The Protective Role of Heat Shock Proteins against Stresses in Animal Breeding
Abstract
:1. Heat Shock Proteins
2. The Role of HSPs in Stress Damage in Animal Breeding
2.1. Protective Roles of Heat Shock Proteins in Broilers
| Stress Type | Species | Organ/Tissue/Cell | Involved HSPs |
|---|---|---|---|
| Heat stress | Chicken | Heart | HSP27/HSP70/HSP90 [31,32,33] |
| Intestinal mucosa | HSP70 [38] | ||
| Fibroblast | HSP70/HSP60/HSP47 [37] | ||
| Bovine | Mammary epithelial cells | HSP27/HSP70/HSP90 [40] | |
| Granulosa cells | HSP32 [41] | ||
| Porcine | Heart/liver/kidney/brain | HSP90 [42] | |
| Mouse | Testes | HSP90α [43] | |
| TM4 cells | CryAB/HSP27/HSP70/HSP110 [44] | ||
| Sertoli cells | HSP72 [45] | ||
| Rat | Testes | HSP60 [46] | |
| Transportation stress | Porcine | Heart | HSP27/HSP70/HSP90 [47,48] |
| Liver | HSP60/HSP70 [49,50] | ||
| Skeletal muscle | HSP70/HSP90 [51] | ||
| Longissimus dorsi muscle | HSP27/HSP70/HSP90 [50,52] |
2.2. The Role of Heat Shock Proteins in Protecting Mammalian Testicular Function
| HSPs | Species | Function in the Genital System |
|---|---|---|
| CryAB/HSP27/HSP70/HSP110 | Mouse | Reduce the activity of MDA and LDH [44] |
| HSP90α | Mouse | Promote spermatogenesis [43] |
| HSP32 | Bovine | Reduce ROS production and activate the antioxidant response [41] |
| HSP72 | Bovine | Reduce caspase-3 activity and the proportion of apoptotic cells [45] |
| HSP70 | Rabbit | Maintain cell integrity [60] |
2.3. The Protective Role of Heat Shock Proteins in Pigs under Transportation-Induced Stress
| HSPs | Species | Organ/Tissue/Cell | Function in Apoptosis |
|---|---|---|---|
| HSP70 | Fish | Hepatocytes | Regulate signal-regulating kinase-1 (ASK1) [84] |
| HSP70 | Chicken | Heart | Inhibit mitochondrial apoptosis pathway [31] |
| HSP90 | Human | U937 cells | Inhibit activation of Apaf-1 [85] |
| HSP27 | Rat | PC12 cells | Promote BIM phosphorylation and degradation [86] |
| Human | 293T cells | Prevent the interaction of Daxx with ASK1 [87] | |
| Promote the interaction of AKT with BAX [88] | |||
| HUVECs | Reduce ROS production and inhibit mitochondrial apoptosis pathway [89] | ||
| Upregulate Bcl-2 and downregulate cleaved caspase-3 and Bax [90] | |||
| U937 cells | Bind to cytochrome c and prevent interaction of Apaf-1 with procaspase-9 [91] | ||
| Inhibit cytochrome c-dependent activation of procaspase 3 [92] |
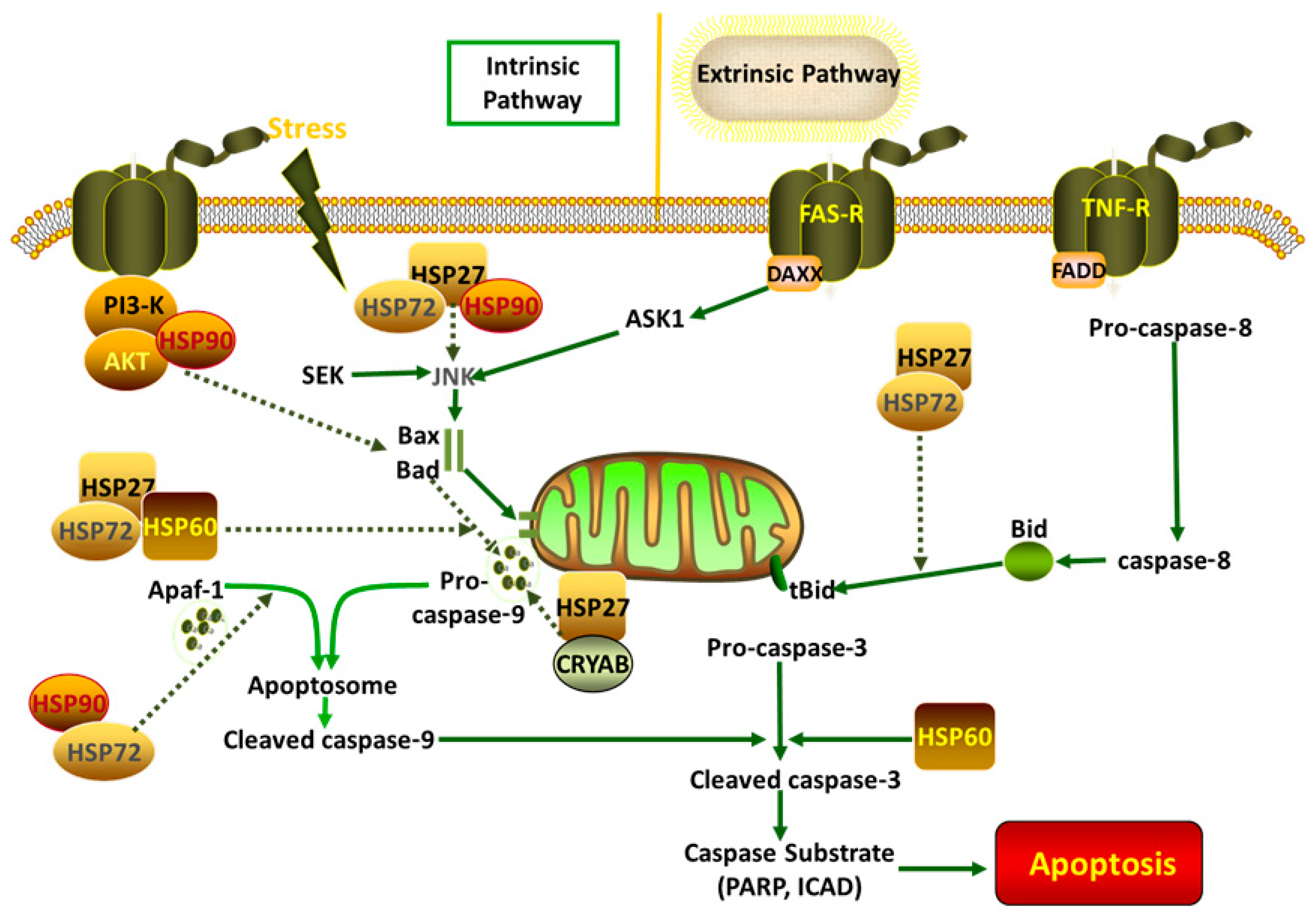
3. The Role of HSPs in Anti-Apoptotic Effect in Animals
4. The Role of HSPs in Veterinary Cancer Diagnosis and Treatments
4.1. Prognostic Significance of Heat Shock Proteins in Cancer and Chemotherapy Resistance
4.2. Inhibition of Heat Shock Proteins as Clinical Treatment for Cancer
5. Feed Supplements Induce HSP Expression in Animal Breeding Industry
6. Conclusions
Funding
Conflicts of Interest
Abbreviations
References
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat Shock Proteins: Biological Functions, Pathological Roles, and Therapeutic Opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Acunzo, J.; Katsogiannou, M.; Rocchi, P. Small Heat Shock Proteins HSP27 (HspB1), αB-Crystallin (HspB5) and HSP22 (HspB8) as Regulators of Cell Death. Int. J. Biochem. Cell Biol. 2012, 44, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xiao, H.; Cao, L. Recent Advances in Heat Shock Proteins in Cancer Diagnosis, Prognosis, Metabolism and Treatment. Biomed. Pharmacother. 2021, 142, 112074. [Google Scholar] [CrossRef] [PubMed]
- Szyller, J.; Bil-Lula, I. Heat Shock Proteins in Oxidative Stress and Ischemia/Reperfusion Injury and Benefits from Physical Exercises: A Review to the Current Knowledge. Oxidative Med. Cell Longev. 2021, 2021, 6678457. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.J.; Guerrero, M.E.; Prince, T.L.; Okusha, Y.; Bonorino, C.; Calderwood, S.K. The Functions and Regulation of Heat Shock Proteins; Key Orchestrators of Proteostasis and the Heat Shock Response. Arch. Toxicol. 2021, 95, 1943–1970. [Google Scholar] [CrossRef]
- Saglam, A.; Calof, A.L.; Wray, S. Novel Factor in Olfactory Ensheathing Cell-Astrocyte Crosstalk: Anti-Inflammatory Protein A-Crystallin B. Glia 2021, 69, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Nakai, A. New Aspects in the Vertebrate Heat Shock Factor System: Hsf3 and Hsf4. Cell Stress Chaperones 1999, 4, 86. [Google Scholar] [CrossRef]
- Knowlton, A.A.; Sun, L. Heat-Shock Factor-1, Steroid Hormones, and Regulation of Heat-Shock Protein Expression in the Heart. Am. J. Physiol.-Heart Circ. Physiol. 2001, 280, H455–H464. [Google Scholar] [CrossRef] [PubMed]
