Genome-Wide Identification and Analysis of SUS and AGPase Family Members in Sweet Potato: Response to Excessive Nitrogen Stress during Storage Root Formation
Abstract
:1. Introduction
2. Results
2.1. Identification and Analysis of IbSUSs and IbAGPases
2.2. Analysis on Conserved Motif and Gene Structure of IbSUSs and IbAGPases
2.3. Analysis of Cis-Acting Element in the Promoters of IbSUSs and IbAGPases
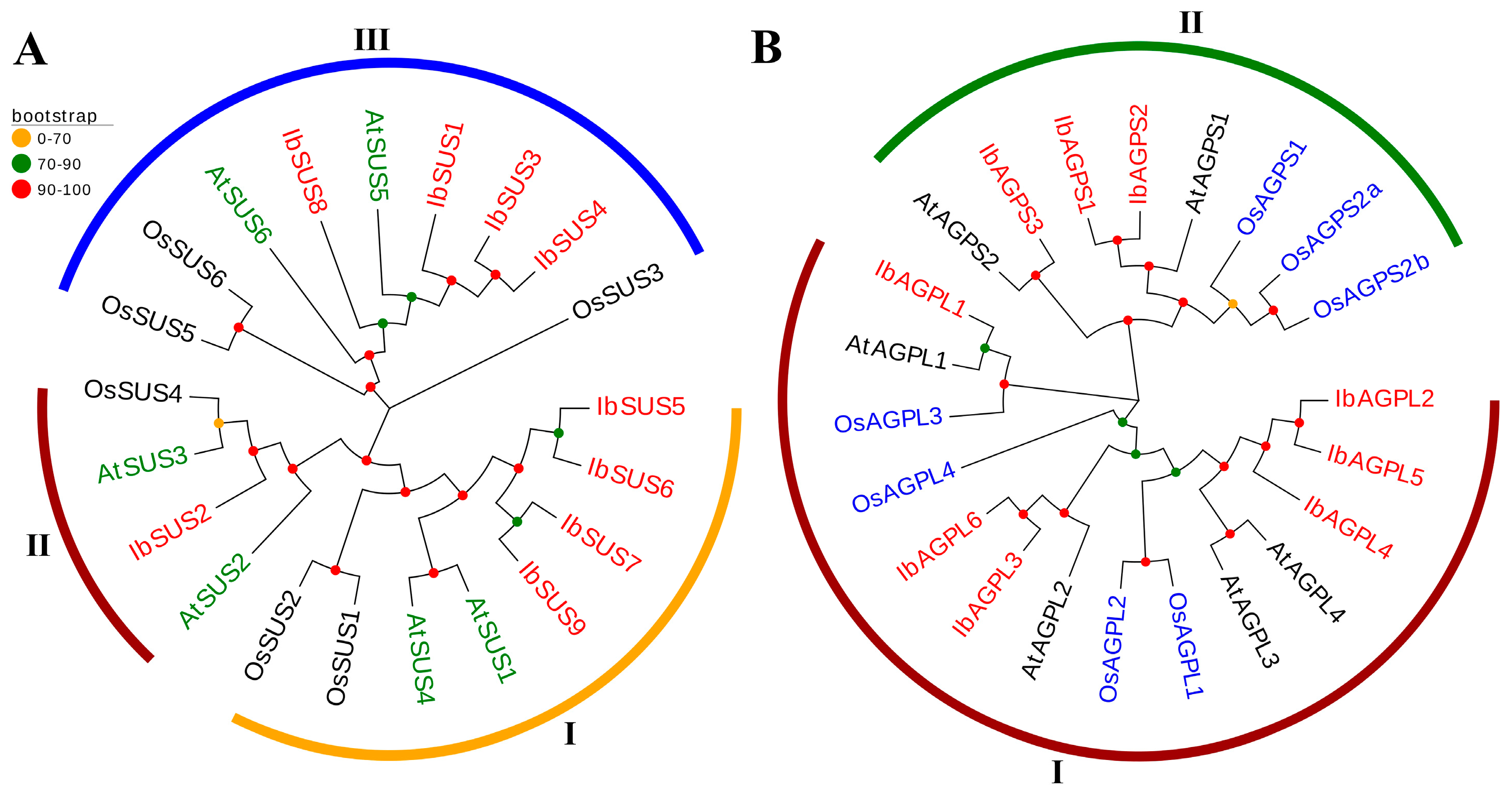
2.4. Analysis of Phylogenetic Relationship of SUSs and AGPases
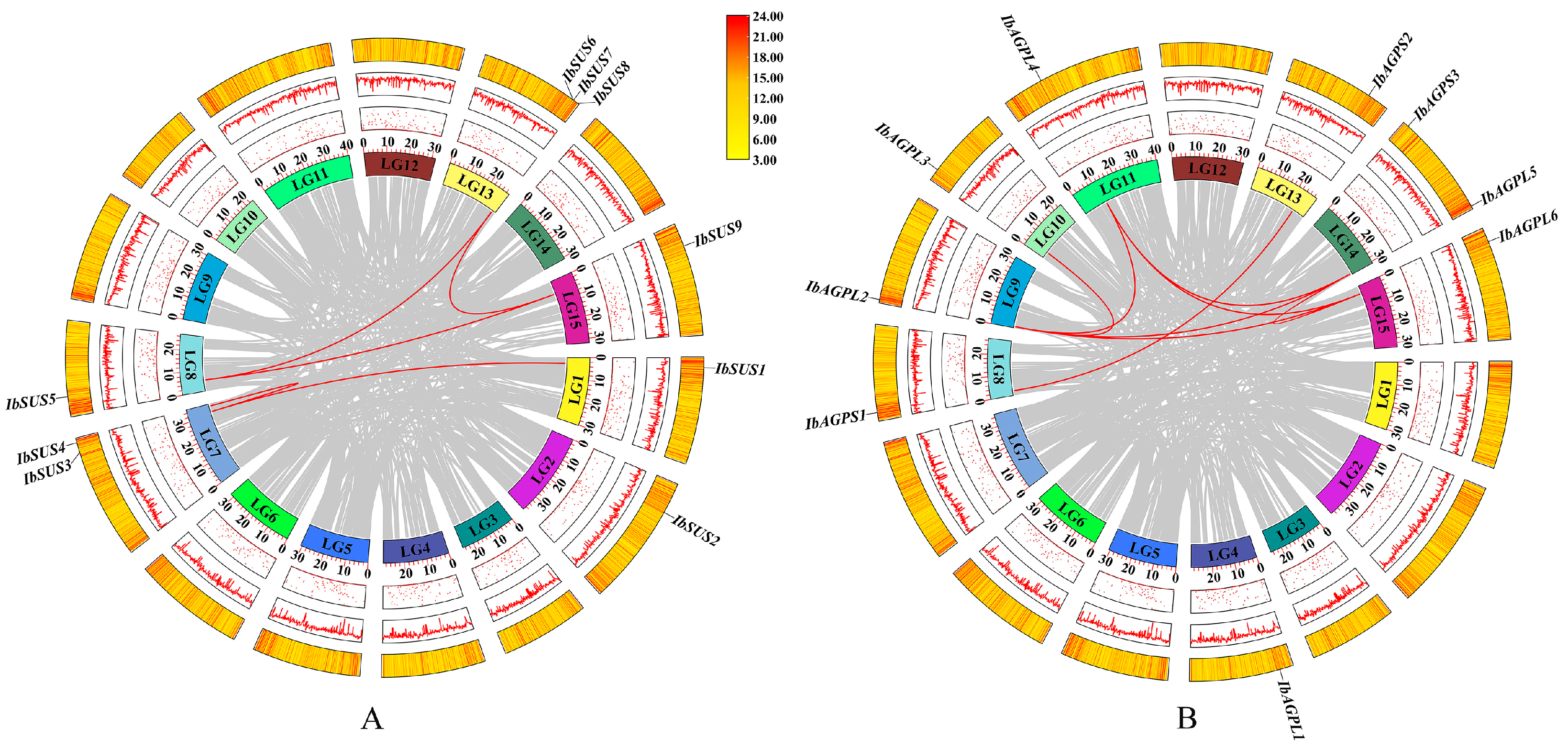
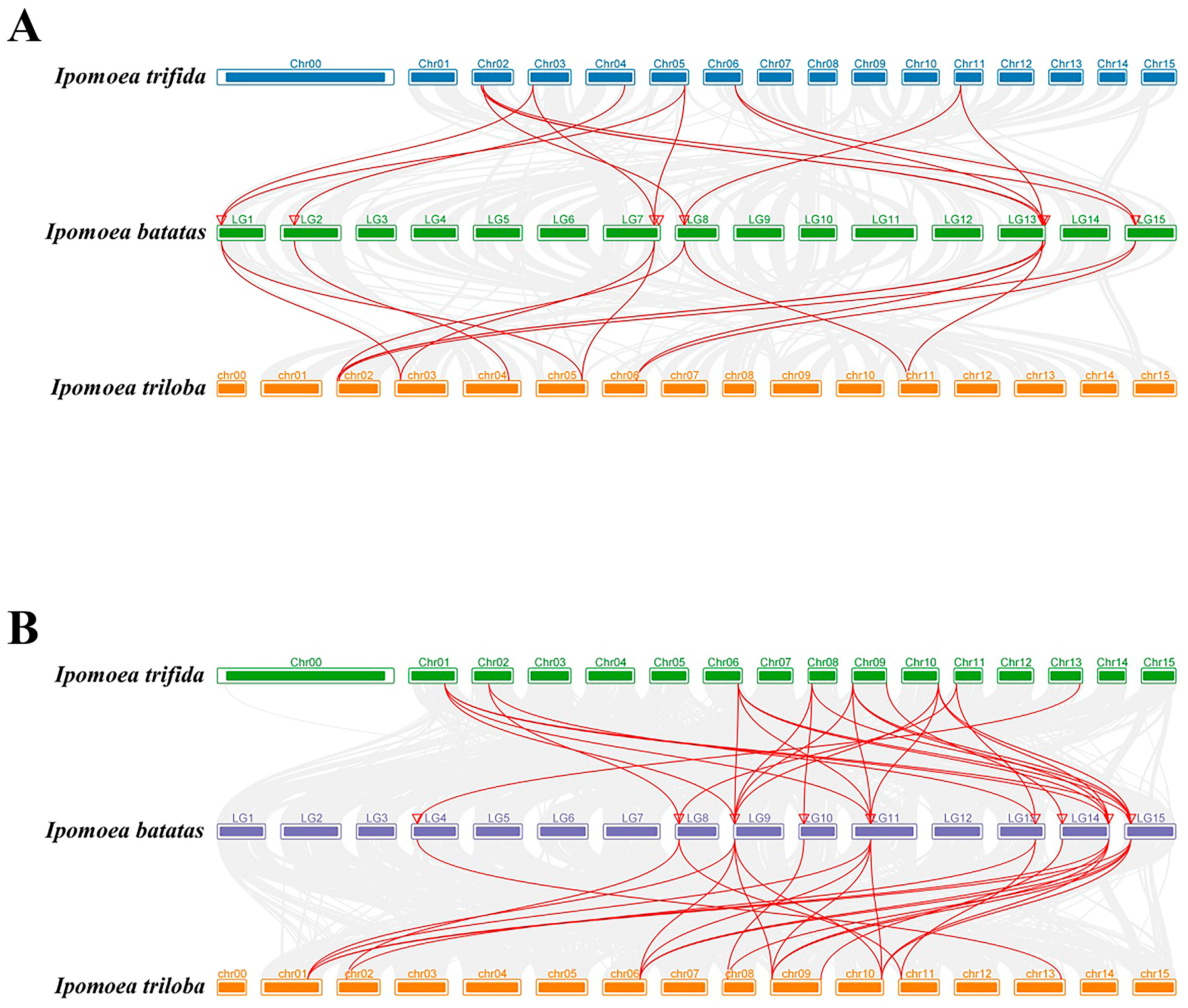
2.5. Synteny Analysis of SUSs and AGPases
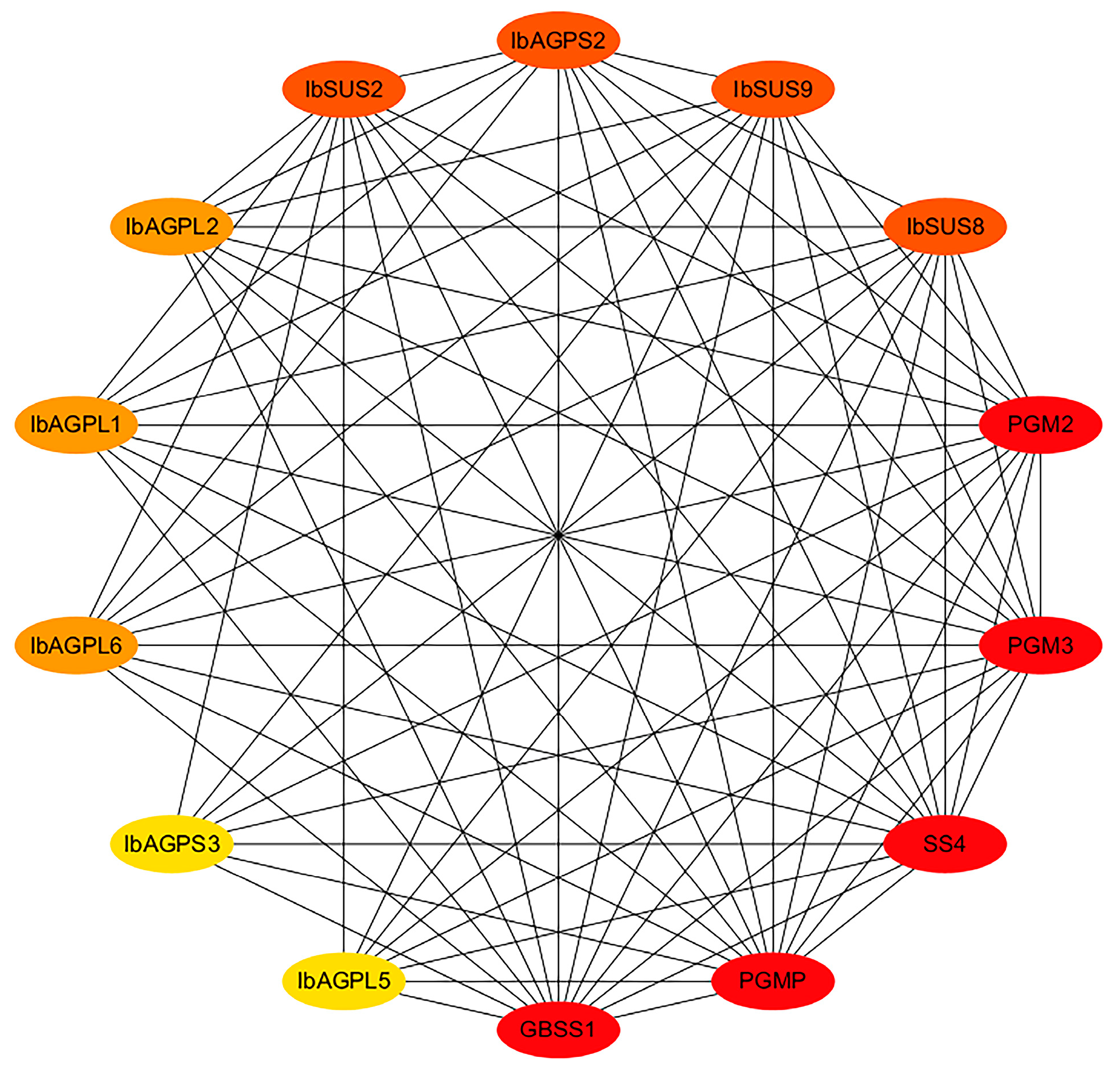
2.6. Protein Interaction Network of IbSUSs and IbAGPases
2.7. Prediction of Secondary and Tertiary Structure of SUSs and AGPases
2.8. Starch Synthesis
2.9. Agronomic Traits under Enclosure Condition
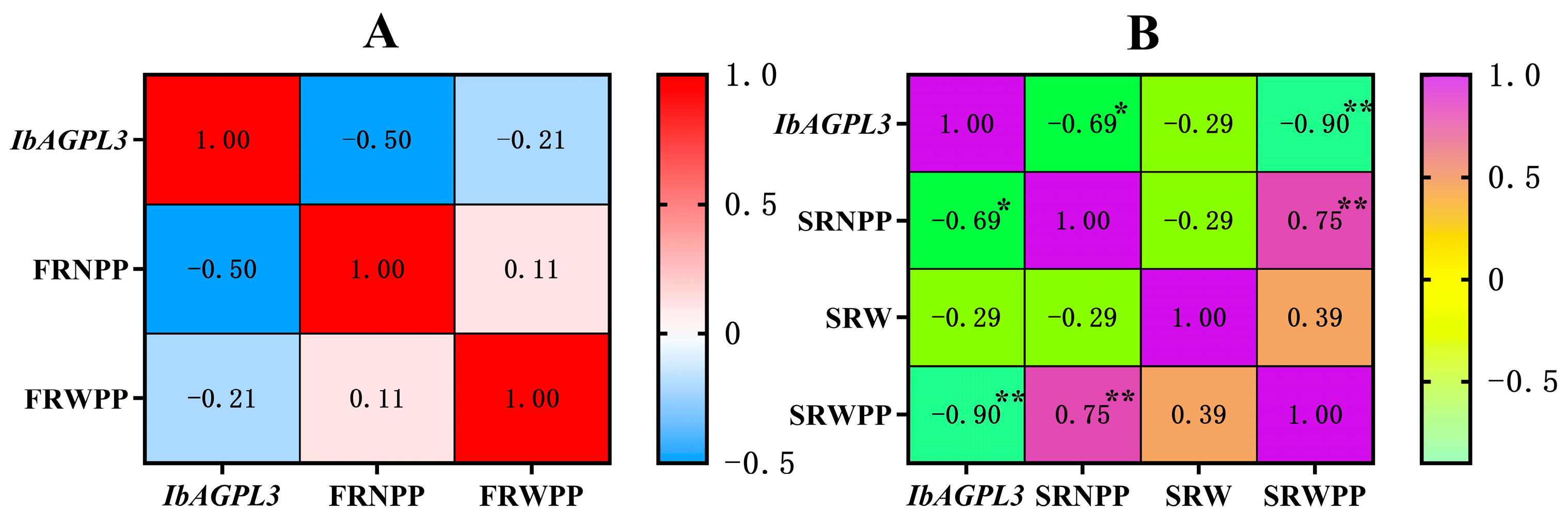
2.10. Relationship between IbAGPL3 Gene Expression Level and Agronomic Traits
3. Discussion
3.1. Evolution of IbSUSs and IbAGPases in Sweet potato
3.2. Response of IbSUSs and IbAGPases to Excessive Nitrogen Stress during Storage Root Development
4. Materials and Methods
4.1. Identification of SUSs and AGPases
4.2. Chromosomal Distribution and Gene Structure of SUSs and AGPases
4.3. Property Prediction of SUSs and AGPases
4.4. Domain Identification and Conserved Motif Analysis of SUSs and AGPases
4.5. Promoter Analyses of SUSs and AGPases
4.6. Phylogenetic Analysis of SUSs and AGPases
4.7. Ka/Ks Analyses and Gene Collinearity of SUSs and AGPases
4.8. Protein Interaction Network Analysis of SUS and AGPase Regulated Proteins
4.9. Secondary and Tertiary Structure Analysis of SUS and AGPase Proteins
4.10. Pot Experiments and Plant Sampling
4.11. Starch Content in Fibrous Root and Storage Root
4.12. Sucrose Synthase (SUS) and ADP-Glucose Pyrophosphorylase (AGPase) Activity Assay
4.13. qRT-PCR Analysis of SUSs and AGPases
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jiang, Z.; Zhang, H.; Gao, S.; Zhai, H.; He, S.; Zhao, N.; Liu, Q. Genome-wide identification and expression analysis of the sucrose synthase gene family in sweet potato and its Two Diploid Relatives. Int. J. Mol. Sci. 2023, 24, 12493. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Kim, S.; Kim, J.; Kwon, S.Y.; Kim, S.H. Comparative analysis of AGPase proteins and conserved domains in sweetpotato (Ipomoea batatas (L.) Lam.) and its two wild relatives. J. Plant Biotechnol. 2022, 49, 39–45. [Google Scholar] [CrossRef]
