Frequent Acquisition of Glycoside Hydrolase Family 32 (GH32) Genes from Bacteria via Horizontal Gene Transfer Drives Adaptation of Invertebrates to Diverse Sources of Food and Living Habitats
Abstract
:1. Introduction
2. Results
2.1. GH32 Genes in Diverse Animals
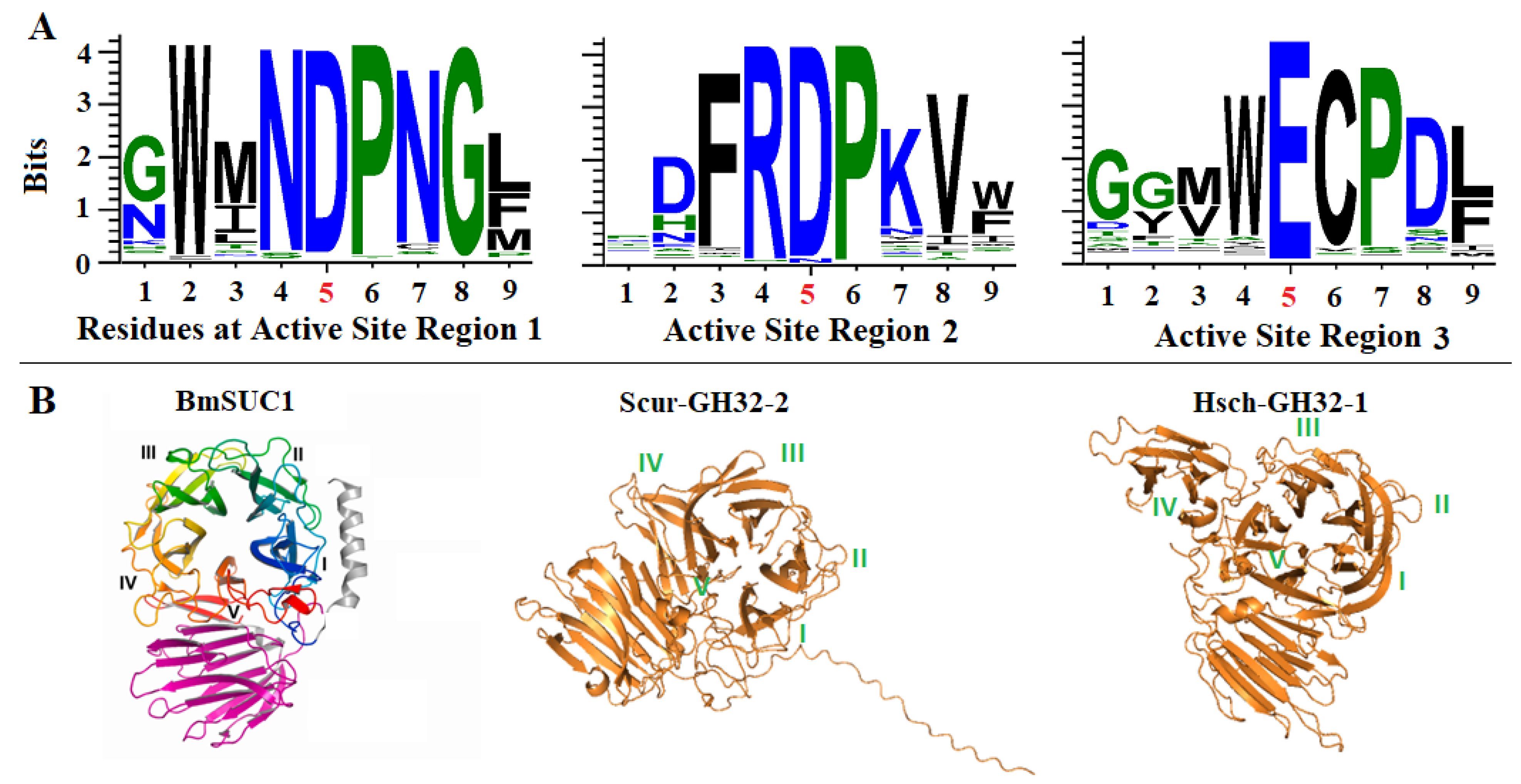
2.2. Conservation of the Catalytic Triads and 3D Structures
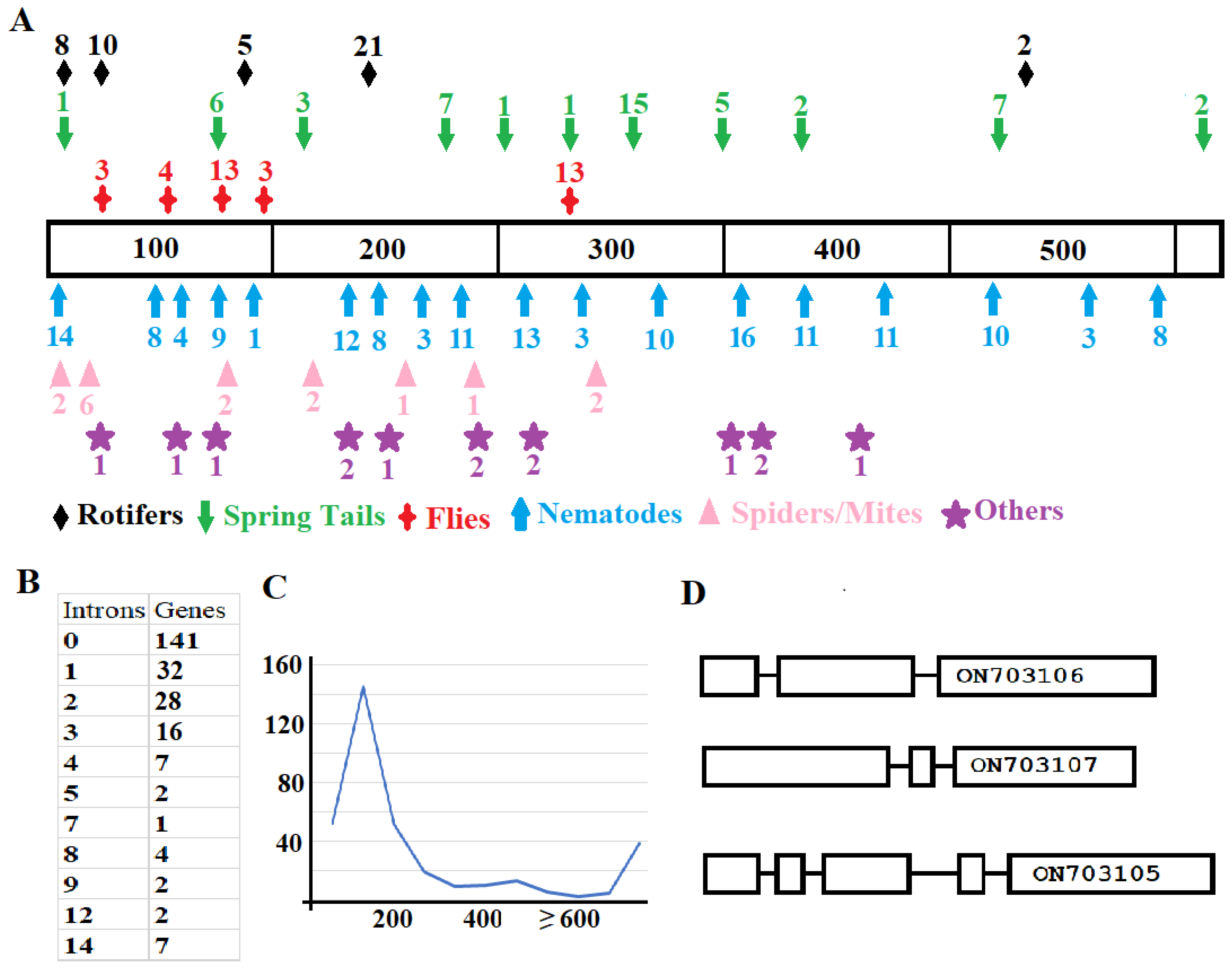
2.3. Structural Variation among Genes from Different Animals and Genes from the Same Animal
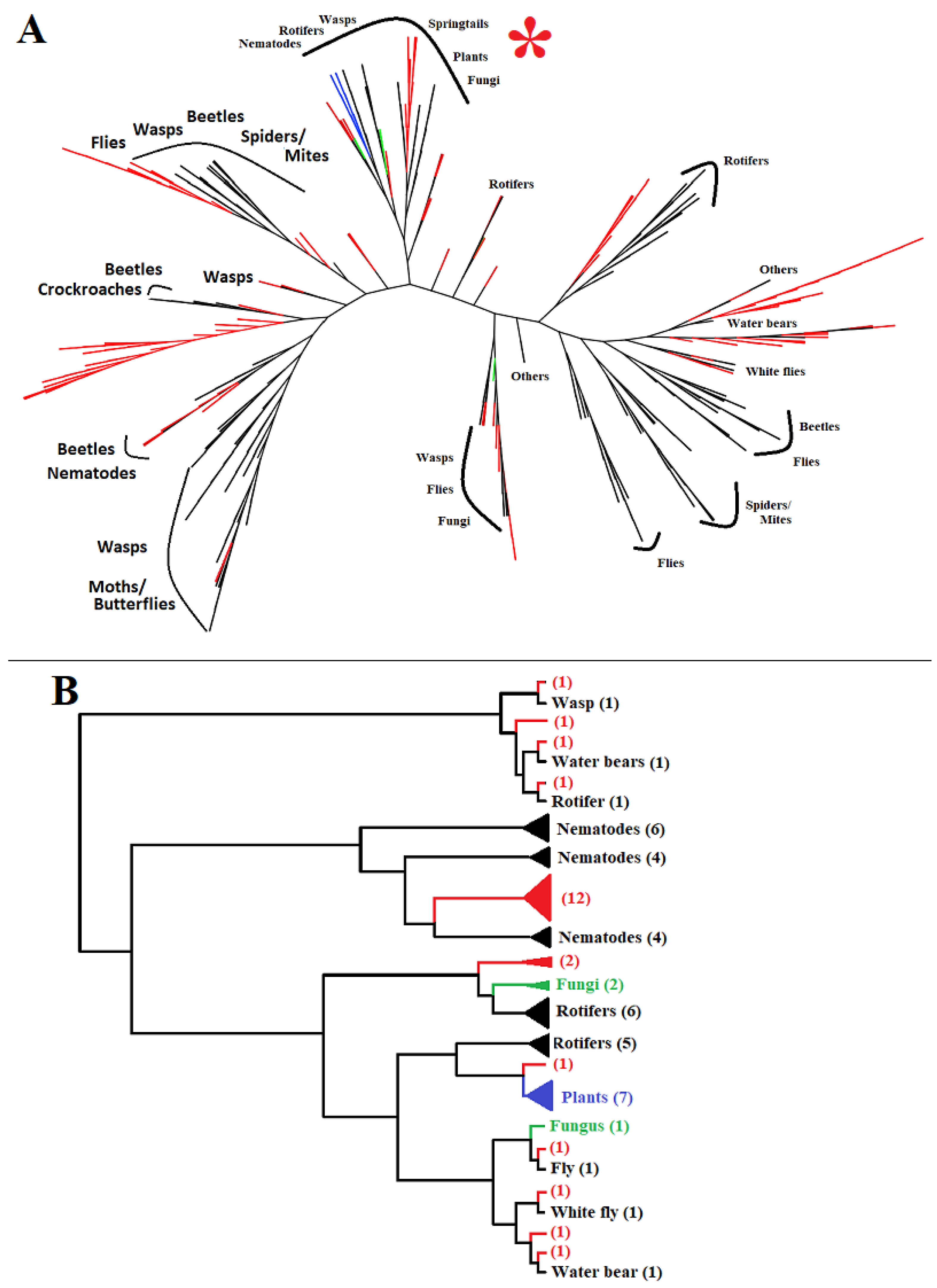
2.4. Faster Diversification Once GH32 Genes Transferred from Bacteria to Animals
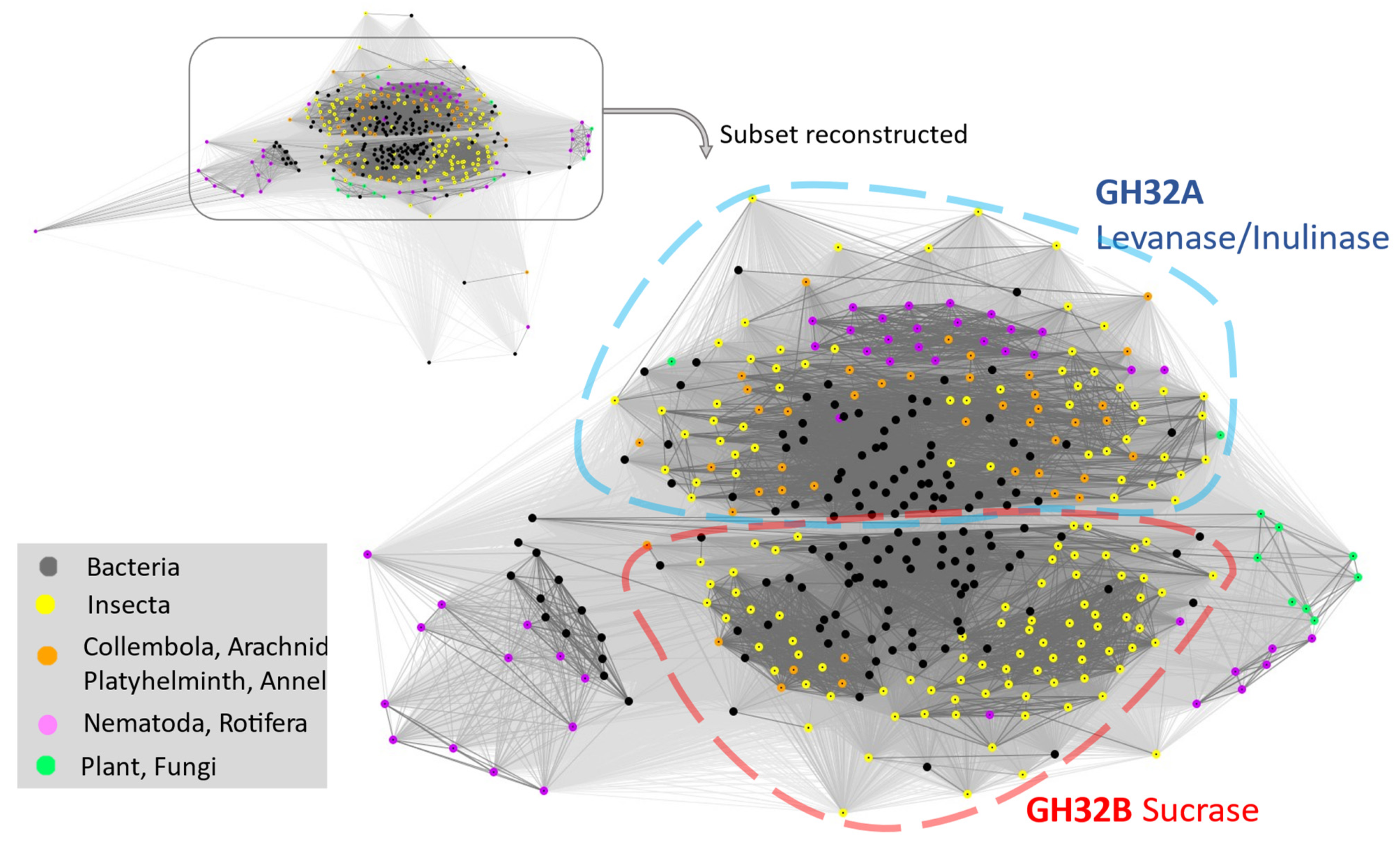
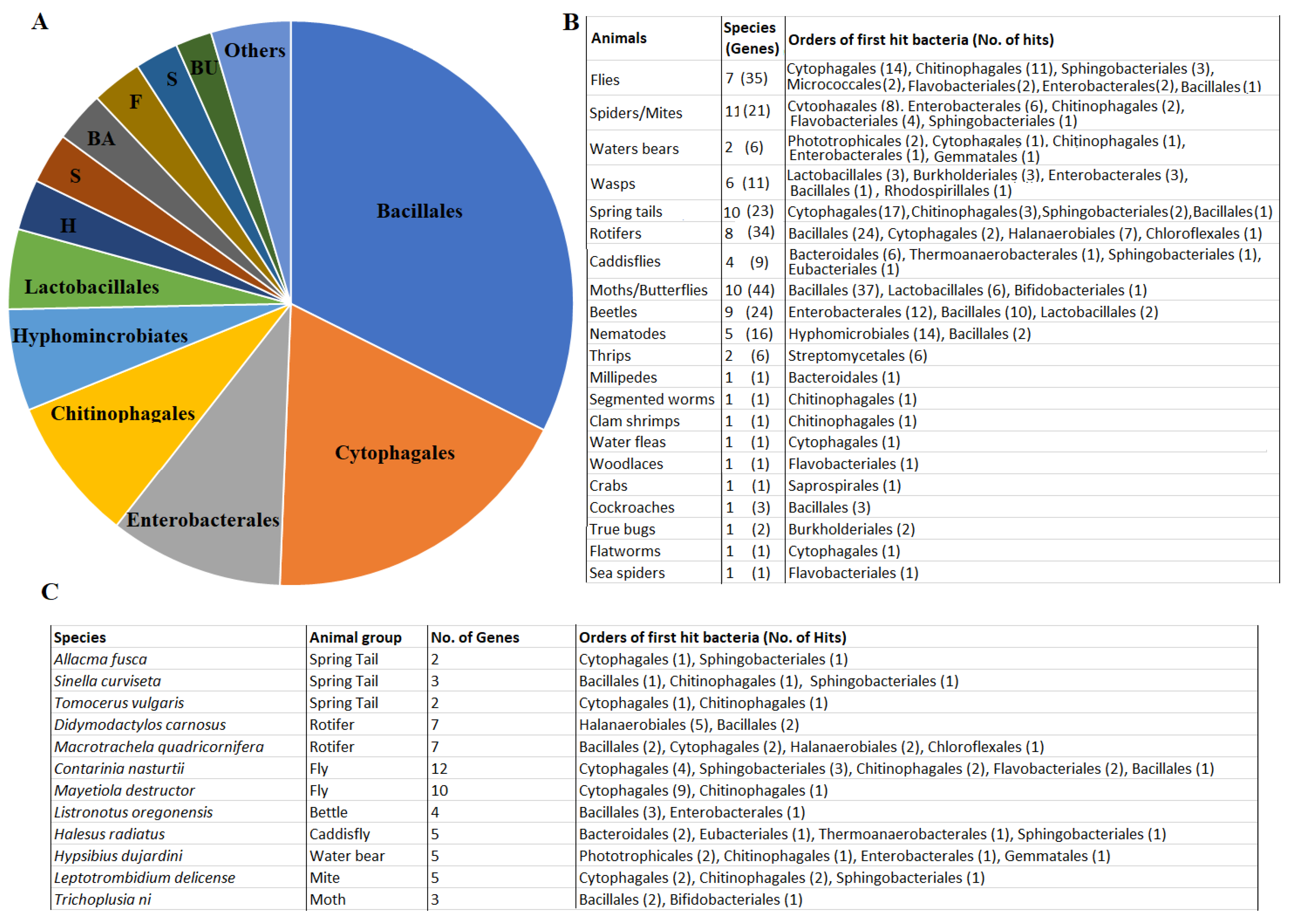
2.5. Ultiple GH32-Gene Transfer Events to Animal Genomes from Diverse Bacteria
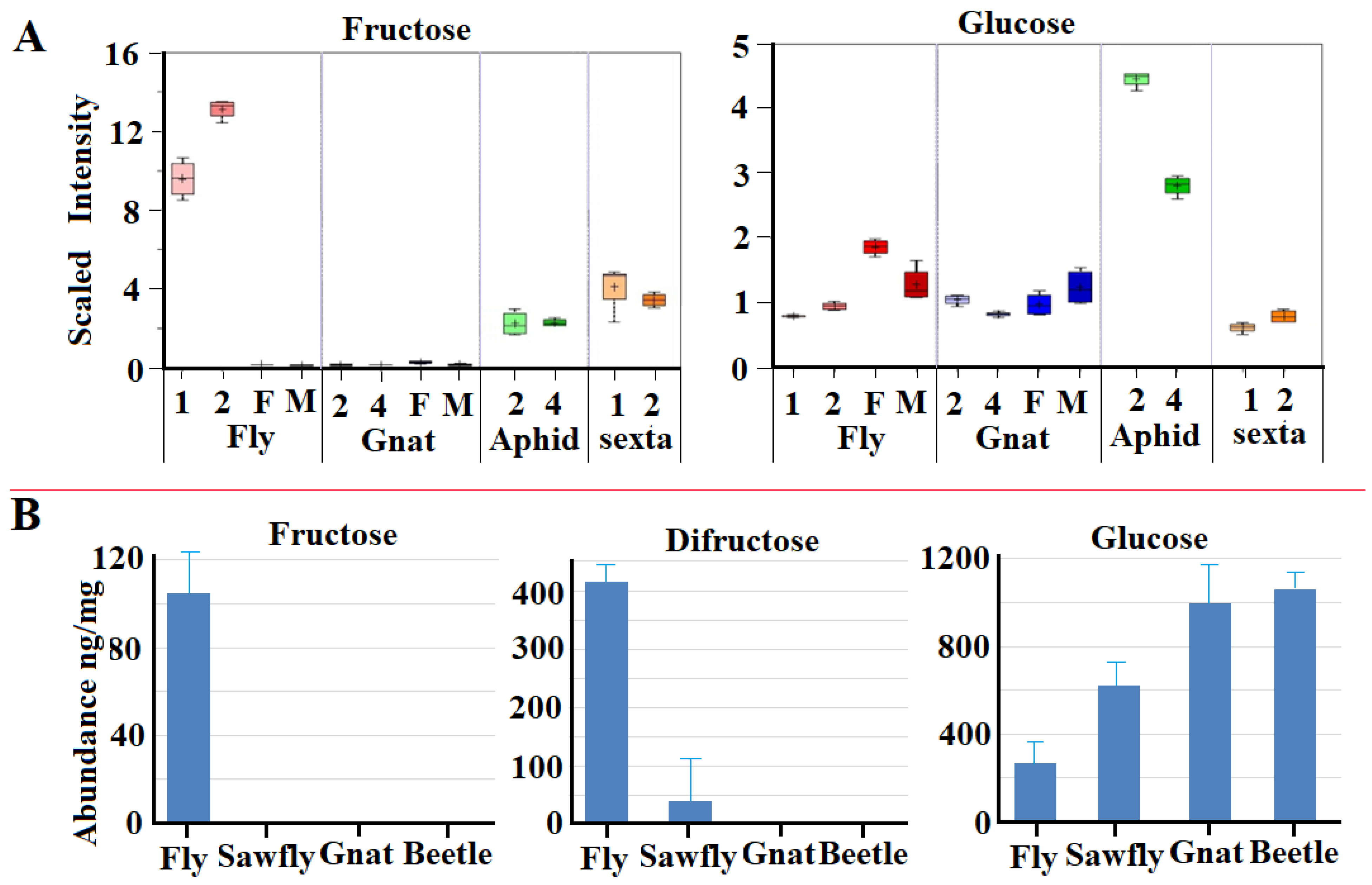
2.6. Impact of Derived GH32 Genes on Sugar Metabolism in the Recipient Animals
3. Discussion
4. Materials and Methods
4.1. Gene Identification
4.2. Identification of the Catalytic Triads
4.3. Prediction of Three-Dimensional Structures of the Identified Proteins
4.4. Best Bacterial Protein Matches of the Identified Animal GH32 Proteins
4.5. CLANS and Phylogenetic Analyses
4.6. Metabolic Profiling and Concentration Determination
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henrissat, B.; Callebaut, I.; Mornon, J.P.; Fabrega, S.; Lehn, P.; Davies, G. Conserved Catalytic Machinery and the Prediction of a Common Fold for Several Families of Glycosyl Hydrolases. Proc. Natl. Acad. Sci. USA 1995, 92, 7090–7094. Available online: https://www.jstor.org/stable/2367800 (accessed on 1 May 2024). [CrossRef] [PubMed]
