Conformational Transition of Semiflexible Ring Polyelectrolyte in Tetravalent Salt Solutions: A Simple Numerical Modeling without the Effect of Twisting
Abstract
1. Introduction
2. Results and Discussion
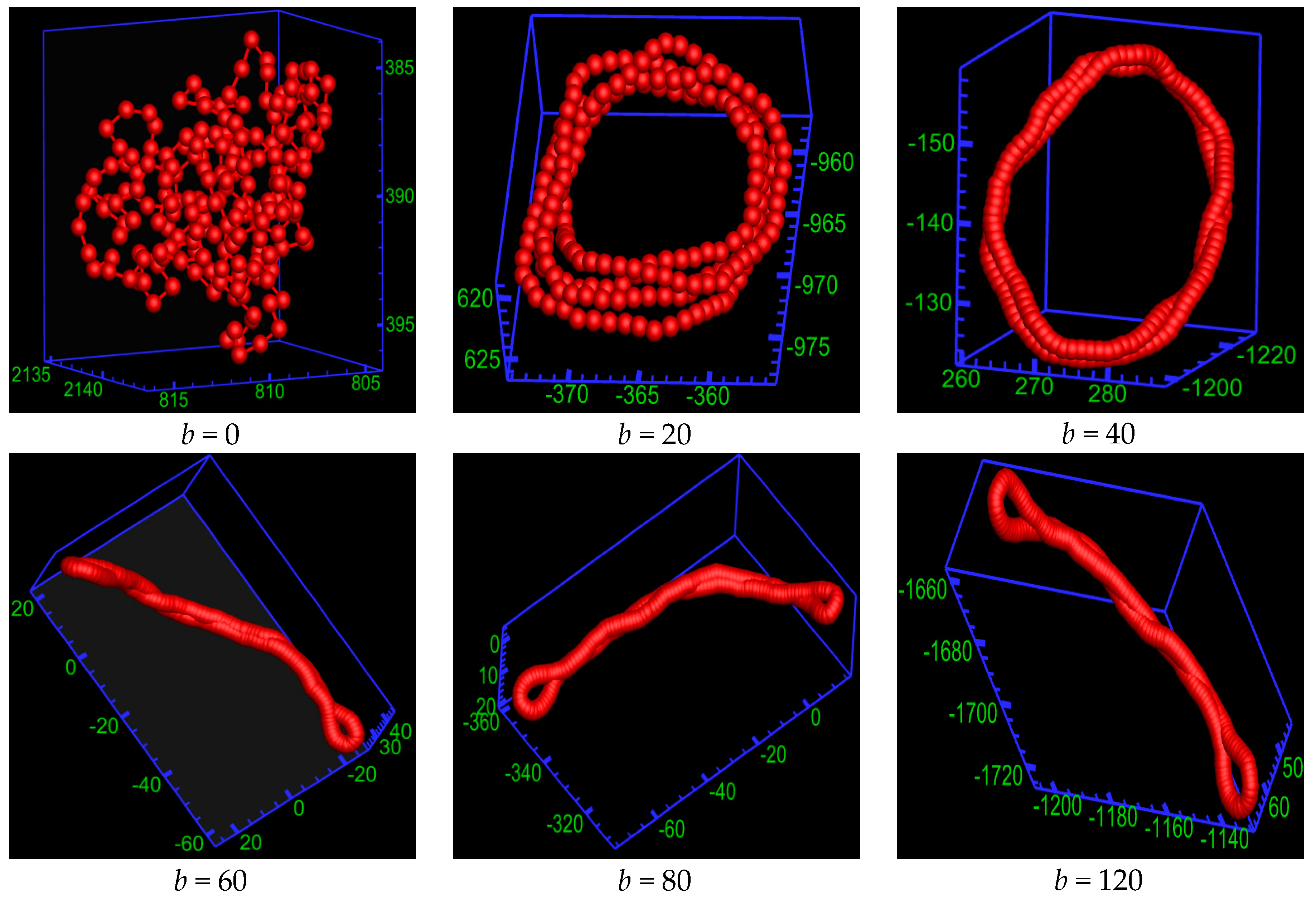
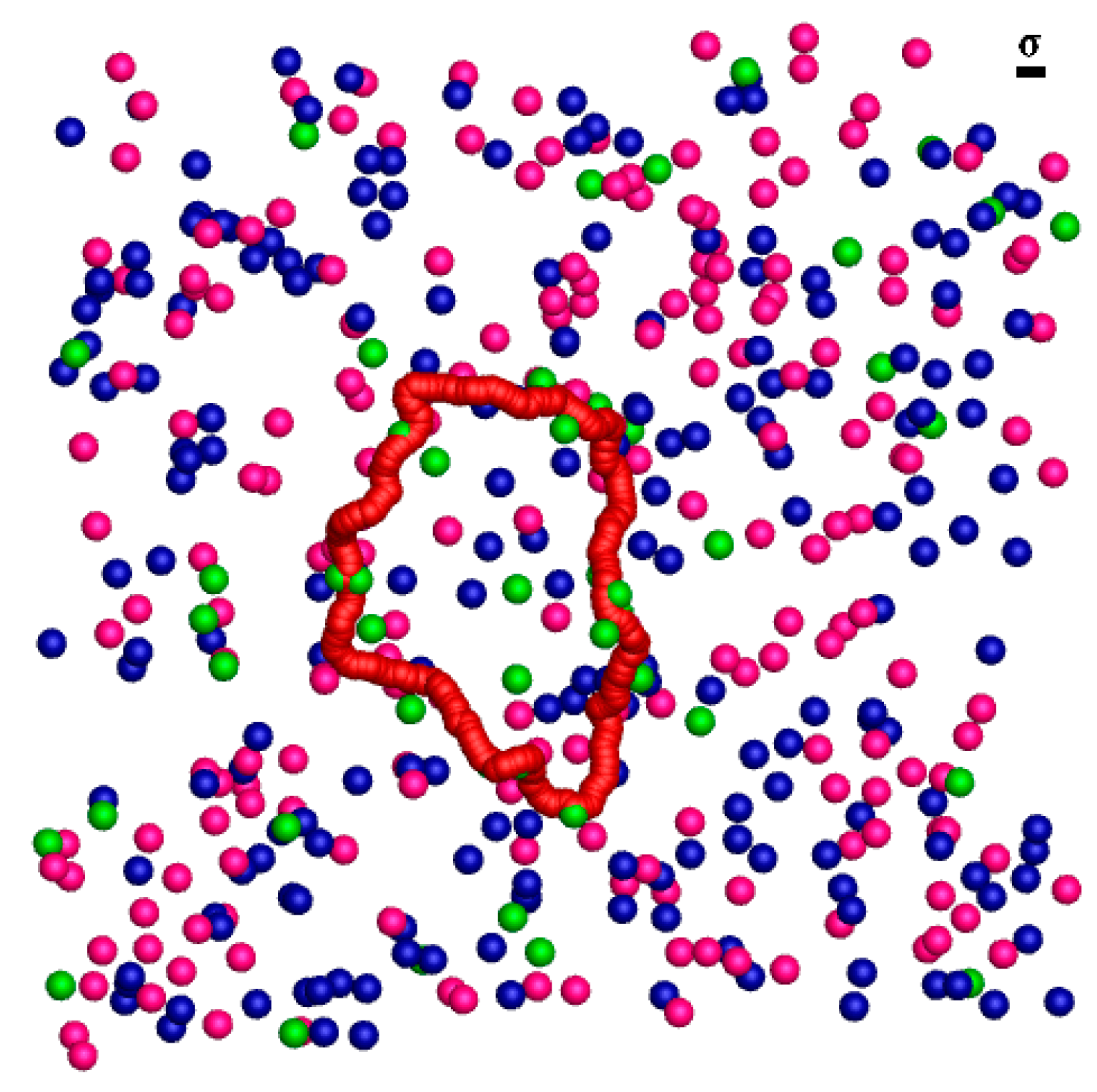
2.1. Equilibrium Phases of the Semiflexible Ring Polyelectrolyte
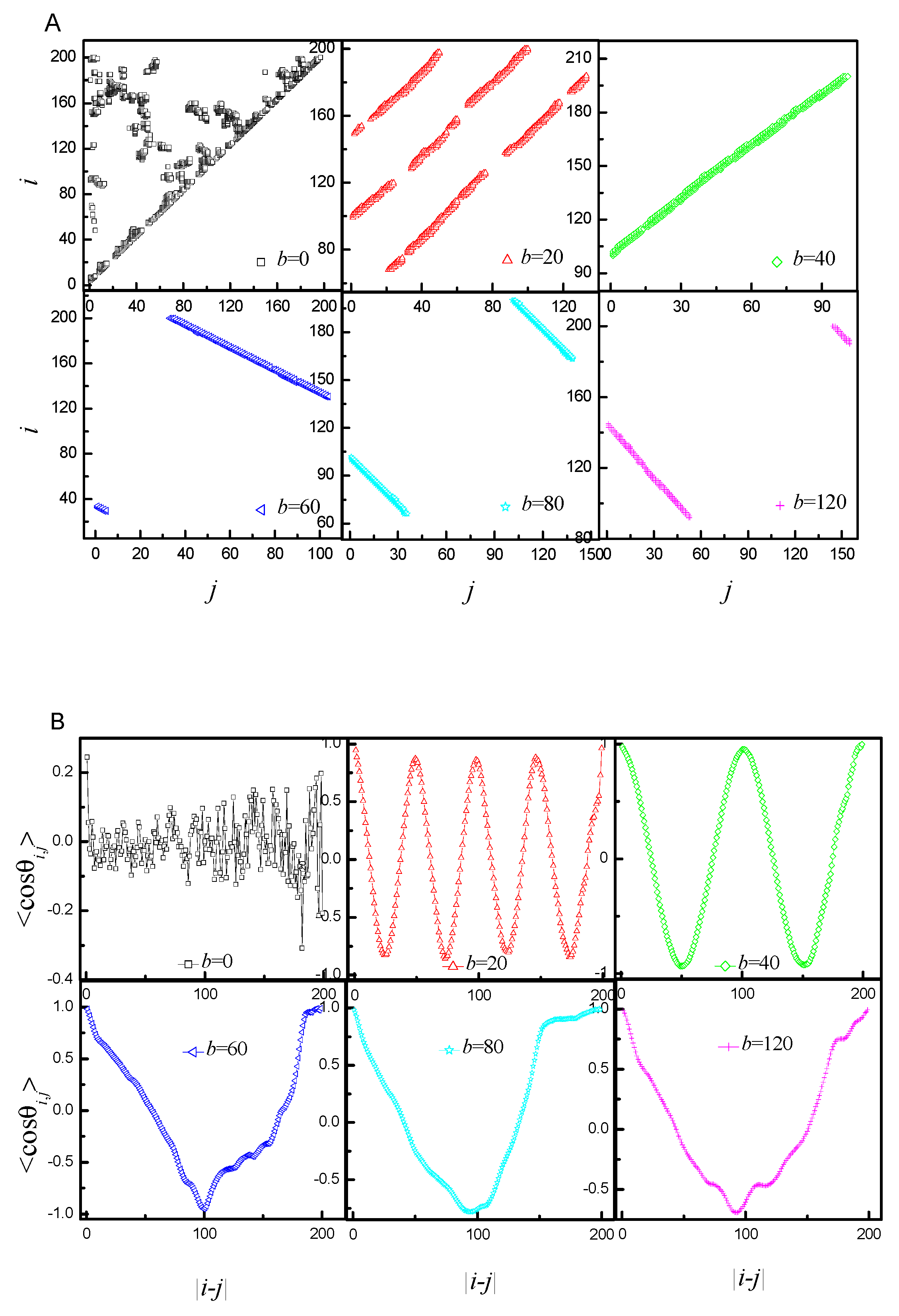
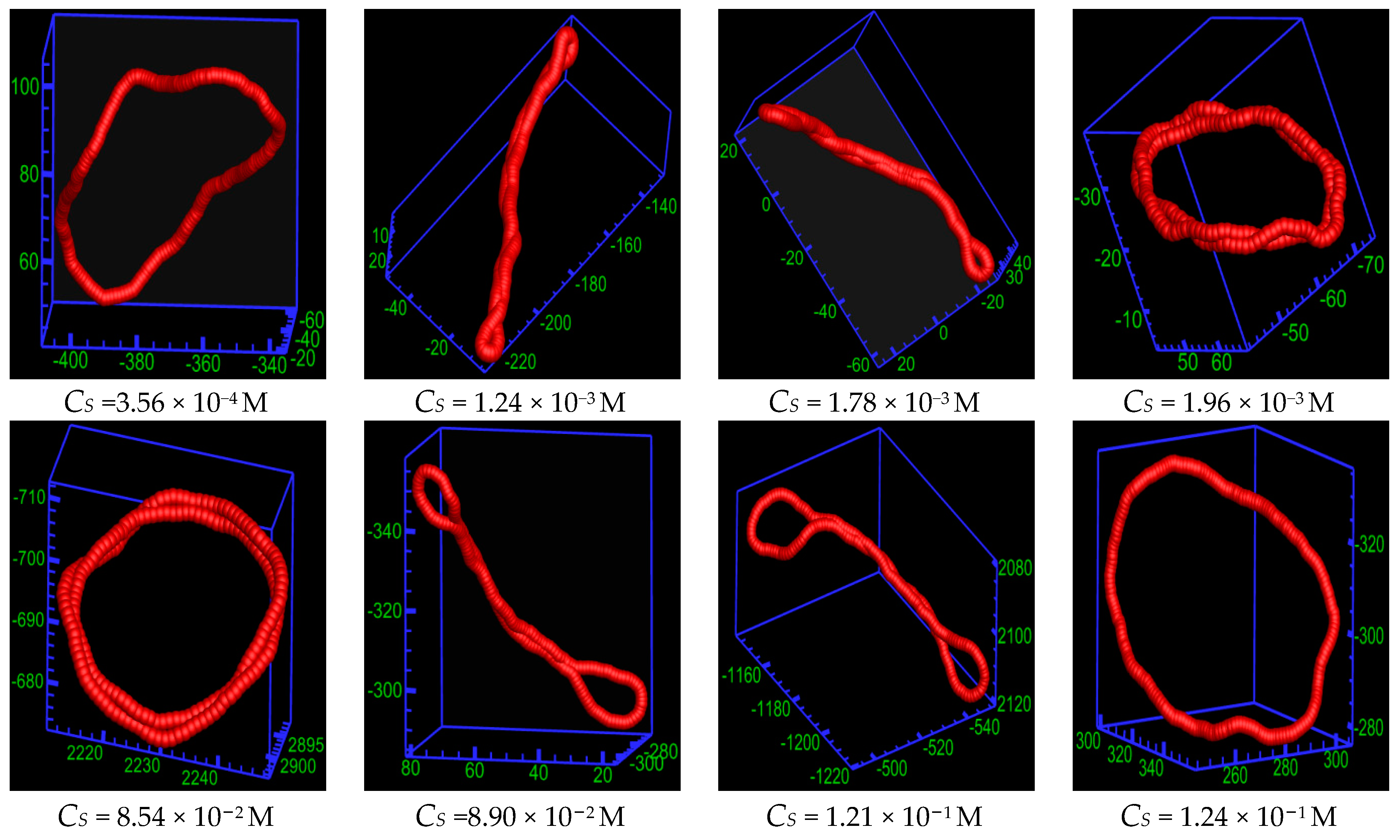
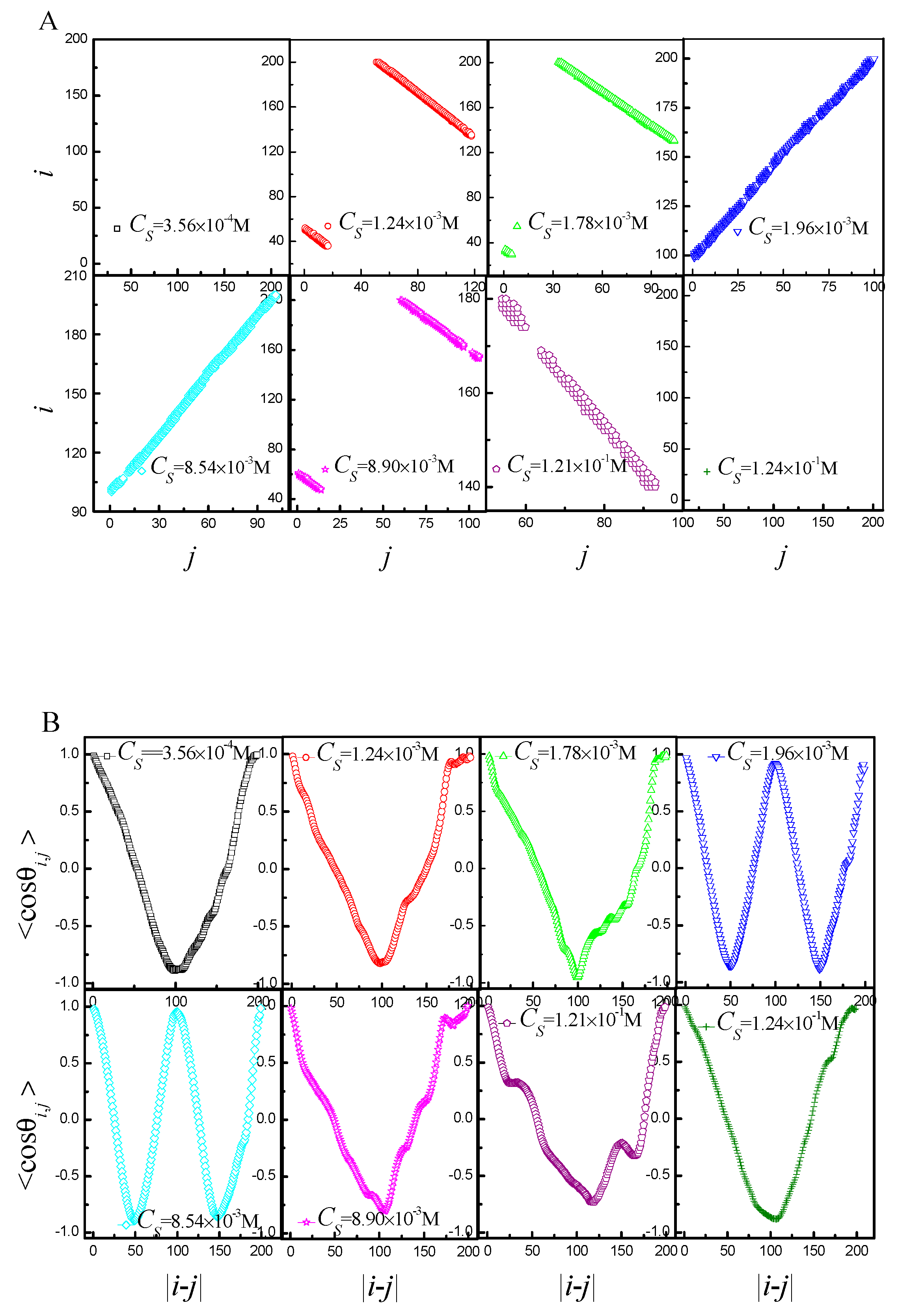
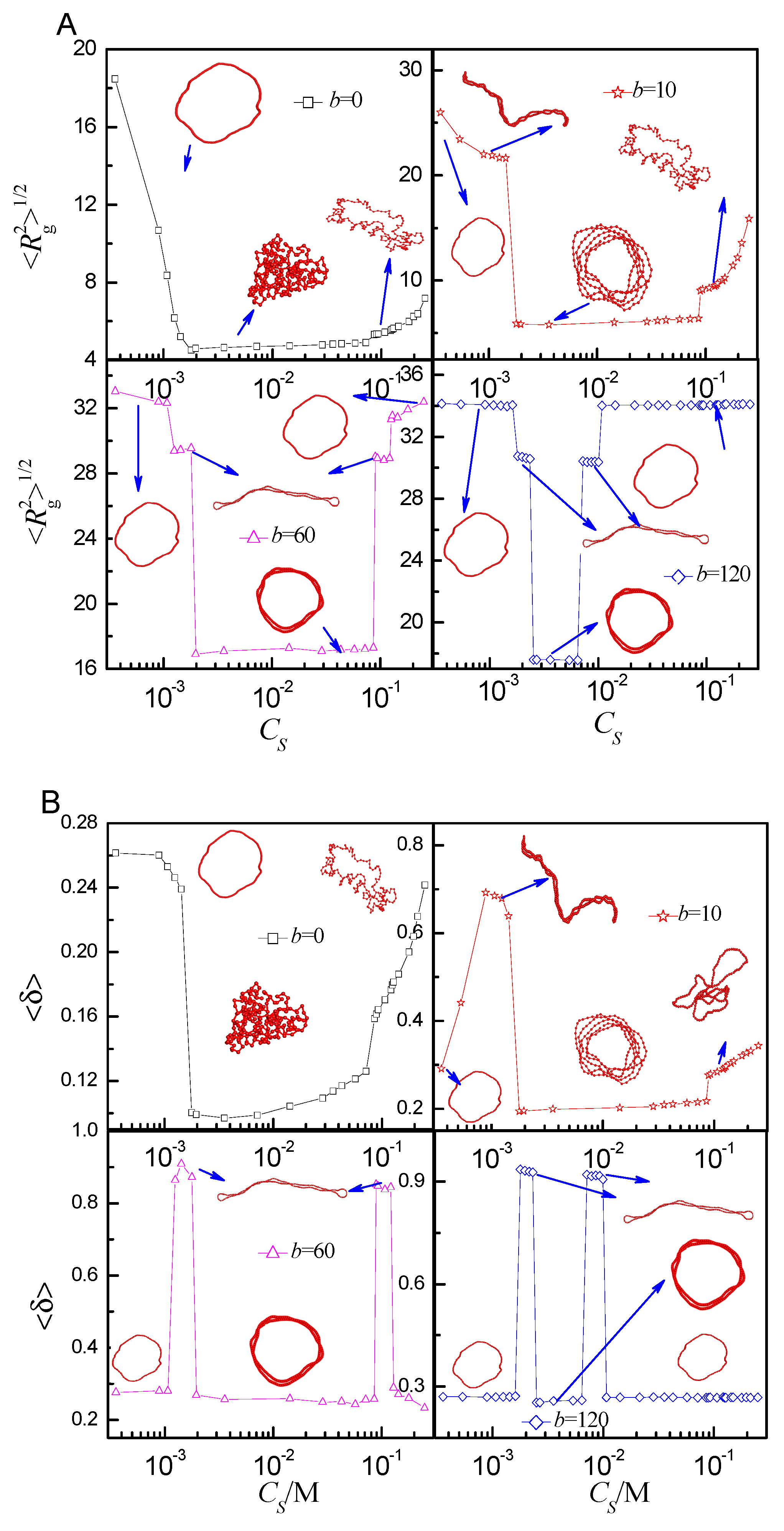
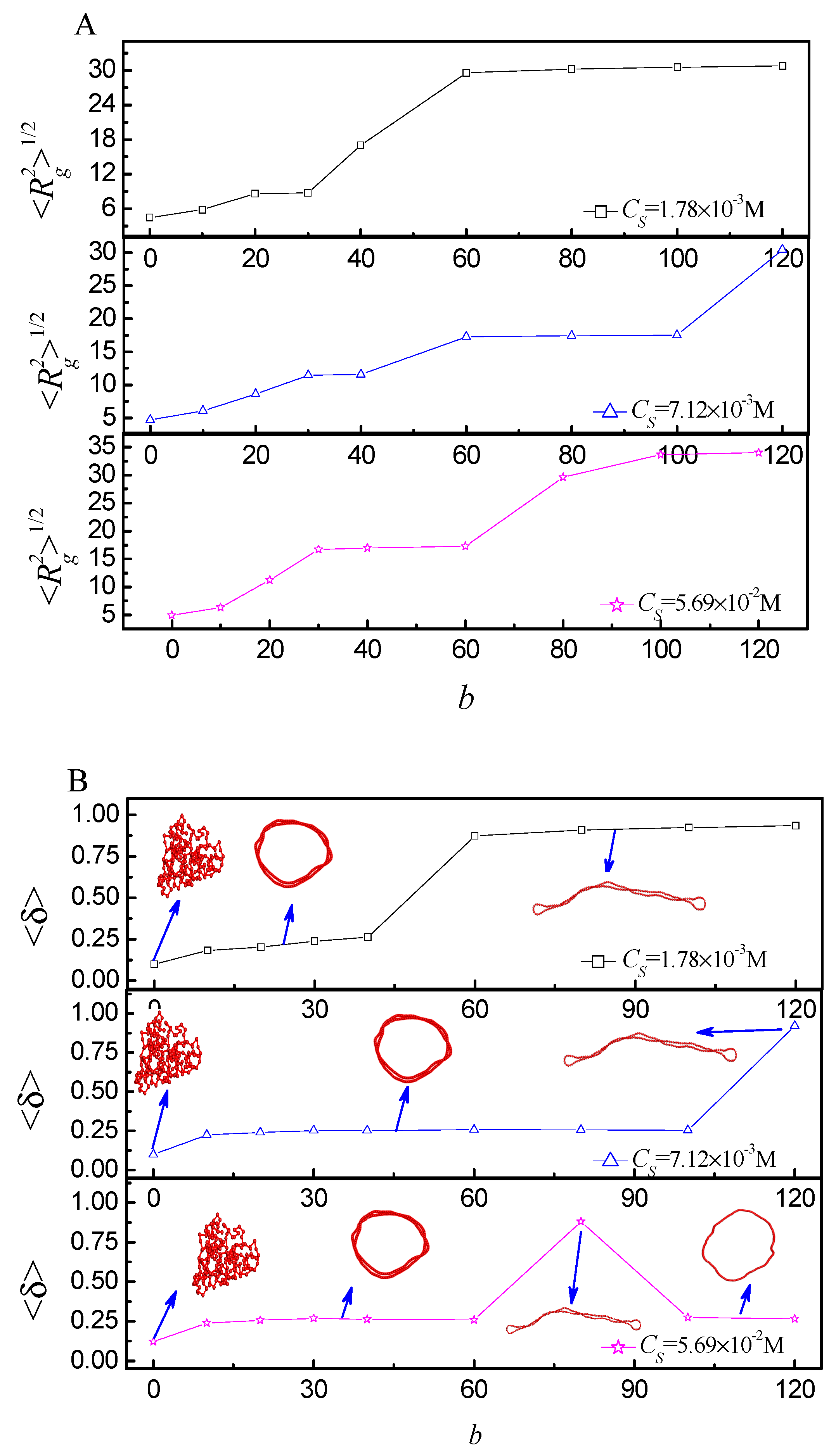
2.2. Statistical Properties of the Ring Polyelectrolyte
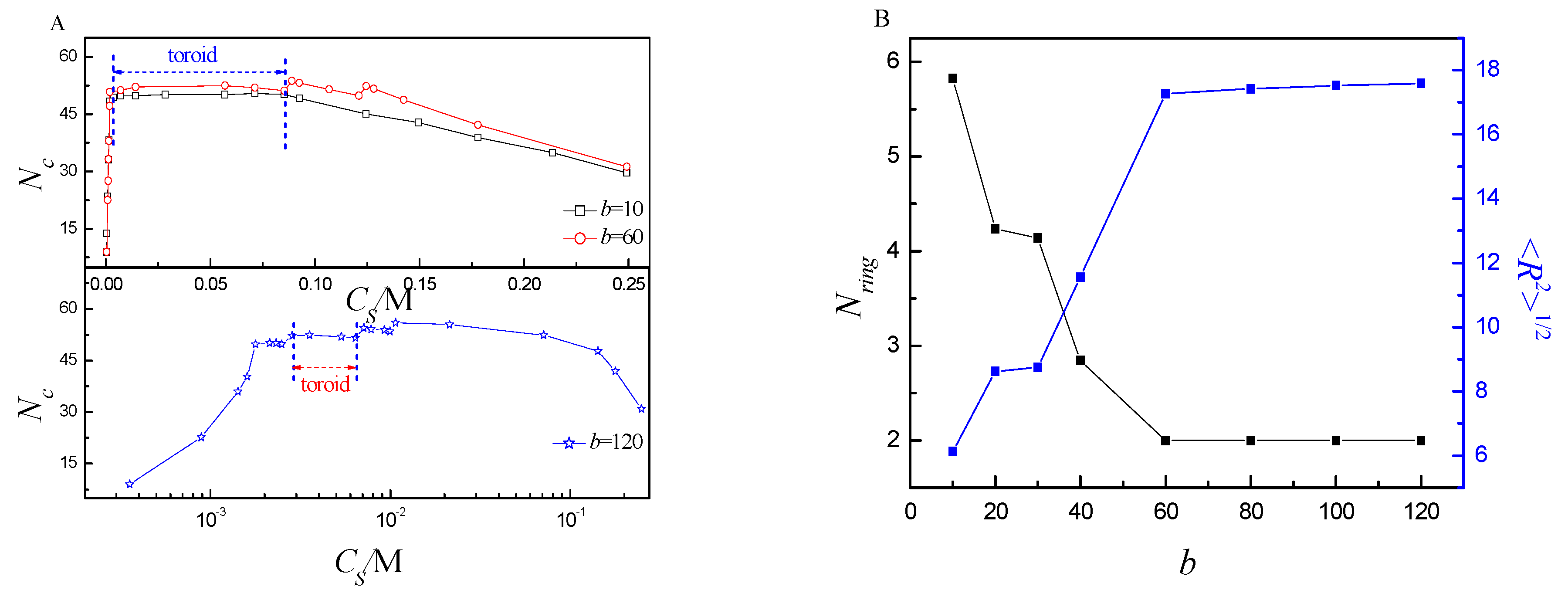
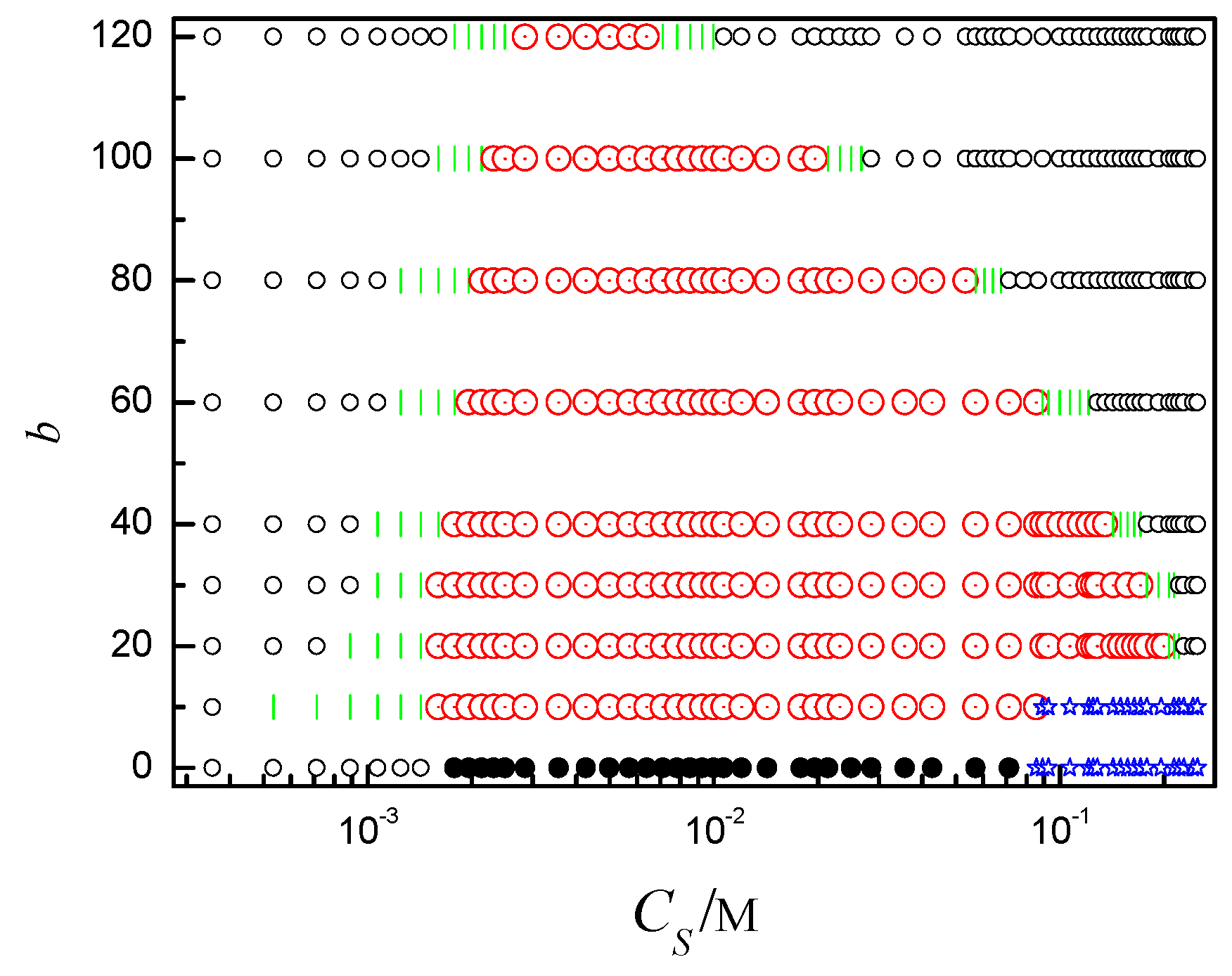
2.3. Phase Diagram of the Ring Polyelectrolyte
3. Model and Methods
3.1. The Coarse-Grained Model of the Semiflexible Ring Polyelectrolyte in Multivalent Salt Solution
3.2. Molecular Dynamics Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kayitmazer, A.B.; Seeman, D.; Minsky, B.B.; Dubin, P.L.; Xu, Y. Protein–polyelectrolyte interactions. Soft Matter 2013, 9, 2553–2583. [Google Scholar] [CrossRef]
- Dessinges, M.N.; Maier, B.; Zhang, Y.; Peliti, M.; Bensimon, D.; Croquette, V. Stretching Single Stranded DNA, a Model Polyelectrolyte. Phys. Rev. Lett. 2002, 89, 8102. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and PH-responsive polymers in drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.S.; Ding, H.M.; Ma, Y.Q. Design strategy of pH-sensitive triblock copolymer micelles for efficient cellular uptake by computer simulations. J. Phys. D Appl. Phys. 2018, 51, 124002. [Google Scholar] [CrossRef]