- Firon, N.; LaBonte, D.; Villordon, A.; Kfir, Y.; Solis, J.; Lapis, E.; Perlman, T.S.; Doron-Faigenboim, A.; Hetzroni, A.; Althan, L.; et al. Transcriptional profiling of sweetpotato (Ipomoea batatas) roots indicates down-regulation of lignin biosynthesis and up-regulation of starch biosynthesis at an early stage of storage root formation. BMC Genom. 2013, 14, 460. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, J.; Sun, L.; Kong, Y.; Chen, J.; Zhu, M.; Xu, T.; Li, Z.; Dong, T. Progress on physiological and molecular mechanisms of storage root formation and development in sweetpotato. Sci. Hortic. 2023, 308, 111588. [Google Scholar] [CrossRef]
- Lyu, R.; Ahmed, S.; Fan, W.; Yang, J.; Wu, X.; Zhou, W.; Zhang, P.; Yuan, L.; Wang, H. Engineering properties of sweet potato starch for industrial applications by biotechnological techniques including genome editing. Int. J. Mol. Sci. 2021, 22, 9533. [Google Scholar] [CrossRef]
- Ravi, V.; Indira, P. Crop physiology of sweetpotato. Hortic. Rev 1998, 23, 277–339. [Google Scholar] [CrossRef]
- Glibert, P.M.; Harrison, J.; Heil, C.; Seitzinger, S. Escalating worldwide use of urea–a global change contributing to coastal eutrophication. Biogeochemistry 2006, 77, 441–463. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Liu, Z.; Hu, Y.; Xia, Z.; Hu, B.; Rennenberg, H. Consequences of excess urea application on photosynthetic characteristics and nitrogen metabolism of Robinia pseudoacacia seedlings. Chemosphere 2024, 346, 140619. [Google Scholar] [CrossRef]
- Chen, Y.; Teng, Z.; Yuan, Y.; Yi, Z.; Zheng, Q.; Yu, H.; Lv, J.; Wang, Y.; Duan, M.; Zhang, J.; et al. Excessive nitrogen in field-grown rice suppresses grain filling of inferior spikelets by reducing the accumulation of cytokinin and auxin. Field Crop. Res. 2022, 283, 108542. [Google Scholar] [CrossRef]
- Duan, W.; Zhang, H.; Xie, B.; Wang, B.; Zhang, L. Impacts of nitrogen fertilization rate on the root yield, starch yield and starch physicochemical properties of the sweet potato cultivar Jishu 25. PLoS ONE 2019, 14, e0221351. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Tang, Z.; Wei, M.; Shi, X.; Zhang, A.; Li, H. Suitable nitrogen rate for storage root yield and quality of sweet potato. J. Plant Nutr. Fertil. 2015, 21, 979–986. [Google Scholar] [CrossRef]
