Expression of Osteopontin and Gremlin 1 Proteins in Cardiomyocytes in Ischemic Heart Failure
Abstract
1. Introduction
2. Results
2.1. OPN Expression
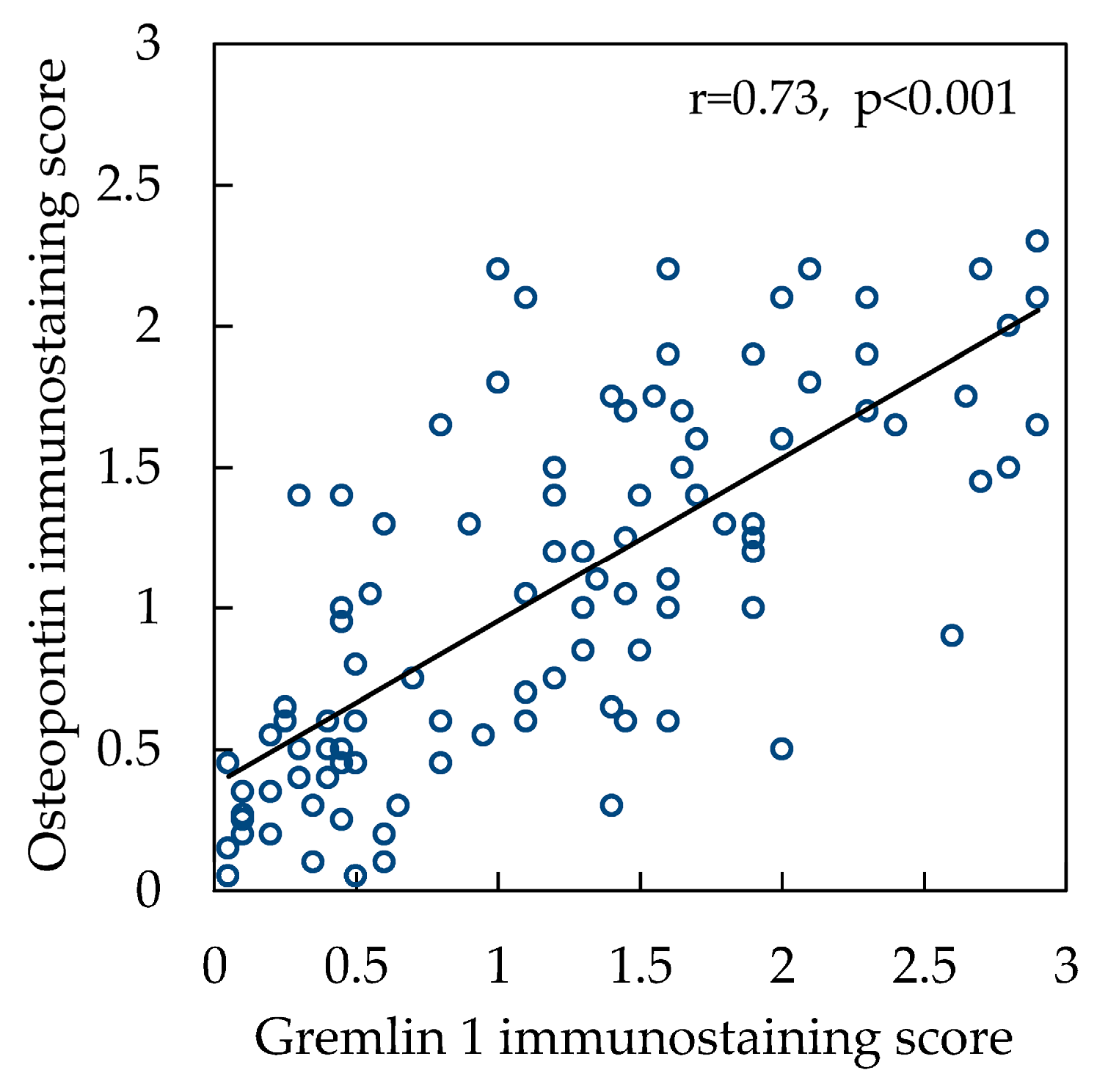
2.2. Grem1 Expression
3. Discussion
4. Materials and Methods
4.1. Study Design and Groups
4.2. Immunohistochemistry
4.3. Analysis of OPN and Grem1 Immunostaining
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Shahim, B.; Kapelios, C.J.; Savarese, G.; Lund, L.H. Global public health burden of heart failure: An updated review. Card Fail Rev. 2023, 9, e11. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart disease and stroke statistics—2023 update: A report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef] [PubMed]
- Szibor, M.; Pöling, J.; Warnecke, H.; Kubin, T.; Braun, T. Remodeling and dedifferentiation of adult cardiomyocytes during disease and regeneration. Cell. Mol. Life Sci. 2014, 71, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Opie, L.H.; Commerford, P.J.; Gersh, B.J.; Pfeffer, M.A. Controversies in ventricular remodelling. Lancet 2006, 367, 356–367. [Google Scholar] [CrossRef]
- Kalogeropoulos, A.; Georgiopoulou, V.; Psaty, B.M.; Rodondi, N.; Smith, A.L.; Harrison, D.G.; Liu, Y.; Hoffman, U.; Bauer, D.C.; Newman, A.B.; et al. Inflammatory markers and incident heart failure risk in older adults: The health ABC (health, aging, and body composition) study. J. Am. Coll. Cardiol. 2010, 55, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Scuricini, A.; Andreozzi, F.; Sgura, C.; Ministrini, S.; Bertolotto, M.; Ramoni, D.; Liberale, L.; Camici, G.G.; Mannino, G.C.; Succurro, E.; et al. Osteopontin levels correlate with severity of diabetic cardiomyopathy in early stage of diabetes. Diabetes Res. Clin. Pract. 2023, 203, 110885. [Google Scholar] [CrossRef] [PubMed]
- Amilca-Seba, K.; Tan, T.Z.; Thiery, J.-P.; Louadj, L.; Thouroude, S.; Bouygues, A.; Sabbah, M.; Larsen, A.K.; Denis, J.A. Osteopontin (OPN/SPP1), a mediator of tumor progression, is regulated by the mesenchymal transcription factor Slug/SNAI2 in colorectal cancer (CRC). Cells 2022, 11, 1808. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Rodriguez, D.; Yousefi, K.; John-Williams, K.; Mendez, A.J.; Goldberg, R.B.; Lymperopoulos, A.; Tamariz, L.J.; Goldberger, J.J.; Myerburg, R.J.; et al. Osteopontin and LDLR are upregulated in hearts of sudden cardiac death victims with heart failure with preserved ejection fraction and diabetes mellitus. Front. Cardiovasc. Med. 2020, 7, 610282. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yousefi, K.; Ding, W.; Singh, J.; Shehadeh, L.A. Osteopontin RNA aptamer can prevent reverse pressure overload-induced heart failure. Cardiovasc. Res. 2017, 113, 633–643. [Google Scholar] [CrossRef]
