Rare Germline Variants in the Adenomatous Polyposis Coli Gene Associated with Dental and Osseous Anomalies
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Literature Search and Keyword Specification
4.2. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanson, C.A.; Miller, J.R. Non-traditional roles for the Adenomatous Polyposis Coli (APC) tumor suppressor protein. Gene 2005, 361, 1–12. [Google Scholar] [CrossRef]
- Smith, K.J.; Johnson, K.A.; Bryan, T.M.; Hill, D.E.; Markowitz, S.; Willson, J.K.; Paraskeva, C.; Petersen, G.M.; Hamilton, S.R.; Vogelstein, B.; et al. The APC gene product in normal and tumor cells. Proc. Natl. Acad. Sci. USA 1993, 90, 2846–2850. [Google Scholar] [CrossRef]
- Faux, M.C.; Ross, J.L.; Meeker, C.; Johns, T.; Ji, H.; Simpson, R.J.; Layton, M.J.; Burgess, A.W. Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion. J. Cell Sci. 2004, 117, 427–439. [Google Scholar] [CrossRef]
- Pollack, A.L.; Barth, A.I.; Altschuler, Y.; Nelson, W.J.; Mostov, K.E. Dynamics of beta-catenin interactions with APC protein regulate epithelial tubulogenesis. J. Cell Biol. 1997, 137, 1651–1662. [Google Scholar] [CrossRef]
- Wen, Y.; Eng, C.H.; Schmoranzer, J.; Cabrera-Poch, N.; Morris, E.J.; Chen, M.; Wallar, B.J.; Alberts, A.S.; Gundersen, G.G. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat. Cell Biol. 2004, 6, 820–830. [Google Scholar] [CrossRef]
- Groden, J.; Thliveris, A.; Samowitz, W.; Carlson, M.; Gelbert, L.; Albertsen, H.; Joslyn, G.; Stevens, J.; Spirio, L.; Robertson, M.; et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991, 66, 589–600. [Google Scholar] [CrossRef]
- Hankey, W.; Frankel, W.L.; Groden, J. Functions of the APC tumor suppressor protein dependent and independent of canonical WNT signaling: Implications for therapeutic targeting. Cancer Metastasis Rev. 2018, 37, 159–172. [Google Scholar] [CrossRef]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer. J. Natl. Cancer Inst. 2017, 109, djw332. [Google Scholar] [CrossRef]
- Al-Tameemi, H.K.; Al-Husseini, R.M.; Al-Mudhafer, R.H.; Abid, H.A.; Al-Gazali, H.R.; Abdullah, D.A.A.; Albaldawy, M.T. Molecular and immunohistochemical study of APC exon 16 and its possible role in colorectal carcinoma development. Heliyon 2024, 10, e23443. [Google Scholar] [CrossRef]
- Bisgaard, M.L.; Fenger, K.; Bulow, S.; Niebuhr, E.; Mohr, J. Familial adenomatous polyposis (FAP): Frequency, penetrance, and mutation rate. Hum. Mutat. 1994, 3, 121–125. [Google Scholar] [CrossRef]
- Aretz, S.; Stienen, D.; Uhlhaas, S.; Pagenstecher, C.; Mangold, E.; Caspari, R.; Propping, P.; Friedl, W. Large submicroscopic genomic APC deletions are a common cause of typical familial adenomatous polyposis. J. Med. Genet. 2005, 42, 185–192. [Google Scholar] [CrossRef]
- Fearnhead, N.S.; Wilding, J.L.; Bodmer, W.F. Genetics of colorectal cancer: Hereditary aspects and overview of colorectal tumorigenesis. Br. Med. Bull. 2002, 64, 27–43. [Google Scholar] [CrossRef]