- Duan, W.; Zhang, H.; Wang, Q.; Xie, B.; Zhang, L. Regulation of root development in nitrogen-susceptible and nitrogen-tolerant sweet potato cultivars under different nitrogen and soil moisture conditions. BMC Plant Biol. 2023, 23, 454. [Google Scholar] [CrossRef] [PubMed]
- Taranet, P.; Harper, S.; Kirchhof, G.; Fujinuma, R.; Menzies, N. Growth and yield response of glasshouse-and field-grown sweetpotato to nitrogen supply. Nutr. Cycl. Agroecosystems 2017, 108, 309–321. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Ribeiro, N.P.; Assunção, N.S.; Nunes, J.G.d.S.; Sorroche, C.P.; Leonel, M. Impact of nitrogen and green manure on yield and quality of sweet potato in sandy soil: A Brazilian case study. J. Agric. Food Res. 2021, 4, 100131. [Google Scholar] [CrossRef]
- Duan, W.; Zhang, H.; Xie, B.; Wang, B.; Hou, F.; Li, A.; Dong, S.; Qin, Z.; Wang, Q.; Zhang, L. Nitrogen utilization characteristics and early storage root development in nitrogen-tolerant and nitrogen-susceptible sweet potato. Physiol. Plant. 2021, 173, 1090–1104. [Google Scholar] [CrossRef]
- Du, X.; Xi, M.; Kong, L. Split application of reduced nitrogen rate improves nitrogen uptake and use efficiency in sweetpotato. Sci. Rep. 2019, 9, 14058. [Google Scholar] [CrossRef] [PubMed]
- Keller, M. Investigation of Cassava Storage Root Initiation and Development for Engineering Increases in Starch and Storage Root Yield. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2014. [Google Scholar] [CrossRef]
- Petreikov, M.; Eisenstein, M.; Yeselson, Y.; Preiss, J.; Schaffer, A.A. Characterization of the AGPase large subunit isoforms from tomato indicates that the recombinant L3 subunit is active as a monomer. Biochem. J. 2010, 428, 201–212. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, D. Gene expression activity and pathway selection for sucrose metabolism in developing storage root of sweet potato. Plant Cell Physiol. 2003, 44, 630–636. [Google Scholar] [CrossRef]
- Chen, A.; He, S.; Li, F.; Li, Z.; Ding, M.; Liu, Q.; Rong, J. Analyses of the sucrose synthase gene family in cotton: Structure, phylogeny and expression patterns. BMC Plant Biol. 2012, 12, 85. [Google Scholar] [CrossRef]