- Singh, K.; Balligand, J.L.; Fischer, T.A.; Smith, T.W.; Kelly, R.A. Glucocorticoids increase osteopontin expression in cardiac muscle cells and microvascular endothelial cells. Role in regulation of inducible nitric oxide synthase. J. Biol. Chem. 1995, 27, 28471–28478. [Google Scholar] [CrossRef]
- Graf, K.; Do, Y.S.; Ashizawa, N.; Meehan, W.P.; Giachelli, C.M.; Marboe, C.C.; Fleck, E.; Hsueh, W.A. Myocardial osteopontin expression is associated with left ventricular hypertrophy. Circulation 1997, 96, 3063–3071. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, J.M.; Schauerte, C.; Hübner, A.; Kölling, M.; Martino, F.; Scherf, K.; Batkai, S.; Zimmer, K.; Foinquinos, A.; Kaucsar, T.; et al. Osteopontin is indispensable for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis. Eur. Heart J. 2015, 36, 2184–2196. [Google Scholar] [CrossRef] [PubMed]
- Behnes, M.; Bertsch, T.; Weiss, C.; Ahmad-Nejad, P.; Akin, I.; Fastner, C.; El-Battrawy, I.; Lang, S.; Neumaier, M.; Borggrefe, M.; et al. Triple head-to-head comparison of fibrotic biomarkers galectin-3, osteopontin and gremlin-1 for long-term prognosis in suspected and proven acute heart failure patients. Int. J. Cardiol. 2016, 203, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Soejima, H.; Irie, A.; Fukunaga, T.; Oe, Y.; Kojima, S.; Kaikita, K.; Kawano, H.; Sugiyama, S.; Yoshimura, M.; Kishikawa, H.; et al. Osteopontin expression of circulating T cells and plasma osteopontin levels are increased in relation to severity of heart failure. Circ. J. 2007, 71, 1879–1884. [Google Scholar] [CrossRef][Green Version]
- Singh, M.; Foster, C.R.; Dalal, S.; Singh, K. Osteopontin: Role in extracellular matrix deposition and myocardial remodeling post-MI. J. Mol. Cell Cardiol. 2010, 48, 538–543. [Google Scholar] [CrossRef]
- Rider, C.C.; Mulloy, B. Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem. J. 2010, 429, 1–12. [Google Scholar] [CrossRef]
- Kami, D.; Shiojima, I.; Makino, H.; Matsumoto, K.; Takahashi, Y.; Ishii, R.; Naito, A.T.; Toyoda, M.; Saito, H.; Watanabe, M.; et al. Gremlin enhances the determined path to cardiomyogenesis. PLoS ONE. 2008, 3, e2407. [Google Scholar] [CrossRef] [PubMed]
- Koli, K.; Sutinen, E.; Rönty, M.; Rantakari, P.; Fortino, V.; Pulkkinen, V.; Greco, D.; Sipilä, P.; Myllärniemi, M. Gremlin-1 overexpression in mouse lung reduces silica-induced lymphocyte recruitment—a link to idiopathic pulmonary fibrosis through negative correlation with CXC10 chemokine. PLoS ONE 2016, 11, e0159010. [Google Scholar] [CrossRef]
- Cahill, E.; Costello, C.M.; Rowan, S.C.; Harkin, S.; Howell, K.; Leonard, M.O.; Southwood, M.; Cummins, E.P.; Fitzpatrick, S.F.; Taylor, C.T.; et al. Gremlin plays a key role in the pathogenesis of pulmonary hypertension. Circulation 2012, 125, 920–930. [Google Scholar] [CrossRef]
