes in COVID-19 patients with a multi-coronavirus protein microarray. Microbiol. Spectr. 2021, 9, e0141621. [Google Scholar] [CrossRef] [PubMed]
- De-Simone, S.G.; Napoleão-Pêgo, P.; Lechuga, G.C.; Carvalho, J.P.R.S.; Monteiro, M.E.; Morel, C.M.; Provance, D.W., Jr. Mapping IgA epitope and cross-reactivity between Severe acute Respiratory Syndrome-Associated Coronavirus 2 and DENV. Vaccines 2023, 11, 1749. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, P.; Steinbeck, F.; Mai, F.; Reisinger, E.C.; Müller-Hilke, B. A linear B-cell epitope close to the furin cleavage site within the S1 domain of SARS-CoV-2 Spike protein discriminates the humoral immune response of nucleic acid- and protein-based vaccine cohorts. Front. Immunol. 2023, 14, 1192395. [Google Scholar] [CrossRef] [PubMed]
- Elko, E.A.; Nelson, G.A.; Mead, H.L.; Zaia, J.A.; Ladner, J.T.; Altin, J.A. COVID-19 vaccination elicits an evolving, cross- reactive antibody response to epitopes conserved with endemic coronavirus spike proteins. CellReports 2022, 40, 111022. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Chin, C.V.; Kenney, D.; Tavares, A.H.; Khan, N.; Conway, H.L.; Liu, G.; Choudhary, M.C.; Gertje, H.P.; O’Connell, A.K.; et al. Spike and nsp6 are key determinants of SARS-CoV-2 Omicron BA.1 attenuation. Nature 2023, 615, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Yang, Y.; Zhang, X.; Li, F. Recent advances in nanotechnology-based COVID-19 vaccines and therapeutic antibodies. Nanoscale 2022, 14, 1054–1074. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, X.; Zhou, H.; Zhu, H.; Jiang, S.; Wang, P. Broadly neutralizing antibodies to SARS-CoV-2 and other human coronaviruses. Nat. Rev. Immunol. 2023, 23, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Han, X.; Yan, J. Structure-based neutralizing mechanisms for SARS-CoV-2 antibodies. Emerg. Microbes Infect. 2022, 11, 2412–2422. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping neutralizing and immunodominant sites on the SARS-CoV-2 Spike receptor-binding domain by structure-guided high-resolution serology. Cell 2020, 183, 1024–1042. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, J.; Schank, M.; Khanal, S.; Dang, X.; Cao, D.; Nguyen, L.N.T.; Zhang, Y.; Wu, X.Y.; Adkins, J.L.; et al. Identification of virus-specific B-cell epitopes by convalescent plasma from COVID-19 patients. Mol. Immunol. 2022, 152, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Peng, W.J.; Kuo, B.S.; Ho, Y.H.; Wang, M.S.; Yang, Y.T.; Chang, P.Y.; Shen, Y.H.; Hwang, K.P. Toward a pan-SARS-CoV-2 vaccine targeting conserved epitopes on Spike and non-spike proteins for potent, broad and durable immune responses. PLoS Pathog. 2023, 19, e1010870. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Sun, H.; Wang, S.; Yuan, L.; Liu, L.; Zhu, Y.; Zhang, J.; Huang, Y.; Qi, R.; Jiang, Y.; et al. The neutralizing breadth of antibodies targeting diverse conserved epitopes between SARS-CoV and SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2022, 119, e2215628119. [Google Scholar] [CrossRef] [PubMed]
- AlKhalifah, J.M.; Seddiq, W.; Alshehri, M.A.; Alhetheel, A.; Albarrag, A.; Meo, S.A.; Al-Tawfiq, J.A.; Barry, M. Impact of MERS-CoV and SARS-CoV-2 viral infection on immunoglobulin-IgG cross-reactivity. Vaccines 2023, 11, 552. [Google Scholar] [CrossRef]
- Mannar, D.; Saville, J.W.; Sun, Z.; Zhu, X.; Marti, M.M.; Srivastava, S.S.; Berezuk, A.M.; Zhou, S.; Tuttle, K.S.; Sobolewski, M.D.; et al. SARS-CoV-2 variants of concern: Spike protein mutational analysis and epitope for broad neutralization. Nat. Commun. 2022, 13, 4696. [Google Scholar] [CrossRef]
- Prakash, S.; Dhanushkodi, N.R.; Zayou, L.; Ibraim, I.C.; Quadiri, A.; Coulon, P.G.; Tifrea, D.F.; Suzer, B.; Shaik, A.M.; Chilukuri, A.; et al. Cross-protection induced by highly conserved human B, CD4+, and CD8+ T-cell epitopes-based vaccine against severe infection, disease, and death caused by multiple SARS-CoV-2 variants of concern. Front. Immunol. 2024, 15, 1328905. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, L.; Misasi, J.; Pegu, A.; Zhang, Y.; Harris, D.R.; Olia, A.S.; Talana, C.A.; Yang, E.S.; Chen, M.; et al. Structural basis for potent antibody neutralization of SARS-CoV-2 variants including B.1.1.529. Science 2022, 376, eabn8897. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, L.B.; Tedla, N.; Bull, R.A. Broadly neutralizing antibodies against emerging SARS-CoV-2 variants. Front. Immunol. 2021, 12, 752003. [Google Scholar] [CrossRef]
- Pinto, D.; Sauer, M.M.; Czudnochowski, N.; Low, J.S.; Tortorici, M.A.; Housley, M.P.; Noack, J.; Walls, A.C.; Bowen, J.E.; Guarino, B.; et al. Broad betacoronavirus neutralization by a stem helix–specific human antibody. Science 2021, 373, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Low, J.S.; Jerak, J.; Tortorici, M.A.; McCallum, M.; Pinto, D.; Cassotta, A.; Foglierini, M.; Mele, F.; Abdelnabi, R.; Weynand, B.; et al. ACE2-binding exposes the SARS-CoV-2 fusion peptide to broadly neutralizing coronavirus antibodies. Science 2022, 377, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Dacon, C.; Tucker, C.; Peng, L.; Lee, C.D.; Lin, T.H.; Yuan, M.; Cong, Y.; Wang, L.; Purser, L.; Williams, J.K.; et al. Broadly neutralizing antibodies target the coronavirus fusion peptide. Science 2022, 377, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Shrock, E.; Fujimura, E.; Kula, T.; Timms, R.T.; Lee, I.H.; Leng, Y.; Robinson, M.L.; Sie, B.M.; Li, M.Z.; Chen, Y.; et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 2022, 370, eabd4250. [Google Scholar] [CrossRef] [PubMed]
- Jaago, M.; Rähni, A.; Pupina, N.; Pihlak, A.; Sadam, H.; Tuvikene, J.; Avarlaid, A.; Planken, A.; Planken, M.; Haring, L.; et al. Differential patterns of cross-reactive antibody response against SARS-CoV-2 spike protein detected for chronically ill and healthy COVID-19 naïve individuals. Sci. Rep. 2022, 12, 16817. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.K.; Bhattacharjee, S. Dengue virus: Epidemiology, biology, and disease etiology. Can. J. Microbiol. 2021, 67, 687–70231. [Google Scholar] [CrossRef] [PubMed]