- Minic, Z. Physiological roles of plant glycoside hydrolases. Planta 2008, 227, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Lammens, W.; Roy, K.L.; Schroeven, L.; Laere, A.V.; Rabijns, A.; Van den Ende, W. Structural insights into glycoside hydrolase family 32 and 68 enzymes: Functional implications. J. Exp. Bot. 2009, 60, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Alberto, F.; Bignon, C.; Sulzenbacher, G.; Henrissat, B.; Czjzek, M. The Three-dimensional Structure of Invertase (β-Fructosidase) from Thermotoga maritima Reveals a Bimodular Arrangement and an Evolutionary Relationship between Retaining and Inverting Glycosidases. J. Biol. Chem. 2004, 279, 18903–18910. [Google Scholar] [CrossRef]
- Sainz-Polo, M.A.; Ramírez-Escudero, M.; Lafraya, A.; González, B.; Marín-Navarro, J.; Polaina, J.; Sanz-Aparicio, J. Three-dimensional structure of Saccharomyces invertase: Role of a non-catalytic domain in oligomerization and substrate specificity. J. Biol. Chem. 2013, 288, 9755–9766. [Google Scholar] [CrossRef] [PubMed]
- Lammens, W.; Le Roy, K.; Van Laere, A.; Rabijns, A.; Van den Ende, W. Crystal structures of Arabidopsis thaliana cell-wall invertase mutants in complex with sucrose. J. Mol. Biol. 2008, 377, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Oba, N.; Park, E.Y. Structural insight into the substrate specificity of Bombyx mori β-fructofuranosidase belonging to the glycoside hydrolase family 32. Insect Biochem. Mol. Biol. 2020, 127, 103494. [Google Scholar] [CrossRef]
- Nagem, R.A.P.; Rojas, A.L.; Golubev, A.M.; Korneeva, O.S.; Eneyskaya, E.V.; Kulminskaya, A.A.; Neustroev, K.N.; Polikarpov, I. Crystal structure of exo-inulinase from Aspergillus awamori: The enzyme fold and structural determinants of substrate recognition. J. Mol. Biol. 2004, 344, 471–480. [Google Scholar] [CrossRef]