- Iansen Irion, C.; Dunkley, J.C.; John-Williams, K.; Condor Capcha, J.M.; Shehadeh, S.A.; Pinto, A.; Loebe, M.; Webster, K.A.; Brozzi, N.A.; Shehadeh, L.A. Nuclear osteopontin is a marker of advanced heart failure and cardiac allograft vasculopathy: Evidence from transplant and retransplant hearts. Front. Physiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Coculescu, B.-I.; Manole, G.; Valeriu Dinca, G.; Coculescu, E.C.; Berteanu, C.; Stocheci, C.M. Osteopontin—a biomarker of disease, but also of stage stratification of the functional myocardial contractile deficit by chronic ischemic heart disease. J. Enzyme Inhib. Med. Chem. 2019, 34, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.-I.; Leu, H.B.; Wu, C.-C.; Yin, W.-H.; Wang, J.-H.; Lin, T.-H.; Tseng, W.-K.; Chang, K.-C.; Chu, S.-H.; Yeh, H.-I.; et al. The clinical significance of osteopontin on the cardiovascular outcomes in patients with stable coronary artery disease. J. Formos. Med. Assoc. 2023, 122, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Schipper, M.E.I.; Scheenstra, M.R.; van Kuik, J.; van Wichen, D.F.; van der Weide, P.; Dullens, H.F.J.; Lahpor, J.; de Jonge, N.; De Weger, R.A. Osteopontin: A potential biomarker for heart failure and reverse remodeling after left ventricular assist device support. J. Heart Lung Transplant. 2011, 30, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Mueller, K.A.L.; Tavlaki, E.; Schneider, M.; Jorbenadze, R.; Gei, T.; Kandolf, R.; Gawaz, M.; Mueller, I.I.; Zuern, C.S. Gremlin-1 identifies fibrosis and predicts adverse outcome patients with heart failure undergoing endomyocardial biopsy. J. Cardiac. Fail. 2013, 19, 6780684. [Google Scholar] [CrossRef] [PubMed]
- Stawowy, P.; Blaschke, F.; Pfautsch, P.; Goetze, S.; Lippek, F.; Wollert-Wulf, B.; Fleck, E.; Graf, K. Increased myocardial expression of osteopontin in patients with advanced heart failure. Eur. J. Heart Fail. 2002, 4, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Müller, I.I.; Schneider, M.; Müller, K.A.L.; Lunov, O.; Borst, O.; Simmet, T.; Gawaz, M. Protective role of Gremlin-1 in myocardial function. Eur. J. Clin. Invest. 2021, 51, e13539. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Meyer, F.J.; Gruenig, E.; Lutz, M.; Lossnitzer, D.; Wipplinger, R.; Katus, H.A.; Frey, N. Osteopontin predicts adverse right ventricular remodelling and dysfunction in pulmonary hypertension. Eur. J. Clin. Invest. 2012, 42, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Krishnamurthy, P.; Singh, K.; Singh, M. Lack of osteopontin improves cardiac function in streptozotocin-induced diabetic mice. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H673–H683. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Behnes, M.; Brueckmann, M.; Lang, S.; Espeter, F.; Weiss, C.; Neumaier, M.; Ahmad-Nejad, P.; Borggrefe, M.; Hoffman, U. Diagnostic and prognostic value of osteopontin in patients with acute congestive heart failure. Eur. J. Heart Fail. 2013, 15, 1390–1400. [Google Scholar] [CrossRef]
