GSK3-Driven Modulation of Inflammation and Tissue Integrity in the Animal Model
Abstract
1. Introduction
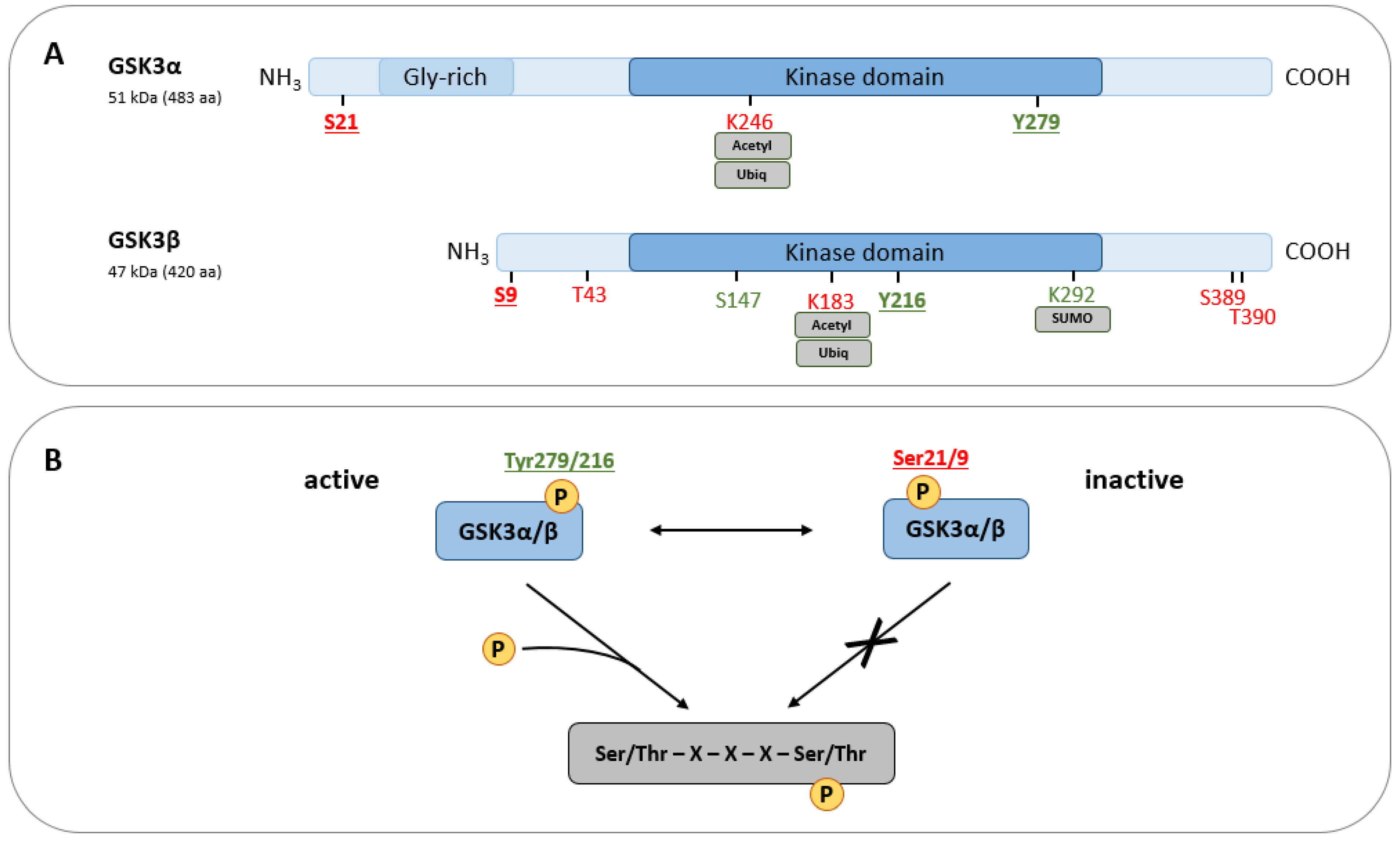
2. GSK3—A Condensed Overview on Structure, Function, and Regulation
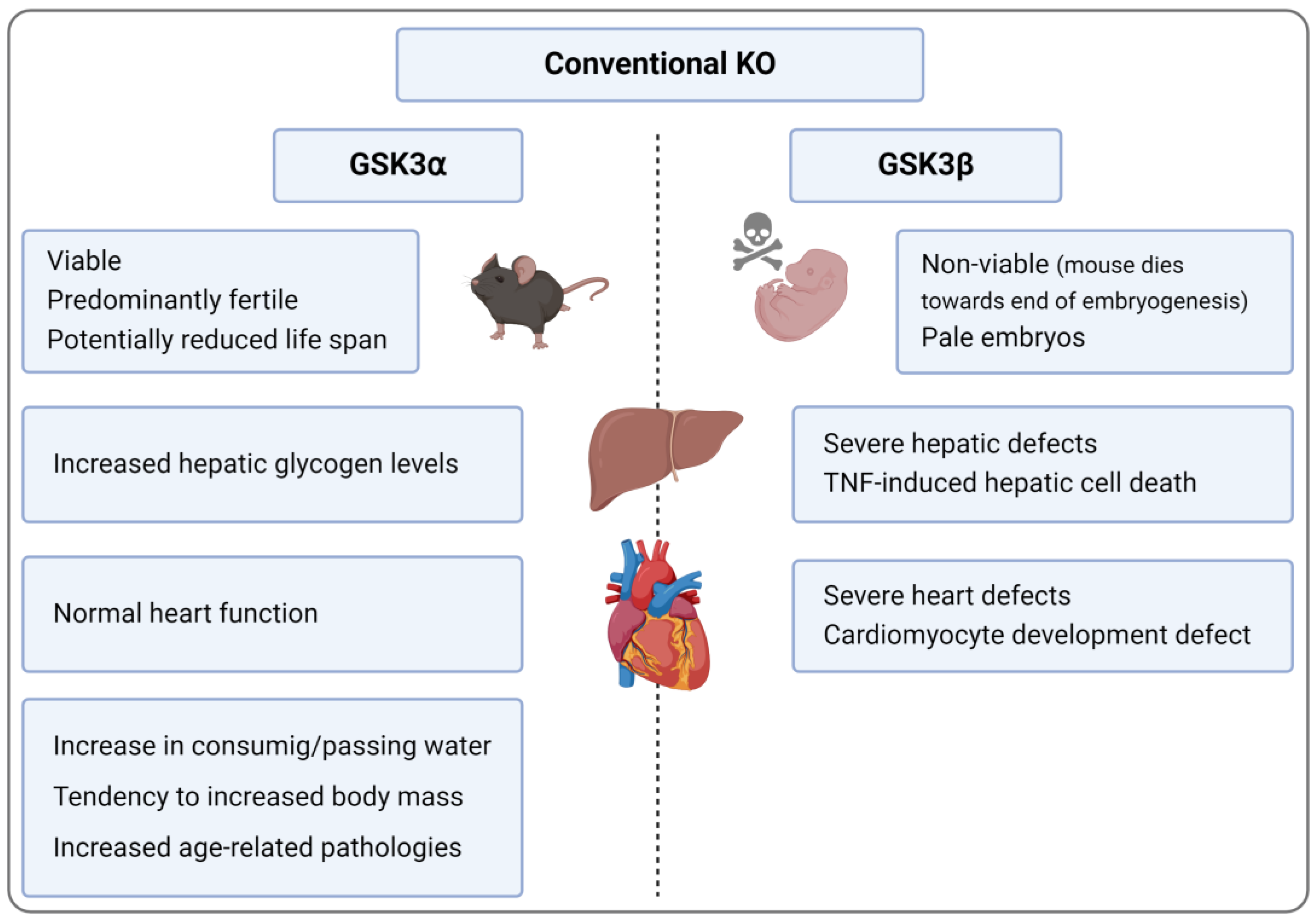
3. KO and KI Models
3.1. Conventional GSK3α KO
3.2. Conditional GSK3α KO
3.3. Conventinal and Conditional GSK3β KO
3.3.1. General Aspects of Conditional GSK3β KO
3.3.2. Inflammation-Associated Aspects of Conditional GSK3β KO
3.4. GSK3α/β Double KO (DKO)
3.5. KI Models
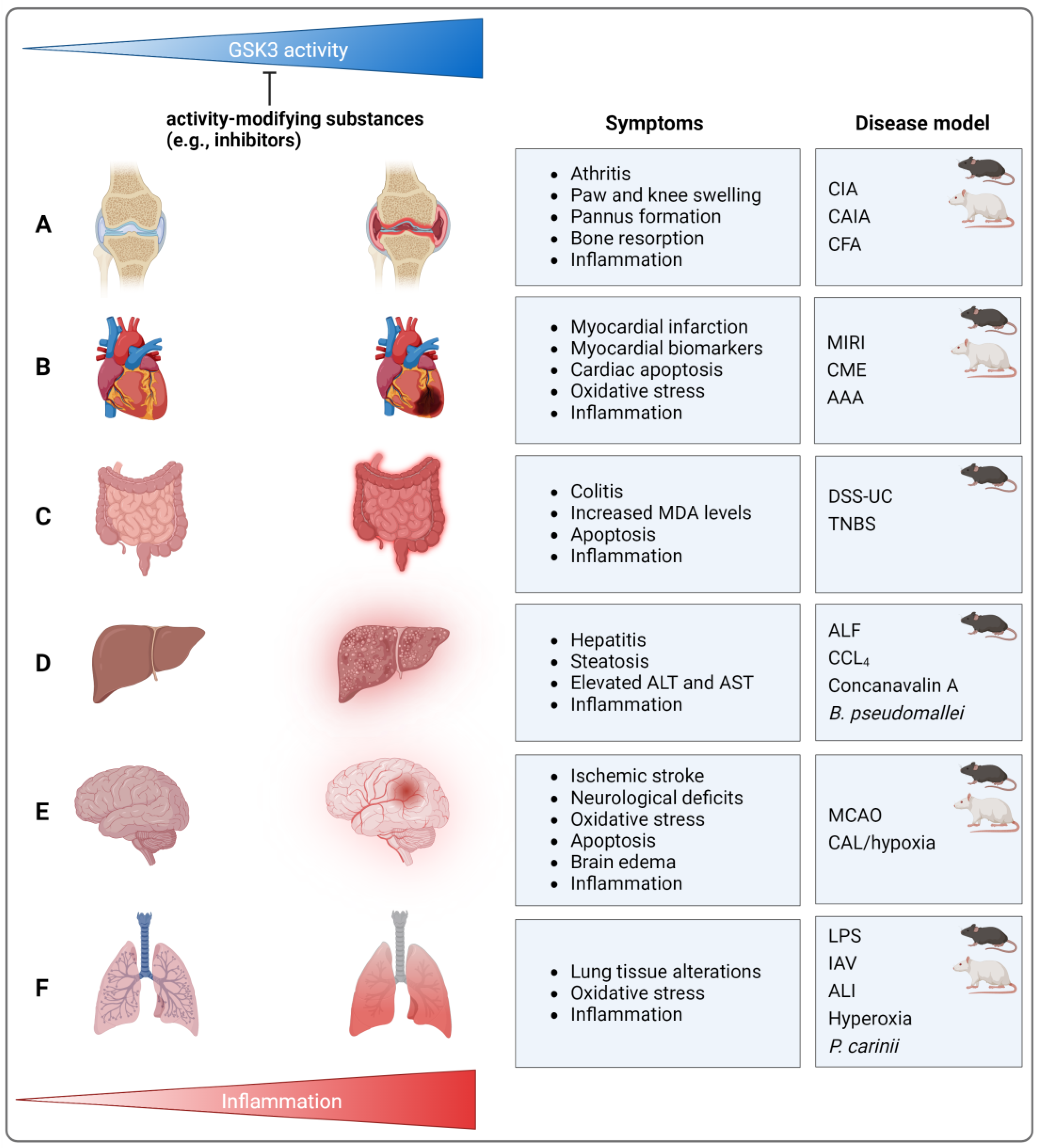
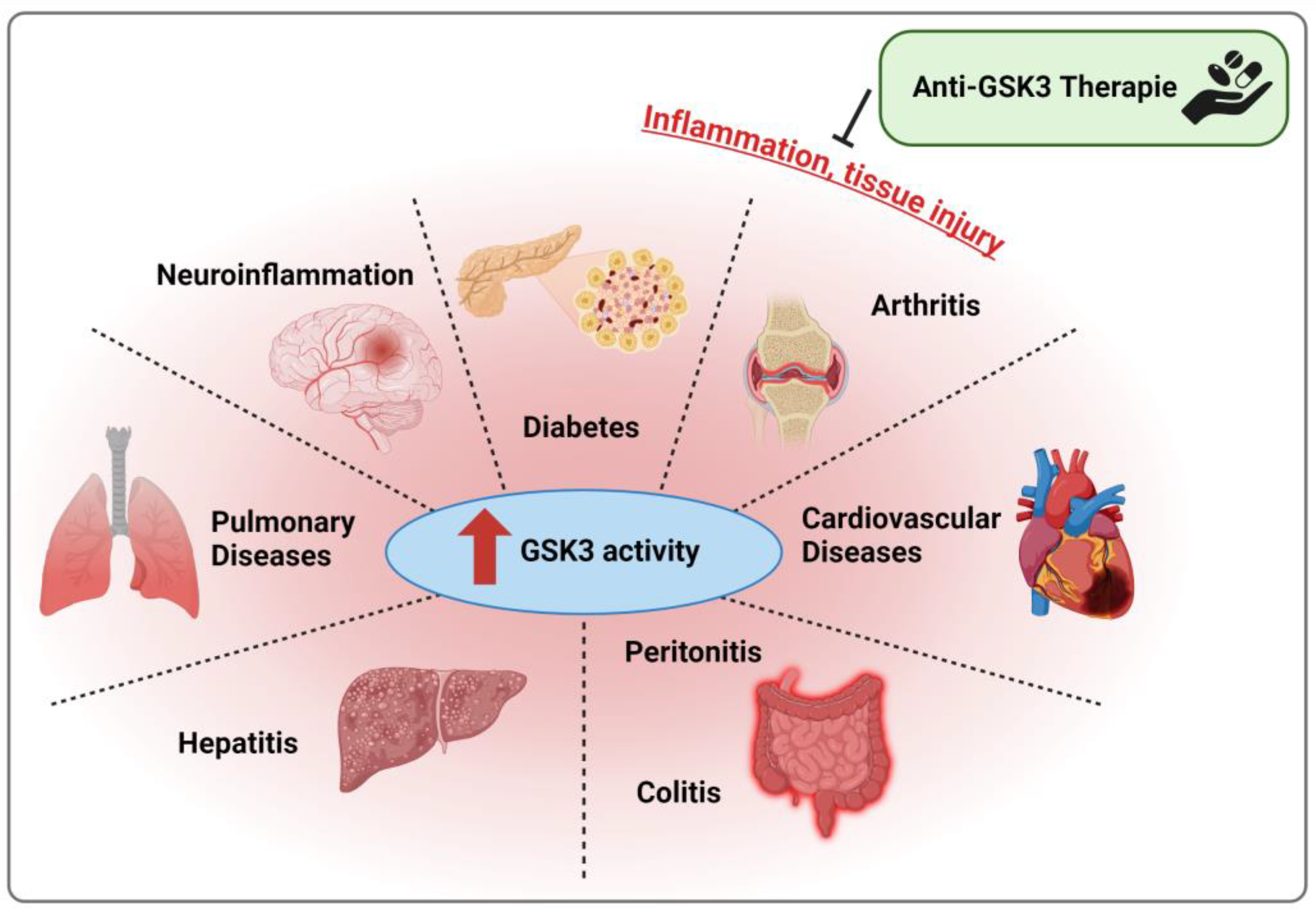
4. Models of Inflammatory/Inflammation-Associated Diseases
4.1. Arthritic Diseases
4.2. Cardiovascular Diseases
4.3. Colitis, Hepatitis, and Peritonitis
4.3.1. Colitis
4.3.2. Hepatitis
4.3.3. Peritonitis
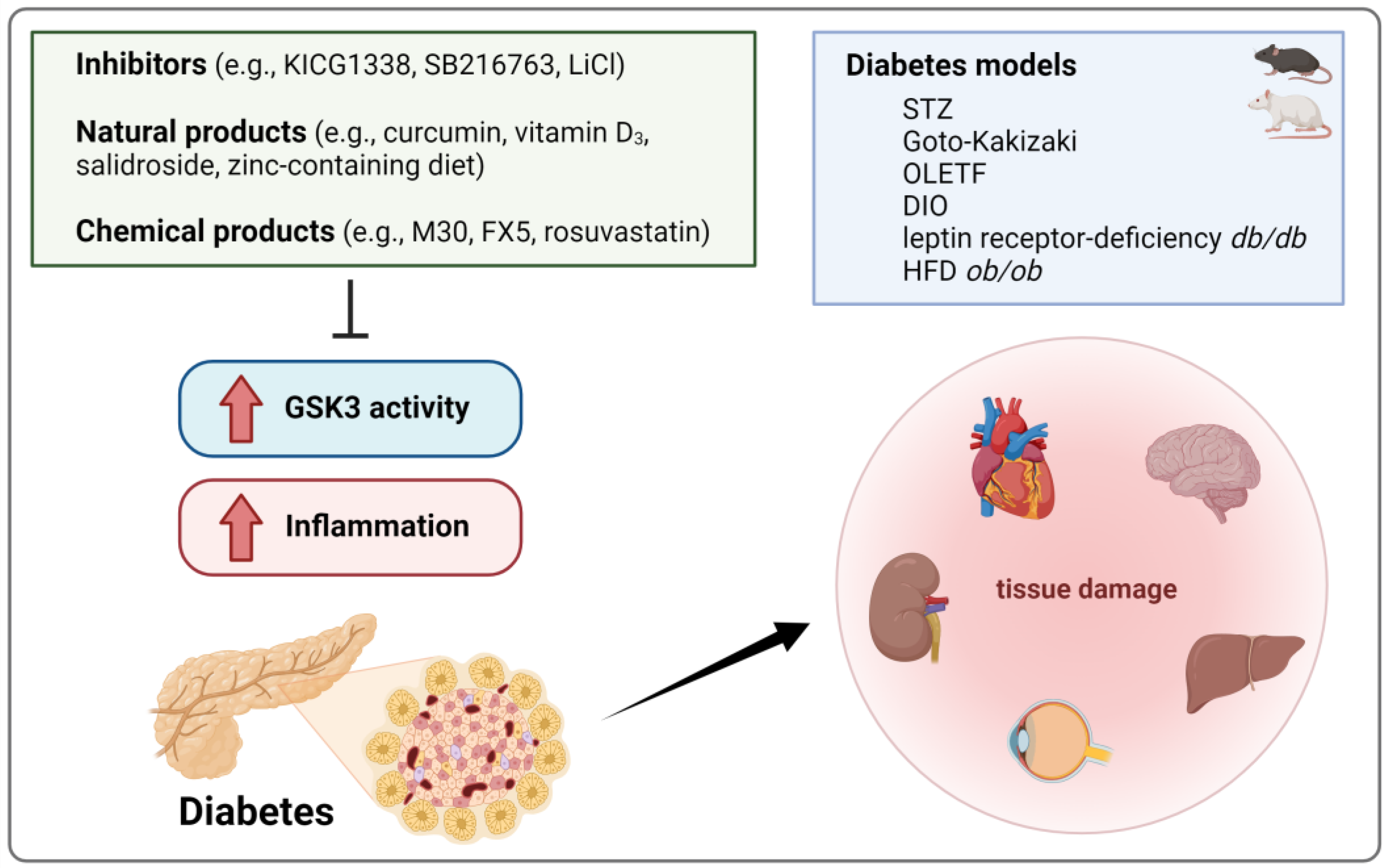
4.4. Diabetes
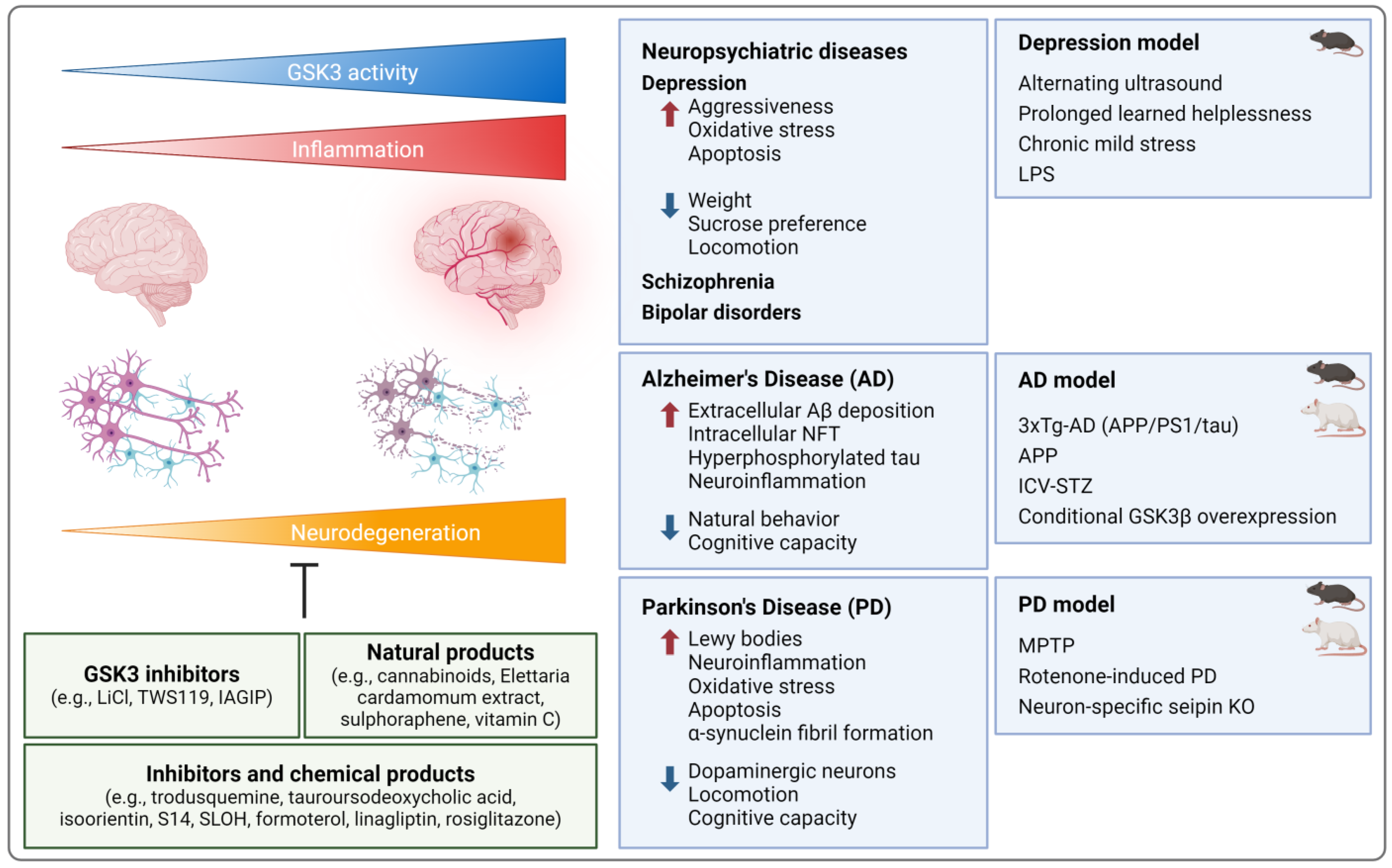
4.5. Neuroinflammation
4.5.1. Neuropsychiatric Diseases
4.5.2. Neurodegenerative Diseases
4.5.3. Ischemic Stroke
4.6. Pulmonary Diseases
4.7. Fibrosis
4.7.1. Liver Fibrosis
4.7.2. Lung Fibrosis
| Inhibitor | Disease | Animal Model | Organ/Tissue Analyzed | References |
|---|---|---|---|---|
| TDZD-8 | Arthritis | CIA (mouse) | Cartilage/bone | [84,86] |
| CIA (rat) | Cartilage/bone | [85] | ||
| CFA (mouse) | Cartilage/bone | [90] | ||
| Colitis | TNBS (mouse) | Intestine | [110] | |
| LiCl | Arthritis | CIA (mouse) | Cartilage/bone | [84] |
| CAIA (mouse) | Cartilage/bone | [67] | ||
| Cardiovascular | AAA (rat) | Aorta | [101] | |
| MIRI (rat) | Heart | [95] | ||
| Colitis | DSS-UC (mouse) | Intestine | [105,106] | |
| Peritonitis | Pam3CSK4 (mouse) | Peritoneum | [67] | |
| Diabetes | Goto-Kakizaki (rat) | Pancreas | [136] | |
| Depression | Chronic mild stress (rat) | Brain | [145] | |
| AD | 3xTg-AD (mouse) | Brain | [165] | |
| VA | Arthritis | CIA (mouse) | Cartilage/bone | [86] |
| SB216763 | Colitis | DSS-UC (mouse) | Intestine | [106] |
| Hepatitis | ALF (mouse) | Liver | [115,116] | |
| Diabetes | STZ (mouse) | Heart | [125] | |
| STZ (mouse) | Brain | [132] | ||
| Ischemic stroke | MCAO (mouse) | Brain | [188] | |
| MCAO (rat) | Brain | [195] | ||
| HIE | CAL/hypoxia (mouse) | Brain | [205] | |
| COPD | LPS (guinea pig) | Lung | [218] | |
| CLF | BDL (mouse) | Liver | [229] | |
| 6-MITC | Colitis | DSS-UC (mouse) | Intestine | [107] |
| KICG1338 | Diabetes | STZ (mouse, rat) | Various (e.g., muscle, liver, pancreas) | [133] |
| TWS119 | AD | APP (mouse) | Microglia | [161] |
| IAGIP | PD | MPTP (mouse) | Brain | [183] |
| inhibitor VIII | Ischemic stroke | MCAO (mouse) | Brain | [187] |
| tideglusib | HIE | CAL/hypoxia (mouse) | Brain | [205] |
| BTZ-6j, -3j | Pulmonary | ALI (mouse) | Lung | [209] |
| 9ING41 | Empyema | S. pneumonia (mouse) | Lung | [233] |
4.8. A Reflection on Contradictory Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Cormier, K.W.; Woodgett, J.R. Recent advances in understanding the cellular roles of GSK-3. F1000Research 2017, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, L.; Diekmann, M.; Brand, K.; Huber, R. GSK3: A Kinase Balancing Promotion and Resolution of Inflammation. Cells 2020, 9, 820. [Google Scholar] [CrossRef] [PubMed]
- Robertson, H.; Hayes, J.D.; Sutherland, C. A partnership with the proteasome; the destructive nature of GSK3. Biochem. Pharmacol. 2018, 147, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Vieyra, R.; Silva-Garcia, O.; Gomez-Garcia, A.; Gutierrez-Castellanos, S.; Alvarez-Aguilar, C.; Baizabal-Aguirre, V.M. Glycogen Synthase Kinase 3beta Modulates the Inflammatory Response Activated by Bacteria, Viruses, and Parasites. Front. Immunol. 2021, 12, 675751. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S.; Cheng, Y.; Lowell, J.A.; Worthen, R.J.; Sitbon, Y.H.; Beurel, E. Stressed and Inflamed, Can GSK3 Be Blamed? Trends Biochem. Sci. 2017, 42, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Arioka, M.; Takahashi-Yanaga, F. Glycogen synthase kinase-3 inhibitor as a multi-targeting anti-rheumatoid drug. Biochem. Pharmacol. 2019, 165, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Moparthi, L.; Koch, S. Wnt signaling in intestinal inflammation. Differentiation 2019, 108, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Emma, M.R.; Augello, G.; Cusimano, A.; Azzolina, A.; Montalto, G.; McCubrey, J.A.; Cervello, M. GSK-3 in liver diseases: Friend or foe? Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118743. [Google Scholar] [CrossRef] [PubMed]
- Teli, D.M.; Gajjar, A.K. Glycogen synthase kinase-3: A potential target for diabetes. Bioorg. Med. Chem. 2023, 92, 117406. [Google Scholar] [CrossRef]
- Yu, H.; Xiong, M.; Zhang, Z. The role of glycogen synthase kinase 3 beta in neurodegenerative diseases. Front. Mol. Neurosci. 2023, 16, 1209703. [Google Scholar] [CrossRef]
- Liang, J.; Yu, M.; Li, Y.; Zhao, L.; Wei, Q. Glycogen synthase kinase-3: A potential immunotherapeutic target in tumor microenvironment. Biomed. Pharmacother. 2024, 173, 116377. [Google Scholar] [CrossRef]
- Ma, Y.; Kroemer, G. The cancer-immune dialogue in the context of stress. Nat. Rev. Immunol. 2024, 24, 264–281. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Bertrand, F.E.; Davis, N.M.; Sokolosky, M.; Abrams, S.L.; Montalto, G.; D’Assoro, A.B.; Libra, M.; Nicoletti, F.; et al. GSK-3 as potential target for therapeutic intervention in cancer. Oncotarget 2014, 5, 2881–2911. [Google Scholar] [CrossRef] [PubMed]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Woodgett, J.R. Glycogen Synthase Kinase 3: A Kinase for All Pathways? Curr. Top Dev. Biol. 2017, 123, 277–302. [Google Scholar]
- Woodgett, J.R.; Cohen, P. Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5). Biochim. Biophys. Acta 1984, 788, 339–347. [Google Scholar] [CrossRef]
- Embi, N.; Rylatt, D.B.; Cohen, P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur. J. Biochem. 1980, 107, 519–527. [Google Scholar] [CrossRef]
- Woodgett, J.R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990, 9, 2431–2438. [Google Scholar] [CrossRef] [PubMed]
- Doble, B.W.; Woodgett, J.R. GSK-3: Tricks of the trade for a multi-tasking kinase. J. Cell Sci. 2003, 116, 1175–1186. [Google Scholar] [CrossRef]
- Ali, A.; Hoeflich, K.P.; Woodgett, J.R. Glycogen synthase kinase-3: Properties, functions, and regulation. Chem. Rev. 2001, 101, 2527–2540. [Google Scholar] [CrossRef]
- Doble, B.W.; Patel, S.; Wood, G.A.; Kockeritz, L.K.; Woodgett, J.R. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev. Cell 2007, 12, 957–971. [Google Scholar] [CrossRef] [PubMed]
- Duda, P.; Akula, S.M.; Abrams, S.L.; Steelman, L.S.; Martelli, A.M.; Cocco, L.; Ratti, S.; Candido, S.; Libra, M.; Montalto, G.; et al. Targeting GSK3 and Associated Signaling Pathways Involved in Cancer. Cells 2020, 9, 1110. [Google Scholar] [CrossRef] [PubMed]
- Kaidanovich-Beilin, O.; Woodgett, J.R. GSK-3: Functional Insights from Cell Biology and Animal Models. Front. Mol. Neurosci. 2011, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, C. What Are the bona fide GSK3 Substrates? Int. J. Alzheimers Dis. 2011, 2011, 505607. [Google Scholar] [CrossRef]
- Fiol, C.J.; Mahrenholz, A.M.; Wang, Y.; Roeske, R.W.; Roach, P.J. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J. Biol. Chem. 1987, 262, 14042–14048. [Google Scholar] [CrossRef] [PubMed]
- Dajani, R.; Fraser, E.; Roe, S.M.; Young, N.; Good, V.; Dale, T.C.; Pearl, L.H. Crystal structure of glycogen synthase kinase 3 beta: Structural basis for phosphate-primed substrate specificity and autoinhibition. Cell 2001, 105, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Dajani, R.; Fraser, E.; Roe, S.M.; Yeo, M.; Good, V.M.; Thompson, V.; Dale, T.C.; Pearl, L.H. Structural basis for recruitment of glycogen synthase kinase 3beta to the axin-APC scaffold complex. EMBO J. 2003, 22, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.; Frame, S.; Cohen, P. Further evidence that the tyrosine phosphorylation of glycogen synthase kinase-3 (GSK3) in mammalian cells is an autophosphorylation event. Biochem. J. 2004, 377, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Lochhead, P.A.; Kinstrie, R.; Sibbet, G.; Rawjee, T.; Morrice, N.; Cleghon, V. A chaperone-dependent GSK3beta transitional intermediate mediates activation-loop autophosphorylation. Mol. Cell 2006, 24, 627–633. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Fitzgerald, T.L.; Yang, L.V.; Lertpiriyapong, K.; Steelman, L.S.; Abrams, S.L.; Montalto, G.; Cervello, M.; Neri, L.M.; Cocco, L.; et al. Roles of GSK-3 and microRNAs on epithelial mesenchymal transition and cancer stem cells. Oncotarget 2017, 8, 14221–14250. [Google Scholar] [CrossRef]
