Sex-Dependent T Cell Dysregulation in Mice with Diet-Induced Obesity
Abstract
:1. Introduction
2. Results
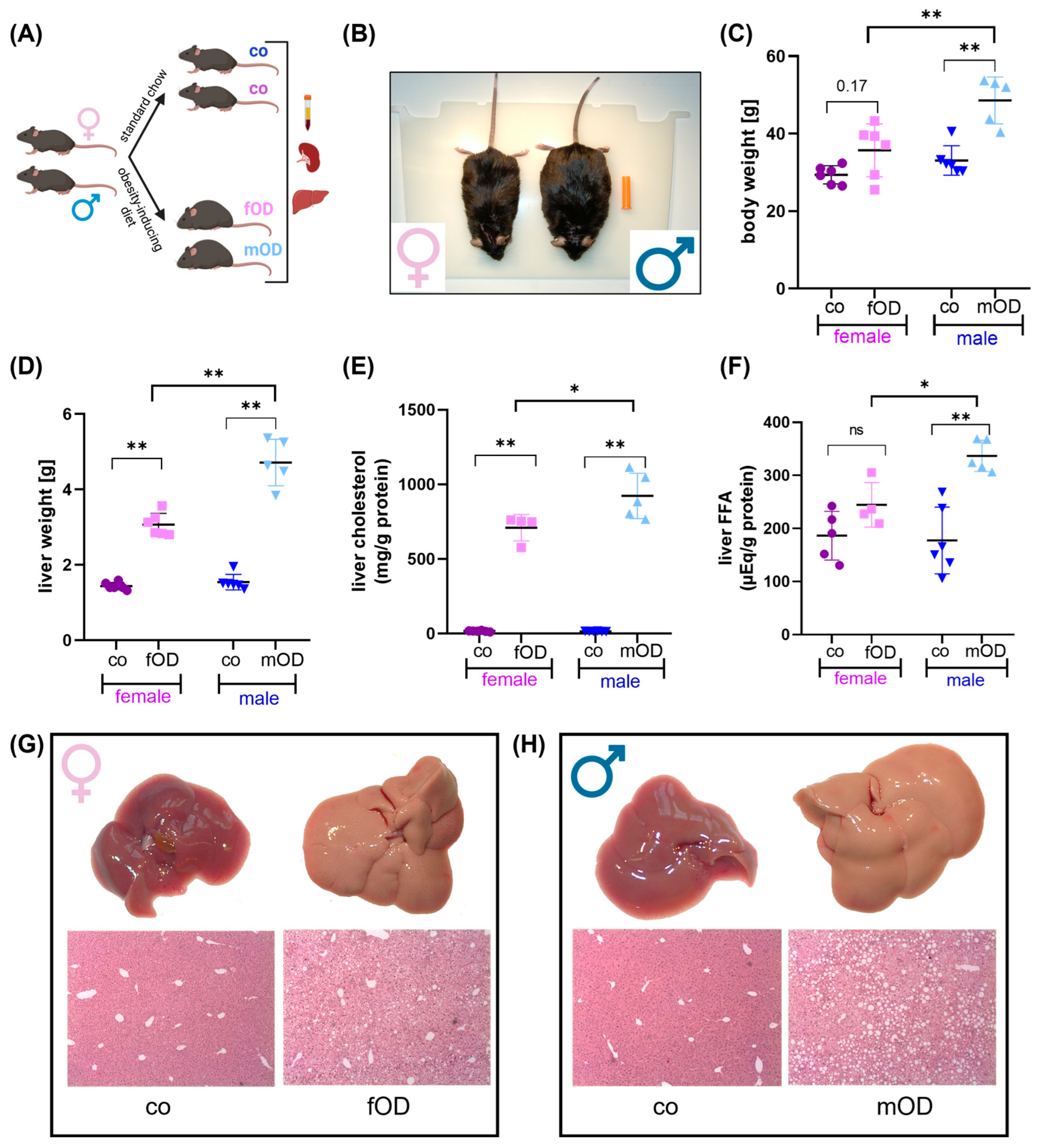
2.1. Male Mice Are More Susceptible to Diet-Induced Obesity and Fatty Liver Disease than Female
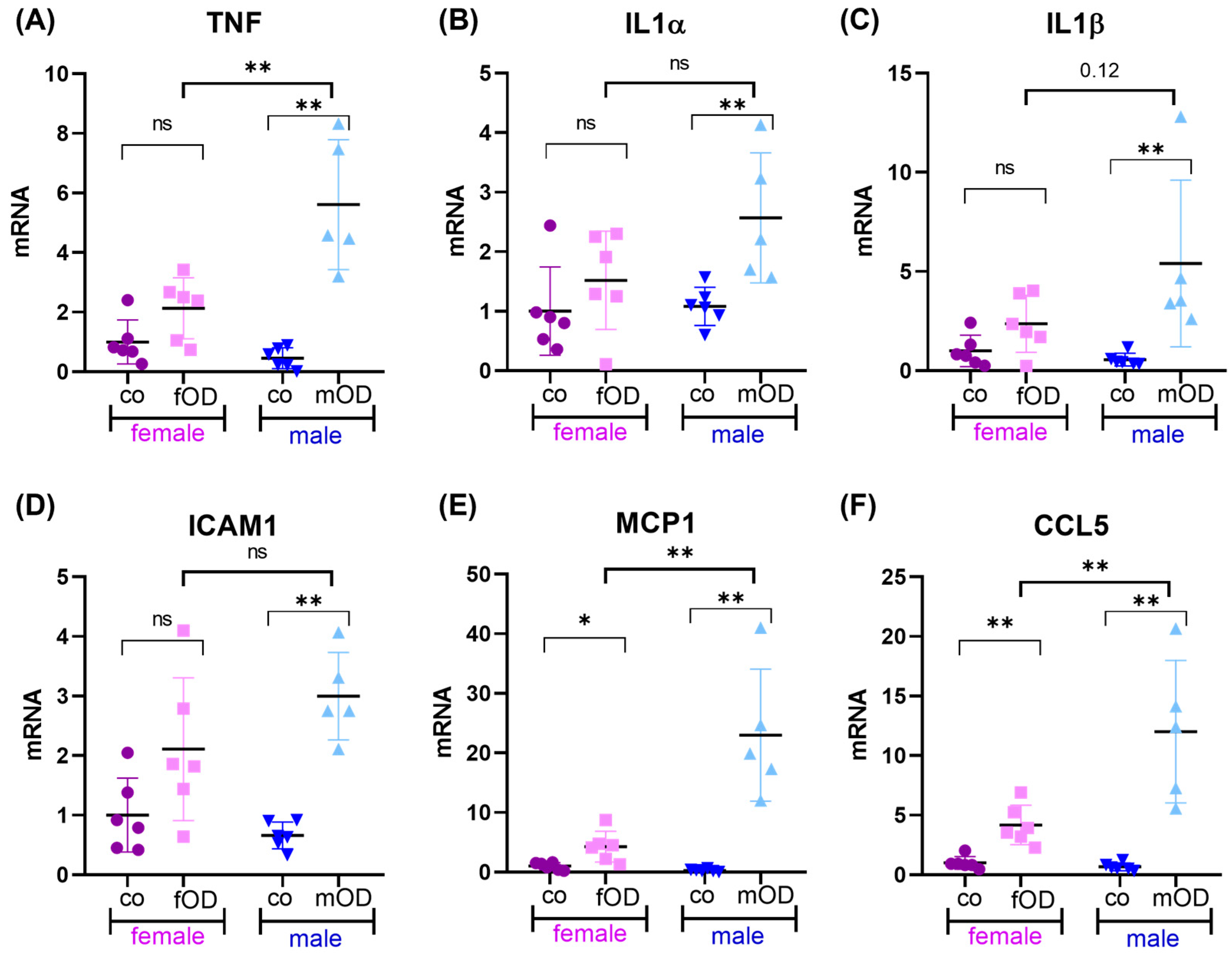
2.2. Obesity-Induced Liver Inflammation Is More Pronounced in Males than Females
