Effects of Nannochloropsis salina Fermented Oil on Proliferation of Human Dermal Papilla Cells and Hair Growth
Abstract
:1. Introduction
2. Results
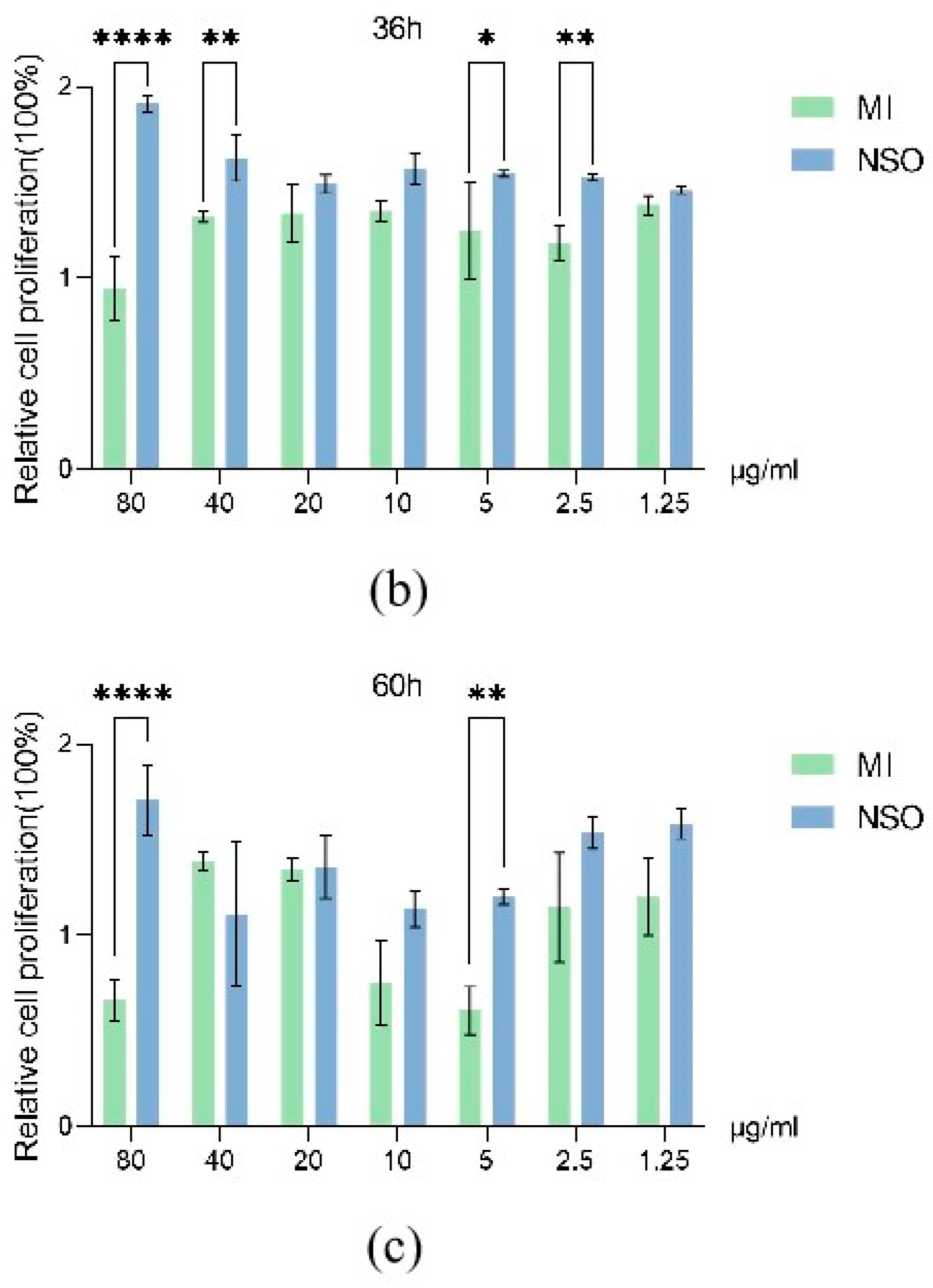
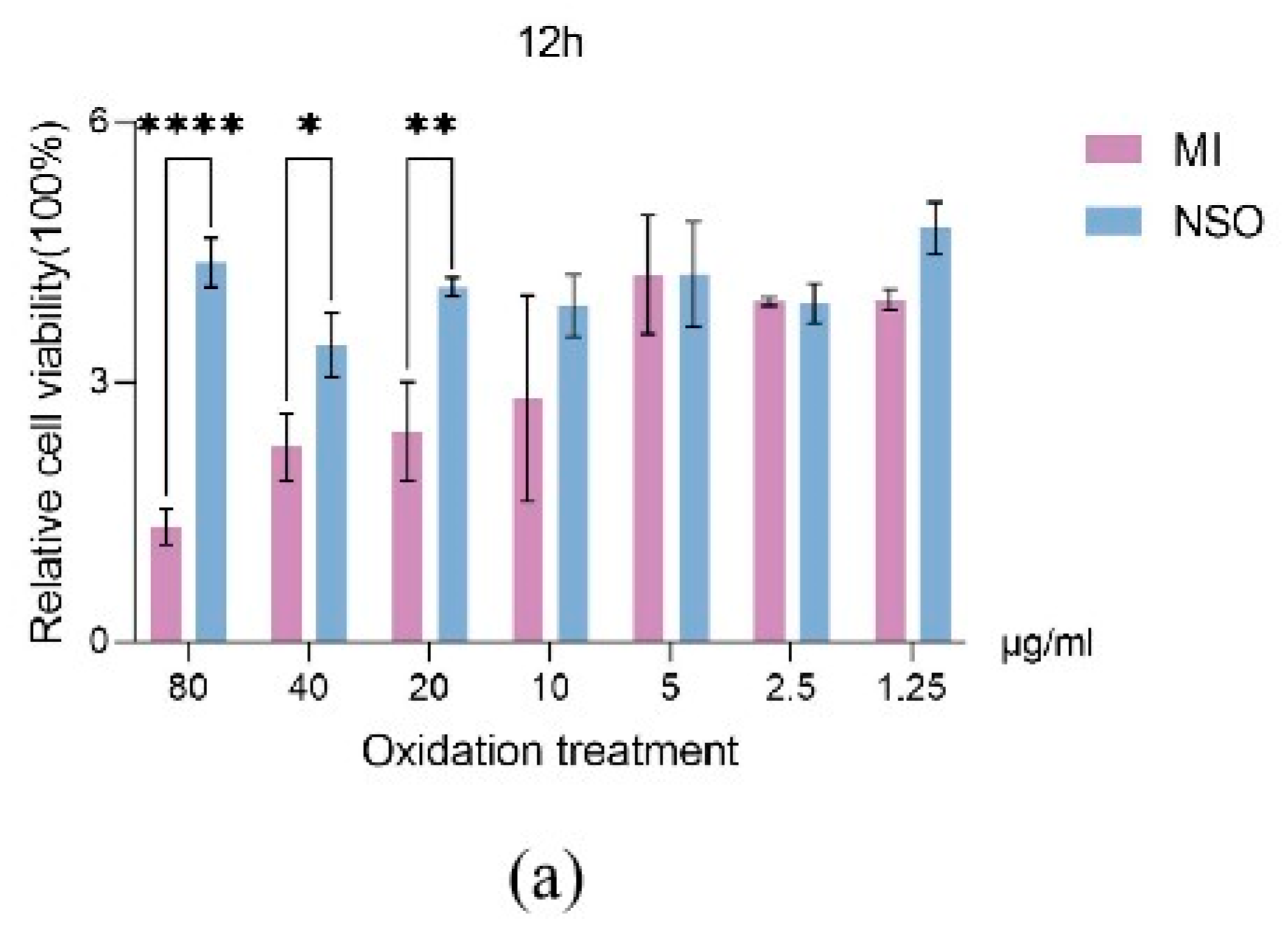
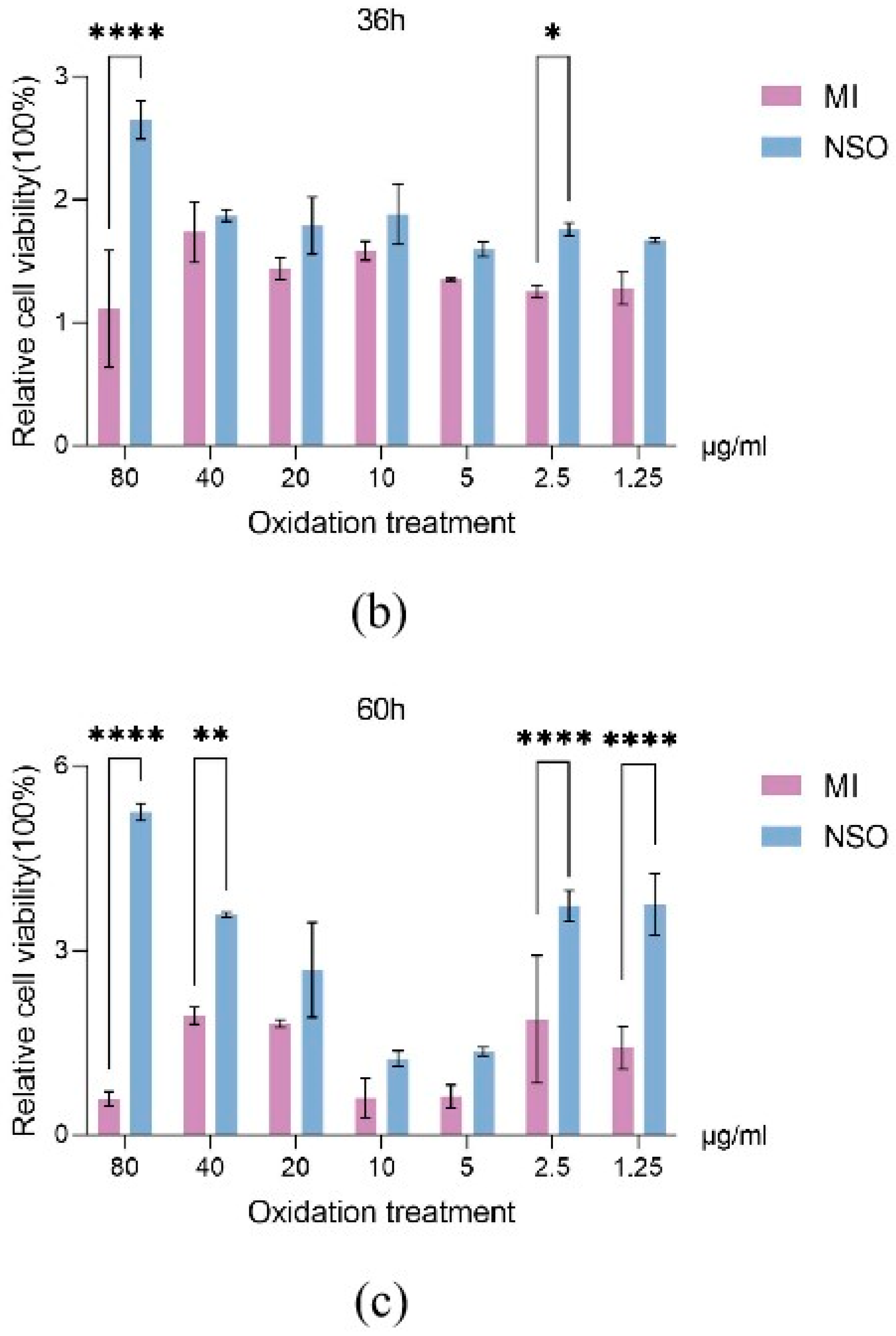
2.1. The Effect of NSO on the Vitality of DPCs with H2O2-Induced Damage
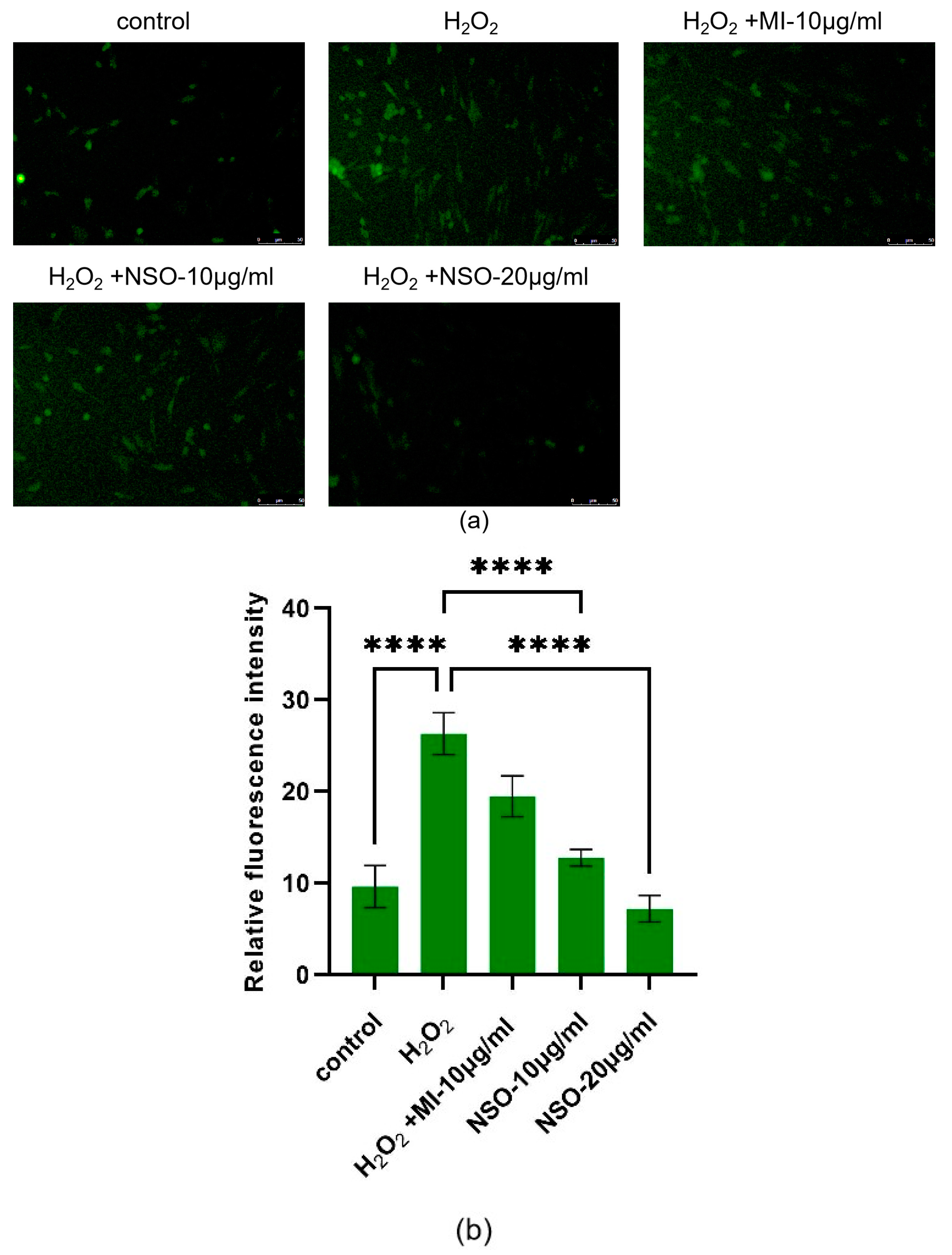
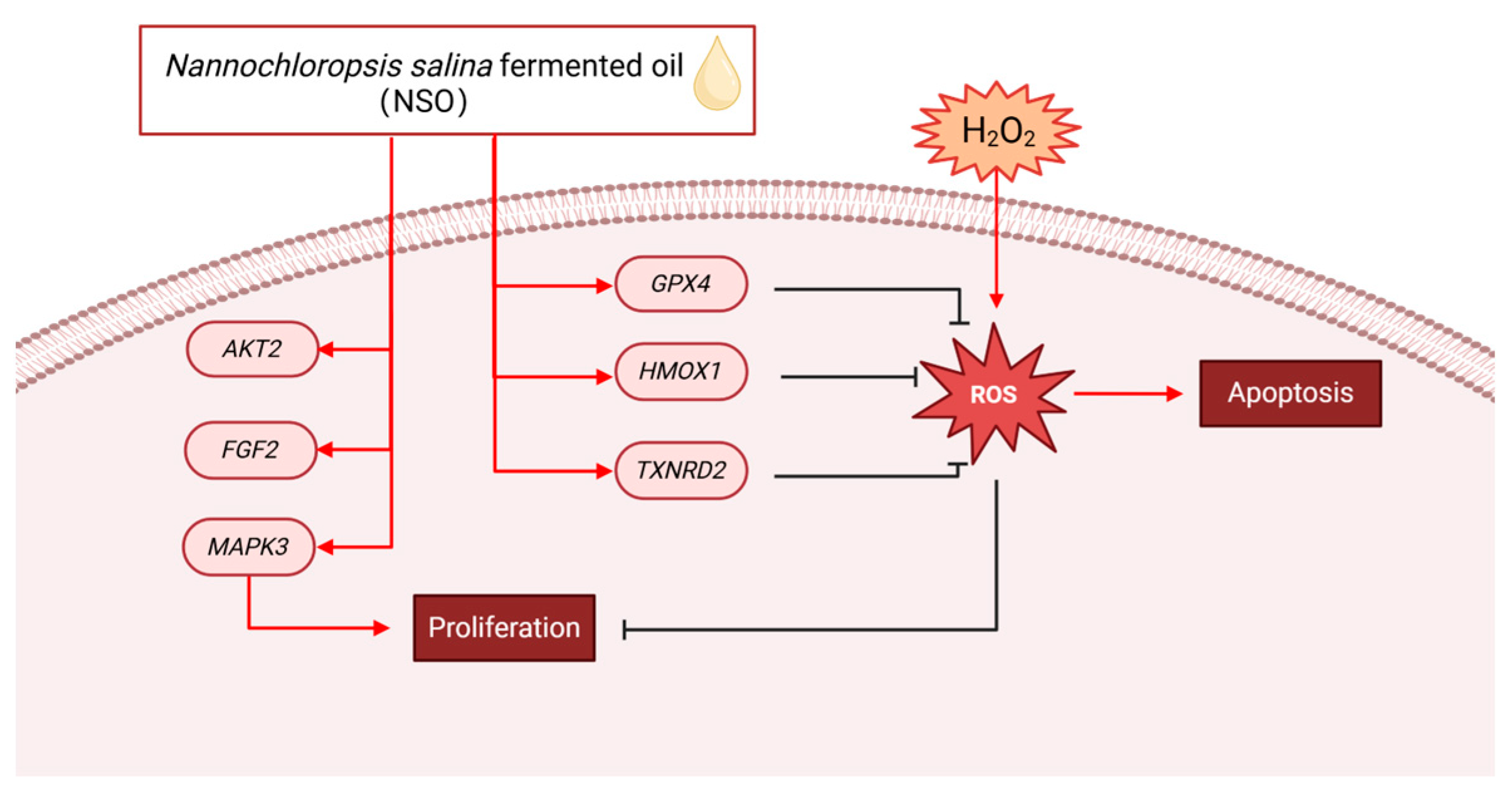
2.2. The Effect of NSO on the H2O2-Induced ROS Production
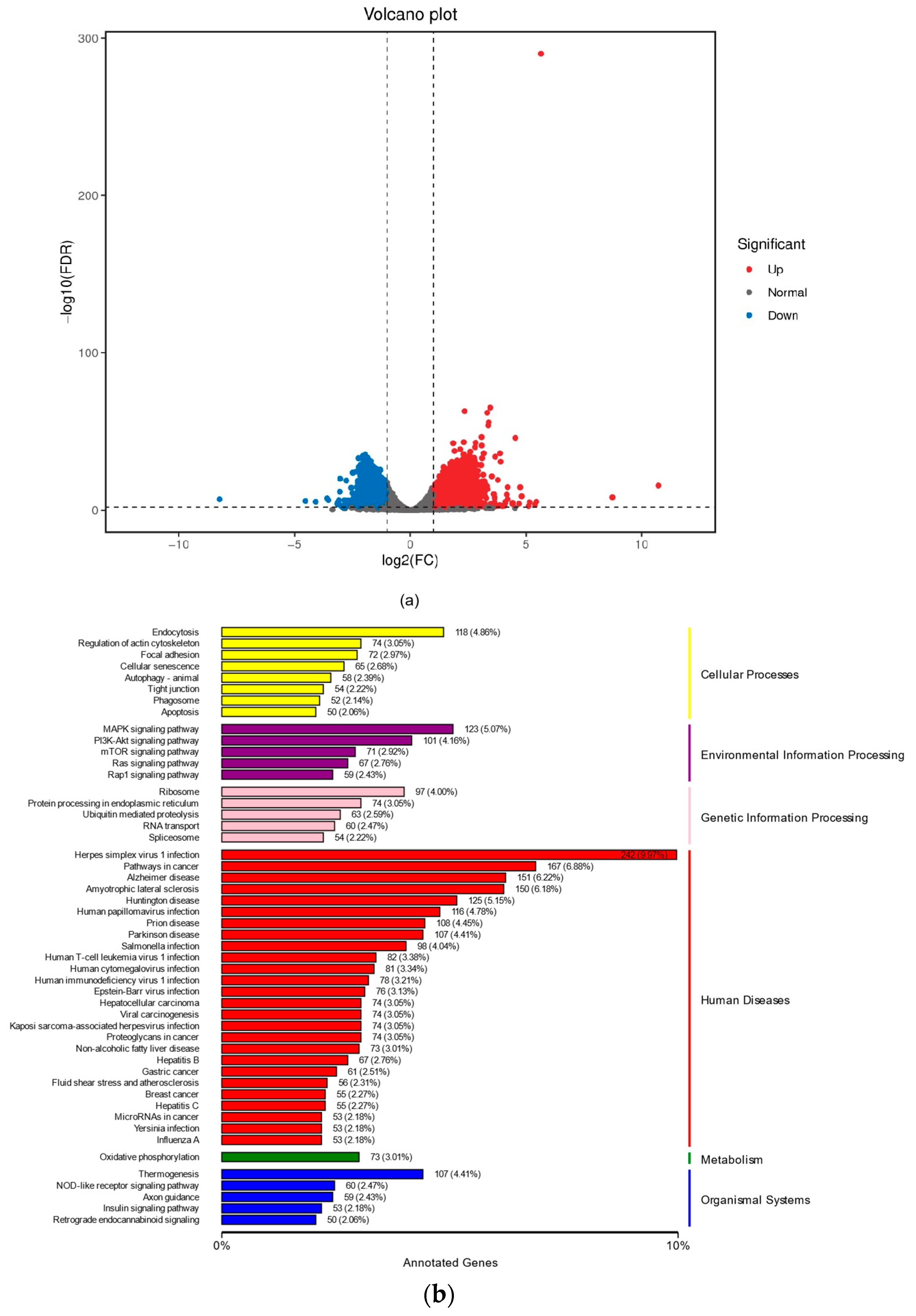
2.3. The Effects of NSO on the Gene Expression Levels of DPC Proliferation and Antioxidation
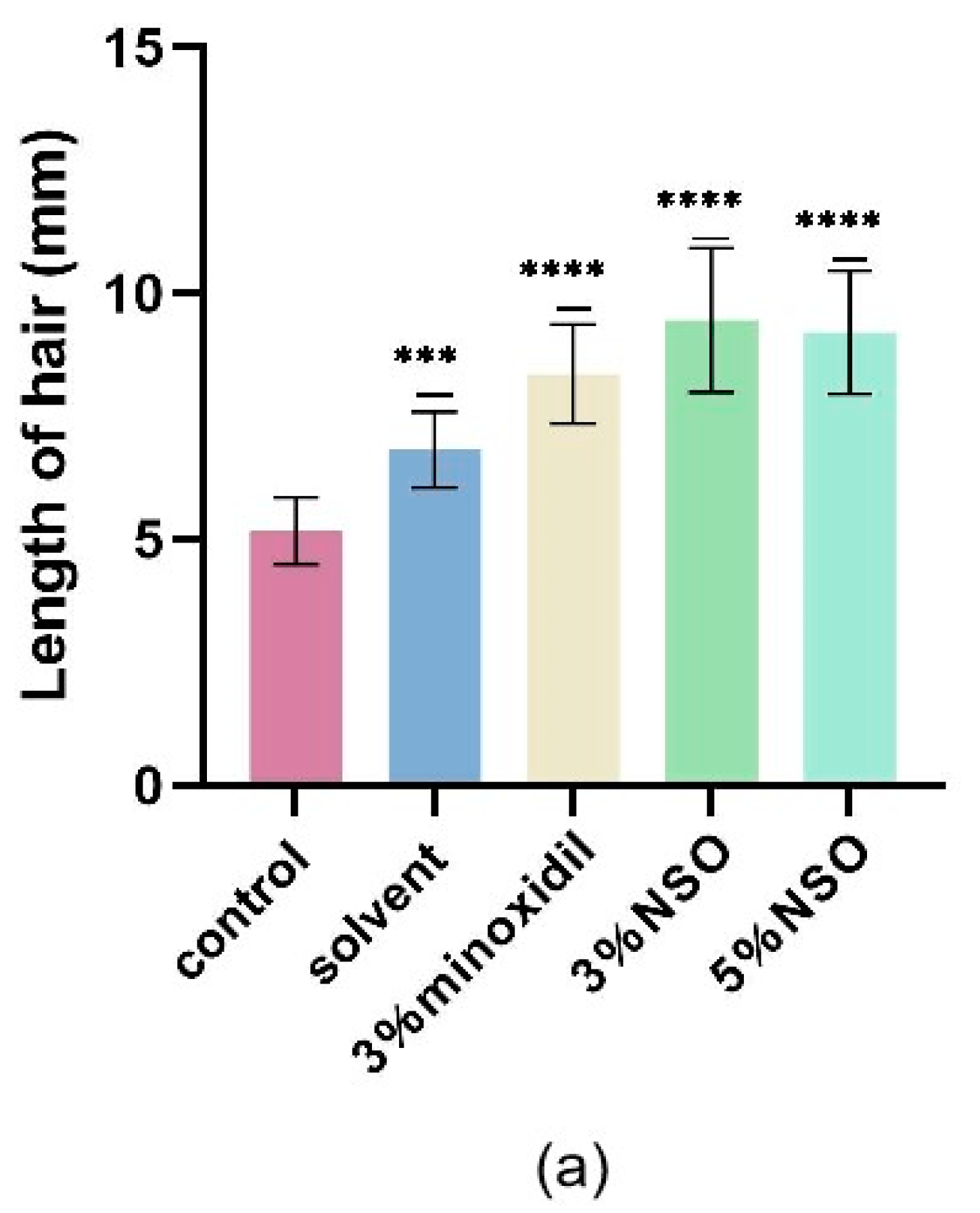
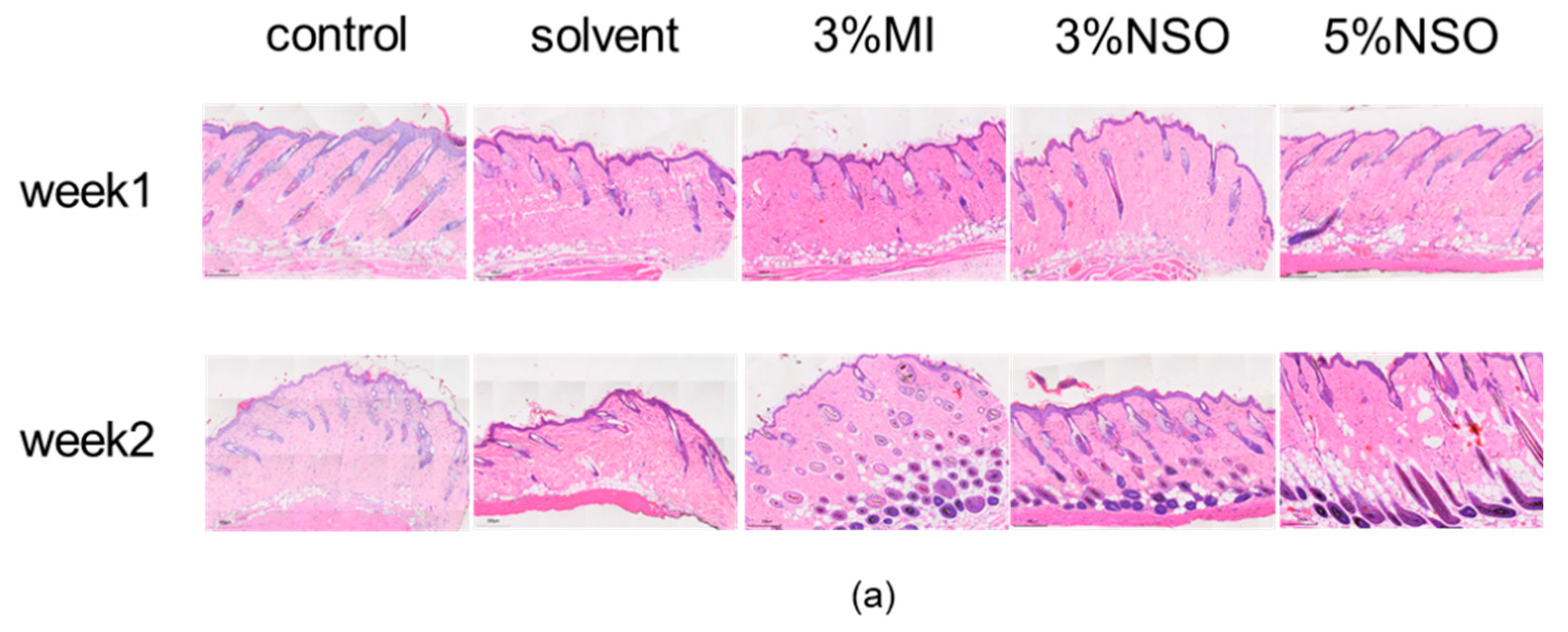
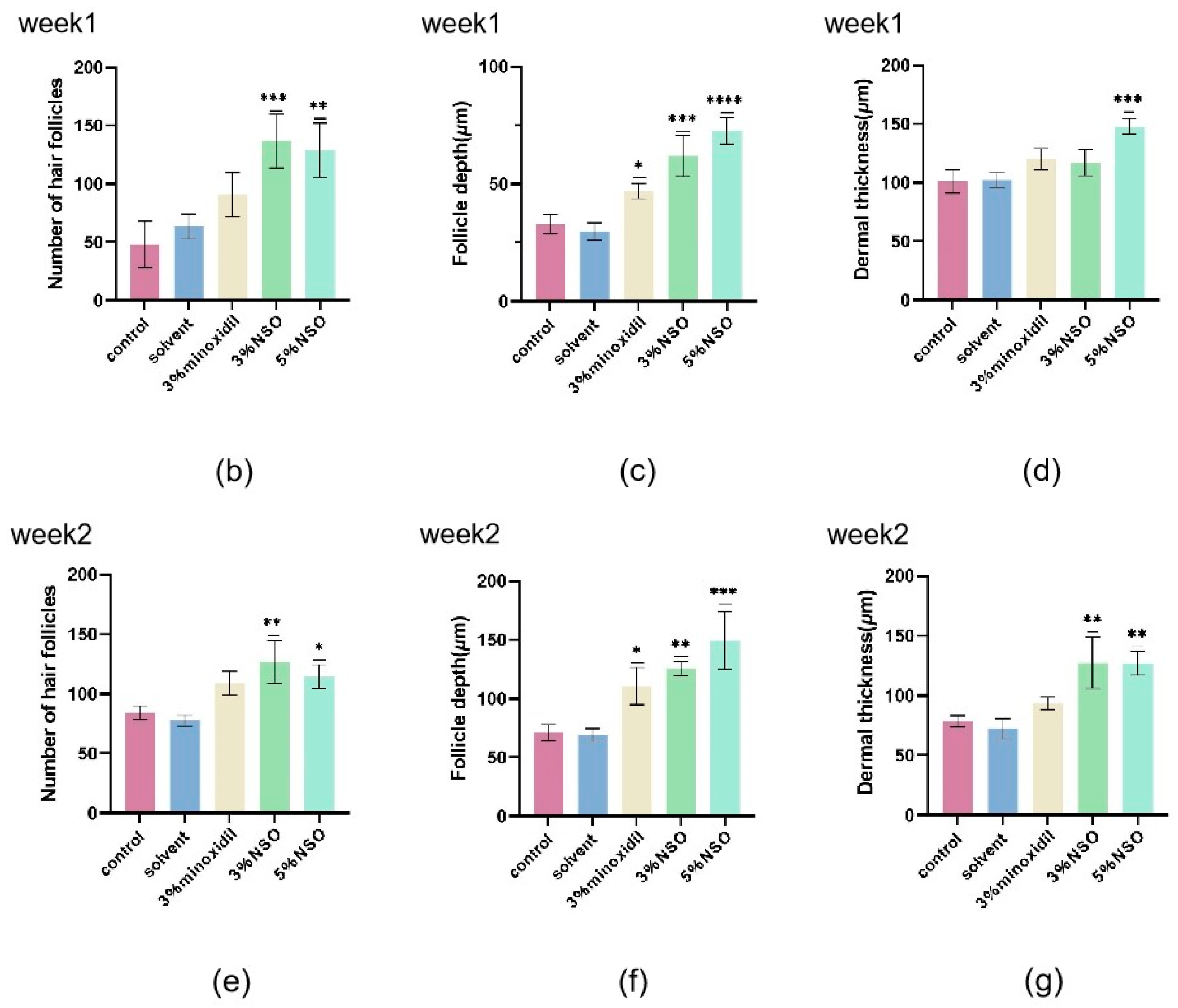
2.4. Hair Growth-Promoting Effect of NSO in C57BL/6 Mice
2.5. GC/MS Profile of NSO Extracts
3. Discussion
4. Materials and Methods
4.1. Nannochloropsis salina Fermented Oil Extract Preparation
GC-MS Analysis
4.2. Cell Culture
4.2.1. Cell Viability Assay
4.2.2. Antioxidant Activity of NSO
4.2.3. Determination of Active Oxygen Level
4.3. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. Experimental Animals
4.4.1. Hair Growth Observation
4.4.2. Histological Analysis
4.4.3. Determination of Hair Length
4.4.4. Determination of Hair Weight
4.4.5. Determination of Hair Follicle Length and Dermal Thickness
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamerson, T.A.; Aguh, C. An Approach to Patients with Alopecia. Med. Clin. N. Am. 2021, 105, 599–610. [Google Scholar] [CrossRef]
- Cho, E.C.; Kim, K. A comprehensive review of biochemical factors in herbs and their constituent compounds in experimental studies on alopecia. J. Ethnopharmacol. 2020, 258, 112907. [Google Scholar] [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. J. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef]