- Bhat, R.V.; Shanley, J.; Correll, M.P.; Fieles, W.E.; Keith, R.A.; Scott, C.W.; Lee, C.M. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc. Natl. Acad. Sci. USA 2000, 97, 11074–11079. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Frame, S. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2001, 2, 769–776. [Google Scholar] [CrossRef]
- Duda, P.; Wisniewski, J.; Wojtowicz, T.; Wojcicka, O.; Jaskiewicz, M.; Drulis-Fajdasz, D.; Rakus, D.; McCubrey, J.A.; Gizak, A. Targeting GSK3 signaling as a potential therapy of neurodegenerative diseases and aging. Expert Opin. Ther. Targets 2018, 22, 833–848. [Google Scholar] [CrossRef]
- Souder, D.C.; Anderson, R.M. An expanding GSK3 network: Implications for aging research. Geroscience 2019, 41, 369–382. [Google Scholar] [CrossRef]
- Kaidanovich-Beilin, O.; Lipina, T.V.; Takao, K.; van Eede, M.; Hattori, S.; Laliberte, C.; Khan, M.; Okamoto, K.; Chambers, J.W.; Fletcher, P.J.; et al. Abnormalities in brain structure and behavior in GSK-3alpha mutant mice. Mol. Brain 2009, 2, 35. [Google Scholar] [CrossRef]
- MacAulay, K.; Doble, B.W.; Patel, S.; Hansotia, T.; Sinclair, E.M.; Drucker, D.J.; Nagy, A.; Woodgett, J.R. Glycogen synthase kinase 3alpha-specific regulation of murine hepatic glycogen metabolism. Cell Metab. 2007, 6, 329–337. [Google Scholar] [CrossRef]
- Barrell, W.B.; Szabo-Rogers, H.L.; Liu, K.J. Novel reporter alleles of GSK-3alpha and GSK-3beta. PLoS ONE 2012, 7, e50422. [Google Scholar] [CrossRef] [PubMed]
- Norregaard, R.; Tao, S.; Nilsson, L.; Woodgett, J.R.; Kakade, V.; Yu, A.S.; Howard, C.; Rao, R. Glycogen synthase kinase 3alpha regulates urine concentrating mechanism in mice. Am. J. Physiol. Renal Physiol. 2015, 308, F650–F660. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maurin, H.; Lechat, B.; Dewachter, I.; Ris, L.; Louis, J.V.; Borghgraef, P.; Devijver, H.; Jaworski, T.; Van Leuven, F. Neurological characterization of mice deficient in GSK3alpha highlight pleiotropic physiological functions in cognition and pathological activity as Tau kinase. Mol. Brain 2013, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Freeman, T.A.; Ahmad, F.; Shang, X.; Mangano, E.; Gao, E.; Farber, J.; Wang, Y.; Ma, X.L.; Woodgett, J.; et al. GSK-3alpha is a central regulator of age-related pathologies in mice. J. Clin. Investig. 2013, 123, 1821–1832. [Google Scholar] [CrossRef]
- Banko, N.S.; McAlpine, C.S.; Venegas-Pino, D.E.; Raja, P.; Shi, Y.; Khan, M.I.; Werstuck, G.H. Glycogen synthase kinase 3alpha deficiency attenuates atherosclerosis and hepatic steatosis in high fat diet-fed low density lipoprotein receptor-deficient mice. Am. J. Pathol. 2014, 184, 3394–3404. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Lal, H.; Zhou, J.; Vagnozzi, R.J.; Yu, J.E.; Shang, X.; Woodgett, J.R.; Gao, E.; Force, T. Cardiomyocyte-specific deletion of Gsk3alpha mitigates post-myocardial infarction remodeling, contractile dysfunction, and heart failure. J. Am. Coll. Cardiol. 2014, 64, 696–706. [Google Scholar] [CrossRef]
- Ahmad, F.; Singh, A.P.; Tomar, D.; Rahmani, M.; Zhang, Q.; Woodgett, J.R.; Tilley, D.G.; Lal, H.; Force, T. Cardiomyocyte-GSK-3alpha promotes mPTP opening and heart failure in mice with chronic pressure overload. J. Mol. Cell Cardiol. 2019, 130, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, R.; Goswami, S.; Dey, S.; Gangoda, M.; Brothag, C.; Eisa, A.; Woodgett, J.; Phiel, C.; Kline, D.; Vijayaraghavan, S. Isoform-specific requirement for GSK3alpha in sperm for male fertility. Biol. Reprod. 2018, 99, 384–394. [Google Scholar] [CrossRef]
- Patel, S.; Werstuck, G. Characterizing the Role of Glycogen Synthase Kinase-3alpha/beta in Macrophage Polarization and the Regulation of Pro-Atherogenic Pathways in Cultured Ldlr(−/−) Macrophages. Front. Immunol. 2021, 12, 676752. [Google Scholar] [CrossRef]
- McAlpine, C.S.; Huang, A.; Emdin, A.; Banko, N.S.; Beriault, D.R.; Shi, Y.; Werstuck, G.H. Deletion of Myeloid GSK3alpha Attenuates Atherosclerosis and Promotes an M2 Macrophage Phenotype. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiacomo, L.; Werstuck, G.H. Investigating the Role of Endothelial Glycogen Synthase Kinase3alpha/beta in Atherogenesis in Low Density Lipoprotein Receptor Knockout Mice. Int. J. Mol. Sci. 2022, 23, 14780. [Google Scholar] [CrossRef]
- Patel, S.; Mastrogiacomo, L.; Fulmer, M.; Shi, Y.; Werstuck, G.H. Deletion of Macrophage-Specific Glycogen Synthase Kinase (GSK)-3alpha Promotes Atherosclerotic Regression in Ldlr(−/−) Mice. Int. J. Mol. Sci. 2022, 23, 9293. [Google Scholar] [CrossRef]
- Hoeflich, K.P.; Luo, J.; Rubie, E.A.; Tsao, M.S.; Jin, O.; Woodgett, J.R. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 2000, 406, 86–90. [Google Scholar] [CrossRef]
- Kerkela, R.; Kockeritz, L.; Macaulay, K.; Zhou, J.; Doble, B.W.; Beahm, C.; Greytak, S.; Woulfe, K.; Trivedi, C.M.; Woodgett, J.R.; et al. Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J. Clin. Investig. 2008, 118, 3609–3618. [Google Scholar] [CrossRef]
- Rao, R.; Patel, S.; Hao, C.; Woodgett, J.; Harris, R. GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity. J. Am. Soc. Nephrol. 2010, 21, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.; Tao, S.; Yang, H.C.; Fogo, A.B.; Woodgett, J.R.; Harris, R.C.; Rao, R. Specific deletion of glycogen synthase kinase-3beta in the renal proximal tubule protects against acute nephrotoxic injury in mice. Kidney Int. 2012, 82, 1000–1009. [Google Scholar] [CrossRef]
- Li, C.; Ge, Y.; Dworkin, L.; Peng, A.; Gong, R. The beta isoform of GSK3 mediates podocyte autonomous injury in proteinuric glomerulopathy. J. Pathol. 2016, 239, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ge, Y.; Peng, A.; Gong, R. The redox sensitive glycogen synthase kinase 3beta suppresses the self-protective antioxidant response in podocytes upon oxidative glomerular injury. Oncotarget 2015, 6, 39493–39506. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, P.; Qiao, Y.; Ge, Y.; Wang, Y.; Quan, S.; Yao, R.; Zhuang, S.; Wang, L.J.; Du, Y.; et al. Genetic and Pharmacologic Targeting of Glycogen Synthase Kinase 3beta Reinforces the Nrf2 Antioxidant Defense against Podocytopathy. J. Am. Soc. Nephrol. 2016, 27, 2289–2308. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Chen, L.; Paul, J.; Yang, S.; Li, F.; Sampson, K.; Woodgett, J.R.; Beaulieu, J.M.; Gamble, K.L.; Li, X. The effects of glycogen synthase kinase-3beta in serotonin neurons. PLoS ONE 2012, 7, e43262. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Brink, L.E.; Maers, K.; Sullivan, M.L.; Bodnar, R.J.; Stolz, D.B.; Cambi, F. Conditional depletion of GSK3b protects oligodendrocytes from apoptosis and lessens demyelination in the acute cuprizone model. Glia 2018, 66, 1999–2012. [Google Scholar] [CrossRef] [PubMed]
- Ochs, S.M.; Dorostkar, M.M.; Aramuni, G.; Schon, C.; Filser, S.; Poschl, J.; Kremer, A.; Van Leuven, F.; Ovsepian, S.V.; Herms, J. Loss of neuronal GSK3beta reduces dendritic spine stability and attenuates excitatory synaptic transmission via beta-catenin. Mol. Psychiatry 2015, 20, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Gupte, M.; Tumuluru, S.; Sui, J.Y.; Singh, A.P.; Umbarkar, P.; Parikh, S.S.; Ahmad, F.; Zhang, Q.; Force, T.; Lal, H. Cardiomyocyte-specific deletion of GSK-3beta leads to cardiac dysfunction in a diet induced obesity model. Int. J. Cardiol. 2018, 259, 145–152. [Google Scholar] [CrossRef]
- Liu, Y.; Tanabe, K.; Baronnier, D.; Patel, S.; Woodgett, J.; Cras-Meneur, C.; Permutt, M.A. Conditional ablation of Gsk-3beta in islet beta cells results in expanded mass and resistance to fat feeding-induced diabetes in mice. Diabetologia 2010, 53, 2600–2610. [Google Scholar] [CrossRef]