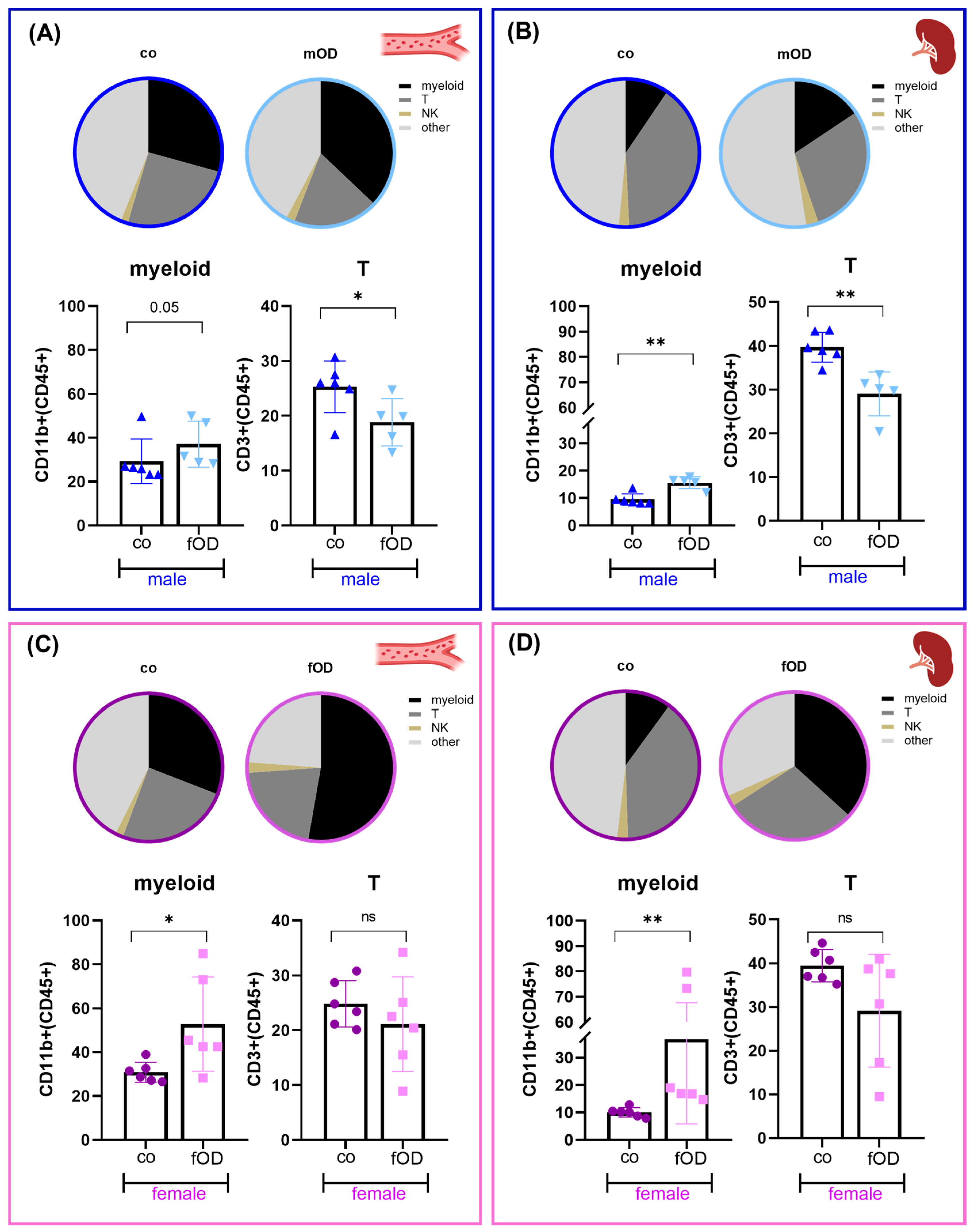
2.3. Obesity-Induced Systemic Immune Cell Alterations in Males Are Only Partly Reflected in Females
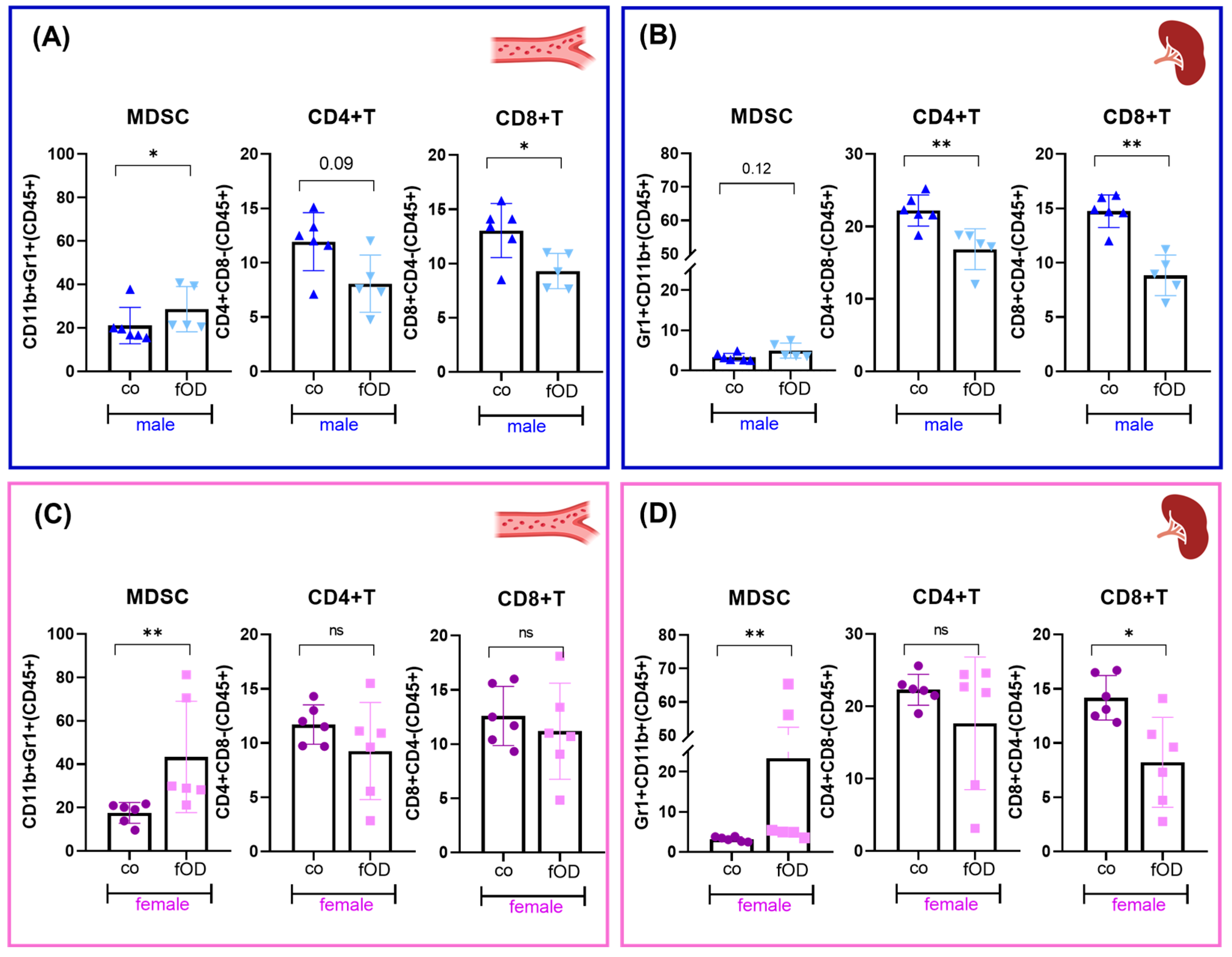
2.4. Males Are More Susceptible to Obesity-Induced T Cell Dysregulation than Females
3. Discussion
4. Materials and Methods
4.1. Experimental Animal Procedures
4.2. Mouse Tissue Processing
4.3. Metabolic Serum Parameter
4.4. Hepatic Lipid Content
4.5. Flow Cytometry
4.6. Isolation and Analysis of RNA
4.7. Visualization and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Mambrini, S.P.; Menichetti, F.; Ravella, S.; Pellizzari, M.; De Amicis, R.; Foppiani, A.; Battezzati, A.; Bertoli, S.; Leone, A. Ultra-Processed Food Consumption and Incidence of Obesity and Cardiometabolic Risk Factors in Adults: A Systematic Review of Prospective Studies. Nutrients 2023, 15, 2583. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity and Overweight—WHO Fact Sheet [Internet]. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 18 June 2024).