- Podzimkova, J.; Palecek, T.; Kuchynka, P.; Marek, J.; Danek, B.A.; Jachymova, M.; Safarikova, M.; Kalousova, M.; Zima, T.; Linhart, A. Plasma osteopontin levels, but not its myocardial expression, reflect heart failure severity in recently diagnosed dilated cardiomyopathy. Herz 2020, 45, S105–S110. [Google Scholar] [CrossRef] [PubMed]
- Georgiadou, P.; Iliodromitis, E.F.; Kolokathis, F.; Varounis, C.; Gizas, V.; Mavroidis, M.; Capetanaki, Y.; Boudoulas, H.; Kremastinos, D.T. Osteopontin as a novel prognostic marker in stable ischaemic heart disease: A 3-year follow-up study. Eur. J. Clin. Invest. 2010, 40, 288–293. [Google Scholar] [CrossRef]
- Mamazhakypov, A.; Sartmyrzaeva, M.; Sarybaev, A.S.; Schermuly, R.; Sydykov, A. Clinical and molecular implications of osteopontin in heart failure. Curr. Issues Mol. Biol. 2022, 44, 3573–3597. [Google Scholar] [CrossRef]
- Mohamed, I.A.; Gadeau, A.P.; Fliegel, L.; Lopaschuk, G.; Mlih, M.; Abdulrahman, N.; Fillmore, N.; Mraiche, F. Na+/H+ exchanger isoform 1-induced osteopontin expression facilitates cardiomyocyte hypertrophy. PLoS ONE 2015, 10, e0123318. [Google Scholar] [CrossRef] [PubMed]
- Dalal, S.; Zha, Q.; Daniels, C.R.; Steagall, R.J.; Joyner, W.L.; Gadeau, A.P.; Singh, M.; Singh, K. Osteopontin stimulates apoptosis in adult cardiac muscle cells via the involvement of CD44 receptors, mitochondrial death pathway, and endoplasmic reticulum stress. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1182–H1191. [Google Scholar] [CrossRef] [PubMed]
- Pollard, C.M.; Desimine, V.L.; Wertz, S.L.; Perez, A.; Parker, B.M.; Maning, J.; McCrink, K.A.; Shehadeh, L.A.; Lymperopoulos, A. Deletion of osteopontin enhances beta (2)-adrenergic receptor-dependent anti-fibrotic signaling in cardiomyocytes. Int. J. Mol. Sci. 2019, 20, 1396. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Moroni, F.; Montone, R.A.; Azzalini, L.; Sanna, T.; Abbate, A. Ischemic cardiomyopathy and heart failure after acute myocardial infarction. Curr. Cardiol. Rep. 2022, 24, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Psarras, S.; Mavroidis, M.; Saboudou, D.; Davos, C.H.; Xanthou, G.; Varela, A.E.; Panoutsakopoulou, V.; Capetanaki, Y. Regulation of adverse remodelling by osteopontin in a genetic heart failure module. Eur. Heart J. 2012, 33, 1954–1963. [Google Scholar] [CrossRef]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Beck, S.; Simmet, T.; Müller, I.; Lang, F.; Gawaz, M. Gremlin-1 C-terminus regulates function of Macrophage Inhibitory Factor (MIF). Cell Physiol. Biochem. 2016, 38, 801–808. [Google Scholar] [CrossRef]
