Local Injection of Stem Cells Can Be a Potential Strategy to Improve Bladder Dysfunction after Outlet Obstruction in Rats
Abstract
:1. Introduction
2. Results
2.1. Post-pBOO Dysfunction as Determined by Cystometry Improved by hAFSCs Treatment
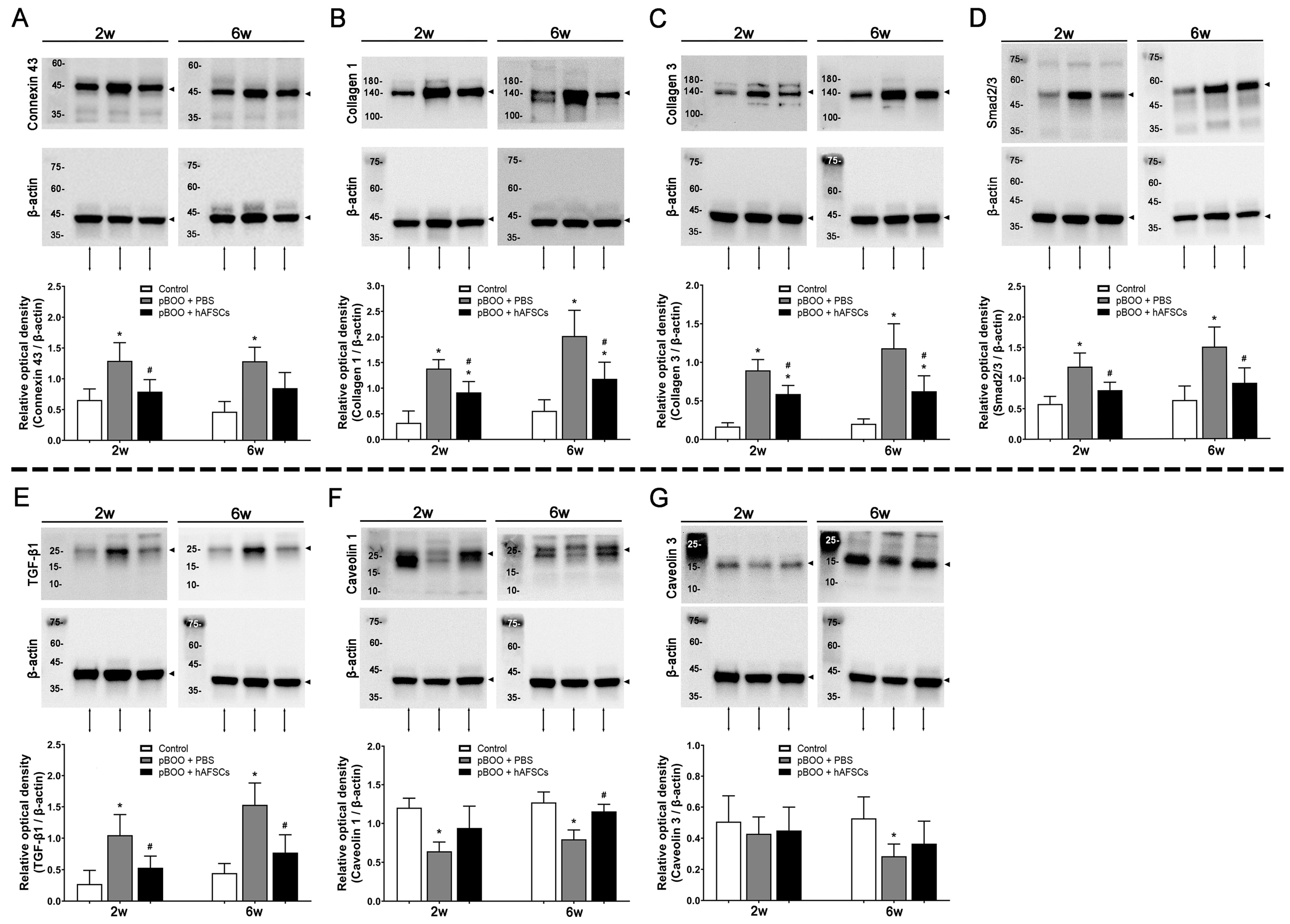
2.2. Expression of TGFβ-Smad Pathway Factors, Bladder Collagen Deposition, and Fibrosis Markers Improved by hAFSCs Treatment
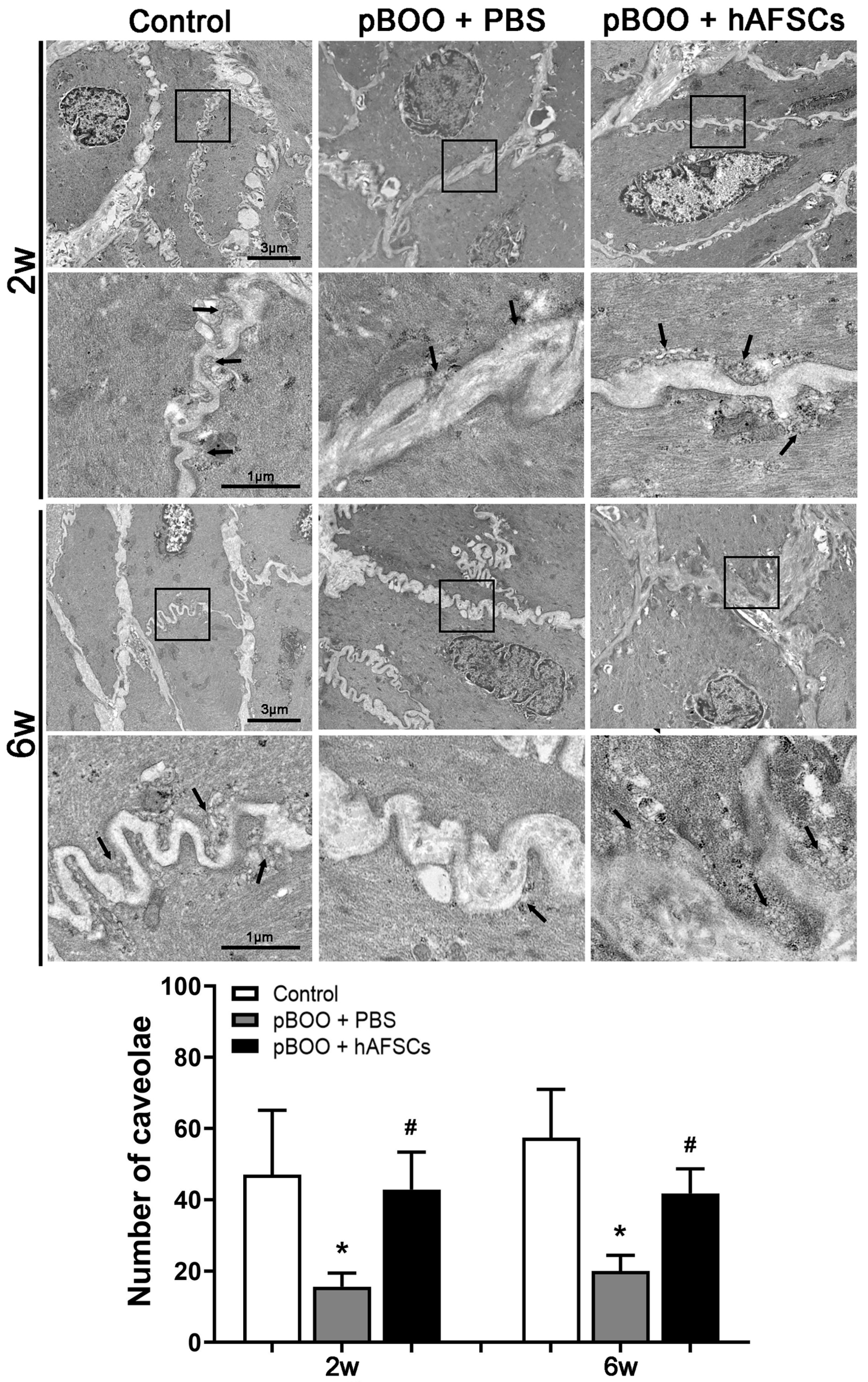
2.3. Electron Microscopy of Caveolae in Detrusor Smooth Muscle Cells (DSMCs) Recovered after hAFSC Treatment
2.4. Survival of hAFSCs in the pBOO Bladder
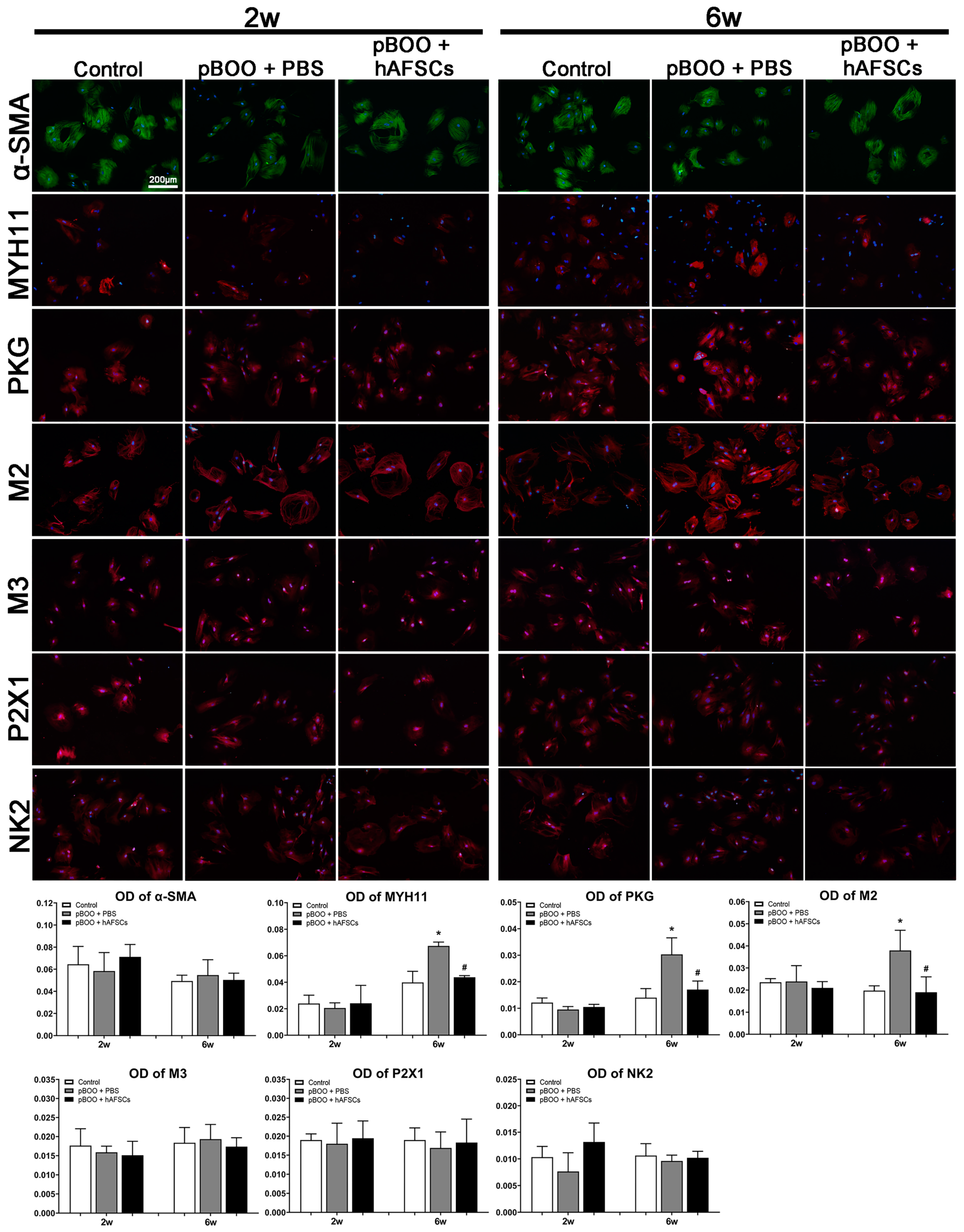
2.5. Immunofluorescent Expression of MYH11 and Receptors Mediating Bladder Contraction on DSMCs
3. Discussion
4. Materials and Methods
4.1. Experiment Design
4.2. Animal Models of pBOO
4.3. Isolation and Characterization of hAFSCs for Treatment
4.4. Conscious Cystometry
4.5. Western Blot Analysis
4.6. Culture of DSMCs
4.7. Immunofluorescence
4.8. Electron Microscopy
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, J.T.; Calhoun, E.; Jacobsen, S.J. Urologic diseases in america project: Benign prostatic hyperplasia. J. Urol. 2008, 179, S75–S80. [Google Scholar] [CrossRef] [PubMed]
- Groutz, A.; Blaivas, J.G.; Chaikin, D.C. Bladder outlet obstruction in women: Definition and characteristics. Neurourol. Urodyn. 2000, 19, 213–220. [Google Scholar] [CrossRef]
- McCrery, R.J.; Appell, R.A. Bladder outlet obstruction in women: Iatrogenic, anatomic, and neurogenic. Curr. Urol. Rep. 2006, 7, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Wiafe, B.; Adesida, A.; Churchill, T.; Adewuyi, E.E.; Li, Z.; Metcalfe, P. Hypoxia-increased expression of genes involved in inflammation, dedifferentiation, pro-fibrosis, and extracellular matrix remodeling of human bladder smooth muscle cells. Vitr. Cell Dev. Biol. Anim. 