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Behary, J.; Amorim, N.; Jiang, X.T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Levings, M.K. Immune regulation in obesity-associated adipose inflammation. J. Immunol. 2013, 191, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Alwarawrah, Y.; Kiernan, K.; MacIver, N.J. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front. Immunol. 2018, 9, 1055. [Google Scholar] [CrossRef]
- Stolarczyk, E. Adipose tissue inflammation in obesity: A metabolic or immune response? Curr. Opin. Pharmacol. 2017, 37, 35–40. [Google Scholar] [CrossRef]
- Touch, S.; Clément, K.; André, S. T Cell Populations and Functions Are Altered in Human Obesity and Type 2 Diabetes. Curr. Diabetes Rep. 2017, 17, 81. [Google Scholar] [CrossRef] [PubMed]
- Van Herck, M.A.; Weyler, J.; Kwanten, W.J.; Dirinck, E.L.; De Winter, B.Y.; Francque, S.M.; Vonghia, L. The Differential Roles of T Cells in Non-alcoholic Fatty Liver Disease and Obesity. Front. Immunol. 2019, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Milner, J.J.; Beck, M.A. The impact of obesity on the immune response to infection. Proc. Nutr. Soc. 2012, 71, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br. J. Nutr. 2011, 106 (Suppl. S3), S5–S78. [Google Scholar] [CrossRef] [PubMed]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [PubMed]
- Tramunt, B.; Smati, S.; Grandgeorge, N.; Lenfant, F.; Arnal, J.F.; Montagner, A.; Gourdy, P. Sex differences in metabolic regulation and diabetes susceptibility. Diabetologia 2020, 63, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Kappert, K.; Böhm, M.; Schmieder, R.; Schumacher, H.; Teo, K.; Yusuf, S.; Sleight, P.; Unger, T. Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: Analysis of the Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects with Cardiovascular Disease (TRANSCEND) and the Ongoing Telmisartan Alone and in Combination with Ramipril Global End Point Trial (ONTARGET). Circulation 2012, 126, 934–941. [Google Scholar]
- Braga Tibaes, J.R.; Barreto Silva, M.I.; Wollin, B.; Vine, D.; Tsai, S.; Richard, C. Sex differences in systemic inflammation and immune function in diet-induced obesity rodent models: A systematic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2024, 25, e13665. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- SantaCruz-Calvo, S.; Bharath, L.; Pugh, G.; SantaCruz-Calvo, L.; Lenin, R.R.; Lutshumba, J.; Liu, R.; Bachstetter, A.D.; Zhu, B.; Nikolajczyk, B.S. Adaptive immune cells shape obesity-associated type 2 diabetes mellitus and less prominent comorbidities. Nat. Rev. Endocrinol. 2022, 18, 23–42. [Google Scholar] [CrossRef]
- Mills, E.L.; Harmon, C.; Jedrychowski, M.P.; Xiao, H.; Gruszczyk, A.V.; Bradshaw, G.A.; Tran, N.; Garrity, R.; Laznik-Bogoslavski, D.; Szpyt, J.; et al. Cysteine 253 of UCP1 regulates energy expenditure and sex-dependent adipose tissue inflammation. Cell Metab. 2022, 34, 140–157.e8. [Google Scholar] [CrossRef]
- Wu, H.; Ghosh, S.; Perrard, X.D.; Feng, L.; Garcia, G.E.; Perrard, J.L.; Sweeney, J.F.; Peterson, L.E.; Chan, L.; Smith, C.W.; et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation 2007, 115, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S. Myeloid derived-suppressor cells: Their role in cancer and obesity. Curr. Opin. Immunol. 2018, 51, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Yang, W.; Tian, Y.; Zeng, X.; Zhou, J.; Mok, M.T.S.; Tang, W.; Feng, Y.; Xu, L.; Chan, A.W.H.; et al. An inflammatory-CCRK circuitry drives mTORC1-dependent metabolic and immunosuppressive reprogramming in obesity-associated hepatocellular carcinoma. Nat. Commun. 2018, 9, 5214. [Google Scholar] [CrossRef]