- Baud, S.; Vaultier, M.N.; Rochat, C. Structure and expression profile of the sucrose synthase multigene family in Arabidopsis. J. Exp. Bot. 2004, 55, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Scofield, G.N.; Terao, T. An expression analysis profile for the entire sucrose synthase gene family in rice. Plant Sci. 2008, 174, 534–543. [Google Scholar] [CrossRef]
- Horst, I.; Welham, T.; Kelly, S.; Kaneko, T.; Sato, S.; Tabata, S.; Parniske, M.; Wang, T.L. TILLING mutants of Lotus japonicus reveal that nitrogen assimilation and fixation can occur in the absence of nodule-enhanced sucrose synthase. Plant Physiol. 2007, 144, 806–820. [Google Scholar] [CrossRef]
- Angeles-Núñez, J.G.; Tiessen, A. Arabidopsis sucrose synthase 2 and 3 modulate metabolic homeostasis and direct carbon towards starch synthesis in developing seeds. Planta 2010, 232, 701–718. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Liu, J.; Ma, Q.; Wu, E.; Gao, J.; Yang, Q.; Feng, B. Transcriptome analysis reveals the mechanism of nitrogen fertilizers in starch synthesis and quality in waxy and non-waxy proso millet. Carbohydr. Polym. 2024, 323, 121372. [Google Scholar] [CrossRef]
- Wang, F.; Ge, S.; Xu, X.; Xing, Y.; Du, X.; Zhang, X.; Lv, M.; Liu, J.; Zhu, Z.; Jiang, Y. Multiomics analysis reveals new insights into the apple fruit quality decline under high nitrogen conditions. J. Agric. Food Chem. 2021, 69, 5559–5572. [Google Scholar] [CrossRef]
- Batra, R.; Saripalli, G.; Mohan, A.; Gupta, S.; Gill, K.S.; Varadwaj, P.K.; Balyan, H.S.; Gupta, P.K. Comparative analysis of AGPase genes and encoded proteins in eight monocots and three dicots with emphasis on wheat. Front. Plant Sci. 2017, 8, 19. [Google Scholar] [CrossRef]
- Crevillén, P.; Ventriglia, T.; Pinto, F.; Orea, A.; Mérida, Á.; Romero, J.M. Differential pattern of expression and sugar regulation of Arabidopsis thaliana ADP-glucose pyrophosphorylase-encoding genes. J. Biol. Chem. 2005, 280, 8143–8149. [Google Scholar] [CrossRef]
- Lu, F.H.; Park, Y.J. Sequence variations in OsAGPase significantly associated with amylose content and viscosity properties in rice (Oryza sativa L.). Genet. Res. 2012, 94, 179–189. [Google Scholar] [CrossRef]