- Bronner, M.P. Gastrointestinal inherited polyposis syndromes. Mod. Pathol. 2003, 16, 359–365. [Google Scholar] [CrossRef]
- Lamlum, H.; Papadopoulou, A.; Ilyas, M.; Rowan, A.; Gillet, C.; Hanby, A.; Talbot, I.; Bodmer, W.; Tomlinson, I. APC mutations are sufficient for the growth of early colorectal adenomas. Proc. Natl. Acad. Sci. USA 2000, 97, 2225–2228. [Google Scholar] [CrossRef]
- Debinski, H.S.; Love, S.; Spigelman, A.D.; Phillips, R.K. Colorectal polyp counts and cancer risk in familial adenomatous polyposis. Gastroenterology 1996, 110, 1028–1030. [Google Scholar] [CrossRef]
- Antohi, C.; Haba, D.; Caba, L.; Ciofu, M.L.; Drug, V.L.; Barboi, O.B.; Dobrovat, B.I.; Panzaru, M.C.; Gorduza, N.C.; Lupu, V.V.; et al. Novel Mutation in APC Gene Associated with Multiple Osteomas in a Family and Review of Genotype-Phenotype Correlations of Extracolonic Manifestations in Gardner Syndrome. Diagnostics 2021, 11, 1560. [Google Scholar] [CrossRef]
- Fearnhead, N.S.; Britton, M.P.; Bodmer, W.F. The ABC of APC. Hum. Mol. Genet. 2001, 10, 721–733. [Google Scholar] [CrossRef]
- Galiatsatos, P.; Foulkes, W.D. Familial adenomatous polyposis. Am. J. Gastroenterol. 2006, 101, 385–398. [Google Scholar] [CrossRef]
- Spirio, L.; Olschwang, S.; Groden, J.; Robertson, M.; Samowitz, W.; Joslyn, G.; Gelbert, L.; Thliveris, A.; Carlson, M.; Otterud, B.; et al. Alleles of the APC gene: An attenuated form of familial polyposis. Cell 1993, 75, 951–957. [Google Scholar] [CrossRef]
- Hernegger, G.S.; Moore, H.G.; Guillem, J.G. Attenuated familial adenomatous polyposis: An evolving and poorly understood entity. Dis. Colon. Rectum 2002, 45, 127–134, discussion 134–126. [Google Scholar] [CrossRef]
- Gonzalez, L.; Alvarez, J.; Weinstein, E.; Korenis, P. Familial adenomatous polyposis in an adolescent with coexisting schizophrenia: Treatment strategies and implications. Mol. Genet. Genom. Med. 2015, 3, 391–395. [Google Scholar] [CrossRef][Green Version]
- Mohn, J.L.; Alexander, J.; Pirone, A.; Palka, C.D.; Lee, S.Y.; Mebane, L.; Haydon, P.G.; Jacob, M.H. Adenomatous polyposis coli protein deletion leads to cognitive and autism-like disabilities. Mol. Psychiatry 2014, 19, 1133–1142. [Google Scholar] [CrossRef]
- Onouchi, T.; Kobayashi, K.; Sakai, K.; Shimomura, A.; Smits, R.; Sumi-Ichinose, C.; Kurosumi, M.; Takao, K.; Nomura, R.; Iizuka-Kogo, A.; et al. Targeted deletion of the C-terminus of the mouse adenomatous polyposis coli tumor suppressor results in neurologic phenotypes related to schizophrenia. Mol. Brain 2014, 7, 21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gardner, E.J. A genetic and clinical study of intestinal polyposis, a predisposing factor for carcinoma of the colon and rectum. Am. J. Hum. Genet. 1951, 3, 167–176. [Google Scholar]
- Avila, S.A.; Nguyen, G.; Wojno, T.; Kim, H.J. Orbital osteomas associated with Gardner’s syndrome: A case presentation and review of literature. Orbit 2024, 43, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Yao, X.; Pan, Y.; Gu, H.; Xiong, F.; Yin, X.; Wu, B.; Chen, T. A novel APC mutation associated with Gardner syndrome in a Chinese family. Gene 2024, 896, 148051. [Google Scholar] [CrossRef] [PubMed]