- Pincus, D. Regulation of Hsf1 and the Heat Shock Response. Adv. Exp. Med. Biol. 2020, 1243, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Tang, S.; Bao, E.; Zhang, M.; Hao, Q.; Yue, Z. The Effect of Transportation on the Expression of Heat Shock Proteins and Meat Quality of M. Longissimus Dorsi in Pigs. Meat Sci. 2009, 83, 474–478. [Google Scholar] [CrossRef]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2017, 38, 226–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Edens, F.W. Heat Conditioning Induces Heat Shock Proteins in Broiler Chickens and Turkey Poults. Poult. Sci. 1998, 77, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Sandercock, D.A.; Hunter, R.R.; Nute, G.R.; Mitchell, M.A.; Hocking, P.M. Acute Heat Stress-Induced Alterations in Blood Acid-Base Status and Skeletal Muscle Membrane Integrity in Broiler Chickens at Two Ages: Implications for Meat Quality. Poult. Sci. 2001, 80, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Seibert, P.; Anklam, C.F.V.; Costa-Beber, L.C.; Sulzbacher, L.M.; Sulzbacher, M.M.; Sangiovo, A.M.B.; Dos Santos, F.K.; Goettems-Fiorin, P.B.; Heck, T.G.; Frizzo, M.N. Increased eHSP70-to-iHSP70 Ratio in Prediabetic and Diabetic Postmenopausal Women: A Biomarker of Cardiometabolic Risk. Cell Stress Chaperones 2022, 27, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.Ž.; Cincović, M.; Starič, J.; Djoković, R.; Belić, B.; Radinović, M.; Majkić, M.; Ilić, Z.Ž. The Correlation between Extracellular Heat Shock Protein 70 and Lipid Metabolism in a Ruminant Model. Metabolites 2021, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Kol, A.; Lichtman, A.H.; Finberg, R.W.; Libby, P.; Kurtjones, E.A. Cutting Edge: Heat Shock Protein (HSP) 60 Activates the Innate Immune Response: CD14 Is an Essential Receptor for HSP60 Activation of Mononuclear Cells. J. Immunol. 2000, 164, 13. [Google Scholar] [CrossRef] [PubMed]
- Rashed, E.; Lizano, P.; Dai, H.; Thomas, A.; Suzuki, C.K.; Depre, C.; Qiu, H. Heat Shock Protein 22 (Hsp22) Regulates Oxidative Phosphorylation upon Its Mitochondrial Translocation with the Inducible Nitric Oxide Synthase in Mammalian Heart. PLoS ONE 2015, 10, e0119537. [Google Scholar] [CrossRef] [PubMed]
- Bruening, W.; Roy, J.; Giasson, B.; Figlewicz, D.A.; Mushynski, W.E.; Durham, H.D. Up-Regulation of Protein Chaperones Preserves Viability of Cells Expressing Toxic Cu/Zn-Superoxide Dismutase Mutants Associated with Amyotrophic Lateral Sclerosis. J. Neurochem. 1999, 72, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.A.; Brecher, P.; Apstein, C.S. Rapid Expression of Heat Shock Protein in the Rabbit after Brief Cardiac Ischemia. J. Clin. Investig. 1991, 87, 139. [Google Scholar] [CrossRef]
- Koyama, S.; Arawaka, S.; Ren, C.H.; Wada, M.; Kawanami, T.; Kurita, K.; Kato, M.; Nagai, M.; Aoki, M.; Itoyama, Y. Alteration of Familial ALS-Linked Mutant SOD1 Solubility with Disease Progression: Its Modulation by the Proteasome and Hsp70. Biochem. Biophys. Res. Commun. 2006, 343, 719–730. [Google Scholar] [CrossRef]
- Mehta, H.B.; Popovich, B.K.; Dillmann, W.H. Ischemia Induces Changes in the Level of mRNAs Coding for Stress Protein 71 and Creatine Kinase M. Circ. Res. 1988, 63, 512. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Reiser, G. The Small Heat Shock Proteins, Especially HspB4 and HspB5 Are Promising Protectants in Neurodegenerative Diseases. Neurochem. Int. 2018, 115, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Impacts of Heat Stress on Global Cattle Production during the 21st Century: A Modelling Study. Lancet Planet. Health 2022, 6, e192–e201. [Google Scholar] [CrossRef] [PubMed]
- Roushdy, E.M.; Zaglool, A.W.; Hassan, F.A.M. Thermal Stress Consequences on Growth Performance, Immunological Response, Antioxidant Status, and Profitability of Finishing Broilers: Transcriptomic Profile Change of Stress-Related Genes. Trop. Anim. Health Prod. 2020, 52, 3685–3696. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ren, M.; Ren, K.; Jin, Y.; Yan, M. Heat Stress Impacts on Broiler Performance: A Systematic Review and Meta-Analysis. Poult. Sci. 2020, 99, 6205–6211. [Google Scholar] [CrossRef]
- Bartlett, J.R.; Smith, M.O. Effects of Different Levels of Zinc on the Performance and Immunocompetence of Broilers under Heat Stress. Poult. Sci. 2003, 82, 1580. [Google Scholar] [CrossRef]
- Vandana, G.D.; Sejian, V.; Lees, A.M.; Pragna, P.; Silpa, M.V.; Maloney, S.K. Heat Stress and Poultry Production: Impact and Amelioration. Int. J. Biometeorol. 2021, 65, 163–179. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, J.; Goldberg, A.L.; Voellmy, R. Abnormal Proteins Serve as Eukaryotic Stress Signals and Trigger the Activation of Heat Shock Genes. Science 1986, 232, 522–524. [Google Scholar] [CrossRef]