- Nasar, S.; Rashid, N.; Iftikhar, S. Dengue proteins with their role in pathogenesis, and strategies for developing an effective anti-dengue treatment: A review. J. Med. Virol. 2020, 92, 941–955. [Google Scholar] [CrossRef] [PubMed]
- Vanroye, F.; Bossche, D.V.D.; Brosius, I.; Tack, B.; Esbroeck, M.V.; Jacobs, J. COVID-19 antibody detecting rapid diagnostic tests show high cross-reactivity when challenged with pre-pandemic malaria, schistosomiasis, and dengue samples. Diagnostics 2021, 11, 1163. [Google Scholar] [CrossRef]
- Shurrab, F.M.; Al-Sadeq, D.W.; Amanullah, F.H.; Al-Absi, E.S.; Qotba, H.; Yassine, H.M.; Abu-Raddad, L.J.; Nasrallah, G.K. Low Risk of Serological Cross-Reactivity between the Dengue Virus and SARS-CoV-2-IgG antibodies using advanced detection assays. Intervirology 2022, 65, 224–229. [Google Scholar] [CrossRef]
- Hunsawong, T.; Buddhari, D.; Rungrojcharoenkit, K.; Suthangkornkul, R.; Mongkolsirichaikul, D.; Lohachanakul, J.; Tayong, K.; Sirikajornpan, K.; Rodpradit, P.; Poolpanichupatam, Y. Anti-Arbovirus Antibodies Cross-React With Severe Acute Respiratory Syndrome Coronavirus 2. Microbiol. Spectr. 2022, 10, e0263922. [Google Scholar] [CrossRef] [PubMed]
- Khairunisa, S.Q.; Amarullah, I.H.; Churrotin, S.; Fitria, A.L.; Amin, M.; Lusida, M.I.; Soegijanto, S. Potential misdiagnosis between COVID-19 and dengue infection using rapid serological test. Infect. Dis. Rep. 2021, 13, 540–551. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Nath, H.; Mallick, A.; Roy, S.; Sukla, S.; Basu, K.; De, A.; Biswas, S. Archived dengue serum samples produced false-positive results in SARS-CoV-2 lateral flow-based rapid antibody tests. J. Med. Microbiol. 2021, 70, 001369. [Google Scholar] [CrossRef] [PubMed]
- Katzelnick, L.C.; Gresh, L.; Halloran, M.E.; Mercado, J.C.; Kuan, G.; Gordon, A.; Balmaseda, A.; Harris, E. Antibody-dependent enhancement of severe dengue disease in humans. Science 2017, 358, 929–932. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, A.; Imad, H.A.; Phumratanaprapin, W.; Phadungsombat, J.; Konishi, E.; Shioda, T. Antibody-dependent enhancement representing in vitro infective progeny virus titer correlates with the viremia level in dengue patients. Sci. Rep. 2021, 11, 12354. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, O.; Nechipurenko, Y.; Lagutkin, D.; Yegorov, Y.E.; Kzhyshkowska, J. SARS-CoV-2 infection of phagocytic immune cells and COVID-19 pathology: Antibody-dependent as well as independent cell entry. Front. Immunol. 2022, 13, 1050478. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Parry, B.; Ravid, S.; Clark, S.; Schrimpf, M.R.; et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 2022, 606, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, E.E.; Shioda, T. SARS-CoV-2 Related antibody-dependent enhancement phenomena in vitro and in vivo. Microorganisms 2023, 11, 1015. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J.; Yu, X.; Jiang, W.; Chen, S.; Wang, R.; Wang, M.; Jiao, S.; Yang, Y.; Wang, W.; et al. Antibody-dependent enhancement (ADE) of SARS-CoV-2 pseudoviral infection requires FcγRIIB and virus-antibody complex with bivalent interaction. Commun. Biol. 2022, 5, 262. [Google Scholar] [CrossRef] [PubMed]
- Ajmeriya, S.; Kumar, A.; Karmakar, S.; Rana, S.; Singh, H. Neutralizing antibodies and antibody-dependent enhancement in COVID-19: A perspective. J. Indian. Inst. Sci. 2022, 102, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Edwards, R.J.; Manne, K.; Martinez, D.R.; Schäfer, A.; Alam, S.M.; Wiehe, K.; Lu, X.; Parks, R.; Sutherland, L.L.; et al. In vitro and in vivo functions of SARS-CoV-2 infection-enhancing and neutralizing antibodies. Cell 2021, 184, 4203–4219. [Google Scholar] [CrossRef] [PubMed]
- Matveev, A.; Pyankov, O.; Khlusevich, Y.; Tyazhelkova, O.; Emelyanova, L.; Timofeeva, A.; Shipovalov, A.; Chechushkov, A.; Zaitseva, N.; Kudrov, G.; et al. Antibodies Capable of Enhancing SARS-CoV-2 Infection Can Circulate in Patients with Severe COVID-19. Int. J. Mol. Sci. 2023, 24, 10799. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Smatti, M.K.; Alsulaiti, H.; Zedan, H.T.; Eid, A.H.; Hssain, A.A.; Abu Raddad, L.J.; Gentilcore, G.; Ouhtit, A.; Althani, A.A. Antibody-dependent enhancement (ADE) of SARS-CoV-2 in patients exposed to MERS-CoV and SARS-CoV-2 antigens. J. Med. Virol. 2024, 96, e29628. [Google Scholar] [CrossRef]