- Shin, J.Y.; Choi, Y.H.; Kim, J.; Park, S.Y.; Nam, Y.J.; Lee, S.Y.; Jeon, J.H.; Jin, M.H.; Lee, S. Polygonum multiflorum extract support hair growth by elongating anagen phase and abrogating the effect of androgen in cultured human dermal papilla cells. BMC Complement. Med. Ther. 2020, 20, 144. [Google Scholar] [CrossRef]
- Park, A.M.; Khan, S.; Rawnsley, J. Hair Biology: Growth and Pigmentation. Facial Plast. Surg. Clin. N. Am. 2018, 26, 415–424. [Google Scholar] [CrossRef]
- Amin, S.S.; Sachdeva, S. Alopecia areata: A review. J. Saudi Soc. Dermatol. Dermatol. Surg. 2013, 17, 37–45. [Google Scholar] [CrossRef]
- Upton, J.H.; Hannen, R.F.; Bahta, A.W.; Farjo, N.; Farjo, B.; Philpott, M.P. Oxidative Stress–Associated Senescence in Dermal Papilla Cells of Men with Androgenetic Alopecia. J. Investig. Dermatol. 2015, 135, 1244–1252. [Google Scholar] [CrossRef]
- Trüeb, R.M. Oxidative stress in ageing of hair. Int. J. Trichol. 2009, 1, 6–14. [Google Scholar] [CrossRef]
- Bakry, O.A.; Elshazly, R.M.; Shoeib, M.A.; Gooda, A. Oxidative stress in alopecia areata: A case-control study. Am. J. Clin. Dermatol. 2014, 15, 57–64. [Google Scholar] [CrossRef]
- Seo, J.A.; Bae, I.H.; Jang, W.H.; Kim, J.H.; Bak, S.Y.; Han, S.H.; Park, Y.H.; Lim, K.M. Hydrogen peroxide and monoethanolamine are the key causative ingredients for hair dye-induced dermatitis and hair loss. J. Dermatol. Sci. 2012, 66, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Yoo, H.G.; Inui, S.; Itami, S.; Kim, I.G.; Cho, A.R.; Lee, D.H.; Park, W.S.; Kwon, O.; Cho, K.H.; et al. Induction of transforming growth factor-beta 1 by androgen is mediated by reactive oxygen species in hair follicle dermal papilla cells. BMB Rep. 2013, 46, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.M.; An, S.; Lee, O.K.; Lee, M.J.; Lee, J.P.; Lee, K.S.; Lee, G.T.; Lee, K.K.; Bae, S. Analysis of changes in microRNA expression profiles in response to the troxerutin-mediated antioxidant effect in human dermal papilla cells. Mol. Med. Rep. 2015, 12, 2650–2660. [Google Scholar] [CrossRef]