2017, 53, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Wiafe, B.; Adesida, A.; Churchill, T.; Metcalfe, P. Mesenchymal stem cells inhibit hypoxia-induced inflammatory and fibrotic pathways in bladder smooth muscle cells. World J. Urol. 2018, 36, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, P.D.; Wang, J.; Jiao, H.; Huang, Y.; Hori, K.; Moore, R.B.; Tredget, E.E. Bladder outlet obstruction: Progression from inflammation to fibrosis. BJU Int. 2010, 106, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Huang, W.C.; Shaw, S.W.; Huang, Y.H.; Lee, T.H. Human amniotic fluid stem cells can alleviate detrusor dysfunction caused by bladder outlet obstruction in rats. Sci. Rep. 2022, 12, 6679. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Smith, P.P.; Kuchel, G.A.; de Groat, W.C.; Birder, L.A.; Chermansky, C.J.; Adam, R.M.; Tse, V.; Chancellor, M.B.; Yoshimura, N. Pathophysiology and animal modeling of underactive bladder. Int. Urol. Nephrol. 2014, 46, S11–S21. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, Y.; Zhu, H.; Wang, B.; Qu, P.; Chen, R.; Sun, X. Sodium Tanshinone IIA sulfonate ameliorates bladder fibrosis in a rat model of partial bladder outlet obstruction by inhibiting the TGF-β/Smad pathway activation. PLoS ONE 2015, 10, e0129655. [Google Scholar] [CrossRef] [PubMed]
- Fullhase, C.; Hennenberg, M.; Sandner, P.; Strittmatter, F.; Niedworok, C.; Bauer, R.M.; Gratzke, C.; Soler, R.; Stief, C.; Andersson, K.E. Reduction of obstruction related bladder overactivity by the guanylyl cyclase modulators BAY 41–2272 and BAY 60–2770 alone or in combination with a phosphodiesterase type 5 inhibitor. Neurourol. Urodyn. 2015, 34, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Won, J.H.; Doo, S.H.; Kim, J.H.; Song, K.Y.; Lee, S.J.; Lim, I.; Chang, K.T.; Song, Y.S.; Kim, S.U. Inhibition of collagen deposit in obstructed rat bladder outlet by transplantation of superparamagnetic iron oxide-labeled human mesenchymal stem cells as monitored by molecular magnetic resonance imaging (MRI). Cell Transplant. 