- Arya, R.; Mallik, M.; Lakhotia, S.C. Heat Shock Genes-Integrating Cell Survival and Death. J. Biosci. 2007, 32, 595–610. [Google Scholar] [CrossRef]
- Jie, X.Y.; Ling, H.M.; Hui, Z.L.; Qi, W.; Quan, Z.X.; Bin, L.Q. Effect of HSPB9 on Apoptosis of DF-1 Cells. Biomed. Environ. Sci. 2019, 32, 107–120. [Google Scholar] [CrossRef]
- Xu, J.; Tang, S.; Song, E.; Yin, B.; Bao, E. Inhibition of Heat Shock Protein 70 Intensifies Heat-Stressed Damage and Apoptosis of Chicken Primary Myocardial Cells in Vitro. Mol. Med. Rep. 2017, 15, 2881. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, Z.; Zhu, H.; Tang, S.; Wu, D.; Zhang, M.; Kemper, N.; Hartung, J.; Bao, E. HSP90 Gene Expression Induced by Aspirin Is Associated with Damage Remission in a Chicken Myocardial Cell Culture Exposed to Heat Stress. Br. Poult. Sci. 2016, 57, 462–473. [Google Scholar] [CrossRef]
- Tang, S.; Buriro, R.; Liu, Z.; Zhang, M.; Ali, I.; Adam, A.; Hartung, J.; Bao, E. Localization and Expression of Hsp27 and αB-Crystallin in Rat Primary Myocardial Cells during Heat Stress In Vitro. PLoS ONE 2013, 8, e69066. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Lu, Y.; Tang, S.; Kemper, N.; Hartung, J.; Bao, E. Aspirin-Induced Heat Stress Resistance in Chicken Myocardial Cells Can Be Suppressed by BAPTA-AM in Vitro. Cell Stress Chaperones 2016, 21, 817–827. [Google Scholar] [CrossRef] [PubMed]
- Al-Aqil, A.; Zulkifli, I.; Bejo, M.H.; Sazili, A.Q.; Rajion, M.A.; Somchit, M.N. Changes in Heat Shock Protein 70, Blood Parameters, and Fear-Related Behavior in Broiler Chickens as Affected by Pleasant and Unpleasant Human Contact. Poult. Sci. 2013, 92, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, I.; Alaqil, A.; Omar, A.R.; Sazili, A.Q.; Rajion, M.A. Crating and Heat Stress Influence Blood Parameters and Heat Shock Protein 70 Expression in Broiler Chickens Showing Short or Long Tonic Immobility Reactions. Poult. Sci. 2009, 88, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.H.; Subramaniyan, S.A.; Kang, D.; Park, J.; Khan, M.; Choi, H.W.; Shim, K. Direct Exposure to Mild Heat Stress Stimulates Cell Viability and Heat Shock Protein Expression in Primary Cultured Broiler Fibroblasts. Cell Stress Chaperones 2020, 25, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.H.; Hao, Y.; Wang, X.L. Overexpression of Heat Shock Protein 70 and Its Relationship to Intestine under Acute Heat Stress in Broilers: 2. Intestinal Oxidative Stress. Poult. Sci. 2010, 91, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Cheng, C.Y.; Tang, P.C.; Chen, C.F.; Chen, H.H.; Lee, Y.P.; Huang, S.Y. Acute Heat Stress Induces Differential Gene Expressions in the Testes of a Broiler-Type Strain of Taiwan Country Chickens. PLoS ONE 2015, 10, e0125816. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Y.; Zheng, N.; Cheng, J.; Wang, J. The Effect of Heat Stress on Gene Expression and Synthesis of Heat-shock and Milk Proteins in Bovine Mammary Epithelial Cells. Anim. Sci. J. 2016, 87, 84–91. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Elsheikh, N.A.H.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 Reduces Heat Stress-Induced Apoptosis in Bovine Granulosa Cells by Suppressing Oxidative Stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef] [PubMed]
- Huau, G.; Liaubet, L.; Gourdine, J.-L.; Riquet, J.; Renaudeau, D. Multi-Tissue Metabolic and Transcriptomic Responses to a Short-Term Heat Stress in Swine. BMC Genom. 2024, 25, 99. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chang, C.; Hao, M.; Chen, M.; Woodley, D.T.; Schönthal, A.H.; Li, W. Heat Shock Protein-90alpha (Hsp90α) Stabilizes Hypoxia-Inducible Factor-1α (HIF-1α) in Support of Spermatogenesis and Tumorigenesis. Cancer Gene Ther. 2021, 28, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Yin, B.; Tang, S.; Zhang, X.; Xu, J.; Bao, E. Vitamin C Mitigates Heat Damage by Reducing Oxidative Stress, Inducing HSP Expression in TM4 Sertoli Cells. Mol. Reprod. Dev. 2019, 86, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Cong, X.; Zhang, Q.; Li, H.; Jiang, Z.; Cao, R.; Gao, S.; Tian, W. Puerarin Ameliorates Heat Stress–Induced Oxidative Damage and Apoptosis in Bovine Sertoli Cells by Suppressing ROS Production and Upregulating Hsp72 Expression. Theriogenology 2017, 88, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, A.; Parvinen, M.; Bacher, M.; Aumüller, G.; Hakovirta, H.; Yagi, A.; Seitz, J. Expression of Mitochondrial Heat Shock Protein 60 in Distinct Cell Types and Defined Stages of Rat Seminiferous Epithelium. Biol. Reprod. 1995, 52, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Bao, E.; Sultan, K.R.; Nowak, B.; Hartung, J. Expression and Distribution of Heat Shock Proteins in the Heart of Transported Pigs. Cell Stress Chaperones 2008, 13, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Bao, E.; Zhang, M.; Yue, Z.; Hartung, J. Variation in the Expression of Hsp27, Hsp70, Hsp90 and Their Corresponding mRNA Transcripts in the Hearts of Pigs during Different Transportation Durations. Livest. Sci. 2010, 129, 88–94. [Google Scholar] [CrossRef]
