Revisiting the Most Stable Structures of the Benzene Dimer
Abstract
:1. Introduction
2. Results
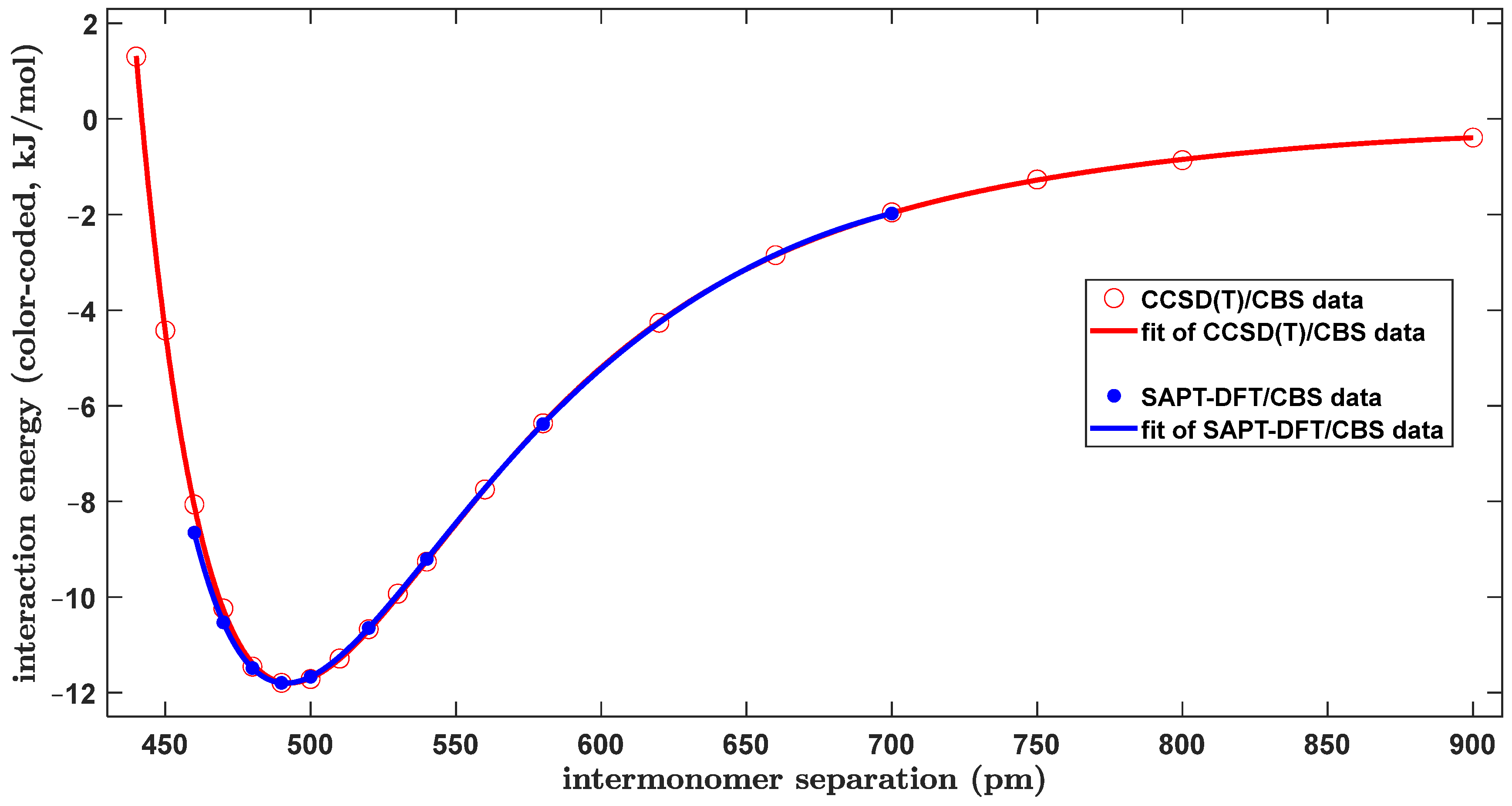
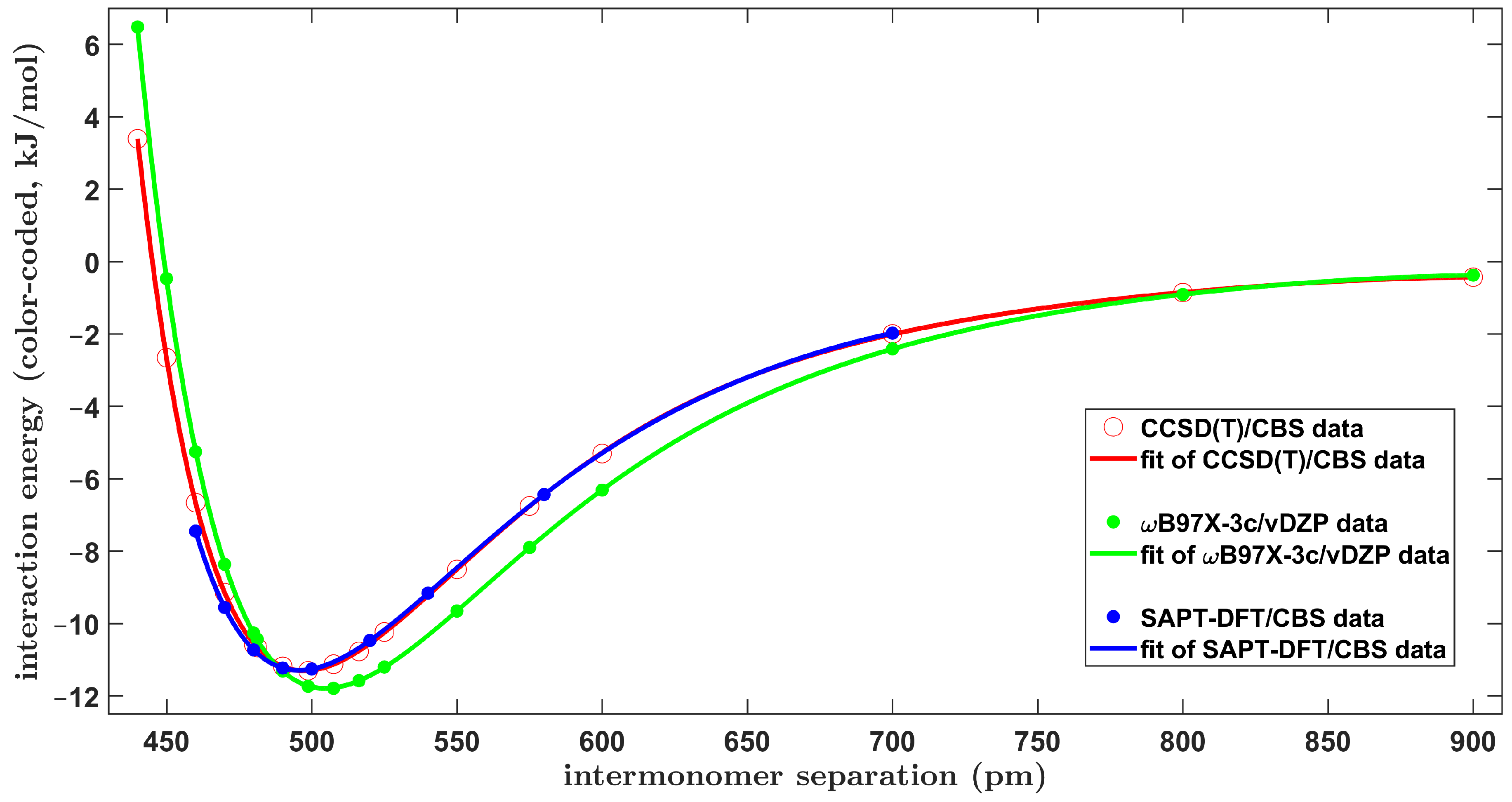
2.1. Confrontation with Previous Data
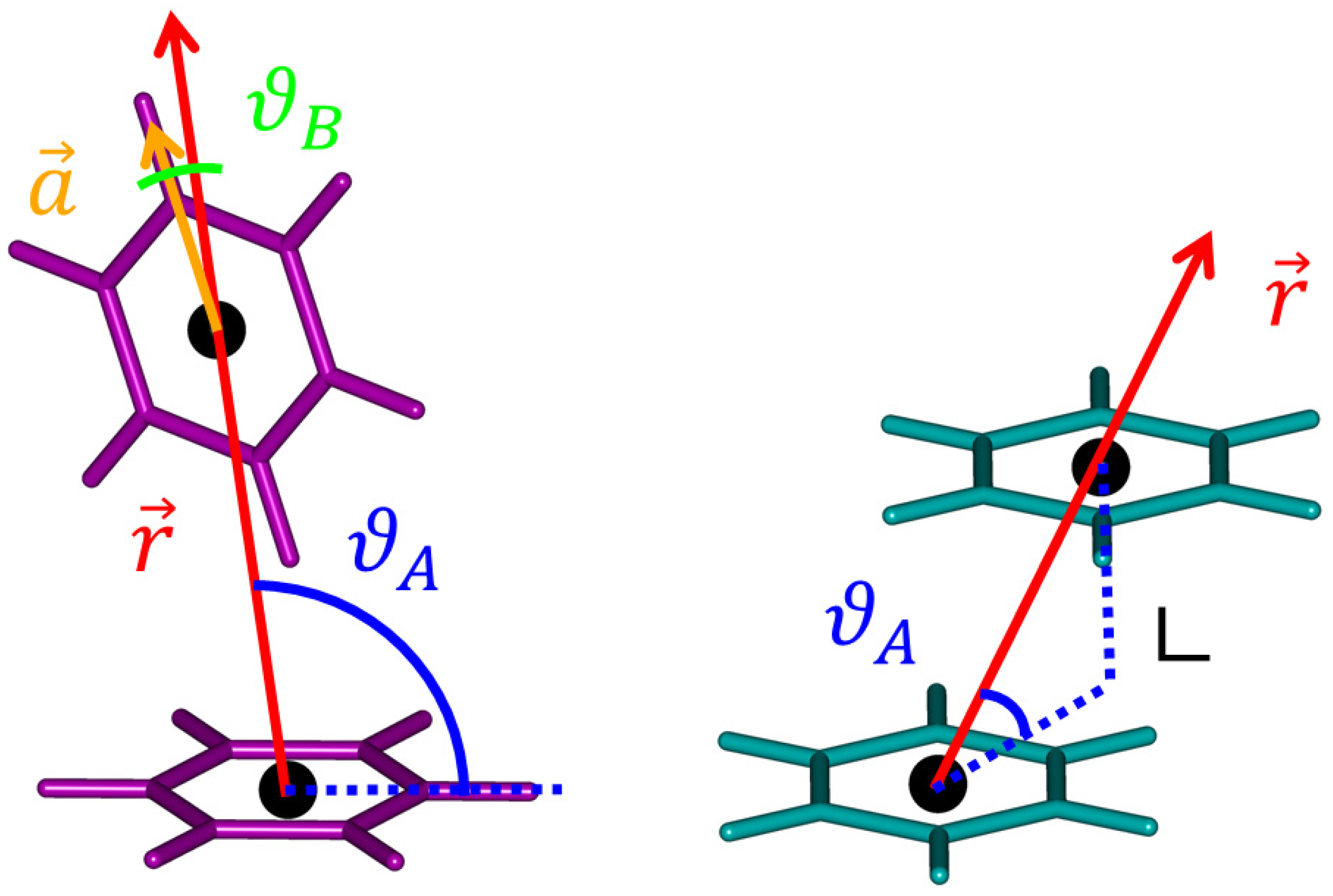
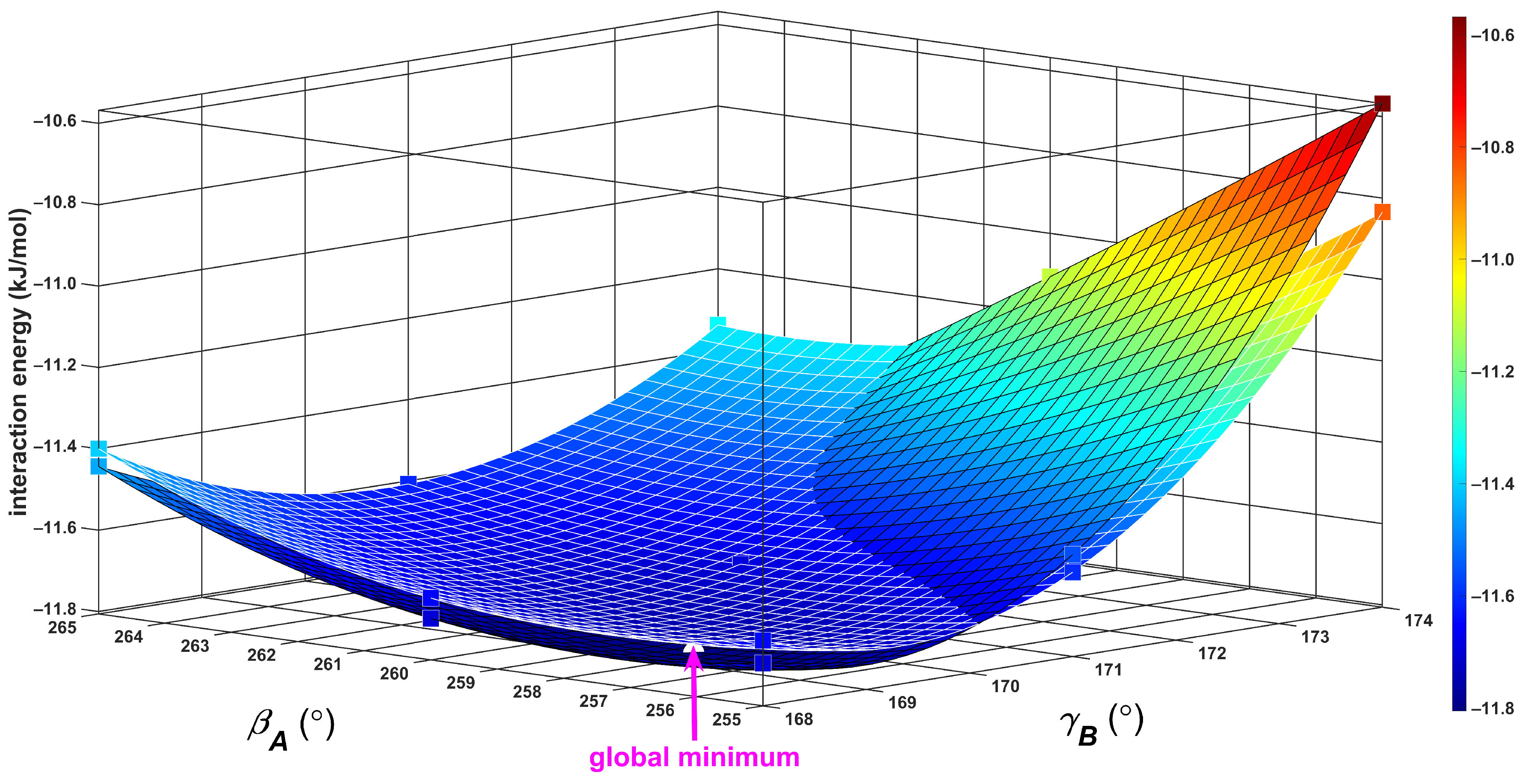
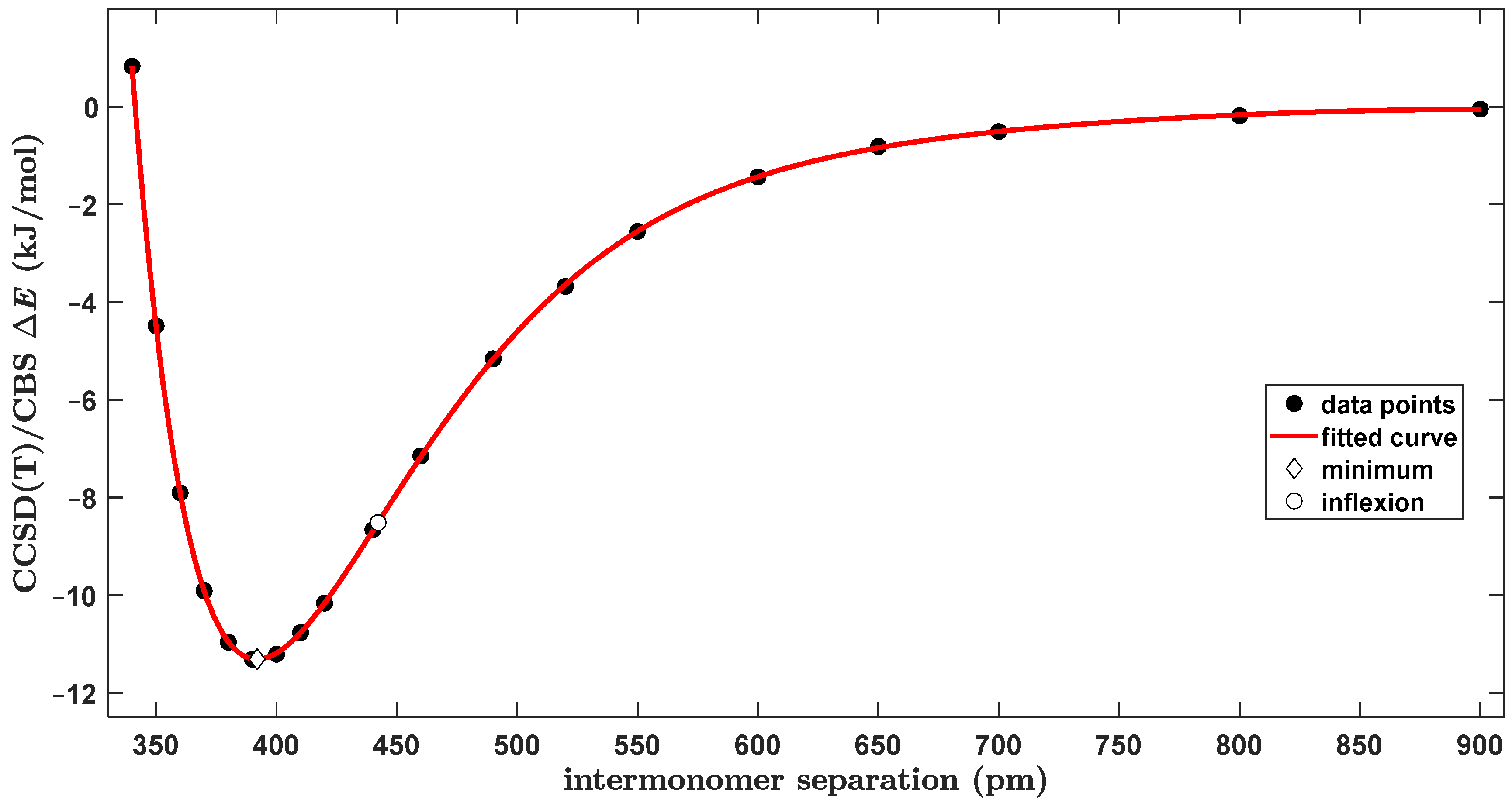
2.2. The Tilted T-Shaped Structure
2.3. The Canonical T-Shaped Structure
2.4. The Parallel-Displaced Structure
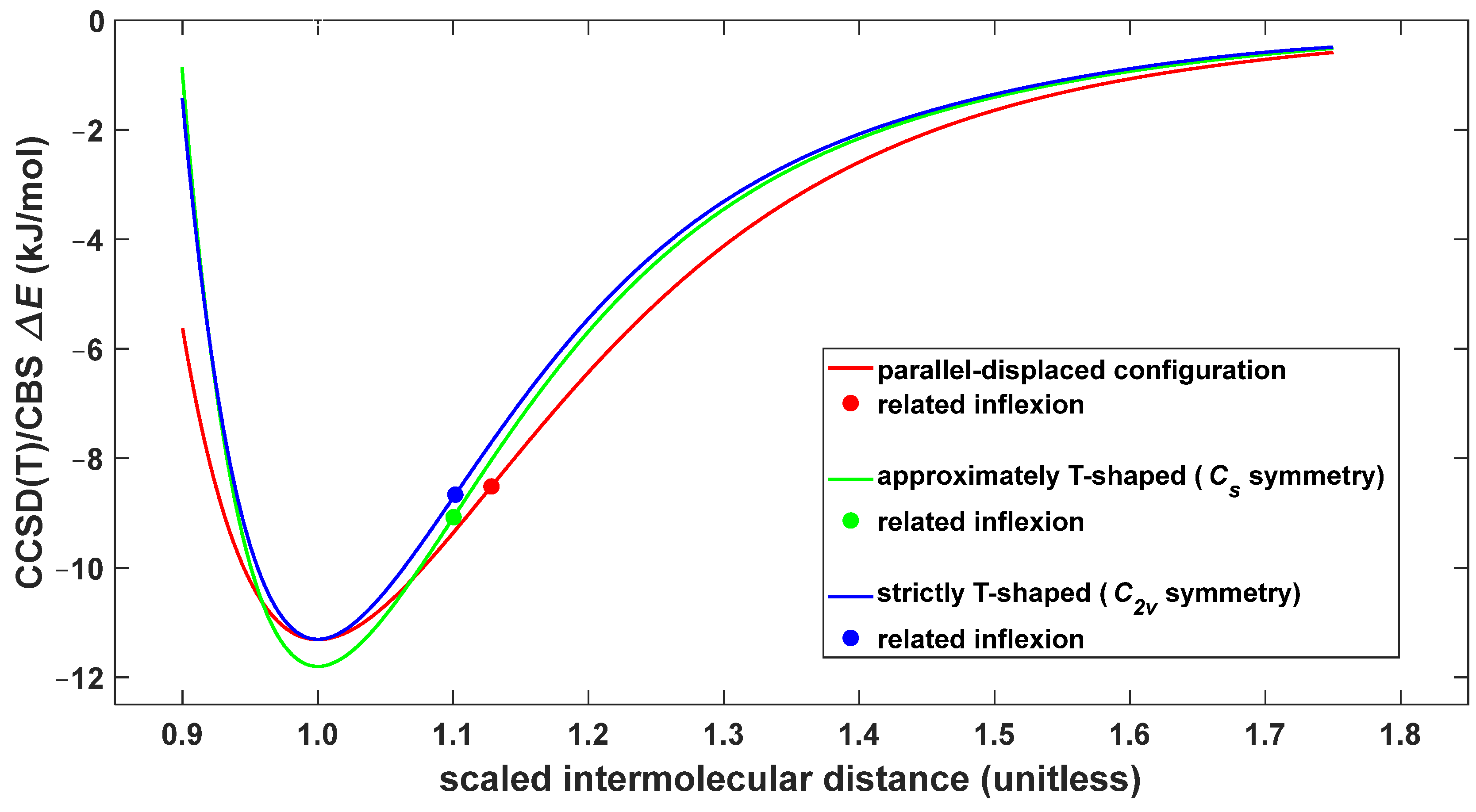
3. Discussion
3.1. The Origin of the Global Minimum
3.2. The Intrinsic Strength of Intermolecular Interactions
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karshikoff, A. Non-Covalent Interactions in Proteins, 2nd ed.; World Scientific: Singapore, 2021. [Google Scholar] [CrossRef]
- Cerveri, A.; Scarica, G.; Sparascio, S.; Hoch, M.; Chiminelli, M.; Tegoni, M.; Protti, S.; Maestri, G. Boosting Energy-Transfer Processes via Dispersion Interactions. Chem. Eur. J. 2023, 29, e202304010. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Jayakumar, S.; Luo, M.; Kong, X.; Li, C.; Li, H.; Chen, J. The promotion effect of π-π interactions in Pd NPs catalysed selective hydrogenation. Nat. Commun. 2022, 13, 1770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Zhang, Y.N.; Ding, R.; Zhang, K.; Guo, H.Y.; Lin, Y.Y. Self-Assembled Nanocarrier Delivery Systems for Bioactive Compounds. Small 2024, 20, 2310838. [Google Scholar] [CrossRef] [PubMed]
- Savastano, M.; de la Torre, M.D.L.; Pagliai, M.; Poggi, G.; Ridi, F.; Bazzicalupi, C.; Melguizo, M.; Bianchi, A. Crystal engineering of high explosives through lone pair-π interactions: Insights for improving thermal safety. iScience 2023, 26, 107330. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Montes, E.; Rojas, W.Y.; Lawson, B.; Vázquez, H.; Kamentska, M. Cooperative Self-Assembly of Dimer Junctions Driven by π Stacking Leads to Conductance Enhancement. Nano Lett. 2023, 23, 6937–6943. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, M.R.; Davis, S.T.; Zhang, S. Synergistic Effect of Hydrogen Bonding and π–π Stacking Enables Long Cycle Life in Organic Electrode Materials. ACS Energy Lett. 2021, 6, 643–649. [Google Scholar] [CrossRef]