- Dawlatly, E.D.; Al-Qurain, A.A.; Salih-Mahmud, M. Gardner’s syndrome presenting with a giant osteoma. Ann. Saudi Med. 1997, 17, 542–544. [Google Scholar] [CrossRef]
- Madani, M.; Madani, F. Gardner’s syndrome presenting with dental complaints. Arch. Iran. Med. 2007, 10, 535–539. [Google Scholar]
- Fotiadis, C.; Tsekouras, D.K.; Antonakis, P.; Sfiniadakis, J.; Genetzakis, M.; Zografos, G.C. Gardner’s syndrome: A case report and review of the literature. World J. Gastroenterol. 2005, 11, 5408–5411. [Google Scholar] [CrossRef]
- Dinarvand, P.; Davaro, E.P.; Doan, J.V.; Ising, M.E.; Evans, N.R.; Phillips, N.J.; Lai, J.; Guzman, M.A. Familial Adenomatous Polyposis Syndrome: An Update and Review of Extraintestinal Manifestations. Arch. Pathol. Lab. Med. 2019, 143, 1382–1398. [Google Scholar] [CrossRef]
- Davies, A.S. Gardner’s syndrome--a case report. Br. J. Oral Surg. 1970, 8, 51–57. [Google Scholar] [CrossRef]
- Gardner, E.J.; Richards, R.C. Multiple cutaneous and subcutaneous lesions occurring simultaneously with hereditary polyposis and osteomatosis. Am. J. Hum. Genet. 1953, 5, 139–147. [Google Scholar]
- Yu, D.; Ng Cw, B.; Zhu, H.; Liu, J.; Lin, Y. Bone and dental abnormalities as first signs of familial Gardner’s syndrome in a Chinese family: A literature review and a case report. Med. Sci. (Paris) 2018, 34, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Cristofaro, M.G.; Giudice, A.; Amantea, M.; Riccelli, U.; Giudice, M. Gardner’s syndrome: A clinical and genetic study of a family. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e1-6. [Google Scholar] [CrossRef] [PubMed]
- Ida, M.; Nakamura, T.; Utsunomiya, J. Osteomatous changes and tooth abnormalities found in the jaw of patients with adenomatosis coli. Oral Surg. Oral Med. Oral Pathol. 1981, 52, 2–11. [Google Scholar] [CrossRef]
- Nandakumar, G.; Morgan, J.A.; Silverberg, D.; Steinhagen, R.M. Familial polyposis coli: Clinical manifestations, evaluation, management and treatment. Mt. Sinai J. Med. 2004, 71, 384–391. [Google Scholar] [PubMed]
- Newman, C.A.; Reuther, W.L., 3rd; Wakabayashi, M.N.; Payette, M.M.; Plavsic, B.M. Gastrointestinal case of the day. Gardner syndrome. Radiographics 1999, 19, 546–548. [Google Scholar] [CrossRef][Green Version]
- Beroud, C.; Soussi, T. APC gene: Database of germline and somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1996, 24, 121–124. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Nagase, H.; Ando, H.; Horii, A.; Ichii, S.; Nakatsuru, S.; Aoki, T.; Miki, Y.; Mori, T.; Nakamura, Y. Somatic mutations of the APC gene in colorectal tumors: Mutation cluster region in the APC gene. Hum. Mol. Genet. 1992, 1, 229–233. [Google Scholar] [CrossRef]