- Verhaest, M.; Van den Ende, W.; Roy, K.L.; Ranter, C.J.D.; Van Laere, A.; Rabijns, A. X-ray diffraction structure of a plant glycosyl hydrolase family 32 protein: Fructan 1-exohydrolase IIa of Cichorium intybus. Plant J. 2005, 41, 400–411. [Google Scholar] [CrossRef]
- Ernits, K.; Eek, P.; Lukk, T.; Visnapuu, T.; Alamäe, T. First crystal structure of an endo-levanase–the BT1760 from a human gut commensal Bacteroides thetaiotaomicron. Sci. Rep. 2019, 9, 8443. [Google Scholar] [CrossRef]
- Hendry, G.A.F. Evolutionary origins and Natural Functions of Fructans-a Climatological, Biogeographicand Mechanistic Appraisal. New Phytol. 1993, 123, 3–14. Available online: https://www.jstor.org/stable/2557765 (accessed on 1 May 2024). [CrossRef]
- Muir, J.G.; Shepherd, S.J.; Rosella, O.; Rose, R.; Barrett, J.S.; Gibson, P.R. Fructan and free fructose content of common Australian vegetables and fruit. J. Agric. Food Chem. 2007, 55, 6619–6627. [Google Scholar] [CrossRef]
- Keeling, P.; Palmer, J. Horizontal gene transfer in eukaryotic evolution. Nat. Rev. Genet. 2008, 9, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.A.; Lougheed, S.C.; Ewart, K.V.; Davies, P.L. Lateral transfer of a lectin-like antifreeze protein gene in fishes. PLoS ONE 2008, 3, e2616. [Google Scholar] [CrossRef] [PubMed]
- van Hoek, A.H.A.M.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J.M. Acquired antibiotic resistance genes: An overview. Front Microbiol. 2011, 2, 203. [Google Scholar] [CrossRef] [PubMed]
- Druzhinina, I.S.; Chenthamara, K.; Zhang, J.; Atanasova, L.; Yang, D.; Miao, Y. Massive lateral transfer of genes encoding plant cell wall-degrading enzymes to the mycoparasitic fungus Trichoderma from its plant-associated hosts. PLOS Genet. 2018, 14, e1007322. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.S.L.; Nemchinov, L.G.; Mota, M.; Eisenback, J.D.; Kamo, K.; Vieira, P. Identification and characterization of the first pectin methylesterase gene discovered in the root lesion nematode Pratylenchus penetrans. PLoS ONE 2019, 14, e0212540. [Google Scholar] [CrossRef] [PubMed]