- Zhu, L.; Bao, E.; Zhao, R.; Hartung, J. Expression of Heat Shock Protein 60 in the Tissues of Transported Piglets. Cell Stress Chaperones 2009, 14, 61–69. [Google Scholar] [CrossRef]
- Lei, L.; Yu, J.; Bao, E. Expression of Heat Shock Protein 90 (Hsp90) and Transcription of Its Corresponding mRNA in Broilers Exposed to High Temperature. Br. Poult. Sci. 2009, 50, 504–511. [Google Scholar] [CrossRef]
- Tang, S.; Bao, E.; Sultan, K.R.; Nowak, B.; Hartung, J. Transportation Stress and Expression of Heat Shock Protein Affecting Pork Quality. Pak. Vet. J. 2014, 34, 112–115. [Google Scholar]
- Zhang, M.; Yue, Z.; Liu, Z.; Islam, A.; Rehana, B.; Tang, S.; Bao, E.; Hartung, J. Hsp70 and HSF-1 Expression Is Altered in the Tissues of Pigs Transported for Various Periods of Times. J. Vet. Sci. 2012, 13, 253–259. [Google Scholar] [CrossRef]
- de Wit, N.J.; Verschuure, P.; Kappé, G.; King, S.M.; de Jong, W.W.; van Muijen, G.N.; Boelens, W.C. Testis-Specific Human Small Heat Shock Protein HSPB9 Is a Cancer/Testis Antigen, and Potentially Interacts with the Dynein Subunit TC℡1. Eur. J. Cell Biol. 2004, 83, 337–345. [Google Scholar] [CrossRef]
- Shahat, A.M.; Rizzoto, G.; Kastelic, J.P. Amelioration of Heat Stress-Induced Damage to Testes and Sperm Quality. Theriogenology 2020, 158, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.R.; Mendes, C.M.; de Castro, L.S.; de Assis, P.M.; Siqueira, A.F.; Delgado, J.C.; Goissis, M.D.; Muiño-Blanco, T.; Cebrián-Pérez, J.Á.; Nichi, M. Evaluation of Lasting Effects of Heat Stress on Sperm Profile and Oxidative Status of Ram Semen and Epididymal Sperm. Oxidative Med. Cell Longev. 2016, 2016, 1687657. [Google Scholar] [CrossRef] [PubMed]
- Adly, M.A.; Assaf, H.A.; Hussein, M.R.A. Heat Shock Protein 27 Expression in the Human Testis Showing Normal and Abnormal Spermatogenesis. Cell Biol. Int. 2008, 32, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Eng, F.; Wiebe, J.P.; Alima, L.H. Long-term Alterations in the Permeability of the Blood-Testis Barrier Following a Single Intratesticular Injection of Dilute Aqueous Glycerol. J. Androl. 1994, 15, 311–317. [Google Scholar] [CrossRef]
- He, C.; Sun, J.; Yang, D.; He, W.; Wang, J.; Qin, D.; Zhang, H.; Cai, H.; Liu, Y.; Li, N. Nrf2 Activation Mediates the Protection of Mouse Sertoli Cells Damage under Acute Heat Stress Conditions. Theriogenology 2022, 177, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Luo, N.J.; Gan, L.; Xue, H.Y.; Luo, K.Y.; Zhang, J.J.; Wang, X.Z. Heat Stress Upregulates Arachidonic Acid to Trigger Autophagy in Sertoli Cells via Dysfunctional Mitochondrial Respiratory Chain Function. J. Transl. Med. 2024, 22, 501. [Google Scholar] [CrossRef]
- Jiang, Z.L. Effect of Heat Stress on the Expression of Hsp70 in Mice Testis Tissue. J. Anhui Agric. Sci. 2009, 30, 106648–106659. [Google Scholar]
- Yadav, V.P.; Dangi, S.S.; Chouhan, V.S.; Gupta, M.; Dangi, S.K.; Singh, G.; Maurya, V.P.; Kumar, P.; Sarkar, M. Expression Analysis of NOS Family and HSP Genes during Thermal Stress in Goat (Capra Hircus). Int. J. Biometeorol. 2016, 60, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Martin, J.W. Neupert Protein Folding in the Cell: The Role of Molecular Chaperones Hsp70 and Hsp60. Annu. Rev. Biophys. Biomol. Struct. 1992, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Ağababaoğlu, İ. ; Önen A; Demir, A. B.; Aktaş, S.; Altun, Z.; Ersöz, H.; Şanl A; Özdemir N; Akkoçlu, A. Chaperonin (HSP60) and Annexin-2 Are Candidate Biomarkers for Non-Small Cell Lung Carcinoma: Erratum: Medicine (Baltimore) 2017, 96, e5903. [Google Scholar]
- Werner, A.; Seitz, J.; Meinhardt, A.; Bergmann, M. Distribution Pattern of HSP60 Immunoreactivity in the Testicular Tissue of Infertile Men. Ann. Anat.-Anat. Anz. 1996, 178, 81–82. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Wu, Y.; Qin, Y. Effects of Chronic Heat Stress on the Expressions of Heat Shock Proteins 60, 70, 90, A2, and HSC70 in the Rabbit Testis. Cell Stress Chaperones 2012, 17, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Lejong, M.; Choa-Duterre, M.; Vanmuylder, N.; Louryan, S. Effects of HSP90 Inhibition on Primordial Germ Cells Migration: A Study in the Gonad of the Chick Embryo. Morphol. Bull. Assoc. Anat. 2020, 104, 228–236. [Google Scholar] [CrossRef]