- Carter-Fenk, K.; Liu, M.L.; Pujal, L.; Loipersberger, M.; Tsanai, M.; Vernon, R.M.; Forman-Kay, J.D.; Head-Gordon, M.; Heidar-Zadeh, F.; Head-Gordon, T. The Energetic Origins of Pi-Pi Contacts in Proteins. J. Am. Chem. Soc. 2023, 145, 24836–24851. [Google Scholar] [CrossRef] [PubMed]
- Samaroo, S.; Hengesbach, C.; Bruggeman, C.; Carducci, N.G.G.; Mtemeri, L.; Staples, R.J.; Guarr, T.; Hickey, D.P. C–H···π interactions disrupt electrostatic interactions between non-aqueous electrolytes to increase solubility. Nat. Chem. 2023, 15, 1365–1373. [Google Scholar] [CrossRef]
- Herman, K.M.; Aprà, E.; Xantheas, S.S. A critical comparison of CH···π versus π···π interactions in the benzene dimer: Obtaining benchmarks at the CCSD(T) level and assessing the accuracy of lower scaling methods. Phys. Chem. Chem. Phys. 2023, 25, 4824–4838. [Google Scholar] [CrossRef]
- Tummanapelli, A.K.; Vasudevan, S. Communication: Benzene dimer—The free energy landscape. J. Chem. Phys. 2013, 139, 201102. [Google Scholar] [CrossRef]
- Van der Avoird, A.; Podeszwa, R.; Ensing, B.; Szalewicz, K. Comment on “Communication: Benzene dimer—The free energy landscape” [J. Chem. Phys. 139, 201102 (2013)]. J. Chem. Phys. 2014, 140, 227101. [Google Scholar] [CrossRef] [PubMed]
- Tummanapelli, A.K.; Vasudevan, S. Response to “Comment on ‘Communication: Benzene dimer—The free energy landscape’” [J. Chem. Phys. 140, 227101 (2014)]. J. Chem. Phys. 2014, 140, 227102. [Google Scholar] [CrossRef] [PubMed]
- Law, K.S.; Schauer, M.; Bernstein, E.R. Dimers of aromatic molecules: (Benzene)2, (toluene)2, and benzene–toluene. J. Chem. Phys. 1984, 81, 4871–4882. [Google Scholar] [CrossRef]