- Lee, G.; Franklin, J.; Gupta, K.; Liu, R.; Zhou, L.; Ryder, C.; Sobieraj, L.; Molitor, L.; Abiona, O.; Meyerson, H.; et al. Loss of GSK3beta in hematopoietic stem cells results in normal hematopoiesis in mice. Blood Adv. 2023, 7, 7185–7189. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Doble, B.W.; MacAulay, K.; Sinclair, E.M.; Drucker, D.J.; Woodgett, J.R. Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol. Cell Biol. 2008, 28, 6314–6328. [Google Scholar] [CrossRef] [PubMed]
- Theeuwes, W.F.; Gosker, H.R.; Langen, R.C.J.; Pansters, N.A.M.; Schols, A.; Remels, A.H.V. Inactivation of glycogen synthase kinase 3beta (GSK-3beta) enhances mitochondrial biogenesis during myogenesis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2913–2926. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, H.; Ni, M.; Yue, S.; Xia, Y.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Lu, L.; Wang, X.; Zhai, Y. Glycogen synthase kinase 3beta promotes liver innate immune activation by restraining AMP-activated protein kinase activation. J. Hepatol. 2018, 69, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ni, M.; Wang, H.; Zhang, J.; Jin, D.; Busuttil, R.W.; Kupiec-Weglinski, J.W.; Li, W.; Wang, X.; Zhai, Y. Gsk3beta regulates the resolution of liver ischemia/reperfusion injury via MerTK. JCI Insight 2023, 8, e151819. [Google Scholar] [CrossRef]
- Sinha, S.; Dwivedi, N.; Woodgett, J.; Tao, S.; Howard, C.; Fields, T.A.; Jamadar, A.; Rao, R. Glycogen synthase kinase-3beta inhibits tubular regeneration in acute kidney injury by a FoxM1-dependent mechanism. FASEB J. 2020, 34, 13597–13608. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Paik, P.K.; Chen, J.; Yarilina, A.; Kockeritz, L.; Lu, T.T.; Woodgett, J.R.; Ivashkiv, L.B. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity 2006, 24, 563–574. [Google Scholar] [CrossRef]
- Kim, W.Y.; Wang, X.; Wu, Y.; Doble, B.W.; Patel, S.; Woodgett, J.R.; Snider, W.D. GSK-3 is a master regulator of neural progenitor homeostasis. Nat. Neurosci. 2009, 12, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.M.; Ka, M.; Kim, W.Y. Loss of GSK-3 Causes Abnormal Astrogenesis and Behavior in Mice. Mol. Neurobiol. 2016, 53, 3954–3966. [Google Scholar] [CrossRef]
- Bali, S.K.; Bryce, D.; Prein, C.; Woodgett, J.R.; Beier, F. Glycogen synthase kinase 3 alpha/beta deletion induces precocious growth plate remodeling in mice. J. Mol. Med. 2021, 99, 831–844. [Google Scholar] [CrossRef]
- McManus, E.J.; Sakamoto, K.; Armit, L.J.; Ronaldson, L.; Shpiro, N.; Marquez, R.; Alessi, D.R. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J. 2005, 24, 1571–1583. [Google Scholar] [CrossRef]
- Tan, M.; Ma, S.; Huang, Q.; Hu, K.; Song, B.; Li, M. GSK-3alpha/beta-mediated phosphorylation of CRMP-2 regulates activity-dependent dendritic growth. J. Neurochem. 2013, 125, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.F.; Agbani, E.O.; Wersall, A.; Poole, A.W.; Williams, C.M.; Zhao, X.; Li, Y.; Hutchinson, J.L.; Hunter, R.W.; Hers, I. Opposing Roles of GSK3alpha and GSK3beta Phosphorylation in Platelet Function and Thrombosis. Int. J. Mol. Sci. 2021, 22, 10656. [Google Scholar] [CrossRef]
- Chen, H.; Fajol, A.; Hoene, M.; Zhang, B.; Schleicher, E.D.; Lin, Y.; Calaminus, C.; Pichler, B.J.; Weigert, C.; Haring, H.U.; et al. PI3K-resistant GSK3 controls adiponectin formation and protects from metabolic syndrome. Proc. Natl. Acad. Sci. USA 2016, 113, 5754–5759. [Google Scholar] [CrossRef]
- Hey, F.; Giblett, S.; Forrest, S.; Herbert, C.; Pritchard, C. Phosphorylations of Serines 21/9 in Glycogen Synthase Kinase 3alpha/beta Are Not Required for Cell Lineage Commitment or WNT Signaling in the Normal Mouse Intestine. PLoS ONE 2016, 11, e0156877. [Google Scholar] [CrossRef]
- Pearce, N.J.; Arch, J.R.; Clapham, J.C.; Coghlan, M.P.; Corcoran, S.L.; Lister, C.A.; Llano, A.; Moore, G.B.; Murphy, G.J.; Smith, S.A.; et al. Development of glucose intolerance in male transgenic mice overexpressing human glycogen synthase kinase-3beta on a muscle-specific promoter. Metabolism 2004, 53, 1322–1330. [Google Scholar] [CrossRef]
- Spittaels, K.; Van den Haute, C.; Van Dorpe, J.; Geerts, H.; Mercken, M.; Bruynseels, K.; Lasrado, R.; Vandezande, K.; Laenen, I.; Boon, T.; et al. Glycogen synthase kinase-3beta phosphorylates protein tau and rescues the axonopathy in the central nervous system of human four-repeat tau transgenic mice. J. Biol. Chem. 2000, 275, 41340–41349. [Google Scholar] [CrossRef] [PubMed]
- Lucas, J.J.; Hernandez, F.; Gomez-Ramos, P.; Moran, M.A.; Hen, R.; Avila, J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001, 20, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.; Borrell, J.; Guaza, C.; Avila, J.; Lucas, J.J. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J. Neurochem. 2002, 83, 1529–1533. [Google Scholar] [CrossRef]
- Engel, T.; Hernandez, F.; Avila, J.; Lucas, J.J. Full reversal of Alzheimer’s disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. J. Neurosci. 2006, 26, 5083–5090. [Google Scholar] [CrossRef]
- Spittaels, K.; Van den Haute, C.; Van Dorpe, J.; Terwel, D.; Vandezande, K.; Lasrado, R.; Bruynseels, K.; Irizarry, M.; Verhoye, M.; Van Lint, J.; et al. Neonatal neuronal overexpression of glycogen synthase kinase-3 beta reduces brain size in transgenic mice. Neuroscience 2002, 113, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tanabe, K.; Bernal-Mizrachi, E.; Permutt, M.A. Mice with beta cell overexpression of glycogen synthase kinase-3beta have reduced beta cell mass and proliferation. Diabetologia 2008, 51, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Caplazi, P.; Baca, M.; Barck, K.; Carano, R.A.; DeVoss, J.; Lee, W.P.; Bolon, B.; Diehl, L. Mouse Models of Rheumatoid Arthritis. Vet. Pathol. 2015, 52, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Yoon, C.H.; Lee, S.W.; Park, Y.B.; Lee, S.K.; Park, M.C. Inhibition of glycogen synthase kinase-3beta suppresses inflammatory responses in rheumatoid arthritis fibroblast-like synoviocytes and collagen-induced arthritis. Jt. Bone Spine 2014, 81, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, J.; Zeng, J.; Hu, B.; Fang, X.; Li, L. Inhibition of GSK-3beta Alleviates Collagen II-Induced Rheumatoid Arthritis in Rats. Med. Sci. Monit. 2016, 22, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.J.; Yang, Y.Y.; Liu, H.C.; Cheng, C.Y.; Wu, Y.C.; Huang, M.C.; Lee, Y.L.; Chen, C.C.; Shen, W.W.; Liu, K.J. Valproic Acid and Lithium Meditate Anti-Inflammatory Effects by Differentially Modulating Dendritic Cell Differentiation and Function. J. Cell Physiol. 2017, 232, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Peng, Y.; Li, H.; Li, C.; Wu, Y.; Wang, X.; Chang, J.; Miao, C. Wilforine inhibits rheumatoid arthritis pathology through the Wnt11/beta-catenin signaling pathway axis. Arthritis Res. Ther. 2023, 25, 243. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yue, Y.; Wang, B.; Chen, S.; Li, D.; Zhen, F.; Liu, L.; Zhu, H.; Xie, M. Anemoside B4 alleviates arthritis pain via suppressing ferroptosis-mediated inflammation. J. Cell Mol. Med. 2024, 28, e18136. [Google Scholar] [CrossRef]
- Louie, J.S. Lessons from Carl M. Pearson 1919–1981. Rheum Dis. Clin. N. Am. 2024, 50, 73–77. [Google Scholar] [CrossRef]
- Yang, H.Y.; Sun, X.; Zhen, S.Q.; Yu, L.Z.; Ding, J.Q.; Liu, L.; Xie, M.; Zhu, H.L. GSK-3beta inhibition alleviates arthritis pain via reducing spinal mitochondrial reactive oxygen species level and inflammation. PLoS ONE 2023, 18, e0284332. [Google Scholar]