- Tang, X.; Peng, C.; Zhang, J.; Cai, Y.; You, X.; Kong, F.; Yan, H.; Wang, G.; Wang, L.; Jin, J.; et al. ADP-glucose pyrophosphorylase large subunit 2 is essential for storage substance accumulation and subunit interactions in rice endosperm. Plant Sci. 2016, 249, 70–83. [Google Scholar] [CrossRef]
- Wang, M.; Xiao, X.; Ying, D.; Wang, Q.; Li, L.; Zhang, R.; Ye, J. Bioinformatics and expression of AGPase gene family in cassava. J. South. Agric. 2019, 50, 222–229. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.; Wan, C.; Wang, P.; Eeckhout, M.; Gao, J. Suitable nitrogen fertilizer application drives the endosperm development and starch synthesis to improve the physicochemical properties of common buckwheat grain. Int. J. Biol. Macromol. 2023, 235, 123837. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, Y.; Tao, X.; Cheng, X.; Wang, H. Isolation and characterization of cDNAs and genomic DNAs encoding ADP-glucose pyrophosphorylase large and small subunits from sweet potato. Mol. Genet. Genom. 2016, 291, 609–620. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, N.; Zhang, H.; Xu, R.; Si, C. Genome-wide analysis of sweet potato ammonium transporter (AMT): Influence on nitrogen utilization, storage root development and yield. Int. J. Mol. Sci. 2023, 24, 17424. [Google Scholar] [CrossRef]
- Jiang, Q.; Du, Y.; Tian, X.; Wang, Q.; Xiong, R.; Xu, G.; Yan, C.; Ding, Y. Effect of panicle nitrogen on grain filling characteristics of high-yielding rice cultivars. Eur. J. Agron. 2016, 74, 185–192. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Chen, Y.; Jiang, Y.; Shi, Y.; Zhao, L.; Liao, P.; Wang, W.; Xu, K.; Dai, Q.; et al. Excessive nitrogen application leads to lower rice yield and grain quality by inhibiting the grain filling of inferior grains. Agriculture 2022, 12, 962. [Google Scholar] [CrossRef]
- Singletary, G.W.; Doehlert, D.C.; Wilson, C.M.; Muhitch, M.J.; Below, F.E. Response of enzymes and storage proteins of maize endosperm to nitrogen supply. Plant Physiol. 1990, 94, 858–864. [Google Scholar] [CrossRef]
- Bellini, C.; Pacurar, D.I.; Perrone, I. Adventitious roots and lateral roots: Similarities and differences. Annu. Rev. Plant Biol. 2014, 65, 639–666. [Google Scholar] [CrossRef]