- Huang, S.Y.; Kuo, Y.H.; Lee, W.C.; Tsou, H.L.; Lee, Y.P.; Chang, H.L.; Wu, J.J.; Yang, P.C. Substantial Decrease of Heat-Shock Protein 90 Precedes the Decline of Sperm Motility during Cooling of Boar Spermatozoa. Theriogenology 1999, 51, 1007. [Google Scholar] [CrossRef] [PubMed]
- Adamovic´, M.; Šamanc, H.; Vujanac, I.; Valčic´, O.; Kirovski, D. Effects of Mineral Substances with a Buffering Effect on Milk Production and Milk Composition in Heat Stress Conditions. Maced. J. Anim. Sci. 2013, 67, 920–925. [Google Scholar] [CrossRef]
- Bohmanova, J.; Misztal, I.; Cole, J.B. Temperature-Humidity Indices as Indicators of Milk Production Losses Due to Heat Stress. J. Dairy Sci. 2007, 90, 1947–1956. [Google Scholar] [CrossRef]
- Peana, I.; Fois, G.; Cannas, A. Effects of Heat Stress and Diet on Milk Production and Feed and Energy Intake of Sarda Ewes. Ital. J. Anim. Sci. 2010, 6, 577–579. [Google Scholar] [CrossRef]
- Adeola, O.; Ball, R.O. Hypothalamic Neurotransmitter Concentrations and Meat Quality in Stressed Pigs Offered Excess Dietary Tryptophan and Tyrosine. J. Anim. Sci. 1992, 70, 1888–1894. [Google Scholar] [CrossRef] [PubMed]
- Driessen, B.; Van Beirendonck, S.; Buyse, J. Effects of Housing, Short Distance Transport and Lairage on Meat Quality of Finisher Pigs. Animals 2020, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sang, Z.; Zhuo, Y.; Wang, X.; Guo, Z.; He, L.; Zeng, C.; Dai, H. Transport Stress Induces Pig Jejunum Tissue Oxidative Damage and Results in Autophagy/Mitophagy Activation. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Jabiry-Zieniewicz, Z.; Bobrowska, K.; Kaminski, P.; Wielgos, M.; Zieniewicz, K.; Krawczyk, M. Low-Dose Hormonal Contraception After Liver Transplantation. Transplant. Proc. 2007, 39, 1530. [Google Scholar] [CrossRef] [PubMed]
- Miranda-de la Lama, G.C.; Rivero, L.; Chacón, G.; Garcia-Belenguer, S.; Villarroel, M.; Maria, G.A. Effect of the Pre-Slaughter Logistic Chain on Some Indicators of Welfare in Lambs. Livest. Sci. 2010, 128, 52–59. [Google Scholar] [CrossRef]
- Yu, J.; Bao, E.; Yan, J.; Lei, L. Expression and Localization of Hsps in the Heart and Blood Vessel of Heat-Stressed Broilers. Cell Stress Chaperones 2008, 13, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Stella, A.M.; Butterfield, D.A.; Scapagnini, G. Redox Regulation in Neurodegeneration and Longevity: Role of the Heme Oxygenase and HSP70 Systems in Brain Stress Tolerance. Antioxid. Redox Signal. 2004, 6, 895–913. [Google Scholar] [PubMed]
- Cumming, R.C.; Andon, N.L.; Haynes, P.A.; Park, M.; Fischer, W.H.; Schubert, D. Protein Disulfide Bond Formation in the Cytoplasm during Oxidative Stress. J. Biol. Chem. 2004, 279, 21749–21758. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, M.M. Heme-Oxygenase-1. Antioxid. Redox Signal. 2020, 32, 1239–1242. [Google Scholar] [CrossRef]
- Musch, M.W.; Kapil, A.; Chang, E.B. Heat Shock Protein 72 Binds and Protects Dihydrofolate Reductase against Oxidative Injury. Biochem Biophys Res Commun 2004, 313, 185–192. [Google Scholar] [CrossRef]
- Hu, H.; Zheng, N.; Gao, H.; Dai, W.; Zhang, Y.; Li, S.; Wang, J. Immortalized Bovine Mammary Epithelial Cells Express Stem Cell Markers and Differentiate in Vitro. Cell Biol. Int. 2016, 40, 861–872. [Google Scholar] [CrossRef]
- Cedraz, H.; Gromboni, J.G.G.; Garcia, A.A.P.; Farias Filho, R.V.; Souza, T.M.; Oliveira, E.R.D.; Oliveira, E.B.D.; Nascimento, C.S.D.; Meneghetti, C.; Wenceslau, A.A. Heat Stress Induces Expression of HSP Genes in Genetically Divergent Chickens. PLoS ONE 2017, 12, e0186083. [Google Scholar] [CrossRef]
- Yan, J.; Bao, E.; Yu, J. Heat Shock Protein 60 Expression in Heart, Liver and Kidney of Broilers Exposed to High Temperature. Res. Vet. Sci. 2009, 86, 533–538. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, K.P.; Singh, M.K.; Saini, N.; Palta, P.; Manik, R.S.; Singla, S.K.; Upadhyay, R.C.; Chauhan, M.S. Effect of Physiologically Relevant Heat Shock on Development, Apoptosis and Expression of Some Genes in Buffalo (Bubalus Bubalis) Embryos Produced In Vitro. Reprod. Domest. Anim. 2013, 48, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Saleh, A.; Nakazawa, A.; Kumar, S.; Srinivasula, S.M.; Kumar, V.; Weichselbaum, R.; Nalin, C.; Alnemri, E.S.; Kufe, D. Negative Regulation of cytochrome C-Mediated Oligomerization of Apaf-1 and Activation of Procaspase-9 by Heat Shock protein 90. Embo J. 2000, 19, 4310. [Google Scholar] [CrossRef]
- Kennedy, D.; Mnich, K.; Oommen, D.; Chakravarthy, R.; Almeida-Souza, L.; Krols, M.; Saveljeva, S.; Doyle, K.; Gupta, S.; Timmerman, V.; et al. HSPB1 Facilitates ERK-Mediated Phosphorylation and Degradation of BIM to Attenuate Endoplasmic Reticulum Stress-Induced Apoptosis. Cell Death Dis. 2017, 8, e3026–e3026. [Google Scholar] [CrossRef] [PubMed]