- Busch, A.; Danchin, E.G.J.; Pauchet, Y. Functional diversification of horizontally acquired glycoside hydrolase family 45 (GH45) proteins in Phytophaga beetles. BMC Evol. Biol. 2019, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Danchin, E.G.; Guzeeva, E.A.; Mantelin, S.; Berepiki, A.; Jones, J.T. Horizontal gene transfer from bacteria has enabled the plant-parasitic nematode Globodera pallida to feed on host-derived sucrose. Mol. Biol. Evol. 2016, 33, 1571–1579. [Google Scholar] [CrossRef]
- Cheng, X.; Garcés-Carrera, S.; Whitworth, R.J.; Fellers, J.P.; Park, Y.; Chen, M.S. A horizontal gene transfer led to the acquisition of a fructan metabolic pathway in a gall midge. Adv. Biosyst. 2020, 4, 1900275. [Google Scholar] [CrossRef]
- Frickey, T.; Lupas, A. CLANS: A Java application for visualizing protein families based on pairwise similarity. Bioinformatics 2004, 20, 3702–3704. [Google Scholar] [CrossRef] [PubMed]
- The International Aphid Genomics Consortium. Genome Sequence of the Pea Aphid Acyrthosiphon pisum. PLoS Biol. 2010, 8, e1000313. [Google Scholar] [CrossRef]
- Robertson, H.M.; Waterhouse, R.M.; Walden, K.O.; Ruzzante, L.; Maarten, J.M.; Reijnders, F.; Coates, B.S.; Legeai, F.; Gress, J.C.; Biyiklioglu, S.; et al. Genome sequence of the wheat stem sawfly, Cephus cinctus, representing an early-branching lineage of the Hymenoptera, illuminates evolution of hymeopteran cChemoreceptors. Genome Biol. Evol. 2018, 10, 2997–3011. [Google Scholar] [CrossRef] [PubMed]