- Shkel, I.A.; Record, M.T. Coulombic free energy and salt ion association per phosphate of all-atom models of DNA oligomer: Dependence on oligomer size. Soft Matter 2012, 8, 9345–9355. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, V.M.; Muthukumar, M.; Wignall, G.D.; Melnichenko, Y.B. Polyelectrolyte chain dimensions and concentration fluctuations near phase boundaries. J. Chem. Phys. 2003, 119, 4085–4098. [Google Scholar] [CrossRef]
- Kanai, S.; Muthukumar, M. Phase separation kinetics of polyelectrolyte solutions. J. Chem. Phys. 2007, 127, 244908. [Google Scholar] [CrossRef] [PubMed]
- Sharratt, W.N.; O’Connell, R.; Rogers, S.E.; Lopez, C.G.; Cabral, J.T. Conformation and Phase Behavior of Sodium Carboxymethyl Cellulose in the Presence of Mono- and Divalent Salts. Macromolecules 2020, 53, 1451–1463. [Google Scholar] [CrossRef]
- Drifford, M.; Polyelectrolyte, M.D. Solutions with Multivalent Added Salts: Stability, Structure, and Dynamics. In Physical Chemistry of Polyelectrolytes, 1st ed.; Radeva, T., Ed.; Dekker: New York, NY, USA, 2001; p. 135. [Google Scholar]
- Ullner, M. Comments on the scaling behavior of flexible polyelectrolytes within the Debye-Huckel approximation. J. Phys. Chem. B 2003, 107, 8097–8110. [Google Scholar] [CrossRef]
- Volk, N.; Vollmer, D.; Schmidt, M.; Oppermann, W.; Huber, K. Conformation and phase diagrams of flexible polyelectrolytes. Adv. Polym. Sci. 2004, 166, 29–65. [Google Scholar] [CrossRef]