- Peng, J.; Hu, Q.; Chen, X.; Wang, C.; Zhang, J.; Ren, X.; Wang, Y.; Tao, X.; Li, H.; Song, M.; et al. Diet-induced obesity accelerates oral carcinogenesis by recruitment and functional enhancement of myeloid-derived suppressor cells. Cell Death Dis. 2021, 12, 946. [Google Scholar] [CrossRef] [PubMed]
- Hale, M.; Itani, F.; Buchta, C.M.; Wald, G.; Bing, M.; Norian, L.A. Obesity triggers enhanced MDSC accumulation in murine renal tumors via elevated local production of CCL2. PLoS ONE 2015, 10, e0118784. [Google Scholar] [CrossRef]
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016, 6, 852–869. [Google Scholar] [CrossRef] [PubMed]
- Turbitt, W.J.; Collins, S.D.; Meng, H.; Rogers, C.J. Increased Adiposity Enhances the Accumulation of MDSCs in the Tumor Microenvironment and Adipose Tissue of Pancreatic Tumor-Bearing Mice and in Immune Organs of Tumor-Free Hosts. Nutrients 2019, 11, 3012. [Google Scholar] [CrossRef]
- Jianmin, P.; Qinchao, H.; Chunyang, W.; Jiayu, Z.; Siyu, W.; Li, W.; Juan, X.; Bin, C. Depletion of Gr1+ myeloid cells attenuates high-fat-diet-aggravated esophageal squamous cell carcinoma in mice. Carcinogenesis 2023, 44, 587–595. [Google Scholar] [CrossRef]
- Yang, Q.; Yu, B.; Kang, J.; Li, A.; Sun, J. Obesity Promotes Tumor Immune Evasion in Ovarian Cancer through Increased Production of Myeloid-Derived Suppressor Cells via IL-6. Cancer Manag. Res. 2021, 13, 7355–7363. [Google Scholar] [CrossRef] [PubMed]
- Clements, V.K.; Long, T.; Long, R.; Figley, C.; Smith, D.M.C.; Ostrand-Rosenberg, S. Frontline Science: High fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J. Leukoc. Biol. 2018, 103, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.T.; Orlandella, R.M.; Turbitt, W.J.; Behring, M.; Manne, U.; Sorge, R.E.; Norian, L.A. Obesity-Associated Myeloid-Derived Suppressor Cells Promote Apoptosis of Tumor-Infiltrating CD8 T Cells and Immunotherapy Resistance in Breast Cancer. Front. Immunol. 2020, 11, 590794. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Sha, H.; Yang, L.; Ji, Y.; Ostrand-Rosenberg, S.; Qi, L. Gr-1+ CD11b+ myeloid-derived suppressor cells suppress inflammation and promote insulin sensitivity in obesity. J. Biol. Chem. 2011, 286, 23591–23599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hinkle, C.C.; O’Neill, S.M.; Shi, J.; Caughey, J.; Lynch, E.; Lynch, G.; Gerelus, M.; Tsai, A.S.D.; Shah, R.; et al. Synergistic Modulation of Inflammatory but not Metabolic Effects of High-Fat Feeding by CCR2 and CX3CR1. Obesity 2017, 25, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Borek, F. Previously unrecognized functions of the spleen: Development and maintenance of immune competence and regulation. Crit. Rev. Immunol. 1986, 6, 287–293. [Google Scholar] [PubMed]
- Tarantino, G.; Citro, V.; Balsano, C. Liver-spleen axis in nonalcoholic fatty liver disease. Expert. Rev. Gastroenterol. Hepatol. 2021, 15, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Di Somma, C.; Muscogiuri, G.; Tarantino, G.; Tenore, G.C.; Orio, F.; Colao, A.; Savastano, S. Nutrition, inflammation and liver-spleen axis. Crit. Rev. Food Sci. Nutr. 2018, 58, 3141–3158. [Google Scholar] [CrossRef] [PubMed]
- Braga Tibaes, J.R.; Azarcoya-Barrera, J.; Wollin, B.; Veida-Silva, H.; Makarowski, A.; Vine, D.; Tsai, S.; Jacobs, R.; Richard, C. Sex Differences Distinctly Impact High-Fat Diet-Induced Immune Dysfunction in Wistar Rats. J. Nutr. 2022, 152, 1347–1357. [Google Scholar] [CrossRef]