- Davies, D.R.; Armstrong, J.G.; Thakker, N.; Horner, K.; Guy, S.P.; Clancy, T.; Sloan, P.; Blair, V.; Dodd, C.; Warnes, T.W.; et al. Severe Gardner syndrome in families with mutations restricted to a specific region of the APC gene. Am. J. Hum. Genet. 1995, 57, 1151–1158. [Google Scholar]
- Newton, K.F.; Mallinson, E.K.; Bowen, J.; Lalloo, F.; Clancy, T.; Hill, J.; Evans, D.G. Genotype-phenotype correlation in colorectal polyposis. Clin. Genet. 2012, 81, 521–531. [Google Scholar] [CrossRef]
- Septer, S.; Bohaty, B.; Onikul, R.; Kumar, V.; Williams, K.B.; Attard, T.M.; Friesen, C.A.; Friesen, L.R. Dental anomalies in pediatric patients with familial adenomatous polyposis. Fam. Cancer 2018, 17, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.T.; Leite, A.F.; de Souza Figueiredo, P.T.; Dos Santos, P.A.C.; Rosa, E.; Mazzeu, J.F.; Sousa, J.B.; Pogue, R.; Acevedo, A.C.; Guerra, E.N.S. Dento-osseous anomalies in patients with familial adenomatous polyposis: A follow-up study. Clin. Oral Investig. 2020, 24, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Seehra, J.; Patel, S.; Bryant, C. Gardner’s Syndrome revisited: A clinical case and overview of the literature. J. Orthod. 2016, 43, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Kerr, S.E.; Thomas, C.B.; Thibodeau, S.N.; Ferber, M.J.; Halling, K.C. APC germline mutations in individuals being evaluated for familial adenomatous polyposis: A review of the Mayo Clinic experience with 1591 consecutive tests. J Mol Diagn 2013, 15, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Nishisho, I.; Nakamura, Y.; Miyoshi, Y.; Miki, Y.; Ando, H.; Horii, A.; Koyama, K.; Utsunomiya, J.; Baba, S.; Hedge, P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science 1991, 253, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Arruda, K.A.R.; Normando, A.G.C.; Pacheco-Pereira, C.; Amorim Dos Santos, J.; Yamaguti, P.M.; Mazzeu, J.F.; Almeida, F.T.; Acevedo, A.C.; Guerra, E.N.S. Phenotypic dento-osseous characterization of a Brazilian family with Familial Adenomatous Polyposis. Arch. Oral Biol. 2021, 129, 105206. [Google Scholar] [CrossRef] [PubMed]
- Eccles, D.M.; Lunt, P.W.; Wallis, Y.; Griffiths, M.; Sandhu, B.; McKay, S.; Morton, D.; Shea-Simonds, J.; Macdonald, F. An unusually severe phenotype for familial adenomatous polyposis. Arch. Dis. Child. 1997, 77, 431–435. [Google Scholar] [CrossRef]
- Soravia, C.; Sugg, S.L.; Berk, T.; Mitri, A.; Cheng, H.; Gallinger, S.; Cohen, Z.; Asa, S.L.; Bapat, B.V. Familial adenomatous polyposis-associated thyroid cancer: A clinical, pathological, and molecular genetics study. Am. J. Pathol. 1999, 154, 127–135. [Google Scholar] [CrossRef]
- Panyarat, C.; Nakornchai, S.; Chintakanon, K.; Leelaadisorn, N.; Intachai, W.; Olsen, B.; Tongsima, S.; Adisornkanj, P.; Ngamphiw, C.; Cox, T.C.; et al. Rare Genetic Variants in Human APC Are Implicated in Mesiodens and Isolated Supernumerary Teeth. Int. J. Mol. Sci. 2023, 24, 4255. [Google Scholar] [CrossRef]