- Ikewaki, N.; Kurosawa, G.; Levy, G.A.; Preethy, S.; Abraham, S.J.K. Antibody-dependent disease enhancement (ADE) after COVID-19 vaccination and beta-glucans as a safer strategy in management. Vaccine 2023, 41, 2427–2429. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.E.S.; Lechuga, G.C.; Napoleão-Pêgo, P.; Carvalho, J.P.R.S.; Gomes, L.R.; Morel, C.M.; Provance, D.W.; De-Simone, S.G. Humoral immune response to SARS-CoV-2 spike protein receptor-binding motif linear epitopes. Vaccines 2024, 12, 342. [Google Scholar] [CrossRef]
- De-Simone, S.G.; Gomes, L.R.; Napoleão-Pêgo, P.; Lechuga, G.C.; Pina, J.C.; Silva, F.R. Identification of linear B epitopes liable for the protective immunity of diphtheria toxin. Vaccines 2021, 9, 313. [Google Scholar] [CrossRef]
- Napoleao-Pego, P.; Carneiro, F.G.; Durans, A.M.; Gomes, L.R.; Morel, C.M.; Provance, D.W., Jr.; De-Simone, S.G. Performance assessment of a multi-epitope chimeric antigen for the serodiagnosis of acute phase of Mayaro fever. Sci. Rep. 2021, 11, 15374. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef] [PubMed]
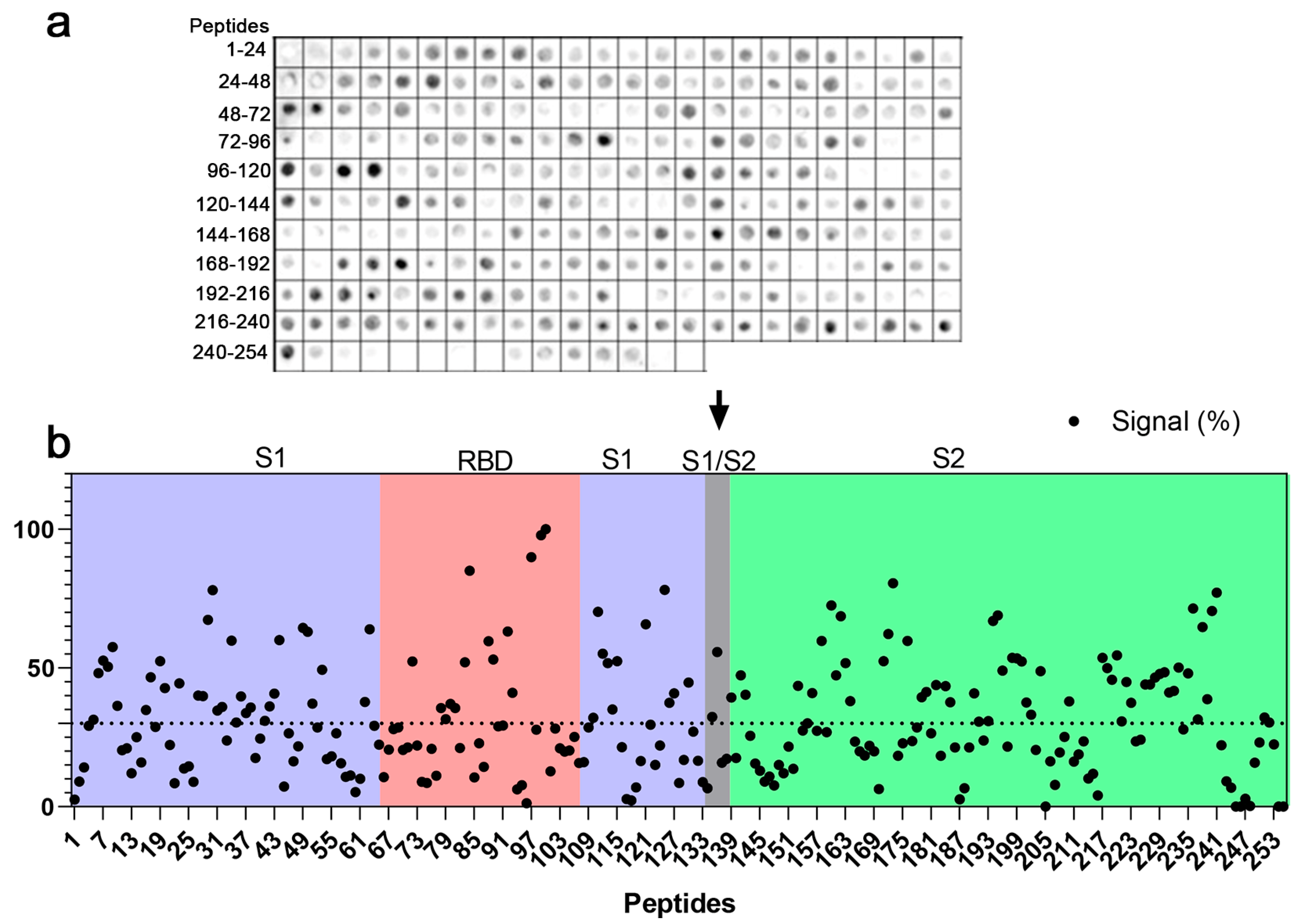
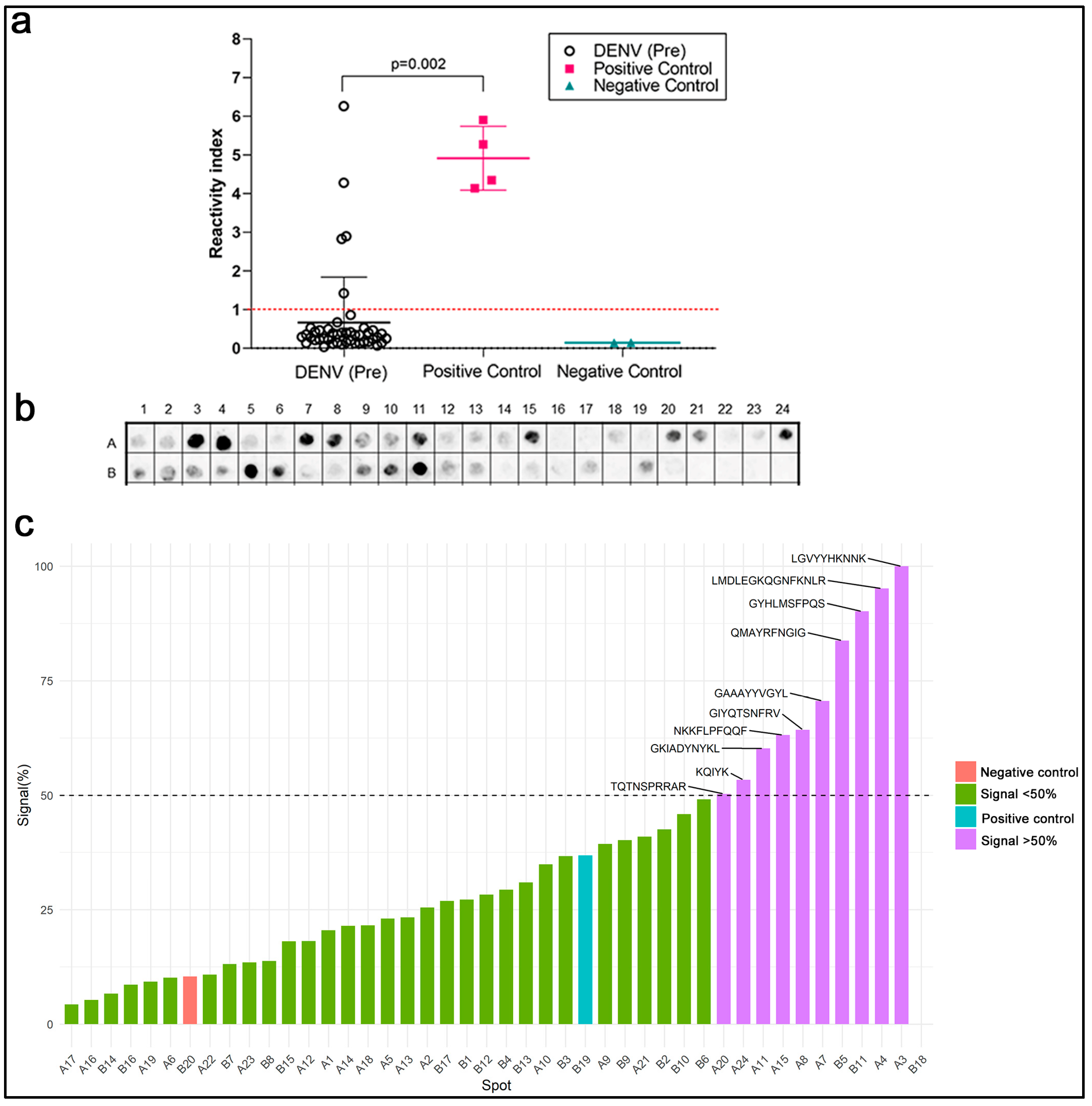


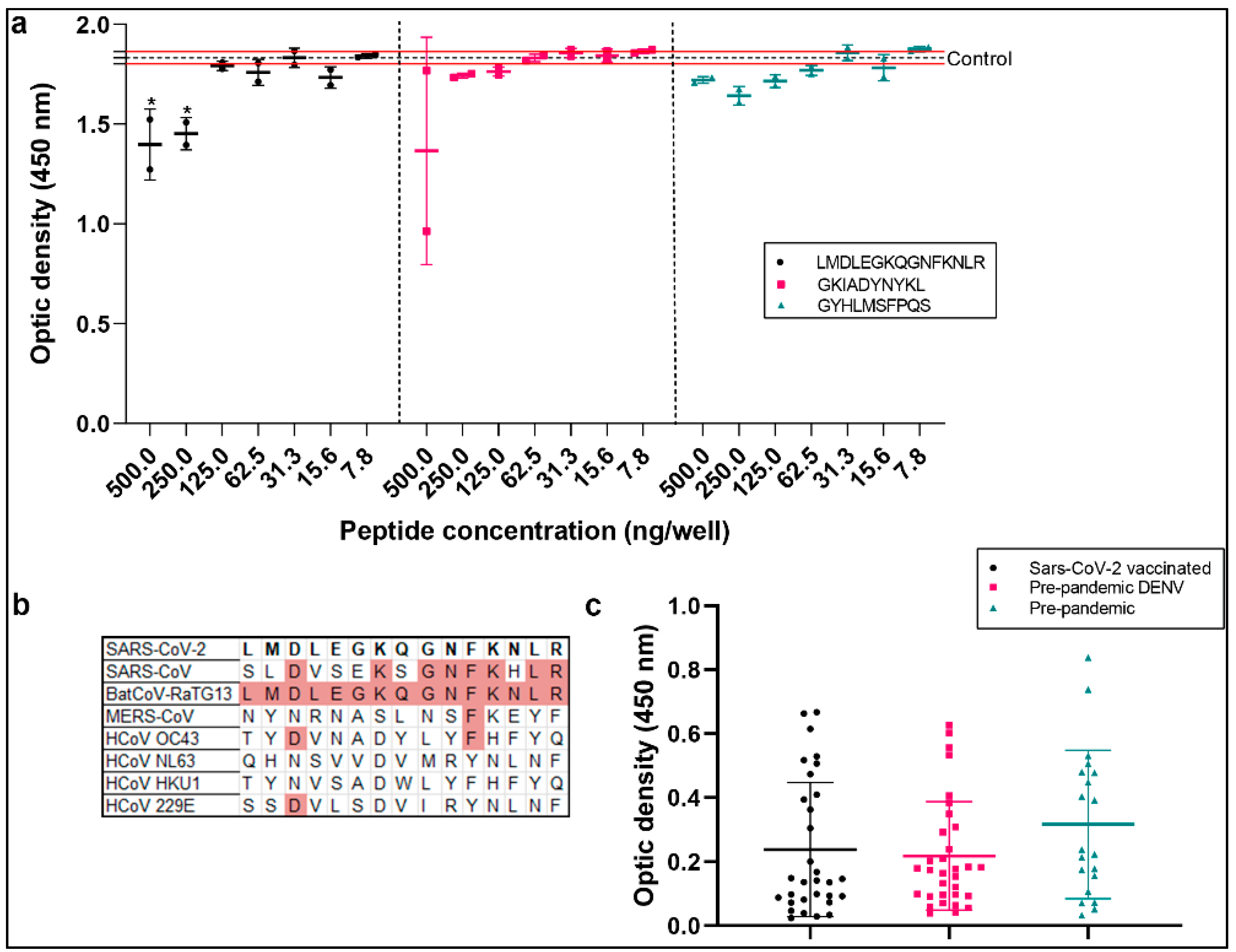
| Code | aa Position | Sequence | Domain |
|---|---|---|---|
| CV19/SG/01huG | 86–100 | FNDGVYFASTEKSNI | S1/NTD |
| CV19/SG/02huG | 111–125 | DSKTQSLLIVNNATN | S1/NTD |
| CV19/SG/03hu1G | 141–150 | LGVYYHKNNK | S1/NTD |
| CV19/SG/04huG | 176–190 | LMDLEGKQGNFKNLR | S1/NTD |
| CV19/SG/05huG | 211–220 | NLVRDLPQGF | S1/NTD |
| CV19/SG/06huG | 246–256 | RSYLTPGDSSS | S1/NTD |
| CV19/SG/07huG | 261–270 | GARVEY | S1/NTD |
| CV19/SG/08huG | 311–320 | GIYQTSNFRV | S1/RBD |
| CV19/SG/09huG | 355–364 | KRISNCVADYSVLYN | S1/RBD |
| CV19/SG/10huG | 396–404 | YADSFVIRGD | S1/RBD |
| CV19/SG/11huG | 416–425 | GKIADYNYKL | S1/RBD |