- Arunan, E.; Gutowsky, H.S. The rotational spectrum, structure and dynamics of a benzene dimer. J. Chem. Phys. 1993, 98, 4294–4296. [Google Scholar] [CrossRef]
- Erlekam, U.; Frankowski, M.; Meijer, G.; von Helden, G. An experimental value for the B1u C–H stretch mode in benzene. J. Chem. Phys. 2006, 124, 171101. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.C.; Kim, D.; Jurečka, P.; Tarakeshwar, P.; Hobza, P.; Kim, K.S. Understanding of Assembly Phenomena by Aromatic−Aromatic Interactions: Benzene Dimer and the Substituted Systems. J. Phys. Chem. A 2007, 111, 3446–3457. [Google Scholar] [CrossRef] [PubMed]
- Van der Avoird, A.; Podeszwa, R.; Szalewicz, K.; Leforestier, C.; van Harrevelt, R.; Bunker, P.R.; Schnell, M.; von Helden, G.; Meijer, G. Vibration–rotation-tunneling states of the benzene dimer: An ab initio study. Phys. Chem. Chem. Phys. 2010, 12, 8219–8240. [Google Scholar] [CrossRef] [PubMed]
- Schnell, M.; Erlekam, U.; Bunker, P.R.; von Helden, G.; Grabow, J.-U.; Meijer, G.; van der Avoird, A. Structure of the Benzene Dimer—Governed by Dynamics. Angew. Chem. Int. Ed. 2013, 52, 5180–5183. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Steber, A.L.; Poblotzki, A.; Pérez, C.; Zinn, S.; Schnell, M. Rotational Signatures of Dispersive Stacking in the Formation of Aromatic Dimers. Angew. Chem. Int. Ed. 2019, 58, 3108–3113. [Google Scholar] [CrossRef]
- Grover, J.R.; Walters, E.A.; Hui, E.T. Dissociation Energies of the Benzene Dimer and Dimer Cation. J. Phys. Chem. 1987, 91, 3233–3237. [Google Scholar] [CrossRef]
- Krause, H.; Ernstberger, B.; Neusser, H.J. Binding energies of small benzene clusters. Chem. Phys. Lett. 1991, 184, 411–417. [Google Scholar] [CrossRef]
- Calvin, J.A.; Peng, C.; Rishi, V.; Kumar, A.; Valeev, E.F. Many-Body Quantum Chemistry on Massively Parallel Computers. Chem. Rev. 2021, 121, 1203–1231. [Google Scholar] [CrossRef] [PubMed]
- Patkowski, K. Recent developments in symmetry-adapted perturbation theory. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020, 10, e1452. [Google Scholar] [CrossRef]