- Pattanayek, S.K.; Pereira, G.G. Shapes of strongly absorbed polyelectrolytes in poor solvents. Phys. Rev. E 2007, 75, 051802. [Google Scholar] [CrossRef] [PubMed]
- Dobrynin, A.V. Electrostatic persistence length of semiflexible and flexible polyelectrolytes. Macromolecules 2005, 38, 9304–9314. [Google Scholar] [CrossRef]
- Klos, J.; Pakula, T. Computer simulations of a polyelectrolyte chain with a mixture of multivalent salts. J. Phys. Condens. Matter 2005, 17, 5635–5645. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.; Shang, Y.; Liu, H.; Hu, Y.; Jiang, J. Salt effect on the interactions between gemini surfactant and oppositely charged polyelectrolyte in aqueous solution. J. Colloid Interface Sci. 2007, 306, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.J.; Hansen, J.C. Nucleosomes and the chromatin fiber. Curr. Opin. Genet. Dev. 2001, 11, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Belyi, V.A.; Muthukumar, M. Electrostatic origin of the genome packing in viruses. Proc. Natl. Acad. Sci. USA 2006, 103, 17174–17178. [Google Scholar] [CrossRef] [PubMed]
- Trotsenko, O.; Roiter, Y.; Minko, S. Conformational transitions of flexible hydrophobic polyelectrolytes in solutions of monovalent and multivalent salts and their mixtures. Langmuir 2012, 28, 6037–6044. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.G.; Richtering, W. Influence of divalent counterions on the solution rheology and supramolecular aggregation of carboxylmethyl cellulose. Cellulose 2019, 26, 1517–1534. [Google Scholar] [CrossRef]