- Pereira, D.L.; Carvalho, P.A.; Achatz, M.I.; Rocha, A.; TardinTorrezan, G.; Alves, F.A. Oral and maxillofacial considerations in Gardner’s syndrome: A report of two cases. Ecancermedicalscience 2016, 10, 623. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Cai, W.; Jiang, B.; Xu, L.; Liu, S.; Zhao, S. A novel mutation of adenomatous polyposis coli (APC) gene results in the formation of supernumerary teeth. J. Cell Mol. Med. 2018, 22, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Martin-Denavit, T.; Duthel, S.; Giraud, S.; Olschwang, S.; Saurin, J.C.; Plauchu, H. Phenotype variability of two FAP families with an identical APC germline mutation at codon 1465: A potential modifier effect? Clin. Genet. 2001, 60, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; He, F.; Xu, X.; Xiong, F.; Zhang, L. APC c.4621C>T variant causing Gardner’s syndrome in a Han Chinese family may be inherited through maternal mosaicism. Exp. Ther. Med. 2021, 21, 488. [Google Scholar] [CrossRef] [PubMed]
- Nilbert, M.; Fernebro, J.; Kristoffersson, U. Novel germline APC mutations in Swedish patients with familial adenomatous polyposis and Gardner syndrome. Scand. J. Gastroenterol. 2000, 35, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- Oku, T.; Takayama, T.; Sato, Y.; Sato, Y.; Takada, K.; Hayashi, T.; Takahashi, M.; Kuroda, M.; Kato, J.; Niitsu, Y. A case of Gardner syndrome with a mutation at codon 1556 of APC: A suggested case of genotype-phenotype correlation in dental abnormality. Eur. J. Gastroenterol. Hepatol. 2004, 16, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Antal, G.; Zsigmond, A.; Till, A.; Orsi, E.; Szanto, I.; Buki, G.; Kereskai, L.; Herbert, Z.; Hadzsiev, K.; Bene, J. Case report: Initial atypical skeletal symptoms and dental anomalies as first signs of Gardner syndrome: The importance of genetic analysis in the early diagnosis. Pathol. Oncol. Res. 2024, 30, 1611768. [Google Scholar] [CrossRef]
- Aggarwal, V.R.; Sloan, P.; Horner, K.; Macfarlane, T.V.; Clancy, T.; Evans, G.; Thakker, N. Dento-osseous changes as diagnostic markers in familial adenomatous polyposis families. Oral Dis. 2003, 9, 29–33. [Google Scholar] [CrossRef]
- D’Agostino, S.; Dell’Olio, F.; Tempesta, A.; Cervinara, F.; D’Amati, A.; Dolci, M.; Favia, G.; Capodiferro, S.; Limongelli, L. Osteoma of the Jaw as First Clinical Sign of Gardner’s Syndrome: The Experience of Two Italian Centers and Review. J. Clin. Med. 2023, 12, 1496. [Google Scholar] [CrossRef]
- Thakker, N.; Davies, R.; Horner, K.; Armstrong, J.; Clancy, T.; Guy, S.; Harris, R.; Sloan, P.; Evans, G. The dental phenotype in familial adenomatous polyposis: Diagnostic application of a weighted scoring system for changes on dental panoramic radiographs. J. Med. Genet. 1995, 32, 458–464. [Google Scholar] [CrossRef][Green Version]