- Shahbaz, M.; Szalewicz, K. Evaluation of methods for obtaining dispersion energies used in density functional calculations of intermolecular interactions. Theor. Chem. Acc. 2019, 138, 25. [Google Scholar] [CrossRef]
- Carter-Fenk, C.; Lao, K.U.; Herbert, J.M. Predicting and Understanding Non-Covalent Interactions Using Novel Forms of Symmetry-Adapted Perturbation Theory. Acc. Chem. Res. 2021, 54, 3679–3690. [Google Scholar] [CrossRef] [PubMed]
- Morales-Silva, M.A.; Jordan, K.D.; Shulenburger, L.; Wagner, L.K. Frontiers of stochastic electronic structure calculations. J. Chem. Phys. 2021, 154, 170401. [Google Scholar] [CrossRef]
- Al-Hamdani, Y.S.; Nagy, P.R.; Zen, A.; Barton, D.; Kállay, M.; Bradenburg, J.G.; Tkatchenko, A. Interactions between large molecules pose a puzzle for reference quantum mechanical methods. Nat. Commun. 2021, 12, 3927. [Google Scholar] [CrossRef]
- Grimme, S.; Goerigk, L.; Fink, R.F. Spin-component-scaled electron correlation methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 886–906. [Google Scholar] [CrossRef]
- Miliordos, E.; Aprà, E.; Xantheas, S.S. Benchmark Theoretical Study of the π–π Binding Energy in the Benzene Dimer. J. Phys. Chem. A 2014, 118, 7568–7578. [Google Scholar] [CrossRef]
- Carter-Fenk, K.; Herbert, J.M. Electrostatics does not dictate the slip-stacked arrangement of aromatic π–π interactions. Chem. Sci. 2020, 11, 6758–6765. [Google Scholar] [CrossRef]
- Czernek, J.; Brus, J.; Czerneková, V.; Kobera, L. Quantifying the Intrinsic Strength of C–H⋯O Intermolecular Interactions. Molecules 2023, 28, 4478. [Google Scholar] [CrossRef] [PubMed]
- Sauceda, H.E.; Vassilev-Galindo, V.; Chmiela, S.; Müller, K.-R.; Tkatchenko, A. Dynamical strengthening of covalent and non-covalent molecular interactions by nuclear quantum effects at finite temperature. Nat. Commun. 2021, 12, 442. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Nozawa, T.; Matsumoto, T.; Yagihashi, F.; Kikuchi, T.; Sato, K. Parallel-stacked aromatic molecules in hydrogen-bonded inorganic frameworks. Nat. Commun. 2021, 12, 7025. [Google Scholar] [CrossRef] [PubMed]