| CV19/SG/12huG | 441–450 | LDSKVGGNYN | S1/RBD-RBM |
| CV19/SG/13huG | 461–470 | LKPFERDIST | S1/RBD-RBM |
| CV19/SG/14huG | 491–505 | PLQSYGFQPT | S1/RBD-RBM |
| CV19/SG/15huG | 556–564 | NKKFLPFQQF | S1/SD1 |
| CV19/SG/16huG | 571–575 | DTTDAVRDPQ | S1/SD1 |
| CV19/SG/17huG | 606–615 | NQVAVLYQDV | S1/SD2 |
| CV19/SG/18huG | 626–635 | ADQLTPTWRV | S1/SD2 |
| CV19/SG/19huG | 651–660 | IGAEHVNNSY | S1/SD2 |
| CV19/SG/20huG | 676–686 | TQTNSPRRAR | Furin cleavage site |
| CV19/SG/21huG | 691–699 | SIIAYTMSL | S2 |
| CV19/SG/22huG | 706–714 | AYSNNSIAIP | S2 |
| CV19/SG/23huG | 771–775 | AVEGD | S2 |
| CV19/SG/24huG | 786–789 | KQIYK | S2 |
| CV19/SG/25huG | 796–800 | DFGGF | S2 |
| CV19/SG/26huG | 806–820 | LPDPSKPSKRSFIED | TMPRSS2 cleavage site and FP1 |
| CV19/SG/27huG | 861–866 | LPPLL | S2 |
| CV19/SG/28huG | 876–890 | ALLAGTITSGWTFGA | S2 |
| CV19/SG/29huG | 901–910 | QMAYRFNGIG | S2 |
| CV19/SG/30huG | 920–929 | KLIANGFNSA | S2/HR1 |
| CV19/SG/31huG | 951–960 | VVNQNAQALN | S2/HR1 |
| CV19/SG/32huG | 971–980 | GAISSVLNDI | S2/HR1 |
| CV19/SG/33huG | 996–1105 | LITGRLQSLQ | S2 |
| CV19/SG/34huG | 1016–1020 | AEIRA | S2 |
| CV19/SG/35huG | 1046–1055 | GYHLMSFPQS | S2 |
| CV19/SG/36huG | 1091–1105 | REGVFVSNGTHW | S2 |
| CV19/SG/37huG | 1111–1115 | EPQII | S2 |
| CV19/SG/38huG | 1136–1145 | TVYDPLQPEL | S2 |
| CV19/SG/39huG | 1181–1190 | KEIDRLNEVK | HR2 |
| CV19/SG/40huG | 1196–1205 | SLIDLQELGK | HR2 |
| CV19/SG/41huG | 1256–1265 | FDEDDSEPVI | CTD |
| Signal (%) | Epitope | aa Position | Sequence | Identity | Serotype | Protein |
|---|---|---|---|---|---|---|
| 100 | LGVYYHKNNK | 141–150 | LGVY | 75% | DENV2 | Polyprotein, RdRp |
| 95.1 | LMDLEGKQGNFKNLR | 176–190 | MDLE | 100% | DENV2 | Envelope protein |
| 70.5 | GAAAYYVGYL | 261–270 | YVGYL | 100% | DENV2 | NS5 |
| 64.3 | GIYQTSNFRV | 311–320 | NFRV | 100% | DENV1 | Polyprotein, Helicase |
| 64.3 | GIYQTSNFRV | 311–320 | YQTS | 71% | DENV2 and 3 | Polyprotein, DEAD domain |
| 60.2 | GKIADYNYKL | 416–425 | GKIA | 100% | DENV1 and 2 | Envelope protein, partial |