- Almeida, F.T.; Pacheco-Pereira, C.; Porporatti, A.L.; Flores-Mir, C.; Leite, A.F.; De Luca Canto, G.; Guerra, E.N. Oral manifestations in patients with familial adenomatous polyposis: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Bulow, S.; Sondergaard, J.O.; Witt, I.; Larsen, E.; Tetens, G. Mandibular osteomas in familial polyposis coli. Dis. Colon Rectum 1984, 27, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Katou, F.; Motegi, K.; Baba, S. Mandibular lesions in patients with adenomatosis coli. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 1989, 17, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Wijn, M.A.; Keller, J.J.; Giardiello, F.M.; Brand, H.S. Oral and maxillofacial manifestations of familial adenomatous polyposis. Oral Dis. 2007, 13, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Budzynska, A. Dentistry anomalies in patients with Lynch syndrome and familial adenomatous polyposis. Ann. Acad. Medicae Stetin. 2008, 54, 106–111. [Google Scholar]
- Bertario, L.; Russo, A.; Sala, P.; Eboli, M.; Giarola, M.; D’Amico, F.; Gismondi, V.; Varesco, L.; Pierotti, M.A.; Radice, P.; et al. Genotype and phenotype factors as determinants of desmoid tumors in patients with familial adenomatous polyposis. Int. J. Cancer 2001, 95, 102–107. [Google Scholar] [CrossRef]
- Bertario, L.; Russo, A.; Sala, P.; Varesco, L.; Giarola, M.; Mondini, P.; Pierotti, M.; Spinelli, P.; Radice, P.; Hereditary Colorectal Tumor, R. Multiple approach to the exploration of genotype-phenotype correlations in familial adenomatous polyposis. J. Clin. Oncol. 2003, 21, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Bisgaard, M.L.; Bulow, S. Familial adenomatous polyposis (FAP): Genotype correlation to FAP phenotype with osteomas and sebaceous cysts. Am. J. Med. Genet. A 2006, 140, 200–204. [Google Scholar] [CrossRef]
- Miyoshi, Y.; Ando, H.; Nagase, H.; Nishisho, I.; Horii, A.; Miki, Y.; Mori, T.; Utsunomiya, J.; Baba, S.; Petersen, G.; et al. Germ-line mutations of the APC gene in 53 familial adenomatous polyposis patients. Proc. Natl. Acad. Sci. USA 1992, 89, 4452–4456. [Google Scholar] [CrossRef]
- Fang, X.; Svitkina, T.M. Adenomatous Polyposis Coli (APC) in cell migration. Eur. J. Cell Biol. 2022, 101, 151228. [Google Scholar] [CrossRef]
- Wang, X.P.; O’Connell, D.J.; Lund, J.J.; Saadi, I.; Kuraguchi, M.; Turbe-Doan, A.; Cavallesco, R.; Kim, H.; Park, P.J.; Harada, H.; et al. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development 2009, 136, 1939–1949. [Google Scholar] [CrossRef] [PubMed]
- Hermans, F.; Hemeryck, L.; Lambrichts, I.; Bronckaers, A.; Vankelecom, H. Intertwined Signaling Pathways Governing Tooth Development: A Give-and-Take Between Canonical Wnt and Shh. Front. Cell Dev. Biol. 2021, 9, 758203. [Google Scholar] [CrossRef] [PubMed]
- Thesleff, I.; Sharpe, P. Signalling networks regulating dental development. Mech. Dev. 1997, 67, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Honma, T.; Matsuda, Y.; Suzuki, Y.; Narisawa, R.; Ajioka, Y.; Asakura, H. Nuclear translocation of beta-catenin in colorectal cancer. Br. J. Cancer 2000, 82, 1689–1693. [Google Scholar] [CrossRef] [PubMed]