- Liu, F.Y.; Wang, M.Q.; Liu, M.M.; Li, T.; Wang, X.H.; Jiang, F.; Wu, X.J.; Cheng, J.; Cai, L.; Li, R. Therapeutic effects of shikonin on adjuvant-induced arthritis in rats and cellular inflammation, migration and invasion of rheumatoid fibroblast-like synoviocytes via blocking the activation of Wnt/beta-catenin pathway. Phytomedicine 2023, 116, 154857. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Meng, Y.; Wang, L.; Sun, J.; Nu, S.; Xia, E.; Ti, T.B.; Luo, L. T-614 attenuates knee osteoarthritis via regulating Wnt/beta-catenin signaling pathway. J. Orthop. Surg. Res. 2021, 16, 403. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Kumar, A.; Sahu, M.; Sharma, G.; Datusalia, A.K.; Rajput, S.K. Exercise preconditioning and low dose copper nanoparticles exhibits cardioprotection through targeting GSK-3beta phosphorylation in ischemia/reperfusion induced myocardial infarction. Microvasc. Res. 2018, 120, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Yang, Q.H.; Ma, X.L.; Li, T.; Xiao, S.; Sun, C.F. Inhibition of TNFAIP1 ameliorates the oxidative stress and inflammatory injury in myocardial ischemia/reperfusion injury through modulation of Akt/GSK-3beta/Nrf2 pathway. Int. Immunopharmacol. 2021, 99, 107993. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhao, B.; Liu, L.; Yuan, Q.; Wu, X.; Xia, Z. Myocardial ischemic post-conditioning protects the lung against myocardial ischemia/reperfusion-induced damage by activating GSK-3beta. Acta Cir. Bras. 2017, 32, 376–387. [Google Scholar] [CrossRef] [PubMed]
- de Hoog, V.C.; Bovens, S.M.; de Jager, S.C.; van Middelaar, B.J.; van Duijvenvoorde, A.; Doevendans, P.A.; Pasterkamp, G.; de Kleijn, D.P.; Timmers, L. BLT1 antagonist LSN2792613 reduces infarct size in a mouse model of myocardial ischaemia-reperfusion injury. Cardiovasc. Res. 2015, 108, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Chen, Z.; Zhou, Y.; Li, H.; Xie, J.; Li, L. Resveratrol Pretreatment Inhibits Myocardial Apoptosis in Rats Following Coronary Microembolization via Inducing the PI3K/Akt/GSK-3beta Signaling Cascade. Drug Des. Dev. Ther. 2021, 15, 3821–3834. [Google Scholar] [CrossRef] [PubMed]
- Anzovino, A.; Chiang, S.; Brown, B.E.; Hawkins, C.L.; Richardson, D.R.; Huang, M.L. Molecular Alterations in a Mouse Cardiac Model of Friedreich Ataxia: An Impaired Nrf2 Response Mediated via Upregulation of Keap1 and Activation of the Gsk3beta Axis. Am. J. Pathol. 2017, 187, 2858–2875. [Google Scholar] [CrossRef] [PubMed]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Bai, Y.; Jiang, X.; Zhou, S.; Wang, Y.; Wintergerst, K.A.; Cui, T.; Ji, H.; Tan, Y.; Cai, L. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3ss/Fyn pathway. Redox Biol. 2018, 15, 405–417. [Google Scholar] [CrossRef]
- Xu, T.; Wang, S.; Li, X.; Li, X.; Qu, K.; Tong, H.; Zhang, R.; Bai, S.; Fan, J. Lithium chloride represses abdominal aortic aneurysm via regulating GSK3beta/SIRT1/NF-kappaB signaling pathway. Free Radic. Biol. Med. 2021, 166, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gallo, D.; Csizmadia, E.; Otterbein, L.E.; Wegiel, B. Carbon monoxide induces chromatin remodelling to facilitate endothelial cell migration. Thromb. Haemost. 2014, 111, 951–959. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, B.D.; Meller, J.; Pryor, A.W.; Kahn, M.; Bortz, P.D.; Wamhoff, B.R.; Blackman, B.R. Hemodynamic activation of beta-catenin and T-cell-specific transcription factor signaling in vascular endothelium regulates fibronectin expression. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Chi, F.; Zhang, G.; Ren, N.; Zhang, J.; Du, F.; Zheng, X.; Zhang, C.; Lin, Z.; Li, R.; Shi, X.; et al. The anti-alcoholism drug disulfiram effectively ameliorates ulcerative colitis through suppressing oxidative stresses-associated pyroptotic cell death and cellular inflammation in colonic cells. Int. Immunopharmacol. 2022, 111, 109117. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Jeong, S.O.; Zheng, M.; Chen, Y.; Cho, G.J.; Chung, H.T.; Joe, Y. Carbon monoxide attenuates dextran sulfate sodium-induced colitis via inhibition of GSK-3beta signaling. Oxid. Med. Cell. Longev. 2013, 2013, 210563. [Google Scholar] [CrossRef]
- Hofmann, C.; Dunger, N.; Scholmerich, J.; Falk, W.; Obermeier, F. Glycogen synthase kinase 3-beta: A master regulator of toll-like receptor-mediated chronic intestinal inflammation. Inflamm. Bowel Dis. 2010, 16, 1850–1858. [Google Scholar] [CrossRef] [PubMed]
- Lohning, A.; Kidachi, Y.; Kamiie, K.; Sasaki, K.; Ryoyama, K.; Yamaguchi, H. 6-(methylsulfinyl)hexyl isothiocyanate (6-MITC) from Wasabia japonica alleviates inflammatory bowel disease (IBD) by potential inhibition of glycogen synthase kinase 3 beta (GSK-3beta). Eur. J. Med. Chem. 2021, 216, 113250. [Google Scholar] [CrossRef] [PubMed]
- Degagne, E.; Degrandmaison, J.; Grbic, D.M.; Vinette, V.; Arguin, G.; Gendron, F.P. P2Y2 receptor promotes intestinal microtubule stabilization and mucosal re-epithelization in experimental colitis. J. Cell Physiol. 2013, 228, 99–109. [Google Scholar] [CrossRef]
- Becker, S.; Wandel, E.; Wobus, M.; Schneider, R.; Amasheh, S.; Sittig, D.; Kerner, C.; Naumann, R.; Hamann, J.; Aust, G. Overexpression of CD97 in intestinal epithelial cells of transgenic mice attenuates colitis by strengthening adherens junctions. PLoS ONE 2010, 5, e8507. [Google Scholar] [CrossRef]
- Mateus, V.; Rocha, J.; Alves, P.; Mota-Filipe, H.; Sepodes, B.; Pinto, R. Thiadiazolidinone-8 Ameliorates Inflammation Associated with Experimental Colitis in Mice. Pharmacology 2018, 101, 35–42. [Google Scholar] [CrossRef]
- Di Gregorio, J.; Sferra, R.; Speca, S.; Vetuschi, A.; Dubuquoy, C.; Desreumaux, P.; Pompili, S.; Cristiano, L.; Gaudio, E.; Flati, V.; et al. Role of glycogen synthase kinase-3beta and PPAR-gamma on epithelial-to-mesenchymal transition in DSS-induced colorectal fibrosis. PLoS ONE 2017, 12, e0171093. [Google Scholar] [CrossRef] [PubMed]
- Fichtner-Feigl, S.; Kesselring, R.; Martin, M.; Obermeier, F.; Ruemmele, P.; Kitani, A.; Brunner, S.M.; Haimerl, M.; Geissler, E.K.; Strober, W.; et al. IL-13 orchestrates resolution of chronic intestinal inflammation via phosphorylation of glycogen synthase kinase-3beta. J. Immunol. 2014, 192, 3969–3980. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Lee, C.G.; Hwang, S.J.; Kim, S.G. Mitigation of carbon tetrachloride-induced hepatic injury by methylene blue, a repurposed drug, is mediated by dual inhibition of GSK3beta downstream of PKA. Br. J. Pharmacol. 2014, 171, 2790–2802. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Chen, D.; Hou, J.; Tan, Z.; Wang, Y.; Huang, X.; Wang, X.; Sun, B. Survival and inflammation promotion effect of PTPRO in fulminant hepatitis is associated with NF-kappaB activation. J. Immunol. 2014, 193, 5161–5170. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ren, F.; Zhang, H.; Wen, T.; Piao, Z.; Zhou, L.; Zheng, S.; Zhang, J.; Chen, Y.; Han, Y.; et al. Inhibition of glycogen synthase kinase 3beta ameliorates D-GalN/LPS-induced liver injury by reducing endoplasmic reticulum stress-triggered apoptosis. PLoS ONE 2012, 7, e45202. [Google Scholar]
- Ren, F.; Zhang, L.; Zhang, X.; Shi, H.; Wen, T.; Bai, L.; Zheng, S.; Chen, Y.; Chen, D.; Li, L.; et al. Inhibition of glycogen synthase kinase 3beta promotes autophagy to protect mice from acute liver failure mediated by peroxisome proliferator-activated receptor alpha. Cell Death Dis. 2016, 7, e2151. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Ren, F.; Zhang, X.; Wen, T.; Shi, H.; Zheng, S.; Zhang, J.; Chen, Y.; Han, Y.; Duan, Z. Oxidative stress promotes D-GalN/LPS-induced acute hepatotoxicity by increasing glycogen synthase kinase 3beta activity. Inflamm. Res. 2014, 63, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Sun, Z.; Zhang, L.; Xu, H. SERP1 reduces inchoate acute hepatic injury through regulation of endoplasmic reticulum stress via the GSK3beta/beta-catenin/TCF/LEF signaling pathway. Mol. Med. Rep. 2022, 25, 193. [Google Scholar] [CrossRef]
- Alhusaini, A.; Fadda, L.; Hasan, I.H.; Zakaria, E.; Alenazi, A.M.; Mahmoud, A.M. Curcumin Ameliorates Lead-Induced Hepatotoxicity by Suppressing Oxidative Stress and Inflammation, and Modulating Akt/GSK-3beta Signaling Pathway. Biomolecules 2019, 9, 703. [Google Scholar] [CrossRef]
- Abdel-Emam, R.A.; Ali, M.F. Effect of l-carnitine supplementation on lead acetate-induced liver cell apoptosis and inflammation: Role of caspase-3 and glycogen synthase kinase-3beta enzymes. Life Sci. 2022, 291, 120277. [Google Scholar] [CrossRef]
- Ganesan, N.; Embi, N.; Hasidah, M.S. The anti-malarial chloroquine modulated cytokine levels and increased animal survivability via Akt-mediated inhibition of GSK3beta in Burkholderia pseudomalleiinfected mice. Trop Biomed. 2018, 35, 709–723. [Google Scholar] [PubMed]