- Di, S.; Wu, Q.; Shi, C.; Zhu, S. Hydroxy-Containing Covalent Organic Framework Combined with Nickel Ferrite as a Platform for the Recognition and Capture of Bisphenols. ACS Appl. Mater. Interfaces 2023, 15, 1827–1842. [Google Scholar] [CrossRef] [PubMed]
- Lao, Z.; Tang, Y.; Dong, X.; Tan, Y.; Li, X.; Liu, X.; Li, L.; Guo, C.; Wei, G. Elucidating the reversible and irreversible self-assembly mechanisms of low-complexity aromatic-rich kinked peptides and steric zipper peptides. Nanoscale 2024, 16, 4025–4038. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Czernek, J.; Brus, J.; Czerneková, V. A Cost Effective Scheme for the Highly Accurate Description of Intermolecular Binding in Large Complexes. Int. J. Mol. Sci. 2022, 23, 15773. [Google Scholar] [CrossRef]
- Czernek, J.; Brus, J. On the Intermolecular Interactions in Thiophene-Cored Single-Stacking Junctions. Int. J. Mol. Sci. 2023, 24, 13349. [Google Scholar] [CrossRef]
- Czernek, J.; Brus, J. Reliable Dimerization Energies for Modeling of Supramolecular Junctions. Int. J. Mol. Sci. 2024, 25, 602. [Google Scholar] [CrossRef] [PubMed]
- Podeszwa, R.; Bukowski, R.; Szalewicz, K. Potential Energy Surface for the Benzene Dimer and Perturbational Analysis of π−π Interactions. J. Phys. Chem. A 2006, 110, 10345–10354. [Google Scholar] [CrossRef] [PubMed]
- Tamagawa, K.; Iijima, T.; Kimura, M. Molecular structure of benzene. J. Mol. Struct. 1976, 30, 243–253. [Google Scholar] [CrossRef]
- Müller, M.; Hansen, A.; Grimme, S. ωB97X-3c: A composite range-separated hybrid DFT method with a molecule-optimized polarized valence double-ζ basis set. J. Chem. Phys. 2023, 158, 014103. [Google Scholar] [CrossRef]