- Ring, C.M.; Finney, R.; Avram, M. Lasers, lights, and compounds for hair loss in aesthetics. Clin. Dermatol. 2022, 40, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Moussa, A.; Kazmi, A.; Bokhari, L.; Sinclair, R.D. Bicalutamide improves minoxidil-induced hypertrichosis in female pattern hair loss: A retrospective review of 35 patients. J. Am. Acad. Dermatol. 2021, 87, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.L.; Montalvão, S.A.L.; Cancela, R.B.B.; Silva, F.A.R.; Urban, A.; Huber, S.C.; Júnior, J.L.R.C.; Lana, J.F.S.D.; Annichinno-Bizzacchi, J.M. Treatment of male pattern alopecia with platelet-rich plasma: A double-blind controlled study with analysis of platelet number and growth factor levels. J. Am. Acad. Dermatol. 2019, 80, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Dubin, D.P.; Lin, M.J.; Leight, H.M.; Farberg, A.S.; Torbeck, R.L.; Burton, W.B.; Khorasani, H. The effect of platelet-rich plasma on female androgenetic alopecia: A randomized controlled trial. J. Am. Acad. Dermatol. 2020, 83, 1294–1297. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, M.; Mazzetti, A.; Moro, L.; Rosette, C.; Gerloni, M. A summary of in vitro, phase I, and phase II studies evaluating the mechanism of action, safety, and efficacy of clascoterone (cortexolone 17a propionate, CB-03-01) in androgenetic alopecia. J. Am. Acad. Dermatol. 2019, 81 (Suppl. 1), AB13. [Google Scholar] [CrossRef]
- Lee, Y.H.; Choi, H.J.; Kim, J.Y.; Kim, J.E.; Lee, J.H.; Cho, S.H.; Yun, M.Y.; An, S.; Song, G.Y.; Bae, S. Ginsenoside Rg4 Enhances the Inductive Effects of Human Dermal Papilla Spheres on Hair Growth Via the AKT/GSK-3β/β-Catenin Signaling Pathway. J. Microbiol. Biotechnol. 2021, 31, 933–941. [Google Scholar] [CrossRef]