- Barua, D.; Hlavacek, W.S. Modeling the effect of APC truncation on destruction complex function in colorectal cancer cells. PLoS Comput. Biol. 2013, 9, e1003217. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.R.; Hocking, A.M.; Brown, J.D.; Moon, R.T. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene 1999, 18, 7860–7872. [Google Scholar] [CrossRef]
- Akiyama, T. Wnt/beta-catenin signaling. Cytokine Growth Factor. Rev. 2000, 11, 273–282. [Google Scholar] [CrossRef]
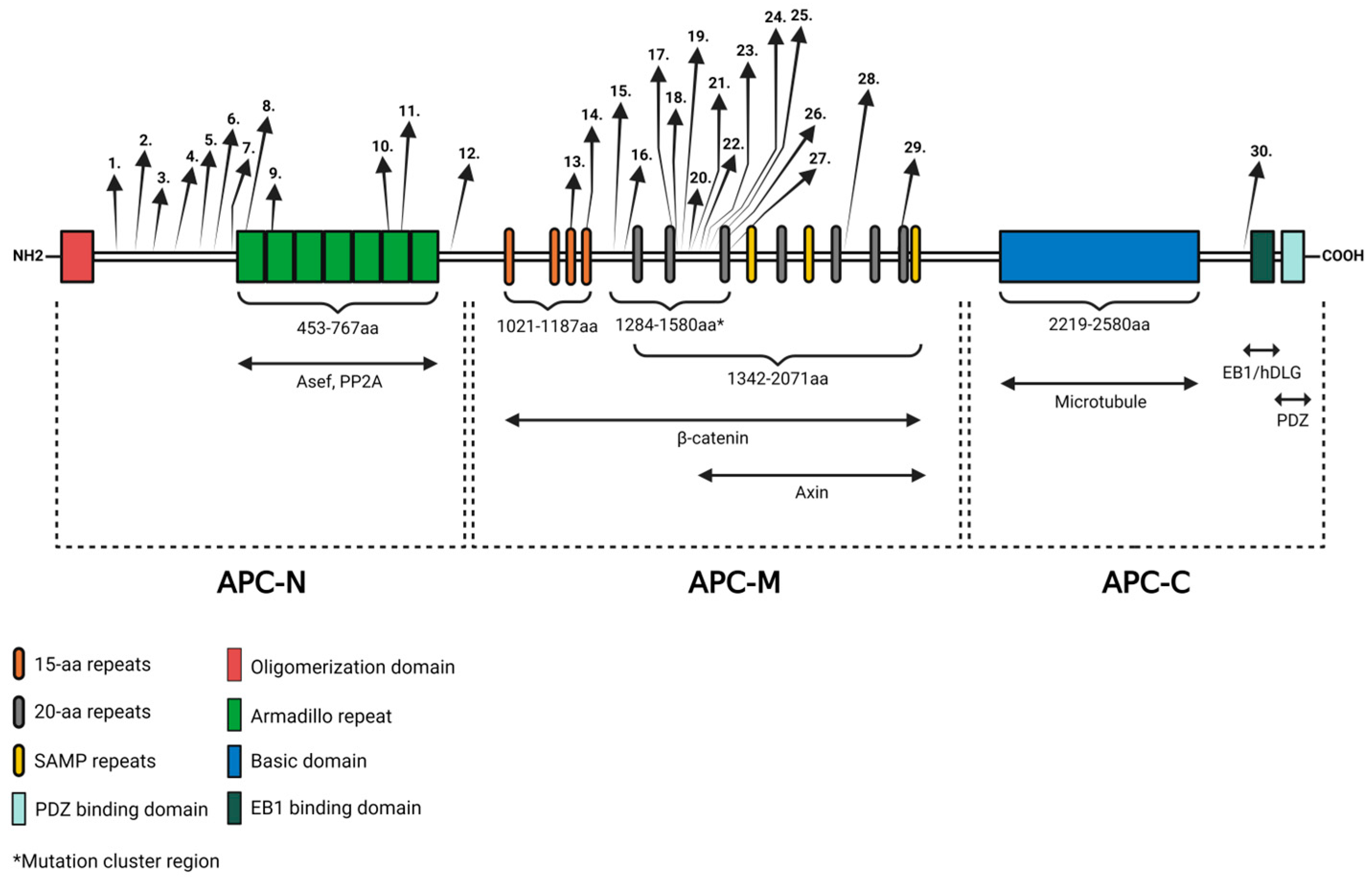

| Mutation | Gender/Age | Polyposis Phenotype | Colorectal Cancer | Extraintestinal Manifestations | Ref. | Localization | |||
|---|---|---|---|---|---|---|---|---|---|
| DNA | Protein | Dental Anomalies | Osseous Anomalies | ||||||
| 1 | c.481C>T a | p.Gln161* | M/54 | Classic FAP | + | - | Hs | [43] | APC-N-terminal region |
| c.481C>T a | p.Gln161* | F/20 | Classic FAP | - | - | Os, Hs | |||
| c.481C>T a | p.Gln161* | M/18 | Classic FAP | - | IT, Od | Dbi | |||
| 2 | c.532-1G>A b | - | M/33 | Classic FAP | + | - | Os, Hs | ||
| c.532-1G>A b | - | F/28 | Classic FAP | + | Od | Os | |||
| c.532-1G>A b | - | M/7 | Classic FAP | - | Od | Os, Hs, Dbi | |||
| 3 | c.646C>T | p.Arg216* | F/12 | ND | ND | IT, Od | - | [44] | |
| 4 | c.761C>G | p.Ser254* | -/- | Attenuated FAP | ND | ST | - | [45] | |
| 5 | c.839C>G | p.Ser280* | -/39 | ND | ND | - | Os | [46] | |
| 6 | c.1240C>T | p.Arg414Cys | -/24 | ND | ND | - | Os | ||
| 7 | c.1354_1355delGT | p.Val452SerfsX7 | -/- | Classic FAP | ND | - | Os | [45] | |
| 8 | c.1370C>G | p.Ser457* | M/39 | Classic FAP | + | - | Os, Dbi | [47] | |
| c.1370C>G c | p.Ser457* | M/32 | Classic FAP | - | - | Os, Hs, Dbi | |||
| c.1370C>G c | p.Ser457* | F/34 | Classic FAP | + | - | Hs, Dbi | |||
| c.1370C>G c | p.Ser457* | M/11 | Classic FAP | - | ST | Hs | |||
| 9 | c.1495C>T | p.Arg499* | F/27 | ND | - | - | Os | [48] | |
| 10 | c.2092T>G | p.Leu698* | M/23 | Classic FAP | + | Dental abnormalities | - | [49] | |
| c.2092T>G d | p.Leu698* | M/48 | Classic FAP | + | - | Os | |||
| c.2092T>G d | p.Leu698* | M/23 | Classic FAP | - | - | Os | |||
| c.2092T>G d | p.Leu698* | F/24 | Classic FAP | - | - | Os | |||
| c.2092T>G d | p.Leu698* | M/21 | Classic FAP | - | - | Os | |||
| 11 | c.2138C>G | p.Ser713* | -/37 | ND | ND | - | Os | [46] | |
| 12 | c. 2740T>G | p.Cys914Gly | M/- | ND | ND | Mesiodens | - | [50] | |
| 13 | c.3199_3202delCAAT | p.Ser1068Glyfs*57 | -/- | Classic FAP | ND | - | Os | [45] | APC-Middle region |
| 14 | c.3374T>C | p.Val1125Ala | F/- | ND | ND | ST | - | [50] | |
| c.3374T>C | p.Val1125Ala | M/- | ND | ND | mesiodens | - | [50] | ||
| 15 | c.3880_3881delCA | p.Gln1294Glyfs*6 | F/30 | ND | + | ST, Od, IT | Os | [51] | |
| 16 | c.3927_3931delAAAGA | p.Glu1309Aspfs*4 | F/18 | Gardner sy | - | - | Os | ||
| 17 | c.4387_4390del | p.Arg1463fs | -/- | Gardner sy | ND | Dental anomalies | Os | [40] | |
| 18 | c.4292_4293delGA e | p.Ser1465Trpfs*3 | M/15 | Gardner sy | ND | ST, Od, IT | - | [52] | |
| c.4292_4293delGA e | p.Ser1465Trpfs*3 | M/66 | Gardner sy | + | IT | - | |||