- Cui, Z.; Jin, N.; Amevor, F.K.; Shu, G.; Du, X.; Kang, X.; Ning, Z.; Deng, X.; Tian, Y.; Zhu, Q.; et al. Dietary supplementation of salidroside alleviates liver lipid metabolism disorder and inflammatory response to promote hepatocyte regeneration via PI3K/AKT/Gsk3-beta pathway. Poult. Sci. 2022, 101, 102034. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, Y.; Li, Y.; Xu, L.; Wang, Y.; Guo, Z.; Song, H.; Yang, M.; Luo, B.; Zheng, A.; et al. Ephedrine hydrochloride inhibits PGN-induced inflammatory responses by promoting IL-10 production and decreasing proinflammatory cytokine secretion via the PI3K/Akt/GSK3beta pathway. Cell Mol. Immunol. 2013, 10, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.N.; Reddy, N.M.; Patil, K.R.; Nakhate, K.T.; Ojha, S.; Patil, C.R.; Agrawal, Y.O. Challenges and issues with streptozotocin-induced diabetes—A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem. Biol. Interact. 2016, 244, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, W.; Xue, W.; Tan, Y.; Hein, D.W.; Li, X.K.; Cai, L. Inactivation of GSK-3beta by metallothionein prevents diabetes-related changes in cardiac energy metabolism, inflammation, nitrosative damage, and remodeling. Diabetes 2009, 58, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, X.; Tan, Y.; Li, B.; Miao, X.; Jin, L.; Shi, X.; Zhang, X.; Miao, L.; Li, X.; et al. Diabetes-induced hepatic pathogenic damage, inflammation, oxidative stress, and insulin resistance was exacerbated in zinc deficient mouse model. PLoS ONE 2012, 7, e49257. [Google Scholar] [CrossRef]
- Yang, F.; Li, B.; Dong, X.; Cui, W.; Luo, P. The beneficial effects of zinc on diabetes-induced kidney damage in murine rodent model of type 1 diabetes mellitus. J. Trace Elem. Med. Biol. 2017, 42, 1–10. [Google Scholar] [CrossRef]
- Sunilkumar, S.; VanCleave, A.M.; McCurry, C.M.; Toro, A.L.; Stevens, S.A.; Kimball, S.R.; Dennis, M.D. REDD1-dependent GSK3beta dephosphorylation promotes NF-kappaB activation and macrophage infiltration in the retina of diabetic mice. J. Biol. Chem. 2023, 299, 104991. [Google Scholar] [CrossRef]
- Gomaa, A.A.; Makboul, R.M.; Al-Mokhtar, M.A.; Nicola, M.A. Polyphenol-rich Boswellia serrata gum prevents cognitive impairment and insulin resistance of diabetic rats through inhibition of GSK3beta activity, oxidative stress and pro-inflammatory cytokines. Biomed. Pharmacother. 2019, 109, 281–292. [Google Scholar] [CrossRef]
- Youssef, M.M.; Abd El-Latif, H.A.; El-Yamany, M.F.; Georgy, G.S. Aliskiren and captopril improve cognitive deficits in poorly controlled STZ-induced diabetic rats via amelioration of the hippocampal P-ERK, GSK3beta, P-GSK3beta pathway. Toxicol. Appl. Pharmacol. 2020, 394, 114954. [Google Scholar] [CrossRef]
- Dusabimana, T.; Park, E.J.; Je, J.; Jeong, K.; Yun, S.P.; Kim, H.J.; Kim, H.; Park, S.W. Geniposide Improves Diabetic Nephropathy by Enhancing ULK1-Mediated Autophagy and Reducing Oxidative Stress through AMPK Activation. Int. J. Mol. Sci. 2021, 22, 1651. [Google Scholar] [CrossRef] [PubMed]
- Datusalia, A.K.; Sharma, S.S. Amelioration of diabetes-induced cognitive deficits by GSK-3beta inhibition is attributed to modulation of neurotransmitters and neuroinflammation. Mol. Neurobiol. 2014, 50, 390–405. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Lee, K.S.; Lee, G.Y.; Jin, H.; Durrance, E.S.; Park, H.S.; Choi, S.H.; Park, K.S.; Kim, Y.B.; Jang, H.C.; et al. Anti-diabetic efficacy of KICG1338, a novel glycogen synthase kinase-3beta inhibitor, and its molecular characterization in animal models of type 2 diabetes and insulin resistance. Mol. Cell Endocrinol. 2015, 409, 1–10. [Google Scholar] [CrossRef]
- Mechlovich, D.; Amit, T.; Bar-Am, O.; Weinreb, O.; Youdim, M.B. Molecular targets of the multifunctional iron-chelating drug, M30, in the brains of mouse models of type 2 diabetes mellitus. Br. J. Pharmacol. 2014, 171, 5636–5649. [Google Scholar] [CrossRef]
- Yu, W.; Wu, J.; Cai, F.; Xiang, J.; Zha, W.; Fan, D.; Guo, S.; Ming, Z.; Liu, C. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS ONE 2012, 7, e52013. [Google Scholar] [CrossRef] [PubMed]
- Pitasi, C.L.; Liu, J.; Gausseres, B.; Pommier, G.; Delangre, E.; Armanet, M.; Cattan, P.; Megarbane, B.; Hanak, A.S.; Maouche, K.; et al. Implication of glycogen synthase kinase 3 in diabetes-associated islet inflammation. J. Endocrinol. 2020, 244, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Muneeb, M.; Mansou, S.M.; Saleh, S.; Mohammed, R.A. Vitamin D and rosuvastatin alleviate type-II diabetes-induced cognitive dysfunction by modulating neuroinflammation and canonical/noncanonical Wnt/beta-catenin signaling. PLoS ONE 2022, 17, e0277457. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Tian, S.; Bao, Y.; Zhang, J.; Xu, D.; Piao, M. Protective effect of salidroside on streptozotocin-induced diabetic nephropathy by inhibiting oxidative stress and inflammation in rats via the Akt/GSK-3beta signalling pathway. Pharm. Biol. 2022, 60, 1732–1738. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Y.; Lu, J.; Xu, R.; Yang, J.Z.; Meng, X.R.; Ou-Yang, X.N.; Yan, Q.Y.; Nie, R.F.; Zhao, T.; Chen, Y.D.; et al. FX5, a non-steroidal glucocorticoid receptor antagonist, ameliorates diabetic cognitive impairment in mice. Acta Pharmacol. Sin. 2022, 43, 2495–2510. [Google Scholar] [CrossRef]
- O’Leary, O.; Nolan, Y. Glycogen synthase kinase-3 as a therapeutic target for cognitive dysfunction in neuropsychiatric disorders. CNS Drugs 2015, 29, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Desse, S.; Martinez, A.; Worthen, R.J.; Jope, R.S.; Beurel, E. TNFalpha disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav. Immun. 2018, 69, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, D.; Bettendorff, L.; Gorlova, A.; Olkhovik, A.; Kalueff, A.V.; Ponomarev, E.D.; Inozemtsev, A.; Chekhonin, V.; Lessmall es, C.K.P.; Anthony, D.C.; et al. Neuroinflammation and aberrant hippocampal plasticity in a mouse model of emotional stress evoked by exposure to ultrasound of alternating frequencies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 90, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Zeng, M.J.; Zhou, L.P.; Li, Y.Q.; Zhao, F.; Shang, Z.Y.; Deng, X.Y.; Ma, Z.Q.; Fu, Q.; Ma, S.P.; et al. Baicalin exerts neuroprotective effects via inhibiting activation of GSK3beta/NF-kappaB/NLRP3 signal pathway in a rat model of depression. Int. Immunopharmacol. 2018, 64, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Yan, P.; Zhu, L.; Yang, H.; Zhao, Y.; Kirby, B.P.; Waddington, J.L.; Zhen, X. Dihydromyricetin exerts a rapid antidepressant-like effect in association with enhancement of BDNF expression and inhibition of neuroinflammation. Psychopharmacology 2018, 235, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Ebeid, M.A.; Habib, M.Z.; Mohamed, A.M.; Faramawy, Y.E.; Saad, S.S.T.; El-Kharashi, O.A.; El Magdoub, H.M.; Abd-Alkhalek, H.A.; Aboul-Fotouh, S.; Abdel-Tawab, A.M. Cognitive effects of the GSK-3 inhibitor “lithium” in LPS/chronic mild stress rat model of depression: Hippocampal and cortical neuroinflammation and tauopathy. Neurotoxicology 2021, 83, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Zhang, K.; Li, Y.; Tang, Z.; Zheng, R.; Ma, Y.; Chen, Z.; Lei, N.; Xiong, L.; Guo, P.; et al. Lipopolysaccharide-induced depression-like model in mice: Meta-analysis and systematic evaluation. Front. Immunol. 2023, 14, 1181973. [Google Scholar] [CrossRef] [PubMed]
- Mello, B.S.F.; Chaves Filho, A.J.M.; Custodio, C.S.; Rodrigues, P.A.; Carletti, J.V.; Vasconcelos, S.M.M.; Sousa, F.C.F.; Sanders, L.L.O.; Macedo, D.S. Doxycycline at subantimicrobial dose combined with escitalopram reverses depressive-like behavior and neuroinflammatory hippocampal alterations in the lipopolysaccharide model of depression. J. Affect. Disord. 2021, 292, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, Y.; Xue, B.; Yuan, P. Low-dose Esketamine suppresses NLRP3-mediated apoptotic and pyroptotic cell death in microglial cells to ameliorate LPS-induced depression via ablating GSK-3beta. Behav. Brain Res. 2024, 459, 114782. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Wang, Y.; Xu, W.; Liu, Y.; Chen, H.; Yu, Z. KCa3.1 deficiency attenuates neuroinflammation by regulating an astrocyte phenotype switch involving the PI3K/AKT/GSK3beta pathway. Neurobiol. Dis. 2019, 132, 104588. [Google Scholar] [CrossRef]