- Gorges, J.; Bädorf, B.; Grimme, S.; Hansen, A. Efficient Computation of the Interaction Energies of Very Large Non-covalently Bound Complexes. Synlett 2023, 34, 1135–1146. [Google Scholar] [CrossRef]
- Řezáč, J.; Riley, K.E.; Hobza, P. S66: A Well-balanced Database of Benchmark Interaction Energies Relevant to Biomolecular Structures. J. Chem. Theory Comput. 2011, 7, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Sargent, C.T.; Kasera, R.; Glick, Z.L.; Sherrill, C.D.; Cheney, D.L. A quantitative assessment of deformation energy in intermolecular interactions: How important is it? J. Chem. Phys. 2023, 158, 244106. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Strengthening of Noncovalent Bonds Caused by Internal Deformations. J. Phys. Chem. A 2024, 128, 2357–2365. [Google Scholar] [CrossRef]
- Stone, A.J. The Theory of Intermolecular Forces, 1st ed.; Clarendon Press: Oxford, UK, 2002; pp. 79–102. [Google Scholar]
- Carter-Fenk, K.; Herbert, J.M. Reinterpreting π-stacking. Phys. Chem. Chem. Phys. 2020, 22, 24870–24886. [Google Scholar] [CrossRef]
- Cabaleiro-Lago, E.M.; Rodríguez-Otero, J.; Vázquez, S.A. Electrostatic penetration effects stand at the heart of aromatic π interactions. Phys. Chem. Chem. Phys. 2022, 24, 8979–8991. [Google Scholar] [CrossRef]
- Vernon, R.M.; Chong, P.A.; Tsang, B.; Kim, T.H.; Bah, A.; Farber, P.; Lin, H.; Forman-Kay, J.D. Pi-Pi contacts are an overlooked protein feature relevant to phase separation. eLife 2018, 7, e31476. [Google Scholar] [CrossRef] [PubMed]
- Halkier, A.; Helgaker, T.; Jørgensen, P.; Klopper, W.; Koch, H.; Olsen, J.; Wilson, A.K. Basis-set convergence in correlated calculations on Ne, N2, and H2O. Chem. Phys. Lett. 1998, 286, 243–252. [Google Scholar] [CrossRef]
- Balasubramani, S.G.; Chen, G.P.; Coriani, S.; Diedenhofen, M.; Frank, M.S.; Franzke, Y.J.; Furche, F.; Grotjahn, R.; Harding, M.E.; Hättig, C.; et al. TURBOMOLE: Modular program suite for ab initio quantum-chemical and condensed-matter simulations. J. Chem. Phys. 2020, 152, 184107. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Häser, M. RI-MP2: First derivatives and global consistency. Theor. Chem. Acc. 1997, 97, 331–340. [Google Scholar] [CrossRef]