- The Tribolium Genome Sequencing Consortium. The genome of the model beetle and pest Tribolium castaneum. Nature 2008, 452, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Pauchet, Y.; Kirsch, R.; Giraud, S.; Vogel, H.; Heckel, D.G. Identification and characterization of plant cell wall degrading enzymes from three glycoside hydrolase families in the cerambycid beetle Apriona japonica. Insect Biochem. Mol. Biol. 2014, 49, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Subramanyam, S.; Nemacheck, J.A.; Bernal-Crespo, V.; Sardesai, N. Insect derived extra oral GH32 plays a role in susceptibility of wheat to Hessian fly. Sci. Rep. 2021, 11, 2081. [Google Scholar] [CrossRef] [PubMed]
- Livingston, D.P.I.I.I.; Hincha, D.K.; Heyer, A.G. Fructan and its relationship to abiotic stress tolerance in plants. Cell. Mol. Life Sci. 2009, 66, 2007–2023. [Google Scholar] [CrossRef]
- Benkeblia, N. Insights on fructans and resistance of plants to drought stress. Front. Sustain. Food Syst. Sect. Crop Biol. Sustain. 2022, 6, 2022. [Google Scholar] [CrossRef]
- Shelomi, M.; Danchin, E.G.J.; Heckel, D.; Wipfler, B.; Bradler, S.; Zhou, X.; Pauchet, Y. Horizontal gene transfer of pectinases from bacteria preceded the diversification of stick and leaf insects. Sci. Rep. 2016, 6, 26388. [Google Scholar] [CrossRef]
- Le, N.G.; van Ulsen, P.; van Spanning, R.; Brouwer, A.; van Straalen, N.M.; Roelofs, D. A functional carbohydrate degrading enzyme potentially acquired by horizontal gene transfer in the genome of the soil invertebrate Folsomia candida. Genes 2022, 13, 1402. [Google Scholar] [CrossRef]
- Wang, M.; Cheong, K.L. Preparation, structural characterisation, and bioactivities of Fructans: A review. Molecules 2023, 28, 1613. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M. Fructan structure and metabolism in overwintering plants. Plants 2021, 10, 933. [Google Scholar] [CrossRef]