- Kišonaitė, M.; Wang, X.; Hyvönen, M. Structure of Gremlin-1 and analysis of its interaction with BMP-2. Biochem. J. 2016, 473, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Stabile, H.; Mitola, S.M.F.; Moroni, E.; Belleri, M.; Nicoli, S.; Coltrini, D.; Peri, F.; Pessi, A.; Orsatti, L.; Talamo, F.; et al. Bone morphogenic protein antagonist Drm/gremlin is a novel proangiogenic factor. Blood 2007, 109, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Mueller, I.; Schönberg, T.; Schneider, M.; Borst, O.; Ziegler, M.; Seizer, P.; Leder, C.; Mueller, K.; Lang, M.; Appenzeller, F.; et al. Gremlin-1 is an inhibitor of macrophage migration inhibitory factor and attenuates atherosclerotic plaque growth in ApoE-/- mice. J. Biol. Chem. 2013, 288, 31635–31645. [Google Scholar] [CrossRef]
- Topol, L.Z.; Bardot, B.; Zhang, Q.; Resau, J.; Huillard, E.; Marx, M.; Calothy, G.; Blair, D.G. Biosynthesis, post-translation modification, and functional characterization of Drm/Gremlin. J. Biol. Chem. 2000, 275, 8785–8793. [Google Scholar] [CrossRef]
- Maciel, T.T.; Melo, R.S.; Schor, N.; Campos, A.H. Gremlin promotes vascular smooth muscle cell proliferation and migration. J. Mol. Cell Cardiol. 2008, 44, 370–379. [Google Scholar] [CrossRef]
- Kaur, G.; Wang, X.; Li, X.; Ong, H.; He, X.; Cai, C. Overexpression of GREM1 improves the survival capacity of aged cardiac mesenchymal progenitor cells via upregulation of the ERK/NRF2-associated antioxidant signal pathway. Cells 2023, 12, 1203. [Google Scholar] [CrossRef]
- Rowan, S.C.; Piouceau, L.; Cornwell, J.; Li, L.; McLoughlin, P. Gremlin 1 blocks vascular endothelial growth factor signaling in the pulmonary microvascular endothelium. Pulm. Circ. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Sun, Z.; Cai, S.; Liu, C.; Cui, Y.; Ji, J.; Jiang, W.G.; Ye, L. Increased expression of Gremlin1 promotes proliferation and epithelial mesenchymal transition in gastric cancer cells and correlates with poor prognosis of patients with gastric cancer. Cancer Genom. Proteom. 2020, 17, 49–60. [Google Scholar] [CrossRef] [PubMed]
- The Human Protein Atlas: Gremlin-1—RNA Single Cell Type Specificity. Available online: https://www.proteinatlas.org/ENSG00000166923-GREM1/single+cell+type (accessed on 1 June 2024).
- Shevde, L.A.; Samant, R.S. Role of osteopontin in the pathophysiology of cancer. Matrix Biol. 2014, 37, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Gooding, S.; Leedham, S.J. Gremlin 1—small protein, big impact: The multiorgan consequences of disrupted BMP antagonism. J. Pathol. 2020, 251, 349–352. [Google Scholar] [CrossRef]
- Leone, O.; Veinot, J.P.; Angelini, A.; Baandrup, U.T.; Basso, C.; Berry, G.; Bruneval, P.; Burke, M.; Butany, J.; Calabrese, F.; et al. 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 2012, 21, 245–274. [Google Scholar] [CrossRef]
- WHO Scientific Group on Sudden Cardiac Death; World Health Organization. Sudden Cardiac Death: Report of a WHO Scientific Group [Meeting Held in Geneva from 24 to 27 October 1984]; World Health Organization: Geneva, Switzerland, 1985; Available online: https://apps.who.int/iris/handle/10665/39554 (accessed on 27 September 2023).