- Weigend, F.; Häser, M.; Patzelt, H.; Ahlrichs, R. RI-MP2: Optimized auxiliary basis sets and demonstration of efficiency. Chem. Phys. Lett. 1998, 294, 143–152. [Google Scholar] [CrossRef]
- Werner, H.J.; Knowles, P.J.; Manby, F.R.; Black, J.A.; Doll, K.; Hesselmann, A.; Kats, D.; Kohn, A.; Korona, T.; Kreplin, D.A.; et al. The Molpro quantum chemistry package. J. Chem. Phys. 2020, 152, 144107. [Google Scholar] [CrossRef] [PubMed]
- Heßelmann, A.; Jansen, G. Density-functional theory-symmetry-adapted intermolecular perturbation theory with density fitting: A new efficient method to study intermolecular interaction energies. J. Chem. Phys. 2005, 122, 014103. [Google Scholar] [CrossRef] [PubMed]
- Czernek, J.; Brus, J.; Czerneková, V. A computational inspection of the dissociation energy of mid-sized organic dimers. J. Chem. Phys. 2022, 156, 204303. [Google Scholar] [CrossRef] [PubMed]
- Heßelmann, A.; Jansen, G. First-order intermolecular interaction energies from Kohn–Sham orbitals. Chem. Phys. Lett. 2002, 357, 464–470. [Google Scholar] [CrossRef]
- Heßelmann, A.; Jansen, G. Intermolecular dispersion energies from time-dependent density functional theory. Chem. Phys. Lett. 2003, 367, 778–784. [Google Scholar] [CrossRef]
- Heßelmann, A.; Jansen, G. Intermolecular induction and exchange-induction energies from coupled-perturbed Kohn–Sham density functional theory. Chem. Phys. Lett. 2002, 362, 319–325. [Google Scholar] [CrossRef]