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of Nonalcoholic Steatohepatitis: An Overview. Hepatol. Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E.; Dudek, M.; Knolle, P. Non-alcoholic fatty liver disease: The interplay between metabolism, microbes and immunity. Nat. Metab. 2021, 3, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.S.; Yi, H.S. Hepatic Immune Microenvironment in Alcoholic and Nonalcoholic Liver Disease. BioMed Res. Int. 2017, 2017, 6862439. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Chung, H.; Softic, S.; Moreno-Fernandez, M.E.; Divanovic, S. The bidirectional immune crosstalk in metabolic dysfunction-associated steatotic liver disease. Cell Metab. 2023, 35, 1852–1871. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, P.; Kam, S.; Heikenwalder, M. T cells: Friends and foes in NASH pathogenesis and hepatocarcinogenesis. Hepatology 2022, 75, 1038–1049. [Google Scholar] [CrossRef]
- Papadopoulos, G.; Giannousi, E.; Avdi, A.P.; Velliou, R.I.; Nikolakopoulou, P.; Chatzigeorgiou, A. Τ cell-mediated adaptive immunity in the transition from metabolic dysfunction-associated steatohepatitis to hepatocellular carcinoma. Front. Cell Dev. Biol. 2024, 12, 1343806. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Soloski, M.J.; Diehl, A.M. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology 2005, 42, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Camporez, J.P.; Lyu, K.; Goldberg, E.L.; Zhang, D.; Cline, G.W.; Jurczak, M.J.; Dixit, V.D.; Petersen, K.F.; Shulman, G.I. Anti-inflammatory effects of oestrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. J. Physiol. 2019, 597, 3885–3903. [Google Scholar] [CrossRef] [PubMed]
- Varlamov, O.; Bethea, C.L.; Roberts, C.T. Sex-specific differences in lipid and glucose metabolism. Front. Endocrinol. 2014, 5, 241. [Google Scholar] [CrossRef]
- Ishikawa, A.; Wada, T.; Nishimura, S.; Ito, T.; Okekawa, A.; Onogi, Y.; Watanabe, E.; Sameshima, A.; Tanaka, T.; Tsuneki, H.; et al. Estrogen regulates sex-specific localization of regulatory T cells in adipose tissue of obese female mice. PLoS ONE 2020, 15, e0230885. [Google Scholar] [CrossRef]
- Adori, M.; Kiss, E.; Barad, Z.; Barabás, K.; Kiszely, E.; Schneider, A.; Sziksz, E.; Ábrahám, I.M.; Matkó, J.; Sármay, G. Estrogen augments the T cell-dependent but not the T-independent immune response. Cell. Mol. Life Sci. 2010, 67, 1661–1674. [Google Scholar] [CrossRef]
- Pettersson, U.S.; Waldén, T.B.; Carlsson, P.O.; Jansson, L.; Phillipson, M. Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS ONE 2012, 7, e46057. [Google Scholar] [CrossRef] [PubMed]
- Imano, N.; Shojima, K.; Tamaki, K.; Shinmura, K. Estrogen contributes to the sex difference in the occurrence of senescence-related T cells during the development of visceral adipose tissue inflammation. Am. J. Physiol. Heart Circ. Physiol. 2023, 324, H662–H674. [Google Scholar] [CrossRef] [PubMed]
- Miras, A.D.; Seyfried, F.; Phinikaridou, A.; Andia, M.E.; Christakis, I.; Spector, A.C.; Botnar, R.M.; le Roux, C.W. Rats fed diets with different energy contribution from fat do not differ in adiposity. Obes. Facts 2014, 7, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, N.; Takamura, T.; Kurita, S.; Misu, H.; Ota, T.; Ando, H.; Yokoyama, M.; Honda, M.; Zen, Y.; Nakanuma, Y.; et al. Lipid-induced oxidative stress causes steatohepatitis in mice fed an atherogenic diet. Hepatology 2007, 46, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Sato Mito, N.; Suzui, M.; Yoshino, H.; Kaburagi, T.; Sato, K. Long term effects of high fat and sucrose diets on obesity and lymphocyte proliferation in mice. J. Nutr. Health Aging 2009, 13, 602–606. [Google Scholar] [CrossRef]
- Jovicic, N.; Jeftic, I.; Jovanovic, I.; Radosavljevic, G.; Arsenijevic, N.; Lukic, M.L.; Pejnovic, N. Differential Immunometabolic Phenotype in Th1 and Th2 Dominant Mouse Strains in Response to High-Fat Feeding. PLoS ONE 2015, 10, e0134089. [Google Scholar] [CrossRef]