- Cole, A.R. Glycogen synthase kinase 3 substrates in mood disorders and schizophrenia. FEBS J. 2013, 280, 5213–5227. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Lauretti, E.; Dincer, O.; Pratico, D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118664. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Buee, L.; Blum, D. Tau and neuroinflammation: What impact for Alzheimer’s Disease and Tauopathies? Biomed. J. 2018, 41, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M. The ordered assembly of tau is the gain-of-toxic function that causes human tauopathies. Alzheimers Dement. 2016, 12, 1040–1050. [Google Scholar] [CrossRef]
- Zaki, M.O.; El-Desouky, S.; Elsherbiny, D.A.; Salama, M.; Azab, S.S. Glimepiride mitigates tauopathy and neuroinflammation in P301S transgenic mice: Role of AKT/GSK3beta signaling. Inflammopharmacology 2022, 30, 1871–1890. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.; Stack, C.; Elipenahli, C.; Jainuddin, S.; Gerges, M.; Starkova, N.; Calingasan, N.Y.; Yang, L.; Tampellini, D.; Starkov, A.A.; et al. Bezafibrate administration improves behavioral deficits and tau pathology in P301S mice. Hum. Mol. Genet. 2012, 21, 5091–5105. [Google Scholar] [CrossRef] [PubMed]
- Di Lauro, C.; Bianchi, C.; Sebastian-Serrano, A.; Soria-Tobar, L.; Alvarez-Castelao, B.; Nicke, A.; Diaz-Hernandez, M. P2X7 receptor blockade reduces tau induced toxicity, therapeutic implications in tauopathies. Prog. Neurobiol. 2022, 208, 102173. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhang, Y.D.; Chen, Q.; Gao, Q.; Zhu, X.C.; Zhou, J.S.; Shi, J.Q.; Lu, H.; Tan, L.; Yu, J.T. TREM2 modifies microglial phenotype and provides neuroprotection in P301S tau transgenic mice. Neuropharmacology 2016, 105, 196–206. [Google Scholar] [CrossRef]
- Martin-Moreno, A.M.; Brera, B.; Spuch, C.; Carro, E.; Garcia-Garcia, L.; Delgado, M.; Pozo, M.A.; Innamorato, N.G.; Cuadrado, A.; de Ceballos, M.L. Prolonged oral cannabinoid administration prevents neuroinflammation, lowers beta-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J. Neuroinflamm. 2012, 9, 8. [Google Scholar] [CrossRef]
- Ricke, K.M.; Cruz, S.A.; Qin, Z.; Farrokhi, K.; Sharmin, F.; Zhang, L.; Zasloff, M.A.; Stewart, A.F.R.; Chen, H.H. Neuronal Protein Tyrosine Phosphatase 1B Hastens Amyloid beta-Associated Alzheimer’s Disease in Mice. J. Neurosci. 2020, 40, 1581–1593. [Google Scholar] [CrossRef]
- Jiang, M.; Zhang, X.; Yan, X.; Mizutani, S.; Kashiwazaki, H.; Ni, J.; Wu, Z. GSK3beta is involved in promoting Alzheimer’s disease pathologies following chronic systemic exposure to Porphyromonas gingivalis lipopolysaccharide in amyloid precursor protein(NL-F/NL-F) knock-in mice. Brain Behav. Immun. 2021, 98, 1–12. [Google Scholar] [CrossRef]
- Dionisio, P.A.; Amaral, J.D.; Ribeiro, M.F.; Lo, A.C.; D’Hooge, R.; Rodrigues, C.M. Amyloid-beta pathology is attenuated by tauroursodeoxycholic acid treatment in APP/PS1 mice after disease onset. Neurobiol. Aging 2015, 36, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liang, Z.; Li, Y.; Zhi, Y.; Yi, L.; Bai, S.; Forest, K.H.; Nichols, R.A.; Dong, Y.; Li, Q.X. Isoorientin, a GSK-3beta inhibitor, rescues synaptic dysfunction, spatial memory deficits and attenuates pathological progression in APP/PS1 model mice. Behav. Brain Res. 2021, 398, 112968. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gonzalez, R.; Pascual, C.; Antequera, D.; Bolos, M.; Redondo, M.; Perez, D.I.; Perez-Grijalba, V.; Krzyzanowska, A.; Sarasa, M.; Gil, C.; et al. Phosphodiesterase 7 inhibitor reduced cognitive impairment and pathological hallmarks in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2133–2145. [Google Scholar] [CrossRef] [PubMed]
- Sy, M.; Kitazawa, M.; Medeiros, R.; Whitman, L.; Cheng, D.; Lane, T.E.; Laferla, F.M. Inflammation induced by infection potentiates tau pathological features in transgenic mice. Am. J. Pathol. 2011, 178, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kosaraju, J.; Tam, K.Y. Anti-neuroinflammatory effects of SLOH in Abeta-induced BV-2 microglial cells and 3xTg-AD mice involve the inhibition of GSK-3beta. Neurosci. Lett. 2018, 687, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Xia, D.; Cai, J.; Gu, B.; Liu, X.; Friedman, V.; Liu, Q.S.; Zhao, L. Treadmill Exercise Reduces Neuroinflammation, Glial Cell Activation and Improves Synaptic Transmission in the Prefrontal Cortex in 3 × Tg-AD Mice. Int. J. Mol. Sci. 2022, 23, 12655. [Google Scholar] [CrossRef] [PubMed]
- Grieb, P. Intracerebroventricular Streptozotocin Injections as a Model of Alzheimer’s Disease: In Search of a Relevant Mechanism. Mol. Neurobiol. 2016, 53, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Abdel Rasheed, N.O.; El Sayed, N.S.; El-Khatib, A.S. Targeting central beta2 receptors ameliorates streptozotocin-induced neuroinflammation via inhibition of glycogen synthase kinase3 pathway in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.A.; Makboul, R.M.; El-Mokhtar, M.A.; Abdel-Rahman, E.A.; Ahmed, I.A.; Nicola, M.A. Terpenoid-rich Elettaria cardamomum extract prevents Alzheimer-like alterations induced in diabetic rats via inhibition of GSK3beta activity, oxidative stress and pro-inflammatory cytokines. Cytokine 2019, 113, 405–416. [Google Scholar] [CrossRef]
- Siddiqui, N.; Ali, J.; Parvez, S.; Najmi, A.K.; Akhtar, M. Neuroprotective Role of DPP-4 Inhibitor Linagliptin Against Neurodegeneration, Neuronal Insulin Resistance and Neuroinflammation Induced by Intracerebroventricular Streptozotocin in Rat Model of Alzheimer’s Disease. Neurochem. Res. 2023, 48, 2714–2730. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Xu, Q.Q.; Xian, Y.F.; Lin, Z.X. Sulforaphene Ameliorates Neuroinflammation and Hyperphosphorylated Tau Protein via Regulating the PI3K/Akt/GSK-3beta Pathway in Experimental Models of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2020, 2020, 4754195. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Chen, Y.; Mao, Y.F.; Zheng, T.; Jiang, Y.; Yan, Y.; Yin, X.; Zhang, B. Long-term treatment with intranasal insulin ameliorates cognitive impairment, tau hyperphosphorylation, and microglial activation in a streptozotocin-induced Alzheimer’s rat model. Sci. Rep. 2017, 7, 45971. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Martin, M.; Jurado-Arjona, J.; Fuster-Matanzo, A.; Hernandez, F.; Rabano, A.; Avila, J. Peripherally triggered and GSK-3beta-driven brain inflammation differentially skew adult hippocampal neurogenesis, behavioral pattern separation and microglial activation in response to ibuprofen. Transl. Psychiatry 2014, 4, e463. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022, 22, 657–673. [Google Scholar] [CrossRef] [PubMed]
- Samim Khan, S.; Janrao, S.; Srivastava, S.; Bala Singh, S.; Vora, L.; Kumar Khatri, D. GSK-3beta: An exuberating neuroinflammatory mediator in Parkinson’s disease. Biochem. Pharmacol. 2023, 210, 115496. [Google Scholar] [CrossRef] [PubMed]
- Lauterbach, E.C.; Fontenelle, L.F.; Teixeira, A.L. The neuroprotective disease-modifying potential of psychotropics in Parkinson’s disease. Park. Dis. 2012, 2012, 753548. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Gutierrez, M.T.; Serrano-Garcia, N.; Orozco-Ibarra, M. Rotenone-Induced Model of Parkinson’s Disease: Beyond Mitochondrial Complex I Inhibition. Mol. Neurobiol. 2023, 60, 1929–1948. [Google Scholar] [CrossRef] [PubMed]
- Hedya, S.A.; Safar, M.M.; Bahgat, A.K. Cilostazol Mediated Nurr1 and Autophagy Enhancement: Neuroprotective Activity in Rat Rotenone PD Model. Mol. Neurobiol. 2018, 55, 7579–7587. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hong, J.; Wu, Y.; Liu, G.; Yu, W.; Chen, L. Seipin deficiency in mice causes loss of dopaminergic neurons via aggregation and phosphorylation of alpha-synuclein and neuroinflammation. Cell Death Dis. 2018, 9, 440. [Google Scholar] [CrossRef]
- Qian, Y.; Yin, J.; Hong, J.; Li, G.; Zhang, B.; Liu, G.; Wan, Q.; Chen, L. Neuronal seipin knockout facilitates Abeta-induced neuroinflammation and neurotoxicity via reduction of PPARgamma in hippocampus of mouse. J. Neuroinflamm. 2016, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, M.; Mat Taib, C.N. MPTP-induced mouse model of Parkinson’s disease: A promising direction of therapeutic strategies. Bosn. J. Basic Med. Sci. 2021, 21, 422–433. [Google Scholar] [PubMed]