- Moszynski, R.; Heijmen, T.G.A.; Jeziorski, B. Symmetry-adapted perturbation theory for the calculation of Hartree–Fock interaction energies. Mol. Phys. 1996, 88, 741–758. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Frish, M.J.; Trucks, J.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Neese, F. Software update: The ORCA program system—Version 5.0. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- ORCA4wB97X-3c. A Fortran Script for Setting Up a ωB97X-3c Calculation with ORCA 5.0.3 or Higher. Available online: https://github.com/grimme-lab/ORCA4wB97X-3c (accessed on 28 June 2024).
- Bende, A.; Farcaş, A.-A. Intermolecular-Type Conical Intersections in Benzene Dimer. Int. J. Mol. Sci. 2023, 24, 2906. [Google Scholar] [CrossRef]
- Pham, H.Q.; Ouyang, R.S.; Lv, D.S. Scalable Quantum Monte Carlo with Direct-Product Trial Wave Functions. J. Chem. Theory Comput. 2024, 20, 3524–3534. [Google Scholar] [CrossRef]
- Vinod, V.; Kleinekathöfer, U.; Zaspel, P. Optimized multifidelity machine learning for quantum chemistry. Mach. Learn. Sci. Technol. 2024, 5, 015054. [Google Scholar] [CrossRef]


| Geometry (Symmetry Group) | Parameter | |||
|---|---|---|---|---|
| /pm | /deg. | /deg. | /kcal/mol | |
| Tilted T-shaped (Cs) | 493.1 a | 100.1 a | 12.6 a | 11.84 a |
| 494.4 b | 99.22 b | 11.75 b | 11.67 b | |
| 492.4 c | 99.19 c | 11.09 c | (11.83 ± 0.04) d | |
| Canonical T-shaped (C2v) | 497.4 a | — | — | 11.34 a |
| 497.0 b 495.0 e | 11.35 b (11.46 ± 0.13) d | |||
| Slipped-parallel (C2h) | 395.4 a | 62.8 a | 62.8 a | 11.21 a |
| 393.7 b 384.0 e | 62.12 b 63.85 e | 62.12 b 63.85 e | 11.24 b (11.09 ± 0.08) d | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czernek, J.; Brus, J. Revisiting the Most Stable Structures of the Benzene Dimer. Int. J. Mol. Sci. 2024, 25, 8272. https://doi.org/10.3390/ijms25158272
Czernek J, Brus J. Revisiting the Most Stable Structures of the Benzene Dimer. International Journal of Molecular Sciences. 2024; 25(15):8272. https://doi.org/10.3390/ijms25158272
Chicago/Turabian StyleCzernek, Jiří, and Jiří Brus. 2024. "Revisiting the Most Stable Structures of the Benzene Dimer" International Journal of Molecular Sciences 25, no. 15: 8272. https://doi.org/10.3390/ijms25158272