| c.4293_4294delAG | p.Ser1465Trpfs*3 | F/31 | Gardner sy | - | - | Os | [53] | ||
| c.4293_4294delAG f | p.Ser1465Trpfs*3 | F/28 | Gardner sy | ND | - | Os | |||
| c.4293_4294delAG f | p.Ser1465Trpfs*3 | F/22 | Gardner sy | ND | - | Os | |||
| 19 | c.4510_4513del | p.Ser1505fs | -/- | Gardner sy | ND | - | Os, Dbi | [40] | |
| 20 | c.4609dup g | p.Thr1537Asnfs*7 | F/16 | Gardner sy | ND | IT | Os | [16] | |
| c.4609dup g | p.Thr1537Asnfs*7 | M/12 | Gardner sy | ND | ST | Os | |||
| 21 | c.4611_4612delAG | p.Glu1538Ilefs*5 | -/- | Gardner sy | ND | ST | - | [40] | |
| 22 | c.4621C>T | p.Gln1541* | M/38 | Gardner sy | - | IT, missing teeth | Os | [54] | |
| 23 | c.4652_4655delAAGA | p.Lys1551Argfs*13 | -/- | Attenuated FAP | ND | ST | - | [45] | |
| 24 | c.4654_4655del | p.Glu1552Glyfs*6 | -/- | Gardner sy | ND | Dental anomalies | Os | [55] | |
| 25 | c.4666del | p.Thr1556Leufs*9 | M/25 | Gardner sy | ND | ST, IT | Os, Dbi | [56] | |
| 26 | c.4668_4669insT | p.Ile1557* | -/- | Gardner sy | ND | - | Os | [40] | |
| 27 | c.4700C>G | p.Ser1567* | F/11 | Gardner sy | ND | IT | - | [57] | |
| c.4700C>G | p.Ser1567* | F/16 | Gardner sy | ND | IT, Od | Os | [57] | ||
| 28 | c.5722A>T | p.Asn1908Tyr | M/- | ND | ND | Mesiodens | - | [50] | |
| 29 | c.6127A>G | p.Ile2043Val | F/- | ND | ND | Mesiodens | - | [50] | |
| 30 | c.8383G>A f | p.Ala2795Thr | M/- | ND | ND | Mesiodens | - | [50] | C-terminal region |
| c.8383G>A f | p.Ala2795Thr | M/- | ND | ND | Mesiodens | - | [50] | ||
| Clinical Features | Frequency in Patients with FAP (%) | Frequency in the General Population (%) | Ref. |
|---|---|---|---|
| Osteomas | 76.1 | 4.3 | [62] |
| 62 | 14 | [63] | |
| 57.7 | 2.6 | [58] | |
| 46–93 | 4–16 | [64] | |
| 40 | 6.6 | [65] | |
| 60–80 | 1–2 | [16] | |
| Odontoma | 26.9 | 0 | [58] |
| 9.4–83.3 | 0–4 | [64] | |
| Supernumerary teeth | 7.7 | 0 | [58] |
| 11–27 | 0–4 | [64] | |
| Impacted teeth | 11.5 | 3.8 | [58] |
| 4–38 | 0–4 | [64] | |
| Dental anomalies | 53.3 | 0 | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büki, G.; Antal, G.; Bene, J. Rare Germline Variants in the Adenomatous Polyposis Coli Gene Associated with Dental and Osseous Anomalies. Int. J. Mol. Sci. 2024, 25, 8189. https://doi.org/10.3390/ijms25158189
Büki G, Antal G, Bene J. Rare Germline Variants in the Adenomatous Polyposis Coli Gene Associated with Dental and Osseous Anomalies. International Journal of Molecular Sciences. 2024; 25(15):8189. https://doi.org/10.3390/ijms25158189
Chicago/Turabian StyleBüki, Gergely, Gréta Antal, and Judit Bene. 2024. "Rare Germline Variants in the Adenomatous Polyposis Coli Gene Associated with Dental and Osseous Anomalies" International Journal of Molecular Sciences 25, no. 15: 8189. https://doi.org/10.3390/ijms25158189
APA StyleBüki, G., Antal, G., & Bene, J. (2024). Rare Germline Variants in the Adenomatous Polyposis Coli Gene Associated with Dental and Osseous Anomalies. International Journal of Molecular Sciences, 25(15), 8189. https://doi.org/10.3390/ijms25158189