- Shi, Z.; Fan, X.; Klaus, D.; Sattemacher, B. Effect of localized nitrogen supply on root morphology in rice and its mechanism. Chin. J. Rice Sci. 2005, 19, 147–152. [Google Scholar]
- Zhang, L.; Sun, S.; Liang, Y.; Li, B.; Ma, S.; Wang, Z.; Ma, B.; Li, M. Nitrogen levels regulate sugar metabolism and transport in the shoot tips of crabapple plants. Front. Plant Sci. 2021, 12, 626149. [Google Scholar] [CrossRef]
- Duan, W.; Wang, Q.; Zhang, H.; Xie, B.; Li, A.; Hou, F.; Dong, S.; Wang, B.; Qin, Z.; Zhang, L. Comparative study on carbon–nitrogen metabolism and endogenous hormone contents in normal and overgrown sweetpotato. S. Afr. J. Bot. 2018, 115, 199–207. [Google Scholar] [CrossRef]
- Cook, F.R.; Fahy, B.; Trafford, K. A rice mutant lacking a large subunit of ADP-glucose pyrophosphorylase has drastically reduced starch content in the culm but normal plant morphology and yield. Funct. Plant Biol. 2012, 39, 1068–1078. [Google Scholar] [CrossRef]
- Rösti, S.; Fahy, B.; Denyer, K. A mutant of rice lacking the leaf large subunit of ADP-glucose pyrophosphorylase has drastically reduced leaf starch content but grows normally. Funct. Plant Biol. 2007, 34, 480–489. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Peer, Y.V.d.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef] [PubMed]



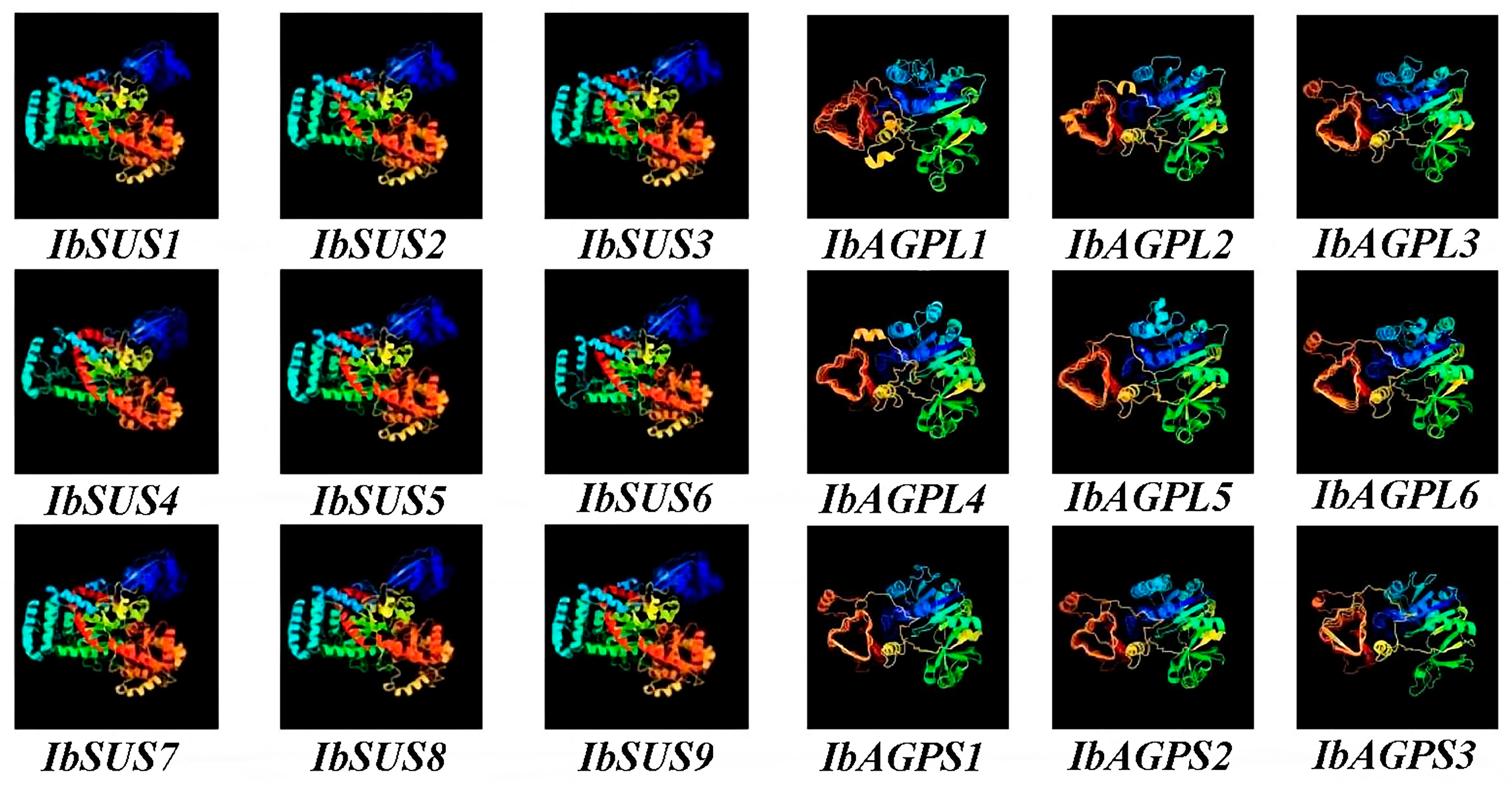


| Gene ID | Gene Name | Protein Size (aa) | MW (Da) | pI | Instability Index | GRAVY | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| g505.t1 | IbSUS1 | 882 | 99,986.35 | 6.21 | 40.26 | −0.312 | cytosol |
| g5497.t1 | IbSUS2 | 811 | 92,130.29 | 6.02 | 38.17 | −0.275 | chloroplast |
| g29617.t1 | IbSUS3 | 837 | 94,763.23 | 6.14 | 36.59 | −0.351 | cytosol |
| g30039.t1 | IbSUS4 | 839 | 94,952.6 | 6.33 | 38.73 | −0.33 | cytosol |
| g31210.t1 | IbSUS5 | 743 | 84,781.48 | 6.11 | 36.16 | −0.202 | plasma membrane |
| g55056.t1 | IbSUS6 | 763 | 87,821.85 | 6.17 | 33.53 | −0.298 | mitochondrion |
| g55074.t1 | IbSUS7 | 851 | 97,494.1 | 6.16 | 38.91 | −0.256 | mitochondrion |
| g55342.t1 | IbSUS8 | 906 | 101,580.88 | 6.99 | 39.82 | −0.214 | chloroplast |
| g60893.t1 | IbSUS9 | 763 | 87,703.25 | 6.18 | 35.82 | −0.261 | cytosol |
| g13324.t1 | IbAGPL1 | 827 | 91,001.22 | 9.08 | 35.39 | −0.164 | endoplasmic reticulum |
| g34068.t1 | IbAGPL2 | 537 | 59,880.73 | 6.5 | 32.44 | −0.173 | cytosol |