- Lee, S.; Hong, D.G.; Yang, S.; Kim, J.; Baek, M.; Kim, S.; Thirumalai, D.; Chung, H.Y.; Chang, S.C.; Lee, J. Anti-Inflammatory Effect of IKK-Activated GSK-3beta Inhibitory Peptide Prevented Nigrostriatal Neurodegeneration in the Rodent Model of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 998. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shen, C.; Li, H.; Tong, J.; Wu, Y.; Ma, Y.; Wang, J.; Wang, Z.; Li, Q.; Zhang, X.; et al. NOD-like receptor NLRC5 promotes neuroinflammation and inhibits neuronal survival in Parkinson’s disease models. J. Neuroinflamm. 2023, 20, 96. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Ju, S.; Yoo, J.W.; Ha, N.C.; Jung, Y. Design and evaluation of IKK-activated GSK3beta inhibitory peptide as an inflammation-responsive anti-colitic therapeutic. Biomater. Sci. 2021, 9, 6584–6596. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, M.; Cianciulli, A.; Calvello, R.; Porro, C.; De Nuccio, F.; Kashyrina, M.; Miraglia, A.; Lofrumento, D.D.; Panaro, M.A. Ser9p-GSK3beta Modulation Contributes to the Protective Effects of Vitamin C in Neuroinflammation. Nutrients 2024, 16, 1121. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.H.; Yoo, A.R.; Chang, D.I.; Hwang, S.J.; Kim, S.H. Inhibition of GSK-3 reduces infarct volume and improves neurobehavioral functions. Biochem. Biophys. Res. Commun. 2008, 371, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, J.; Li, X.; Guo, C.; Fang, T.; Chen, Z. GSK-3beta inhibitors suppressed neuroinflammation in rat cortex by activating autophagy in ischemic brain injury. Biochem. Biophys. Res. Commun. 2011, 411, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Cui, J.; Yang, Z.; Guo, C.; Cao, J.; Xi, M.; Weng, Y.; Yin, Y.; Wang, Y.; Wei, G.; et al. Neuroprotective effect of Apelin 13 on ischemic stroke by activating AMPK/GSK-3beta/Nrf2 signaling. J. Neuroinflamm. 2019, 16, 24. [Google Scholar] [CrossRef]
- Tanioka, M.; Park, W.K.; Park, J.; Lee, J.E.; Lee, B.H. Lipid Emulsion Improves Functional Recovery in an Animal Model of Stroke. Int. J. Mol. Sci. 2020, 21, 7373. [Google Scholar] [CrossRef]
- Han, Y.; Shen, X.; Gao, Z.; Han, P.; Bi, X. Enriched environment treatment promotes neural functional recovery together with microglia polarization and remyelination after cerebral ischemia in rats. Brain Res. Bull. 2024, 209, 110912. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Y.; Zhang, X.; Zhang, Y.; Jiang, D.; Dong, X.; Deng, S.; Yang, L.; Guan, Y.; Zhu, L.; et al. Mindin is a critical mediator of ischemic brain injury in an experimental stroke model. Exp. Neurol. 2013, 247, 506–516. [Google Scholar] [CrossRef]
- Venna, V.R.; Benashski, S.E.; Chauhan, A.; McCullough, L.D. Inhibition of glycogen synthase kinase-3beta enhances cognitive recovery after stroke: The role of TAK1. Learn. Mem. 2015, 22, 336–343. [Google Scholar] [CrossRef]
- Chien, M.Y.; Chuang, C.H.; Chern, C.M.; Liou, K.T.; Liu, D.Z.; Hou, Y.C.; Shen, Y.C. Salvianolic acid A alleviates ischemic brain injury through the inhibition of inflammation and apoptosis and the promotion of neurogenesis in mice. Free Radic. Biol. Med. 2016, 99, 508–519. [Google Scholar] [CrossRef]
- Valerio, A.; Bertolotti, P.; Delbarba, A.; Perego, C.; Dossena, M.; Ragni, M.; Spano, P.; Carruba, M.O.; De Simoni, M.G.; Nisoli, E. Glycogen synthase kinase-3 inhibition reduces ischemic cerebral damage, restores impaired mitochondrial biogenesis and prevents ROS production. J. Neurochem. 2011, 116, 1148–1159. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, X.; Zhao, Y.; Zhang, L.; Bai, X.; Zhang, J.; Zhao, X.; Chen, L.; Wang, L.; Cui, L. Pretreatment by evodiamine is neuroprotective in cerebral ischemia: Up-regulated pAkt, pGSK3beta, down-regulated NF-kappaB expression, and ameliorated BBB permeability. Neurochem. Res. 2014, 39, 1612–1620. [Google Scholar] [CrossRef]
- Chern, C.M.; Lu, C.K.; Liou, K.T.; Wang, Y.H.; Tsai, K.C.; Chang, C.L.; Chang, C.C.; Shen, Y.C. Medicarpin isolated from Radix Hedysari ameliorates brain injury in a murine model of cerebral ischemia. J. Food Drug Anal. 2021, 29, 581–605. [Google Scholar] [CrossRef]
- Chern, C.M.; Wang, Y.H.; Liou, K.T.; Hou, Y.C.; Chen, C.C.; Shen, Y.C. 2-Methoxystypandrone ameliorates brain function through preserving BBB integrity and promoting neurogenesis in mice with acute ischemic stroke. Biochem. Pharmacol. 2014, 87, 502–514. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, H.Y.; Wu, B.; Cheng, C.Y.; Xiao, W.; Wang, Z.Z.; Yang, Y.Y.; Li, P.; Yang, H. Ginkgolide K attenuates neuronal injury after ischemic stroke by inhibiting mitochondrial fission and GSK-3beta-dependent increases in mitochondrial membrane permeability. Oncotarget 2017, 8, 44682–44693. [Google Scholar] [CrossRef]
- Liu, H.; Wu, X.; Luo, J.; Zhao, L.; Li, X.; Guo, H.; Bai, H.; Cui, W.; Guo, W.; Feng, D.; et al. Adiponectin peptide alleviates oxidative stress and NLRP3 inflammasome activation after cerebral ischemia-reperfusion injury by regulating AMPK/GSK-3beta. Exp. Neurol. 2020, 329, 113302. [Google Scholar] [CrossRef]
- Liu, F.; McCullough, L.D. Middle cerebral artery occlusion model in rodents: Methods and potential pitfalls. J. Biomed. Biotechnol. 2011, 2011, 464701. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Wang, J.; Xia, S.; Ran, H.; Gao, L.; Feng, C.; Gui, L.; Zhou, Z.; Yuan, J. Human umbilical cord-derived mesenchymal stem cell transplantation supplemented with curcumin improves the outcomes of ischemic stroke via AKT/GSK-3beta/beta-TrCP/Nrf2 axis. J. Neuroinflamm. 2023, 20, 49. [Google Scholar] [CrossRef]
- Jossin, Y. Reelin Functions, Mechanisms of Action and Signaling Pathways during Brain Development and Maturation. Biomolecules 2020, 10, 964. [Google Scholar] [CrossRef]
- Zhu, X.; Li, J.; You, D.; Xiao, Y.; Huang, Z.; Yu, W. Neuroprotective Effect of E3 Ubiquitin Ligase RNF8 Against Ischemic Stroke via HDAC2 Stability Reduction and Reelin-Dependent GSK3beta Inhibition. Mol. Neurobiol. 2022, 59, 4776–4790. [Google Scholar] [CrossRef]
- Wang, H.; Huang, S.; Yan, K.; Fang, X.; Abussaud, A.; Martinez, A.; Sun, H.S.; Feng, Z.P. Tideglusib, a chemical inhibitor of GSK3beta, attenuates hypoxic-ischemic brain injury in neonatal mice. Biochim. Biophys. Acta 2016, 1860, 2076–2085. [Google Scholar] [CrossRef]
- D’Angelo, B.; Ek, C.J.; Sun, Y.; Zhu, C.; Sandberg, M.; Mallard, C. GSK3beta inhibition protects the immature brain from hypoxic-ischaemic insult via reduced STAT3 signalling. Neuropharmacology 2016, 101, 13–23. [Google Scholar] [CrossRef]
- Li, L.; Xu, W.; Luo, Y.; Lao, C.; Tong, X.; Du, J.; Huang, B.; Li, D.; Chen, J.; Ye, H.; et al. Aloe polymeric acemannan inhibits the cytokine storm in mouse pneumonia models by modulating macrophage metabolism. Carbohydr. Polym. 2022, 297, 120032. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3beta-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, P.; Cui, A.; Ye, D.Y.; Xiang, M.; Chu, Y. Discovery and anti-inflammatory evaluation of benzothiazepinones (BTZs) as novel non-ATP competitive inhibitors of glycogen synthase kinase-3beta (GSK-3beta). Bioorg. Med. Chem. 2018, 26, 5479–5493. [Google Scholar] [CrossRef]
- Kapoor, A.; Shintani, Y.; Collino, M.; Osuchowski, M.F.; Busch, D.; Patel, N.S.; Sepodes, B.; Castiglia, S.; Fantozzi, R.; Bishop-Bailey, D.; et al. Protective role of peroxisome proliferator-activated receptor-beta/delta in septic shock. Am. J. Respir. Crit. Care Med. 2010, 182, 1506–1515. [Google Scholar] [CrossRef]
- Zhang, H.; Sha, J.; Feng, X.; Hu, X.; Chen, Y.; Li, B.; Fan, H. Dexmedetomidine ameliorates LPS induced acute lung injury via GSK-3beta/STAT3-NF-kappaB signaling pathway in rats. Int. Immunopharmacol. 2019, 74, 105717. [Google Scholar] [CrossRef]
- Deng, J.; Wang, X.; Qian, F.; Vogel, S.; Xiao, L.; Ranjan, R.; Park, H.; Karpurapu, M.; Ye, R.D.; Park, G.Y.; et al. Protective role of reactive oxygen species in endotoxin-induced lung inflammation through modulation of IL-10 expression. J. Immunol. 2012, 188, 5734–5740. [Google Scholar] [CrossRef]