- Horkay, F.; Basser, P.J.; Hecht, A.M.; Geissler, E. Ionic effects in semi-dilute biopolymer solutions: A small angle scattering study. J. Chem. Phys. 2018, 149, 163312. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Q.; Colby, R.H. Dynamics of associative polymers. Soft Matter 2018, 14, 2961–2977. [Google Scholar] [CrossRef] [PubMed]
- Sciortino, F.; Mir, S.H.; Pakdel, A.; Oruganti, A.; Abe, H.; Witecka, A.; Shri, D.N.A.; Rydzek, G.; Ariga, K. Saloplastics as multiresponsive ion exchange reservoir and catalyst support. J. Mater. Chem. A 2020, 8, 17713–17724. [Google Scholar] [CrossRef]
- Dressick, W.J.; Wahl, K.J.; Bassim, N.D.; Stroud, R.M.; Petrovykh, D.Y. Divalent-Anon salt effect in polyelectrolyte multilayer depositions. Langmuir 2012, 28, 15831–15843. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.S.; Pais, A.A.C.C.; Miguel, M.G.; Lindman, B. Modeling of DNA compaction by polycations. J. Chem. Phys. 2003, 119, 8150–8157. [Google Scholar] [CrossRef]
- Sarraguça, J.M.G.; Skepo, M.; Pais, A.A.C.C.; Linse, P. Structure of polyelectrolytes in 3:1 salt solutions. J. Chem. Phys. 2003, 119, 12621–12628. [Google Scholar] [CrossRef]
- Klos, J.; Pakula, T. Lattice Monte Carlo simulations of a charged polymer chain: Effect of valence and concentration of the added salt. J. Chem. Phys. 2005, 122, 134908. [Google Scholar] [CrossRef] [PubMed]
- Muthukumar, M. 50th Anniversary Perspective: A Perspective on Polyelectrolyte Solutions. Macromolecules 2017, 50, 9528–9560. [Google Scholar] [CrossRef]