| g38633.t1 | IbAGPL3 | 496 | 55,273.67 | 8.37 | 30.71 | −0.192 | chloroplast |
| g43092.t1 | IbAGPL4 | 484 | 53,623.32 | 5.93 | 34.01 | −0.154 | chloroplast |
| g59752.t1 | IbAGPL5 | 501 | 55,690.41 | 6.37 | 37.47 | −0.254 | cytosol |
| g60496.t1 | IbAGPL6 | 518 | 57,247.42 | 6.68 | 30.05 | −0.184 | cytoskeleton |
| g30656.t1 | IbAGPS1 | 522 | 57,155.24 | 6.74 | 39.79 | −0.178 | cytosol |
| g54359.t1 | IbAGPS2 | 520 | 56,904.82 | 7.58 | 37.85 | −0.203 | chloroplast_cytosol |
| g55556.t1 | IbAGPS3 | 579 | 63,531.23 | 5.78 | 49.69 | −0.287 | chloroplast |
| Gene Pairs | Ka | Ks | Ka/Ks |
|---|---|---|---|
| IbSUS1/IbSUS3 | 0.102 | 0.5658 | 0.1803 |
| IbSUS6/IbSUS9 | 0.0342 | 1.1358 | 0.0301 |
| IbSUS7/IbSUS5 | 0.1389 | 1.0692 | 0.1299 |
| IbSUS3/IbSUS4 | 0.017 | 0.0292 | 0.5796 |
| IbAGPS2/IbAGPS1 | 0.049 | 0.4795 | 0.1021 |
| IbAGPL5/IbAGPL2 | 0.1111 | 0.6767 | 0.1642 |
| Protein | α-Helix | β-Corner | Random Coil | Extended Strand |
|---|---|---|---|---|
| IbSUS1 | 51.13% | 7.14% | 29.59% | 12.13% |
| IbSUS2 | 53.14% | 6.54% | 27.25% | 13.07% |
| IbSUS3 | 49.22% | 6.21% | 32.50% | 12.07% |
| IbSUS4 | 51.49% | 6.56% | 29.56% | 12.40% |
| IbSUS5 | 48.99% | 6.46% | 30.96% | 13.59% |
| IbSUS6 | 53.08% | 7.08% | 26.61% | 13.24% |
| IbSUS7 | 53.35% | 6.58% | 26.67% | 13.40% |
| IbSUS8 | 49.45% | 6.84% | 31.13% | 12.58% |
| IbSUS9 | 52.95% | 6.03% | 28.44% | 12.58% |
| IbAGPL1 | 35.91% | 0.00% | 49.09% | 14.99% |
| IbAGPL2 | 20.67% | 0.00% | 59.40% | 19.93% |
| IbAGPL3 | 20.97% | 0.00% | 57.46% | 21.57% |
| IbAGPL4 | 20.87% | 0.00% | 58.26% | 20.87% |
| IbAGPL5 | 21.16% | 0.00% | 57.88% | 20.96% |
| IbAGPL6 | 19.96% | 0.00% | 61.20% | 19.11% |
| IbAGPS1 | 20.88% | 0.00% | 58.81% | 20.31% |
| IbAGPS2 | 20.38% | 0.00% | 60.77% | 18.85% |
| Days after Planting | Nitrogen Level | FRNPP | FRWPP (g) | SRNPP | SRW (g) | SRWPP (g) |
|---|---|---|---|---|---|---|
| 15 d | MN | 28.50 ± 1.61 a | 1.71 ± 0.23 a | - | - | - |
| EN | 24.00 ± 1.41 a | 1.53 ± 0.24 a | - | - | - | |
| 45 d | MN | - | - | 4.00 ± 0.45 a | 20.11 ± 1.52 a | 77.69 ± 4.60 a |
| EN | - | - | 2.50 ± 0.22 b | 17.41 ± 2.41 a | 41.27 ± 3.38 b |
| Primer Name | Primers Sequence |
|---|---|
| IbSUS1 | CCTTTTGTTCTTCCCATACCACTC |
| CATACCTCTTTCACCTTGCCAG | |
| IbSUS2 | TCAGTGTACCGATGTGCTCATC |
| TCTGGTTGTGGTTGGAGGTTC | |