- Charette, S.J.; Lavoie, J.N.; Lambert, H.; Landry, J. Inhibition of Daxx-Mediated Apoptosis by Heat Shock Protein 27. Mol. Cell. Biol. 2000, 20, 7602–7612. [Google Scholar] [CrossRef]
- Havasi, A.; Li, Z.; Wang, Z.; Martin, J.L.; Botla, V.; Ruchalski, K.; Schwartz, J.H.; Borkan, S.C. Hsp27 Inhibits Bax Activation and Apoptosis via a Phosphatidylinositol 3-Kinase-Dependent Mechanism *. J. Biol. Chem. 2008, 283, 12305–12313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-L.; Jia, K.-Y.; Sun, D.; Yang, M. Protective Effect of HSP27 in Atherosclerosis and Coronary Heart Disease by Inhibiting Reactive Oxygen Species. J. Cell. Biochem. 2019, 120, 2859–2868. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, L.; Song, X.; Yan, Y.; Liu, N.; Li, T.; Yan, B.; Liu, B. HSP27 Inhibits Homocysteine-Induced Endothelial Apoptosis by Modulation of ROS Production and Mitochondrial Caspase-Dependent Apoptotic Pathway. BioMed Res. Int. 2016, 2016, 4847874. [Google Scholar] [CrossRef]
- Bruey, J.-M.; Ducasse, C.; Bonniaud, P.; Ravagnan, L.; Susin, S.A.; Diaz-Latoud, C.; Gurbuxani, S.; Arrigo, A.-P.; Kroemer, G.; Solary, E.; et al. Hsp27 Negatively Regulates Cell Death by Interacting with Cytochrome c. Nat. Cell Biol. 2000, 2, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Farber, R.; Nakazawa, A.; Kumar, S.; Bharti, A.; Nalin, C.; Weichselbaum, R.; Kufe, D.; Kharbanda, S. Hsp27 Functions as a Negative Regulator of Cytochrome C-Dependent Activation of Procaspase-3. Oncogene 2000, 19, 1975–1981. [Google Scholar] [CrossRef] [PubMed]
- Abdelnour, S.A.; Yang, C.-Y.; Swelum, A.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Abdo, M.; Shang, J.-H.; Lu, Y.-Q. Molecular, Functional, and Cellular Alterations of Oocytes and Cumulus Cells Induced by Heat Stress and Shock in Animals. Environ. Sci. Pollut. Res. 2020, 27, 38472–38490. [Google Scholar] [CrossRef] [PubMed]
- Padmini, E.; Tharani, J. Heat-Shock Protein 70 Modulates Apoptosis Signal-Regulating Kinase 1 in Stressed Hepatocytes of Mugil Cephalus. Fish Physiol. Biochem. 2014, 40, 1573–1585. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Liu, N.; Yan, Y.; Liu, B. Apoptosis, Autophagy and Atherosclerosis: Relationships and the Role of Hsp27. Pharmacol. Res. 2021, 166, 105169. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Dixit, V.M. Death Receptors: Signaling and Modulation. Science 1998, 281, 1305–1308. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, J.; Guo, Z.; Shi, X.; Zhang, Y.; Zhang, L.; Yu, Q.; Han, L. Effect of Oxidative Stress on AIF-mediated Apoptosis and Bovine Muscle Tenderness during Postmortem Aging. J. Food Sci. 2020, 85, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Vostakolaei, M.A.; Hatami-Baroogh, L.; Babaei, G.; Molavi, O.; Kordi, S.; Abdolalizadeh, J. Hsp70 in Cancer: A Double Agent in the Battle between Survival and Death. J. Cell. Physiol. 2021, 236, 3420–3444. [Google Scholar] [CrossRef]
- Garrido, C.; Bruey, J.M.; Fromentin, A.; Hammann, A.; Arrigo, A.P.; Solary, E. HSP27 Inhibits Cytochrome C-Dependent Activation of Procaspase-9. Faseb J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1999, 13, 2061–2070. [Google Scholar] [CrossRef]
- Gorman, A.M.; Szegezdi, E.; Quigney, D.J.; Samali, A. Hsp27 Inhibits 6-Hydroxydopamine-Induced Cytochrome c Release and Apoptosis in PC12 Cells. Biochem Biophys Res Commun 2005, 327, 801–810. [Google Scholar] [CrossRef]
- Chaudhary, S.; Chaudhary, S.S.; Rawat, S.; Kaur, S.; Devi, B.; Ahmad, M.M.; Arshad, Z.; Mustafa, M.; Al Jedaie, M.M.; Alam, P. Molecular Mechanism and Role of Translational Values of Heat Shock Protein (HSP27) in Various Disease. J Pharm. Res Int 2020, 32, 110–118. [Google Scholar] [CrossRef]
- Matsumori, Y.; Hong, S.M.; Aoyama, K.; Fan, Y.; Kayama, T.; Sheldon, R.A.; Vexler, Z.S.; Ferriero, D.M.; Weinstein, P.R.; Liu, J. Hsp70 Overexpression Sequesters AIF and Reduces Neonatal Hypoxic/Ischemic Brain Injury. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2005, 25, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.; Xie, Q.; Chen, Z.; Zhang, Y.; Chen, Y.; Li, H.; Lai, W.; Chen, Y.; Huang, M. Involvement of PARP-1/AIF Signaling Pathway in Protective Effects of Gualou Guizhi Decoction Against Ischemia–Reperfusion Injury-Induced Apoptosis. Neurochem. Res. 2020, 45, 278–294. [Google Scholar] [CrossRef]
- Chu, R.M.; Sun, T.J.; Yang, H.Y.; Wang, D.G.; Liao, K.W.; Chuang, T.F.; Lin, C.H.; Lee, W.C. Heat Shock Proteins in Canine Transmissible Venereal Tumor. Vet. Immunol. Immunopathol. 2001, 82, 9. [Google Scholar] [CrossRef]