- Li, R.; Ren, T.; Zeng, J. Mitochondrial Coenzyme Q Protects Sepsis-Induced Acute Lung Injury by Activating PI3K/Akt/GSK-3beta/mTOR Pathway in Rats. Biomed. Res. Int. 2019, 2019, 5240898. [Google Scholar] [CrossRef]
- Li, J.; Shi, J.; Li, P.; Guo, X.; Wang, T.; Liu, A. Genipin attenuates hyperoxia-induced lung injury and pulmonary hypertension via targeting glycogen synthase kinase-3 beta in neonatal rats. Nutrition 2019, 57, 237–244. [Google Scholar] [CrossRef]
- Lasbury, M.E.; Ray, C.A.; Durant, P.J.; Wang, S.H.; Zhang, C.; Liao, C.P.; Tschang, D.; Lee, C.H. Survival pathway signal transduction is reduced in alveolar macrophages during Pneumocystis pneumonia. J. Eukaryot. Microbiol. 2006, 53 (Suppl. 1), S130–S131. [Google Scholar] [CrossRef]
- Yu, Q.; Yu, X.; Zhong, X.; Ma, Y.; Wu, Y.; Bian, T.; Huang, M.; Zeng, X. Melatonin modulates airway smooth muscle cell phenotype by targeting the STAT3/Akt/GSK-3beta pathway in experimental asthma. Cell Tissue Res. 2020, 380, 129–142. [Google Scholar] [CrossRef]
- Huang, X.; Yu, H.; Xie, C.; Zhou, Y.L.; Chen, M.M.; Shi, H.L.; Tang, W.F.; Dong, J.C.; Luo, Q.L. Louki Zupa decoction attenuates the airway inflammation in acute asthma mice induced by ovalbumin through IL-33/ST2-NF-kappaB/GSK3beta/mTOR signalling pathway. Pharm. Biol. 2022, 60, 1520–1532. [Google Scholar] [CrossRef]
- Baarsma, H.A.; Bos, S.; Meurs, H.; Visser, K.H.; Smit, M.; Schols, A.M.; Langen, R.C.; Kerstjens, H.A.; Gosens, R. Pharmacological inhibition of GSK-3 in a guinea pig model of LPS-induced pulmonary inflammation: I. Effects on lung remodeling and pathology. Respir. Res. 2013, 14, 113. [Google Scholar] [CrossRef]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, Z.; Xin, Z.; Yang, Y.; Yu, Y.; Cui, J.; Liu, H.; Chen, F. Glycogen synthase kinase-3beta: A promising candidate in the fight against fibrosis. Theranostics 2020, 10, 11737–11753. [Google Scholar] [CrossRef] [PubMed]
- MadanKumar, P.; NaveenKumar, P.; Manikandan, S.; Devaraj, H.; Niranjali Devaraj, S. Morin ameliorates chemically induced liver fibrosis in vivo and inhibits stellate cell proliferation in vitro by suppressing Wnt/beta-catenin signaling. Toxicol. Appl. Pharmacol. 2014, 277, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Uhm, Y.K.; Lim, Y.J.; Yim, S.V. Human umbilical cord blood-derived mesenchymal stem cells improve glucose homeostasis in rats with liver cirrhosis. Int. J. Oncol. 2011, 39, 137–143. [Google Scholar]
- Liu, W.; Li, J.; Cai, Y.; Wu, Q.; Pan, Y.; Chen, Y.; Chen, Y.; Zheng, X.; Li, W.; Zhang, X.; et al. Hepatic IGF-1R overexpression combined with the activation of GSK-3beta and FOXO3a in the development of liver cirrhosis. Life Sci. 2016, 147, 97–102. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, C.; Song, A.; Lei, X.; Li, G.; Sun, H.; Wang, X.; Geng, Z.; Shu, G.; Deng, X. Water extract of earthworms mitigates mouse liver fibrosis by potentiating hepatic LKB1/Nrf2 axis to inhibit HSC activation and hepatocyte death. J. Ethnopharmacol. 2024, 321, 117495. [Google Scholar] [CrossRef]
- Liao, Z.; Zhang, J.; Liu, B.; Yan, T.; Xu, F.; Xiao, F.; Wu, B.; Bi, K.; Jia, Y. Polysaccharide from Okra (Abelmoschus esculentus (L.) Moench) Improves Antioxidant Capacity via PI3K/AKT Pathways and Nrf2 Translocation in a Type 2 Diabetes Model. Molecules 2019, 24, 1906. [Google Scholar] [CrossRef]
- Cheng, X.; Ma, X.; Zhu, Q.; Song, D.; Ding, X.; Li, L.; Jiang, X.; Wang, X.; Tian, R.; Su, H.; et al. Pacer Is a Mediator of mTORC1 and GSK3-TIP60 Signaling in Regulation of Autophagosome Maturation and Lipid Metabolism. Mol. Cell 2019, 73, 788–802.e787. [Google Scholar] [CrossRef]
- Chen, H.J.; Liu, J. Actein ameliorates hepatic steatosis and fibrosis in high fat diet-induced NAFLD by regulation of insulin and leptin resistant. Biomed. Pharmacother. 2018, 97, 1386–1396. [Google Scholar] [CrossRef]
- Zhuang, S.; Hua, X.; He, K.; Zhou, T.; Zhang, J.; Wu, H.; Ma, X.; Xia, Q.; Zhang, J. Inhibition of GSK-3beta induces AP-1-mediated osteopontin expression to promote cholestatic liver fibrosis. FASEB J. 2018, 32, 4494–4503. [Google Scholar] [CrossRef]
- Du, C.; Dong, J.; Wang, Q.; Xu, C.; Feng, S.; Feng, R.; Lv, X.; Li, J.; Zhang, L.; Huang, C.; et al. Hastatoside attenuatescarbon tetrachloride-induced liver fibrosis by targeting glycogen synthase kinase-3beta. Phytomedicine 2023, 109, 154585. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, J.; Yue, H.; Du, C.; Xiao, Z.; Zhao, W.; Li, N.; Wang, X.; Liu, X.; Li, Y.; et al. Glycogen synthase kinase-3beta promotes radiation-induced lung fibrosis by regulating beta-catenin/lin28 signaling network to determine type II alveolar stem cell transdifferentiation state. FASEB J. 2020, 34, 12466–12480. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Z.; Yan, Z.; Liang, X.; Liu, X.; Liu, Y.; Wang, P.; Bai, C.; Gu, Y.; Zhou, P.K. MiRNA-155-5p inhibits epithelium-to-mesenchymal transition (EMT) by targeting GSK-3beta during radiation-induced pulmonary fibrosis. Arch. Biochem. Biophys. 2021, 697, 108699. [Google Scholar] [CrossRef]
- Boren, J.; Shryock, G.; Fergis, A.; Jeffers, A.; Owens, S.; Qin, W.; Koenig, K.B.; Tsukasaki, Y.; Komatsu, S.; Ikebe, M.; et al. Inhibition of Glycogen Synthase Kinase 3beta Blocks Mesomesenchymal Transition and Attenuates Streptococcus pneumonia-Mediated Pleural Injury in Mice. Am. J. Pathol. 2017, 187, 2461–2472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Shao, R.; Li, W. Cdc42-interacting protein 4 silencing relieves pulmonary fibrosis in STZ-induced diabetic mice via the Wnt/GSK-3beta/beta-catenin pathway. Exp. Cell Res. 2017, 359, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Yang, Y.; Chen, S.; Peng, C.; Lavin, M.F.; Yeo, A.J.; Li, C.; Liu, X.; Guan, Y.; Du, X.; et al. Bone marrow mesenchymal stromal cells attenuate silica-induced pulmonary fibrosis potentially by attenuating Wnt/beta-catenin signaling in rats. Stem Cell Res. Ther. 2018, 9, 311. [Google Scholar] [CrossRef]
- Veening-Griffioen, D.H.; Ferreira, G.S.; van Meer, P.J.K.; Boon, W.P.C.; Gispen-de Wied, C.C.; Moors, E.H.M.; Schellekens, H. Are some animal models more equal than others? A case study on the translational value of animal models of efficacy for Alzheimer’s disease. Eur. J. Pharmacol. 2019, 859, 172524. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.K.; Jucker, M. Developing mouse models of aging: A consideration of strain differences in age-related behavioral and neural parameters. Neurobiol. Aging 1999, 20, 137–145. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Palasca, O.; Yarani, R.; Litman, T.; Anthon, C.; Groenen, M.A.M.; Stadler, P.F.; Pociot, F.; Jensen, L.J.; Gorodkin, J. Human pathways in animal models: Possibilities and limitations. Nucleic Acids Res. 2021, 49, 1859–1871. [Google Scholar] [CrossRef] [PubMed]
- Fallon, P.G. Why does work on same mouse models give different results? Nature 2008, 454, 691. [Google Scholar] [CrossRef]
- Bhat, R.V.; Andersson, U.; Andersson, S.; Knerr, L.; Bauer, U.; Sundgren-Andersson, A.K. The Conundrum of GSK3 Inhibitors: Is it the Dawn of a New Beginning? J. Alzheimers Dis. 2018, 64, S547–S554. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kühl, F.; Brand, K.; Lichtinghagen, R.; Huber, R. GSK3-Driven Modulation of Inflammation and Tissue Integrity in the Animal Model. Int. J. Mol. Sci. 2024, 25, 8263. https://doi.org/10.3390/ijms25158263
Kühl F, Brand K, Lichtinghagen R, Huber R. GSK3-Driven Modulation of Inflammation and Tissue Integrity in the Animal Model. International Journal of Molecular Sciences. 2024; 25(15):8263. https://doi.org/10.3390/ijms25158263
Chicago/Turabian StyleKühl, Friederike, Korbinian Brand, Ralf Lichtinghagen, and René Huber. 2024. "GSK3-Driven Modulation of Inflammation and Tissue Integrity in the Animal Model" International Journal of Molecular Sciences 25, no. 15: 8263. https://doi.org/10.3390/ijms25158263
APA StyleKühl, F., Brand, K., Lichtinghagen, R., & Huber, R. (2024). GSK3-Driven Modulation of Inflammation and Tissue Integrity in the Animal Model. International Journal of Molecular Sciences, 25(15), 8263. https://doi.org/10.3390/ijms25158263