- Jacobs, M.; Lopez, C.G.; Dobrynin, A.V. Quantifying the Effect of Multivalent Ions in Polyelectrolyte Solutions. Macromolecules 2021, 54, 9577–9586. [Google Scholar] [CrossRef]
- Hsiao, P.Y. Chain morphology, swelling exponent, persistence length, like-charge attraction, and charge distribution around a chain in polyelectrolyte solutions: Effects of salt concentration and ion size studied by molecular dynamics simulations. Macromolecules 2006, 39, 7125–7137. [Google Scholar] [CrossRef]
- Hsiao, P.Y. Overcharging, charge inversion, and reentrant condensation: Using highly charged polyelectrolytes in tetravalent salt solutions as an example of study. J. Phys. Chem. B 2008, 112, 7347–7350. [Google Scholar] [CrossRef]
- Wang, F.H.; Wu, Y.Y.; Tan, Z.J. Salt contribution to the flexibility of single-stranded nucleicacid offinite length. Biopolymers 2013, 99, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Jordan, E.; Roosen-Runge, F.; Leibarth, S.; Zhang, F.J.; Sztucki, M.; Hildebrandt, A.; Kohlbacher, O.; Schreiber, F. Competing salt effects on phase behavior of protein solutions: Tailoring of protein interaction by the binding of multivalent ions and charge screening. J. Phys. Chem. B 2014, 118, 11365–11374. [Google Scholar] [CrossRef] [PubMed]