- Park, H.J.; Jin, G.R.; Jung, J.H.; Hwang, S.B.; Lee, S.H.; Lee, B.H. Hair Growth Promotion Effect of Nelumbinis Semen Extract with High Antioxidant Activity. Evid. Based Complement. Altern. Med. 2021, 2021, 6661373. [Google Scholar] [CrossRef]
- Choi, H.; Munkhbayer, S.; Choi, S.; Shin, C.; Kim, K.; Kwon, O. LB1604 The role of polyunsaturated fatty acids on hair growth. J. Investig. Dermatol. 2018, 138, B23. [Google Scholar] [CrossRef]
- Ma, X.N.; Chen, T.P.; Yang, B.; Liu, J.; Chen, F. Lipid Production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Jaseera, K.V.; Ebeneezar, S.; Sayooj, P.; Anusree, V.N.; Kaladharan, P. Dietary supplementation of microalgae, Aurantiochytrium sp. and co-feeding with Artemia enhances the growth, stress tolerance and survival in Penaeus monodon (Fabricius, 1798) post larvae. Aquaculture 2021, 533, 736176. [Google Scholar] [CrossRef]
- Zhao, W.; Fang, H.-H.; Liu, Z.-Z.; Chen, J.-M.; Zhang, C.-W.; Gao, B.-Y.; Niu, J. Responses in growth performance, enzymatic activity, immune function and liver health after dietary supplementation of Porphyridium sp. in juvenile golden pompano (Trachinotus ovatus). Aquac. Nutr. 2021, 27, 679–690. [Google Scholar] [CrossRef]
- Demirbas, A.; Demirbas, M.F. Importance of algae oil as a source of biodiesel. Energy Convers. Manag. 2011, 52, 163–170. [Google Scholar] [CrossRef]
- Mathew, S.A.; Bhonde, R.R. Omega-3 polyunsaturated fatty acids promote angiogenesis in placenta derived mesenchymal stromal cells. Pharmacol. Res. 2018, 132, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.; Santos, R.A.; Iglesias, P.; Couto, A.; Serra, C.R.; Gouvinhas, I.; Barros, A.; Oliva-Teles, A.; Enes, P.; Díaz-Rosales, P. Effect of extraction method and solvent system on the phenolic content and antioxidant activity of selected macro- and microalgae extracts. J. Appl. Phycol. 2020, 32, 349–362. [Google Scholar] [CrossRef]