- Zeppenfield, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Michaud, K.; Basso, C.; d’Amati, G.; Giordano, C.; Kholova, I.; Preston, S.D.; Rizzo, S.; Sabatasso, S.; Sheppard, M.N.; Vink, A.; et al. Diagnosis of myocardial infarction at autopsy: AECVP reappraisal in the light of the current clinical classification. Virchows Arch. 2020, 476, 179–194. [Google Scholar] [CrossRef]
- Kuprytė, M.; Lesauskaitė, V.; Keturakis, V.; Bunevičienė, V.; Utkienė, L.; Jusienė, L.; Pangonytė, D. Remodeling of cardiomyocytes: Study of morphological cellular changes preceding symptomatic ischemic heart failure. Int. J. Mol. Sci. 2023, 24, 14557. [Google Scholar] [CrossRef]
- Uhlen, M.; Bandrowski, A.; Carr, S.; Edwards, A.; Ellenberg, J.; Lundberg, E.; Rimm, D.L.; Rodriguez, H.; Hiltke, T.; Snyder, M.; et al. A proposal for validation of antibodies. Nat Methods 2016, 13, 823–827. [Google Scholar] [CrossRef]
- Edfors, F.; Hober, A.; Linderbäck, K.; Maddalo, G.; Azimi, A.; Sivertsson, Å.; Tegel, H.; Hober, S.; Al-Khalili Szigyarto, C.; Fagerberg, L.; et al. Enhanced validation of antibodies for research applications. Nat Commun. 2018, 9, 4130. [Google Scholar] [CrossRef]
- Weller, M.G. Ten basic rules of antibody validation. Anal Chem Insights. 2018, 13, 1177390118757462. [Google Scholar] [CrossRef]
- The Human Protein Atlas: Assays, Annotation, Reliability Score. Available online: https://www.proteinatlas.org/about/assays+annotation (accessed on 27 September 2023).
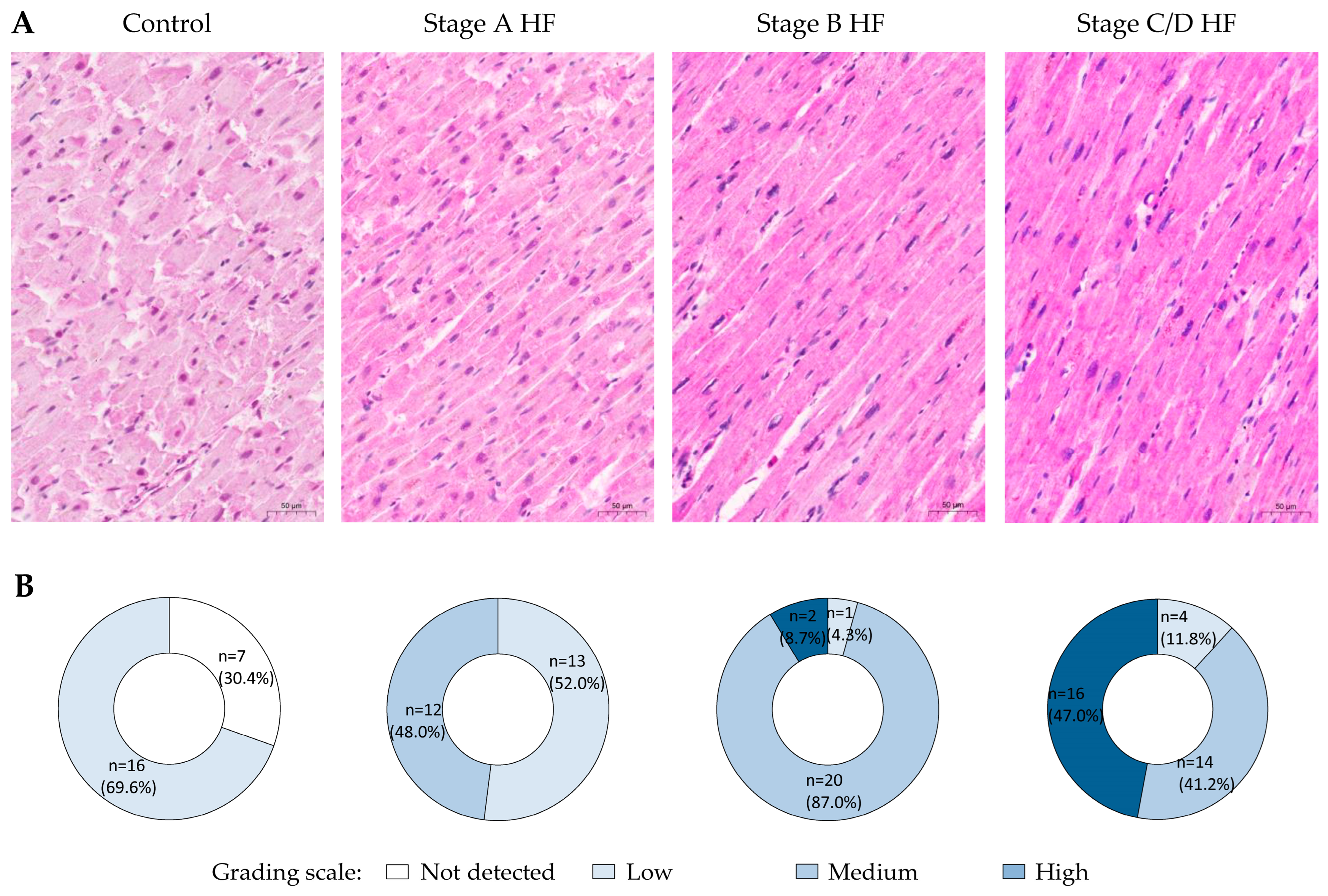



| Characteristic | Control Group n = 23 | Stage A HF Group n = 25 | Stage B HF Group n = 23 | Stages C/D HF Group n = 34 |
|---|---|---|---|---|
| Age (SD), years | 50.5 (7.9) | 53.8 (8.0) | 54.4 (7.7) | 56.2 (7.2) |
| Sex | Male | Male | Male | Male |
| Previous clinical symptoms of HF | No | No | No | Yes |
| Atherosclerotic stenosis ≥ 75% in at least one coronary artery | No | Yes | Yes | Yes |
| Scar after myocardial infarction | No | No | Yes | Yes |
| Mean length (SD) of left ventricular cardiomyocyte, µm [56] | 61.8 (6.3) | 72.2 (5.4) | 78.9 (6.3) | 103.3 (10.7) |
| Mean diameter (SD) of left ventricular cardiomyocyte, µm [56] | 11.7 (1.5) | 14.3 (1.0) | 15.2 (1.3) | 18.9 (2.7) |