- Alarcón, F.; Pére-Hernández, G.; Pérez, E.; Gama Goicochea, A.G. Coarse-grained simulations of the salt dependence of the radius of gyration of polyelectrolytes as models for biomolecules in aqueous solution. Eur. Biophys. J. 2013, 42, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.F.; Hsiao, P.Y. Role of chain stiffness on the conformation of single polyelectrolytes in salt solutions. J. Chem. Phys. 2007, 127, 064901. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.F.; Shendruk, T.N.; Slater, G.W.; Hsiao, P.Y. Structure of polyelectrolyte brushes subject to normal electric fields. Langmuir 2013, 29, 2359–2370. [Google Scholar] [CrossRef] [PubMed]
- Benkova, Z.; Cifra, P. Stiffening transition in semiflexible cyclic macromolecules. Macromol. Theory Simul. 2011, 20, 65–74. [Google Scholar] [CrossRef]
- Dey, A.; Reddy, G. Toroidal condensates by semiflexible polymer chains: Insights into nucleation, growth and packing defects. J. Phys. Chem. B 2017, 121, 9291–9301. [Google Scholar] [CrossRef] [PubMed]
- Zifferer, G. Shape asymmetry of star-branched random walks with many arms. J. Chem. Phys. 1995, 102, 3720–3726. [Google Scholar] [CrossRef]
- Zifferer, G. Shape distribution and correlation between size and shape of tetrahedral lattice chains in athermal and theta systems. J. Chem. Phys. 1998, 109, 3691–3698. [Google Scholar] [CrossRef]
- Manning, G.S. Limiting laws and counterion condensation in polyelectrolyte solutions I. colligative properties. J. Chem. Phys. 1969, 51, 924–933. [Google Scholar] [CrossRef]
- Bloomfield, V.A. DNA Condensation by Multivalent Cations. Biopolymers 1997, 44, 269–282. [Google Scholar] [CrossRef]