| IbSUS3 | AGTGCGGTAGAGTTCGCTGTT |
| TCTGGTTGTGGTTGGAGGTTC | |
| IbSUS4 | AGTGCGGTAGAGTTCGCTGTT |
| TTATCATCACCAGCACTTTCCA | |
| IbSUS5 | GTCAGCCTCTTCTCCTTCTCAG |
| ATGTTGTCATTCTTTCCCCCC | |
| IbSUS6 | CGCTTAATCATCTCACGCTCC |
| GTTCACCAATACAAGGGCAAG | |
| IbSUS7 | GGGTTGGAGGGTCGTTAGATA |
| TGTAGGAGTAGAGGAGGGGCG | |
| IbSUS8 | TAAGTTGGGCTGTGGTTTTGG |
| ATAGCCTCCGTGAGCGTCTT | |
| IbSUS9 | ATCCCCGAGTTTTTCCTTGT |
| TTGCCACCATCAGCCATCACTAAC | |
| IbAGPL1 | TTGCCACCATCAGCCATCACTAAC |
| ATTCGAGACCGGATGCCAACAAC | |
| IbAGPL2 | TCTCCTCGATTCCTACCACCAACC |
| CACCGAAATCTAACCGTGACCTCT | |
| IbAGPL3 | GTTCCCTCAATCGTCACCTTTCTCG |
| GTGGCAGCAAGAACCTCAACAAATC | |
| IbAGPL4 | CAATGATGCTCTTGCTGTGGAACTG |
| GCCGCCTCCCAGTATGATTGC | |
| IbAGPL5 | CCGAGATTCTTCCTGCTGCTGTG |
| ATTGTCCCGATGTCCTCCCAGTAG | |
| IbAGPL6 | GGTGACGCCAAGAACAAGAACATTG |
| GCTCGGCTATCATCCATTGGTACAC | |
| IbAGPS1 | ATGTTAGCAAGGCGTCGTTCCG |
| CGAGGAGGCAATGGCATCAGC | |
| IbAGPS2 | GCATCACTCAATCGTCACCTCTCAC |
| GCCTCACAGCATCAGCAGTACC | |
| IbAGPS3 | CCGCTATACACTTTACCTCGCCATC |
| CCCTTCTCCCACCCTTGTCCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Lin, Y.; Meng, Y.; Si, C. Genome-Wide Identification and Analysis of SUS and AGPase Family Members in Sweet Potato: Response to Excessive Nitrogen Stress during Storage Root Formation. Int. J. Mol. Sci. 2024, 25, 8236. https://doi.org/10.3390/ijms25158236
Han S, Lin Y, Meng Y, Si C. Genome-Wide Identification and Analysis of SUS and AGPase Family Members in Sweet Potato: Response to Excessive Nitrogen Stress during Storage Root Formation. International Journal of Molecular Sciences. 2024; 25(15):8236. https://doi.org/10.3390/ijms25158236
Chicago/Turabian StyleHan, Shaoxuan, Yanhui Lin, Yayi Meng, and Chengcheng Si. 2024. "Genome-Wide Identification and Analysis of SUS and AGPase Family Members in Sweet Potato: Response to Excessive Nitrogen Stress during Storage Root Formation" International Journal of Molecular Sciences 25, no. 15: 8236. https://doi.org/10.3390/ijms25158236
APA StyleHan, S., Lin, Y., Meng, Y., & Si, C. (2024). Genome-Wide Identification and Analysis of SUS and AGPase Family Members in Sweet Potato: Response to Excessive Nitrogen Stress during Storage Root Formation. International Journal of Molecular Sciences, 25(15), 8236. https://doi.org/10.3390/ijms25158236