| Antibody | Species | Immunogen | Dilution | Manufacturer (Catalog Number) | RRID | Lot Number |
|---|---|---|---|---|---|---|
| Osteopontin (OPN) | Mouse monoclonal | Bone protein fractions, immunogen sequence—full length | 1:100 | Developmental Studies Hybridoma Bank, DSHB (MPIIIB10(1)) | AB_2286610 | - |
| Osteopontin (OPN) | Rabbit polyclonal | KLH conjugated synthetic peptide corresponding to amino acids 273 through 301 from the C-terminal region of human SPP1 | 1:50 | Thermo Fisher Scientific (PA5-13494) | AB_2286594 | YI4041893 |
| Gremlin 1 (Grem1) | Rabbit polyclonal | Synthetic peptide, corresponding to amino acids from 52 through 67 | 1:100 | Abcam (ab22138) | AB_446814 | GR3362005-1 |
| Gremlin 1 (Grem1) | Rabbit polyclonal | KLH conjugated synthetic peptide derived from human gremlin 1, corresponding to amino acids from 101 through 184 | 1:200 | GeneTex (GTX03394) | - | 822303719 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuprytė, M.; Lesauskaitė, V.; Siratavičiūtė, V.; Utkienė, L.; Jusienė, L.; Pangonytė, D. Expression of Osteopontin and Gremlin 1 Proteins in Cardiomyocytes in Ischemic Heart Failure. Int. J. Mol. Sci. 2024, 25, 8240. https://doi.org/10.3390/ijms25158240
Kuprytė M, Lesauskaitė V, Siratavičiūtė V, Utkienė L, Jusienė L, Pangonytė D. Expression of Osteopontin and Gremlin 1 Proteins in Cardiomyocytes in Ischemic Heart Failure. International Journal of Molecular Sciences. 2024; 25(15):8240. https://doi.org/10.3390/ijms25158240
Chicago/Turabian StyleKuprytė, Milda, Vaiva Lesauskaitė, Vitalija Siratavičiūtė, Lina Utkienė, Lina Jusienė, and Dalia Pangonytė. 2024. "Expression of Osteopontin and Gremlin 1 Proteins in Cardiomyocytes in Ischemic Heart Failure" International Journal of Molecular Sciences 25, no. 15: 8240. https://doi.org/10.3390/ijms25158240
APA StyleKuprytė, M., Lesauskaitė, V., Siratavičiūtė, V., Utkienė, L., Jusienė, L., & Pangonytė, D. (2024). Expression of Osteopontin and Gremlin 1 Proteins in Cardiomyocytes in Ischemic Heart Failure. International Journal of Molecular Sciences, 25(15), 8240. https://doi.org/10.3390/ijms25158240