- Schiessel, H.; Pincus, P. Counterion-Condensation-Induced Collapse of Highly Charged Polyelectrolytes. Macromolecules 1998, 31, 7953–7959. [Google Scholar] [CrossRef]
- Golan, R.; Pietrasanta, L.L.; Hsieh, W.; Hansma, H.G. DNA Toroids: Stages in Condensation. Biochemistry 1999, 38, 14069–14076. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Lin, W.L.; Chai, A.H.; Lu, D.; Kang, N.Q.; Zhang, L.X. Conformational behavior of single circular semiflexible polyelectrolyte in presence of multivalent counterions. Chin. J. Polym. Sci. 2023, 41, 448–458. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Tom, A.M.; Rajesh, R.; Vemparala, S. Aggregation dynamics of rigid polyelectrolytes. J. Chem. Phys. 2016, 144, 034904. [Google Scholar] [CrossRef]
- Tom, A.M.; Rajesh, R.; Vemparala, S. Aggregation of flexible polyelectrolytes: Phase diagram and dynamics. J. Chem. Phys. 2017, 147, 144903. [Google Scholar] [CrossRef] [PubMed]
- Raafatnia, S.; Hickey, O.A.; Holm, C. Electrophoresis of a spherical polyelectrolyte-grafted colloid in monovalent salt solutions: Comparison of molecular dynamics simulations with theory and numerical calculations. Macromolecules 2015, 48, 775–787. [Google Scholar] [CrossRef]
- Hickey, O.A.; Shendruk, T.N.; Harden, J.L.; Slater, G.W. Simulations of free-solution electrophoresis of polyelectrolytes with a finit debye length using the Debye-Hückel approximation. Phys. Rev. Lett. 2012, 109, 098302. [Google Scholar] [CrossRef]
- LAMMPS Package. Available online: https://lammps.sandia.gov/ (accessed on 24 July 2024).