- Ahn, S.; Anderson, J.A.; Sorrells, M.E.; Tanksley, S.D. Homoeologous relationships of rice, wheat and maize chromosomes. Mol. Gen. Genet. 1993, 241, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, A.; Sato, Y.; Yoshida, M. Genetic engineering of rice capable of synthesizing fructans and enhancing chilling tolerance. J. Exp. Bot. 2008, 59, 793–802. [Google Scholar] [CrossRef]
- Schnyder, H.; Gillenberg, C.; Hinz, J. Fructan contents and dry matter deposition in different tissues of the wheat grain during development. Plant Cell Environ. 1993, 16, 179–187. [Google Scholar] [CrossRef]
- Cimini, S.; Locato, V.; Vergauwen, R.; Paradiso, A.; Cecchini, C.; Vandenpoel, L.; Verspreet, J.; Courtin, C.M.; D’Egidio, M.G.; Van den Ende, W.; et al. Fructan biosynthesis and degradation as part of plant metabolism controlling sugar fluxes during durum wheat kernel maturation. Front. Plant Sci. 2015, 6, 89. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.H.; Birnbaum, M.J. Molecular Aspects of Fructose Metabolism and Metabolic Disease. Cell Metab. 2021, 33, 2329–2354. Available online: https://www.sciencedirect.com/science/article/pii/S1550413121004290 (accessed on 1 May 2024). [CrossRef] [PubMed]
- Johnson, R.J.; Stenvinkel, P.; Andrews, P.; Sánchez-Lozada, L.G.; Nakagawa, T.; Gaucher, E.; Andres-Hernando, A.; Rodriguez-Iturbe, B.; Jimenez, C.R.; Garcia, G.; et al. Fructose metabolism as a common evolutionary pathway of survival associated with climate change, food shortage and droughts (Review-Symposium). J. Intern. Med. 2020, 287, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Bismut, H.; Hers, H.G.; Van Schaftingen, E. Conversion of fructose to glucose in the rabbit small intestine. A reappraisal of the direct pathway. Eur. J. Biochem. 1993, 213, 721–726. [Google Scholar] [CrossRef]