2012, 21, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Al-Saikan, B.; Ding, J.; Tredget, E.; Metcalfe, P. Benefits of mesenchymal stem cells after partial bladder outlet obstruction. Can. Urol. Assoc. J. 2016, 10, E1–E6. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Noguchi, M.; Matsuo, M.; Nomata, K.; Suematsu, T.; Kanetake, H. Decreased cellular membrane expression of gap junctional protein, connexin 43, in rat detrusor muscle with chronic partial bladder outlet obstruction. Urology 2005, 65, 1254–1258. [Google Scholar] [CrossRef] [PubMed]
- Polyak, E.; Boopathi, E.; Mohanan, S.; Deng, M.; Zderic, S.A.; Wein, A.J.; Chacko, S. Alterations in caveolin expression and ultrastructure after bladder smooth muscle hypertrophy. J. Urol. 2009, 182, 2497–2503. [Google Scholar] [CrossRef] [PubMed]
- DiSanto, M.E.; Stein, R.; Chang, S.; Hypolite, J.A.; Zheng, Y.; Zderic, S.; Wein, A.J.; Chacko, S. Alteration in expression of myosin isoforms in detrusor smooth muscle following bladder outlet obstruction. Am. J. Physiol. Cell Physiol. 2003, 285, C1397–C1410. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.T.; Martinez-Ferrer, M.; Makari, J.H.; Wills, M.L.; Thomas, J.C.; Adams, M.C.; Brock, J.W., 3rd; Pope, J.C., 4th; Bhowmick, N.A. Recruitment of bone marrow derived cells to the bladder after bladder outlet obstruction. J. Urol. 2009, 182, 1769–1774. [Google Scholar] [CrossRef]