- Okada, S.; Furuya, M.; Takenaka, S.; Fukui, A.; Matsubayashi, M.; Tani, H.; Sasai, K. Localization of Heat Shock Protein 110 in Canine Mammary Gland Tumors. Vet. Immunol. Immunopathol. 2015, 167, 139. [Google Scholar] [CrossRef]
- Salvermoser, L.; Dressel, S.; Schleißheimer, S.; Stangl, S.; Diederichs, C.; Wergin, M.; Bley, C.R.; Haller, B.; Multhoff, G. 7Hsp70 Serum Levels in Pet Dogs—a Potential Diagnostic Biomarker for Spontaneous Round Cell Tumors. Cell Stress Chaperones 2019, 24, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Akiharu, K.; Kyoichi, O.; Bolag, A.; Takehiko, Y.; Munenori, I.; Erito, M.; Yoshitaka, T.; Norimichi, K.; Toru, Y.; Masaki, S. Nuclear Heat Shock Protein 110 Expression Is Associated with Poor Prognosis and Chemotherapy Resistance in Gastric Cancer. Oncotarget 2016, 7, 18415–18423. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Calderwood, S.K. Heat Shock Proteins in Cancer: Diagnostic, Prognostic, Predictive, and Treatment Implications. Cell Stress Chaperones 2005, 10, 86. [Google Scholar] [CrossRef]
- Ergul, M.; Aktan, F.; Yildiz, M.T.; Tutar, Y. Perturbation of HSP Network in MCF-7 Breast Cancer Cell Line Triggers Inducible HSP70 Expression and Leads to Tumor Suppression. Anticancer Agents Med. Chem. 2020, 20, 1051–1060. [Google Scholar] [CrossRef]
- Lanneau, D.; De, T.A.; Maurel, S.; Didelot, C.; Garrido, C. Apoptosis versus Cell Differentiation: Role of Heat Shock Proteins HSP90, HSP70 and HSP27. Prion 2007, 1, 53. [Google Scholar] [CrossRef]
- Kunachowicz, D.; Król-Kulikowska, M.; Raczycka, W.; Sleziak, J.; Błażejewska, M.; Kulbacka, J. Heat Shock Proteins, a Double-Edged Sword: Significance in Cancer Progression, Chemotherapy Resistance and Novel Therapeutic Perspectives. Cancers 2024, 16, 1500. [Google Scholar] [CrossRef]
- Jakubowiczgil, J.; Langner, E.; Bądziul, D.; Wertel, I.; Rzeski, W. Silencing of Hsp27 and Hsp72 in Glioma Cells as a Tool for Programmed Cell Death Induction upon Temozolomide and Quercetin Treatment. Toxicol. Appl. Pharmacol. 2013, 273, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Lampros, M.; Vlachos, N.; Voulgaris, S.; Alexiou, G.A. The Role of Hsp27 in Chemotherapy Resistance. Biomedicines 2022, 10, 897. [Google Scholar] [CrossRef] [PubMed]
- Jinhee, J.; Jeongok, L.; Jihae, K.; Sookyung, L.; You, G.Y.; Sunhwa, P.; Jiman, P.; Eungkyun, K.; Pannghill, S.; An, J.K. Quercetin Suppresses HeLa Cell Viability via AMPK-Induced HSP70 and EGFR down-Regulation. J. Cell. Physiol. 2010, 223, 408–414. [Google Scholar] [CrossRef]
- Önay Uçar, E.; Şengelen, A.; Mertoğlu, E.; Pekmez, M.; Arda, N. Suppression of HSP70 Expression by Quercetin and Its Ther-apeutic Potential Against Cancer. In HSP70 in Human Diseases and Disorders; Asea, A.A.A., Kaur, P., Eds.; Heat Shock Proteins; Springer International Publishing: Cham, Switzerland, 2018; Volume 14, pp. 361–379. ISBN 978-3-319-89550-5. [Google Scholar]
- Zhou, Y.; Ma, J.; Zhang, J.; He, L.; Gong, J.; Long, C. Pifithrin-μ Is Efficacious against Non-Small Cell Lung Cancer via Inhibition of Heat Shock Protein 70. Oncol. Rep. 2017, 37, 313–322. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, G.; Qiu, Y.; Hu, Y.; Liu, J.; Zhao, J.; Zhang, S.; Zhang, J. HSP90 Inhibitor AUY922 Can Reverse Fulvestrant Induced Feedback Reaction in Human Breast Cancer Cells. Cancer Sci. 2017, 108, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Selvarajah, G.T.; Bonestroo, F.A.; Kirpensteijn, J.; Kik, M.J.; van der Zee, R.; van Eden, W.; Timmermans-Sprang, E.P.; Slob, A.; Mol, J.A. Heat Shock Protein Expression Analysis in Canine Osteosarcoma Reveals HSP60 as a Potentially Relevant Therapeutic Target. Cell Stress Chaperones 2013, 18, 607–622. [Google Scholar] [CrossRef] [PubMed]
- Romanucci, M.; Della Salda, L. Heat Shock Protein Expression and Implications in Spontaneous Animal Tumors: Veterinary and Comparative Aspects. In Heat Shock Proteins in Veterinary Medicine and Sciences; Asea, A.A.A., Kaur, P., Eds.; Springer Interna-tional Publishing: Cham, Switzerland, 2017; Volume 12, pp. 81–101. ISBN 978-3-319-73376-0. [Google Scholar]
- Cappello, F.; Bellafiore, M.; David, S.; Anzalone, R.; Zummo, G. Ten Kilodalton Heat Shock Protein (HSP10) Is Overexpressed during Carcinogenesis of Large Bowel and Uterine Exocervix. Cancer Lett. 2003, 196, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Piura, B.; Rabinovich, A.; Yavelsky, V.; Wolfson, M. Heat shock proteins and malignancies of the female genital tract. Harefuah 2002, 141, 969–972. [Google Scholar]