- Supraja, K.V.; Behera, B.; Balasubramanian, P. Efficacy of microalgal extracts as biostimulants through seed treatment and foliar spray for tomato cultivation. Ind. Crops Prod. 2020, 151, 112453. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Mujeeb, M.; Vedamurthy, A.; Shettar, A.K.; Puranik, S.; Ghagane, S.; Thimmappa, S.C. In vitro Anti-oxidant and Anti-cancer Activity of Tetradesmus acuminatus Microalgae Extract on MCF-7 Human Breast Cancer Cell Line. Int. J. Cancer Res. 2020, 16, 1–9. [Google Scholar] [CrossRef]
- Gürlek, C.; Yarkent, Ç.; Köse, A.; Tuğcu, B.; Gebeloğlu, I.K.; Öncel, S.Ş.; Elibol, M. Screening of antioxidant and cytotoxic activities of several microalgal extracts with pharmaceutical potential. Health Technol. 2020, 10, 111–117. [Google Scholar] [CrossRef]
- Kang, J.I.; Yoon, H.S.; Kim, S.M.; Park, J.E.; Hyun, Y.J.; Ko, A.; Ahn, Y.S.; Koh, Y.S.; Hyun, J.W.; Yoo, E.S.; et al. Mackerel-Derived Fermented Fish Oil Promotes Hair Growth by Anagen-Stimulating Pathways. Int. J. Mol. Sci. 2018, 19, 2770. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Xue, X. Detection of Total Reactive Oxygen Species in Adherent Cells by 2’,7’-Dichlorodihydrofluorescein Diacetate Staining. J. Vis. Exp. 2020, 160, e60682. [Google Scholar] [CrossRef]
- Munkhbayar, S.; Jang, S.; Cho, A.R.; Choi, S.J.; Shin, C.Y.; Eun, H.C.; Kim, K.H.; Kwon, O. Role of Arachidonic Acid in Promoting Hair Growth. Ann. Dermatol. 2016, 28, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.S.; Jeong, J.; Lee, C.M.; Lee, K.S.; Lee, J.N.; Park, S.M.; Lee, Y.M. Activation of Hair Cell Growth Factors by Linoleic Acid in Malva verticillata Seed. Molecules 2021, 26, 2117. [Google Scholar] [CrossRef] [PubMed]
- Amjad Khan, W.; Chun-Mei, H.; Khan, N.; Iqbal, A.; Lyu, S.-W.; Shah, F. Bioengineered Plants Can Be a Useful Source of Omega-3 Fatty Acids. BioMed Res. Int. 2017, 2017, 7348919. [Google Scholar] [CrossRef]