- Song, Y.S.; Lee, H.J.; Doo, S.H.; Lee, S.J.; Lim, I.; Chang, K.T.; Kim, S.U. Mesenchymal stem cells overexpressing hepatocyte growth factor (HGF) inhibit collagen deposit and improve bladder function in rat model of bladder outlet obstruction. Cell Transplant. 2012, 21, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.S.; Mokhtari, R.B.; El Hout, Y.; Azadi, M.A.; Alauddin, M.; Yeger, H.; Farhat, W.A. TGF-beta1 induces EMT reprogramming of porcine bladder urothelial cells into collagen producing fibroblasts-like cells in a Smad2/Smad3-dependent manner. J. Cell Commun. Signal. 2014, 8, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Yoon, J.Y.; Seo, S.I.; Hwang, T.K.; Park, Y.H. Effects of partial bladder outlet obstruction and its relief on type I and II collagen and detrusor contractility in the rat. Neurol. Urodyn. 2000, 19, 29–42. [Google Scholar] [CrossRef]
- Deveaud, C.M.; Macarak, E.J.; Kucich, U.; Ewalt, D.H.; Abrams, W.R.; Howard, P.S. Molecular analysis of collagens in bladder fibrosis. J. Urol. 1998, 160, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Haefliger, J.A.; Tissieres, P.; Tawadros, T.; Formenton, A.; Bény, J.L.; Nicod, P.; Frey, P.; Medaet, P. Connexin 43 and 26 are differentially increased after rat bladder obstruction. Exp. Cell Res. 2002, 274, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Christ, G.J.; Day, N.S.; Day, M.; Zhao, W.; Persson, K.; Pandita, P.K.; Andersson, K.E. Increased connexin43-mediated intercellular communication in a rat model of bladder overactivity in vivo. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1241–R1248. [Google Scholar] [CrossRef] [PubMed]
- Galbiati, F.; Razani, B.; Lisanti, M.P. Emerging themes in lipid rafts and caveolae. Cell 2001, 106, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Cristofaro, V.; Peters, C.A.; Yalla, S.V.; Sullivan, M.P. Smooth muscle caveolae differentially regulate specific agonist induced bladder contractions. Neurourol. Urodyn. 2007, 26, 71–80. [Google Scholar] [CrossRef]
- Cohen, A.W.; Hnasko, R.; Schubert, W.; Lisanti, M.P. Role of caveolae and caveolins in health and disease. Physiol. Rev. 2004, 84, 1341–1379. [Google Scholar] [CrossRef]