- Seternes, T.; Tonheim, T.; Myhr, A.; Dalmo, R.A. A plant 35S CaMV promoter induces long-term expression of luciferase in Atlantic salmon. Sci. Rep. 2016, 6, 25096. [Google Scholar] [CrossRef]
- Palazzo, A.; Caizzi, R.; Moschetti, R.; Marsano, R.M. What Have We Learned in 30 Years of Investigations on Bari Transposons? Cells 2022, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.B.; Senapathy, P. RNA splice junctions of different classes of eukaryotes: Sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987, 15, 7155–7174. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Zhao, L.; Chanon, A.M.; Chattopadhyay, N.; Dami, I.E.; Blakeslee, J.J. Quantification of carbohydrates in grape tissues using capillary zone electrophoresis. Front. Plant Sci. 2016, 7, 818. [Google Scholar] [CrossRef]
- Mack, A.; Wei, T.-C. Analysis of Sugars Using an Agilent InfinityLab Poroshell 120 HILIC-Z Colum. Agilent Application Note 5991-8984EN. 2019. Available online: https://www.agilent.com/cs/library/applications/5991-8984EN_Poroshell%20HILICZ_sugars_application.pdf (accessed on 1 May 2024).
- Dai, Y.; Hsiao, J. Discovery metabolomics LC MS Methods Optimized for Polar Metabolites. Agilent Application Note 5994-1492EN. 2019. Available online: https://hpst.cz/sites/default/files/download/2021/03/application-discovery-metabolomics-hilic-z-5994-1492en-agilent.pdf (accessed on 1 May 2024).
| Animal Group | Scientific Name | Species with GH32 | Genes Per Species | Total Genes |
|---|---|---|---|---|
| Dipterans | Diptera | 7 | 1-12 | 35 |
| Arachnids | Arachnida | 11 | 1-5 | 21 |
| Waters bears | Tardigrada | 2 | 1, 6 | 6 |
| Wasps | Hymenoptera | 6 | 1-4 | 11 |
| Springtails | Collembola | 10 | 1-4 | 23 |
| Rotifers | Bdelloida | 8 | 1-7 | 34 |
| Caddisflies | Trichoptera | 4 | 1-5 | 9 |