- Wu, D.; Xv, J.; Song, E.; Tang, S.; Zhang, X.; Kemper, N.; Hartung, J.; Bao, E. Acetyl Salicylic Acid Protected against Heat Stress Damage in Chicken Myocardial Cells and May Associate with Induced Hsp27 Expression. Cell Stress Chaperones 2015, 20, 687–696. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Xu, J.; Song, E.; Lv, Y.; Tang, S.; Zhang, X.; Kemper, N.; Hartung, J.; Bao, E. In Vitro Evaluation of Aspirin-Induced HspB1 against Heat Stress Damage in Chicken Myocardial Cells. Cell Stress Chaperones 2016, 21, 405–413. [Google Scholar] [CrossRef]
- Tang, S.; Yin, B.; Song, E.; Chen, H.; Cheng, Y.; Zhang, X.; Bao, E.; Hartung, J. Aspirin Upregulates αB-Crystallin to Protect the Myocardium against Heat Stress in Broiler Chickens. Sci. Rep. 2016, 6, 37273. [Google Scholar] [CrossRef]
- Toplu, H.D.O.; Tunca, R.; Aypak, S.U.; Coven, F.; Epikmen, E.T.; Karaarslan, S.; YagiN, O. Effects of Heat Conditioning and Dietary Ascorbic Acid Supplementation on Heat Shock Protein 70 Expression, Blood Parameters and Fear-Related Behavior in Broilers Subjected to Heat Stress. Acta Sci. Vet. 2014, 42, 2305–2307. [Google Scholar]
- Yin, B.; Di, L.; Tang, S.; Bao, E. Vitamin CNa Enhances the Antioxidant Ability of Chicken Myocardium Cells and Induces Heat Shock Proteins to Relieve Heat Stress Injury. Res. Vet. Sci. 2020, 133, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, H.; Cong, X.; Wang, X.; Jiang, Z.; Zhang, Q.; Qi, X.; Gao, S.; Cao, R.; Tian, W. Baicalin Attenuates Lipopolysaccharide Induced Inflammation and Apoptosis of Cow Mammary Epithelial Cells by Regulating NF-κB and HSP72. Int. Immunopharmacol. 2016, 40, 139. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mao, Y.; Guo, K.; Wu, H.; Song, X.; Qu, M.; Lan, L.; Luo, J. The Synergistic Effect of Traditional Chinese Medicine Prescription and Rumen-Protected γ-Aminobutyric Acid on Beef Cattle under Heat Stress. J. Anim. Physiol. Anim. Nutr. 2021, 105, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Xie, C.; Yin, Y.; Li, F.; Li, T.; Huang, R.; Zheng, R.; Deng, Z. Effect of L-Arginine on HSP70 Expression in Liver in Weanling Piglets. Bmc Vet. Res. 2013, 9, 63. [Google Scholar]
- Che, D.; Adams, S.; Zhao, B.; Qin, G.; Jiang, H. Effects of Dietary L-Arginine Supplementation from Conception to Post-Weaning in Piglets. Curr. Protein Pept. Sci. 2019, 20, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Ramesh, K.; Hyder, I.; Uniyal, S.; Yadav, V.P.; Panda, R.P.; Maurya, V.P.; Singh, G.; Kumar, P.; Mitra, A. Effect of Melatonin Administration on Thyroid Hormones, Cortisol and Expression Profile of Heat Shock Proteins in Goats (Capra Hircus) Exposed to Heat Stress. Small Rumin. Res. 2013, 112, 216–223. [Google Scholar] [CrossRef]
- Tonomura, H.; Takahashi, K.A.; Mazda, O.; Arai, Y.; Inoue, A.; Terauchi, R.; Shin-Ya, M.; Kishida, T.; Imanishi, J.; Kubo, T. Glutamine Protects Articular Chondrocytes from Heat Stress and NO-Induced Apoptosis with HSP70 Expression. Osteoarthritis Cartilage 2006, 14, 545. [Google Scholar] [CrossRef]
- Luo, L.; Li, Y.; Shan, H.; Wang, L.; Yuan, F.; Ma, Y.; Li, W.; He, T.; Wang, Y.; Qu, M.; et al. L-glutamine Protects Mouse Brain from Ischemic Injury via Up-regulating Heat Shock Protein 70. CNS Neurosci. Ther. 2019, 25, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Khodagholi, F.; Eftekharzadeh, B.; Maghsoudi, N.; Rezaei, P.F. Chitosan Prevents Oxidative Stress-Induced Amyloid β Formation and Cytotoxicity in NT2 Neurons: Involvement of Transcription Factors Nrf2 and NF-κB. Mol. Cell. Biochem. 2010, 337, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.-K.; Cheng, P.-Y.; Lee, Y.-M.; Liu, Y.-P.; Ding, C.; Liu, W.-H.; Yen, M.-H. The Role of Heat Shock Protein 70 in the Protective Effect of YC-1 on Heat Stroke Rats. Eur. J. Pharmacol. 2013, 699, 67–73. [Google Scholar] [CrossRef]
- Kan, L.; Guo, F.; Liu, Y.; Pham, V.H.; Guo, Y.; Wang, Z. Probiotics Bacillus Licheniformis Improves Intestinal Health of Subclinical Necrotic Enteritis-Challenged Broilers. Front. Microbiol. 2021, 12, 623739. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Liu, Y.; Bao, E.; Tang, S. The Protective Role of Heat Shock Proteins against Stresses in Animal Breeding. Int. J. Mol. Sci. 2024, 25, 8208. https://doi.org/10.3390/ijms25158208
Liu S, Liu Y, Bao E, Tang S. The Protective Role of Heat Shock Proteins against Stresses in Animal Breeding. International Journal of Molecular Sciences. 2024; 25(15):8208. https://doi.org/10.3390/ijms25158208
Chicago/Turabian StyleLiu, Sirui, Yinkun Liu, Endong Bao, and Shu Tang. 2024. "The Protective Role of Heat Shock Proteins against Stresses in Animal Breeding" International Journal of Molecular Sciences 25, no. 15: 8208. https://doi.org/10.3390/ijms25158208