- Kogo, H.; Ito, S.Y.; Moritoki, Y.; Kurahashi, H.; Fujimoto, T. Differential expression of caveolin-3 in mouse smooth muscle cells in vivo. Cell Tissue Res. 2006, 324, 291–300. [Google Scholar] [CrossRef]
- Woodman, S.E.; Cheung, M.W.; Tarr, M.; North, A.C.; Schubert, W.; Lagaud, G.; Marks, C.B.; Russell, R.G.; Hassan, G.S.; Factor, S.M.; et al. Urogenital alterations in aged male caveolin-1 knockout mice. J. Urol. 2004, 171, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Lin, Y.H.; Chen, T.C.; Chang, S.D. How antepartum and postpartum acute urinary retention affects the function and structure of rat bladder. Int. Urogynecol. J. 2014, 25, 1105–1113. [Google Scholar] [CrossRef]
- Teng, Y.D. Functional multipotency of stem cells: Biological traits gleaned from neural progeny studies. Semin. Cell Dev. Biol. 2019, 95, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Weber, W.T.; Wang, S.; Wein, A.J.; Zderic, S.A.; Chacko, S.; DiSanto, M.E. Generation of a cell line with smooth muscle phenotype from hypertrophied urinary bladder. Am. Physiol. Cell Physiol. 2002, 283, C373–C382. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.E.; Gopalakurup, S.K.; Levin, R.M.; Chacko, S. Expression of smooth muscle myosin isoforms in urinary bladder smooth muscle during hypertrophy and regression. Lab. Investig. 1995, 73, 244–251. [Google Scholar] [PubMed]
- Artim, D.E.; Kullmann, F.A.; Daugherty, S.L.; Wu, H.Y.; de Groat, W.C. Activation of the nitric oxide-cGMP pathway reduces phasic contractions in neonatal rat bladder strips via protein kinase G. Am. J. Physiol. Renal. Physiol. 2009, 297, F333–F340. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W.; Jeong, S.W.; Song, K.H. Increased expression of neuregulin 1 in the urothelium of rat bladder with partial bladder outlet obstruction. BMC. Urol. 2017, 17, 115. [Google Scholar] [CrossRef] [PubMed]