- Schneider, T.; Stoll, E.P. Molecular-Dynamics study of a 3-dimensional one component model for distortive phase-transitions. Phys. Rev. B Condens. Matter Mater. Phys. 1978, 17, 1302–1322. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, D.; Chai, A.; Hu, X.; Zhong, P.; Kang, N.; Kuang, X.; Yang, Z. Conformational Transition of Semiflexible Ring Polyelectrolyte in Tetravalent Salt Solutions: A Simple Numerical Modeling without the Effect of Twisting. Int. J. Mol. Sci. 2024, 25, 8268. https://doi.org/10.3390/ijms25158268
Lu D, Chai A, Hu X, Zhong P, Kang N, Kuang X, Yang Z. Conformational Transition of Semiflexible Ring Polyelectrolyte in Tetravalent Salt Solutions: A Simple Numerical Modeling without the Effect of Twisting. International Journal of Molecular Sciences. 2024; 25(15):8268. https://doi.org/10.3390/ijms25158268
Chicago/Turabian StyleLu, Dan, Aihua Chai, Xiuxia Hu, Peihua Zhong, Nianqian Kang, Xianfei Kuang, and Zhiyong Yang. 2024. "Conformational Transition of Semiflexible Ring Polyelectrolyte in Tetravalent Salt Solutions: A Simple Numerical Modeling without the Effect of Twisting" International Journal of Molecular Sciences 25, no. 15: 8268. https://doi.org/10.3390/ijms25158268
APA StyleLu, D., Chai, A., Hu, X., Zhong, P., Kang, N., Kuang, X., & Yang, Z. (2024). Conformational Transition of Semiflexible Ring Polyelectrolyte in Tetravalent Salt Solutions: A Simple Numerical Modeling without the Effect of Twisting. International Journal of Molecular Sciences, 25(15), 8268. https://doi.org/10.3390/ijms25158268