- Le Floc’h, C.; Cheniti, A.; Connétable, S.; Piccardi, N.; Vincenzi, C.; Tosti, A. Effect of a nutritional supplement on hair loss in women. J. Cosmet. Dermatol. 2015, 14, 76–82. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M.; Chaikul, P. Para rubber seed oil: The safe and efficient bio-material for hair loss treatment. J. Cosmet. Dermatol. 2021, 20, 2160–2167. [Google Scholar] [CrossRef]
- Sohn, K.M.; Jeong, K.H.; Kim, J.E.; Park, Y.M.; Kang, H. Hair growth-promotion effects of different alternating current parameter settings are mediated by the activation of Wnt/β-catenin and MAPK pathway. Exp. Dermatol. 2015, 24, 958–963. [Google Scholar] [CrossRef]
- Fingar, D.C.; Richardson, C.J.; Tee, A.R.; Cheatham, L.; Tsou, C.; Blenis, J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol. Cell Biol. 2004, 24, 200–216. [Google Scholar] [CrossRef]
- Lin, W.H.; Xiang, L.J.; Shi, H.X.; Zhang, J.; Jiang, L.P.; Cai, P.T.; Lin, Z.L.; Lin, B.B.; Huang, Y.; Zhang, H.L.; et al. Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. BioMed Res. Int. 2015, 2015, 730139. [Google Scholar] [CrossRef]
- Shi, J.H.; Zuo, K.Y.; Zhang, Y.Y.; Wang, B.; Han, X.; Lian, A.B.; Liu, J.Y. NANOG Alleviates the Damage of Human Hair Follicle Mesenchymal Stem Cells Caused by H2O2 through Activation of AKT Pathway. Biomed. Environ. Sci. 2019, 32, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lai, H.; Cai, X.; Liu, Y.; Hong, L.; Huang, X.; Shao, L. Thioredoxin Reductase 2 Synergizes with Cytochrome c, Somatic to Alleviate Doxorubicin-Induced Oxidative Stress in Cardiomyocytes and Mouse Myocardium. Int. Heart J. 2023, 64, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yu, Y.; Shen, Y.; Huang, H.; Lin, D.; Wang, K.; Yu, Y.; Li, K.; Cao, Y.; Wang, Q.; et al. Ythdf2 promotes pulmonary hypertension by suppressing Hmox1-dependent anti-inflammatory and antioxidant function in alveolar macrophages. Redox Biol. 2023, 61, 102638. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Qin, D.; Zhao, C.; Chai, L.; Yu, Z.; Wang, W.; Tong, L.; Lv, L.; Wang, Y.; Rehwinkel, J.; et al. Redox homeostasis maintained by GPX4 facilitates STING activation. Nat. Immunol. 2020, 21, 727–735. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, Y.; Jiang, Y.; Zhang, L.; Cheng, W. GPX4: The hub of lipid oxidation, ferroptosis, disease and treatment. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188890. [Google Scholar] [CrossRef]
- Xiong, W.; Li, X.; Xiang, J.; Wu, Q. High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl. Microbiol. Biotechnol. 2008, 78, 29–36. [Google Scholar] [CrossRef]
- Liang, S.; Yang, X.; Zhu, X.; Ibrar, M.; Liu, L.; Li, S.; Li, X.; Tian, T.; Li, S. Metabolic Engineering to Improve Docosahexaenoic Acid Production in Marine Protist Aurantiochytrium sp. by Disrupting 2,4-Dienoyl-CoA Reductase. Front. Mar. Sci. 2022, 9, 939716. [Google Scholar] [CrossRef]
- Bejaoui, M.; Taarji, N.; Saito, M.; Nakajima, M.; Isoda, H. Argan (Argania Spinosa) press cake extract enhances cell proliferation and prevents oxidative stress and inflammation of human dermal papilla cells. J. Dermatol. Sci. 2021, 103, 33–40. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, T.; Wang, J.; Jia, J.; Yi, Y.H.; Chen, Y.X.; Miao, Y.; Hu, Z.Q. Hydroxytyrosol prevents dermal papilla cells inflammation under oxidative stress by inducing autophagy. J. Biochem. Mol. Toxicol. 2019, 33, e22377. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, Y.; Bao, Q.; Shen, C.; Ni, D.; Hu, P.; Shi, J. Endogenous Copper for Nanocatalytic Oxidative Damage and Self-Protection Pathway Breakage of Cancer. ACS Nano 2021, 15, 16286–16297. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Kim, D.W.; Lee, S.H. Extract of Allium tuberosum Rottler ex Spreng Promoted the Hair Growth through Regulating the Expression of IGF-1. Evid. Based Complement Alternat. Med. 2015, 2015, 413538. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Park, M.A.; Kim, Y.C. Peppermint Oil Promotes Hair Growth without Toxic Signs. Toxicol. Res. 2014, 30, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Rambwawasvika, H.; Dzomba, P.; Gwatidzo, L. Hair Growth Promoting Effect of Dicerocaryum senecioides Phytochemicals. Int. J. Med. Chem. 2019, 2019, 7105834. [Google Scholar] [CrossRef] [PubMed]




| EPA | (Z)-9-PA | LA | PA | MA |
|---|---|---|---|---|
| 23.25% ± 2.54% | 26.01% ± 0.50% | 15.33% ± 1.39% | 9.92% ± 1.40% | 5.70% ± 0.50% |
| Gene | Primer |
|---|---|
| β-actin | F: 5′-GTGGAGTCATACTGGAACATGTAG-3′ R: 5′-AATGGTGAAGGTCGGTGTG-3′ |
| TXNRD2 | F: 5′-GCGAGTTCCAGAAACCAGGA-3′ R: 5′- AATATGCATGGTAAACATTGCTGT-3′ |
| HMOX1 | F: 5′-CTTCTTCACCTTCCCCAACA-3′ R: 5′-AGCTCCTGCAACTCCTCAAA-3′ |
| GPX4 | F: 5′-CAAAGTCCTAGGAAACGCCC-3′ R: 5′-CCTTGGCTGAGAA TTCGTGC-3′ |
| MAPK3 | F: 5′-TGCTGGACCGGATGTTAACC-3′ R: 5′-CTCATCCGTCGGGTCATAGT-3′ |
| AKT2 | F: 5′-TACATCAAGACCTGGAGGCC-3′ R: 5′-GGGGGTAGAGTCTGATCAGG-3′ |
| FGF2 | F: 5′-TCAAGCTACAACTTCAAGCAGA-3′ R: 5′-AGCCAGTAATCTTCCATCTTCCT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ying, M.; Zhou, J.; Zeng, Z.; Li, S.; Yang, X. Effects of Nannochloropsis salina Fermented Oil on Proliferation of Human Dermal Papilla Cells and Hair Growth. Int. J. Mol. Sci. 2024, 25, 8231. https://doi.org/10.3390/ijms25158231
Ying M, Zhou J, Zeng Z, Li S, Yang X. Effects of Nannochloropsis salina Fermented Oil on Proliferation of Human Dermal Papilla Cells and Hair Growth. International Journal of Molecular Sciences. 2024; 25(15):8231. https://doi.org/10.3390/ijms25158231
Chicago/Turabian StyleYing, Ming, Jialin Zhou, Zuye Zeng, Shuangfei Li, and Xuewei Yang. 2024. "Effects of Nannochloropsis salina Fermented Oil on Proliferation of Human Dermal Papilla Cells and Hair Growth" International Journal of Molecular Sciences 25, no. 15: 8231. https://doi.org/10.3390/ijms25158231