- Bahadory, F.; Moore, K.H.; Liu, L.; Burcher, E. Gene expression of muscarinic, tachykinin, and purinergic receptors in porcine bladder: Comparison with cultured cells. Front. Pharmacol. 2013, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Shaw, S.S.; Lin, Y.H.; Huang, Y.H.; Lee, T.H. Improvement in bladder dysfunction after bladder transplantation of amniotic fluid stem cells in diabetic rats. Sci. Rep. 2018, 8, 2105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, B.; Xu, Z.; Liu, Y.; Zhang, K.; Li, Y.; Wang, H. Are TGF-beta 1 and bFGF correlated with bladder underactivity induced by bladder outlet obstruction? Urol. Int. 2008, 81, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.H.; Higashira-Hoshi, H.; Itoh, Y. Functional muscarinic M2 and M3 receptors and b-adrenoceptor in cultured rat bladder smooth muscle. Life Sci. 2002, 70, 1159–1172. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, C.-C.; Shaw, S.W.; Chen, T.-C.; Lin, Y.-H.; Huang, Y.-H.; Lee, T.-H. Local Injection of Stem Cells Can Be a Potential Strategy to Improve Bladder Dysfunction after Outlet Obstruction in Rats. Int. J. Mol. Sci. 2024, 25, 8310. https://doi.org/10.3390/ijms25158310
Liang C-C, Shaw SW, Chen T-C, Lin Y-H, Huang Y-H, Lee T-H. Local Injection of Stem Cells Can Be a Potential Strategy to Improve Bladder Dysfunction after Outlet Obstruction in Rats. International Journal of Molecular Sciences. 2024; 25(15):8310. https://doi.org/10.3390/ijms25158310
Chicago/Turabian StyleLiang, Ching-Chung, Steven W. Shaw, Tse-Ching Chen, Yi-Hao Lin, Yung-Hsin Huang, and Tsong-Hai Lee. 2024. "Local Injection of Stem Cells Can Be a Potential Strategy to Improve Bladder Dysfunction after Outlet Obstruction in Rats" International Journal of Molecular Sciences 25, no. 15: 8310. https://doi.org/10.3390/ijms25158310