- Krishna, K.B.; Stefanovic-Racic, M.; Dedousis, N.; Sipula, I.; O’Doherty, R.M. Similar degrees of obesity induced by diet or aging cause strikingly different immunologic and metabolic outcomes. Physiol. Rep. 2016, 4, e12708. [Google Scholar] [CrossRef]
- Pérez de Heredia, F.; Garaulet, M.; Portillo, M.P.; Zamora, S. Resistance to dietary obesity in rats given different high-energy diets. Int. J. Vitam. Nutr. Res. Int. Z. Vitam-Ernahrungsforschung J. Int. Vitaminol. Nutr. 2006, 76, 271–279. [Google Scholar] [CrossRef]
- Khan, S.H.; Hemann, E.A.; Legge, K.L.; Norian, L.A.; Badovinac, V.P. Diet-induced obesity does not impact the generation and maintenance of primary memory CD8 T cells. J. Immunol. 2014, 193, 5873–5882. [Google Scholar] [CrossRef]
- Nyambuya, T.M.; Dludla, P.V.; Nkambule, B.B. Diet-Induced Obesity Promotes the Upregulation of Fas Expression on T-cells. Biology 2021, 10, 217. [Google Scholar] [CrossRef]
- Kado, T.; Nawaz, A.; Takikawa, A.; Usui, I.; Tobe, K. Linkage of CD8+ T cell exhaustion with high-fat diet-induced tumourigenesis. Sci. Rep. 2019, 9, 12284. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, K.; Nichols, A.G.; Alwarawrah, Y.; MacIver, N.J. Effects of T cell leptin signaling on systemic glucose tolerance and T cell responses in obesity. PLoS ONE 2023, 18, e0286470. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, Z.; Neuperger, P.; Rákóczi, B.; Gémes, N.; Dukay, B.; Hajdu, P.; Péter, M.; Balogh, G.; Tiszlavicz, L.; Vígh, L.; et al. Characterization of obesity-related diseases and inflammation using single cell immunophenotyping in two different diet-induced obesity models. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Huang, D.; Zheng, Y. Ameliorative effects of α-lipoic acid on high-fat diet-induced oxidative stress and glucose uptake impairment of T cells. Free Radic. Res. 2016, 50, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Zhang, Y.; Yang, L.; Liu, G.; Ye, J.; Wang, H. Effects of a High-Fat Diet on Adipose Tissue CD8+ T Cells in Young vs. Adult Mice. Inflammation 2017, 40, 1944–1958. [Google Scholar] [CrossRef] [PubMed]
- Collins, N. Dietary Regulation of Memory T Cells. Int. J. Mol. Sci. 2020, 21, 4363. [Google Scholar] [CrossRef] [PubMed]
- Cavallari, J.F.; Denou, E.; Foley, K.P.; Khan, W.I.; Schertzer, J.D. Different Th17 immunity in gut, liver, and adipose tissues during obesity: The role of diet, genetics, and microbes. Gut Microbes 2016, 7, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- WHO. Global Recommendations on Physical Activity for Health. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 18 June 2024).
- WHO. Fact Sheet on Healthy Diet. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 18 June 2024).
- Aoki, M.; Sato, N. Fatty Acid Content and Composition of Triacylglycerols of Chlorella kessleri. Bio-Protocol 2018, 8, e2676. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brummer, C.; Singer, K.; Brand, A.; Bruss, C.; Renner, K.; Herr, W.; Pukrop, T.; Dorn, C.; Hellerbrand, C.; Matos, C.; et al. Sex-Dependent T Cell Dysregulation in Mice with Diet-Induced Obesity. Int. J. Mol. Sci. 2024, 25, 8234. https://doi.org/10.3390/ijms25158234
Brummer C, Singer K, Brand A, Bruss C, Renner K, Herr W, Pukrop T, Dorn C, Hellerbrand C, Matos C, et al. Sex-Dependent T Cell Dysregulation in Mice with Diet-Induced Obesity. International Journal of Molecular Sciences. 2024; 25(15):8234. https://doi.org/10.3390/ijms25158234
Chicago/Turabian StyleBrummer, Christina, Katrin Singer, Almut Brand, Christina Bruss, Kathrin Renner, Wolfgang Herr, Tobias Pukrop, Christoph Dorn, Claus Hellerbrand, Carina Matos, and et al. 2024. "Sex-Dependent T Cell Dysregulation in Mice with Diet-Induced Obesity" International Journal of Molecular Sciences 25, no. 15: 8234. https://doi.org/10.3390/ijms25158234