| 60.2 | GKIADYNYKL | 416–425 | KIAD | 100% | DENV1 | Polyprotein, NS5 |
| 63.2 | NKKFLPFQQF | 556–564 | KFLP | 100% | DENV2 | Polyprotein, NS1 |
| 50.3 | TQTNSPRRAR | 676–686 | SPRR | 100% | DENV1 | Polyprotein, NS1 |
| 50.3 | TQTNSPRRAR | 676–686 | PRRA | 100% | DENV1, 2 and 3 | Polyprotein, NS5 and RdRp |
| 53.3 | KQIYK | 786–789 | QIYK | 100% | DENV2 | Polyprotein, NS4B |
| 90.1 | GYHLMSFPQS | 1046–1055 | SFPQS | 100% | DENV1, 2 and 4 | Polyprotein, NS3 |
| 90.1 | GYHLMSFPQS | 1046–1055 | MSFP | 100% | DENV3 | Polyprotein, Envelope protein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lechuga, G.C.; Temerozo, J.R.; Napoleão-Pêgo, P.; Carvalho, J.P.R.S.; Gomes, L.R.; Bou-Habib, D.C.; Morel, C.M.; Provance, D.W., Jr.; Souza, T.M.L.; De-Simone, S.G. Enhanced Assessment of Cross-Reactive Antigenic Determinants within the Spike Protein. Int. J. Mol. Sci. 2024, 25, 8180. https://doi.org/10.3390/ijms25158180
Lechuga GC, Temerozo JR, Napoleão-Pêgo P, Carvalho JPRS, Gomes LR, Bou-Habib DC, Morel CM, Provance DW Jr., Souza TML, De-Simone SG. Enhanced Assessment of Cross-Reactive Antigenic Determinants within the Spike Protein. International Journal of Molecular Sciences. 2024; 25(15):8180. https://doi.org/10.3390/ijms25158180
Chicago/Turabian StyleLechuga, Guilherme C., Jairo R. Temerozo, Paloma Napoleão-Pêgo, João P. R. S. Carvalho, Larissa R. Gomes, Dumith Chequer Bou-Habib, Carlos M. Morel, David W. Provance, Jr., Thiago M. L. Souza, and Salvatore G. De-Simone. 2024. "Enhanced Assessment of Cross-Reactive Antigenic Determinants within the Spike Protein" International Journal of Molecular Sciences 25, no. 15: 8180. https://doi.org/10.3390/ijms25158180
APA StyleLechuga, G. C., Temerozo, J. R., Napoleão-Pêgo, P., Carvalho, J. P. R. S., Gomes, L. R., Bou-Habib, D. C., Morel, C. M., Provance, D. W., Jr., Souza, T. M. L., & De-Simone, S. G. (2024). Enhanced Assessment of Cross-Reactive Antigenic Determinants within the Spike Protein. International Journal of Molecular Sciences, 25(15), 8180. https://doi.org/10.3390/ijms25158180