| Moths/Butterflies | Lepidoptera | 11 | 1-9 | 44 |
| Beetles | Coleoptera | 9 | 1-4 | 24 |
| Nematodes | Nematoda | 5 | 1-7 | 16 |
| Thrips | Thysanoptera | 2 | 2-3 | 6 |
| Millipedes | Diplopoda | 1 | 1 | 1 |
| Segmented worms | Annelida | 1 | 1 | 1 |
| Clam shrimps | Spinicaudata | 1 | 1 | 1 |
| Water fleas | Anomopoda | 1 | 1 | 1 |
| Woodlaces | Isopoda | 1 | 1 | 1 |
| Crabs | Pleocyemata | 1 | 1 | 1 |
| Cockroaches | Blattodea | 1 | 3 | 3 |
| True bugs | Hemiptera | 1 | 2 | 2 |
| Flatworms | Platyhelminthes | 1 | 1 | 1 |
| Sea spiders | Pycnogonida | 1 | 1 | 1 |
| Total | 84 | 242 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Liu, X.; Jordan, K.W.; Yu, J.; Whitworth, R.J.; Park, Y.; Chen, M.-S. Frequent Acquisition of Glycoside Hydrolase Family 32 (GH32) Genes from Bacteria via Horizontal Gene Transfer Drives Adaptation of Invertebrates to Diverse Sources of Food and Living Habitats. Int. J. Mol. Sci. 2024, 25, 8296. https://doi.org/10.3390/ijms25158296
Cheng X, Liu X, Jordan KW, Yu J, Whitworth RJ, Park Y, Chen M-S. Frequent Acquisition of Glycoside Hydrolase Family 32 (GH32) Genes from Bacteria via Horizontal Gene Transfer Drives Adaptation of Invertebrates to Diverse Sources of Food and Living Habitats. International Journal of Molecular Sciences. 2024; 25(15):8296. https://doi.org/10.3390/ijms25158296
Chicago/Turabian StyleCheng, Xiaoyan, Xuming Liu, Katherine W. Jordan, Jingcheng Yu, Robert J. Whitworth, Yoonseong Park, and Ming-Shun Chen. 2024. "Frequent Acquisition of Glycoside Hydrolase Family 32 (GH32) Genes from Bacteria via Horizontal Gene Transfer Drives Adaptation of Invertebrates to Diverse Sources of Food and Living Habitats" International Journal of Molecular Sciences 25, no. 15: 8296. https://doi.org/10.3390/ijms25158296







