Neurotrophins and Their Receptors: BDNF’s Role in GABAergic Neurodevelopment and Disease
Abstract
1. Historical Perspective on Neurotrophins
2. Neurotrophins Expression, Synthesis, Structure, and Secretion in Brain
2.1. Expression in the Developing Brain

2.2. Synthesis, Secretion, and Protein Levels
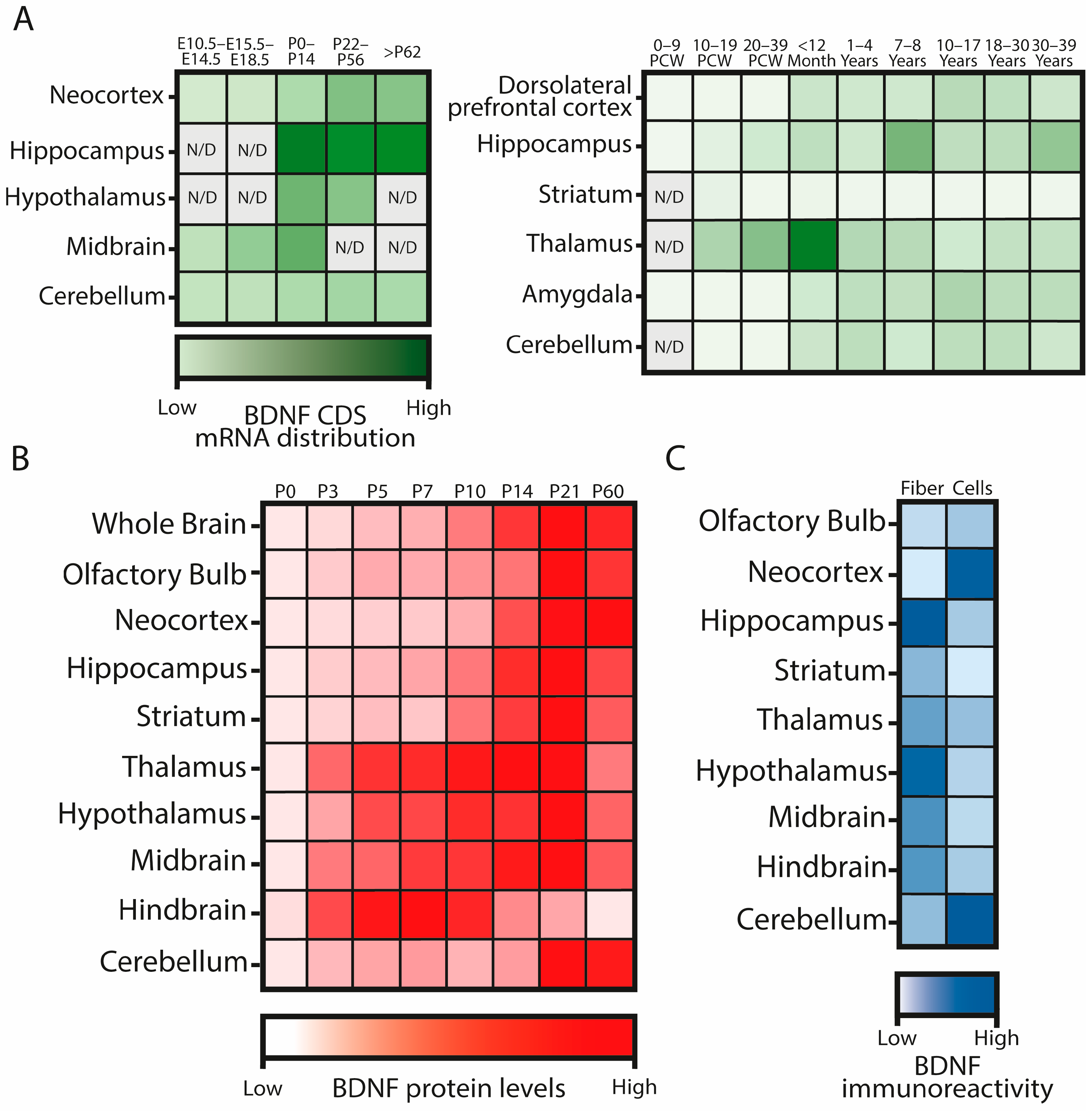
2.3. BDNF Expression and Protein Location during Development
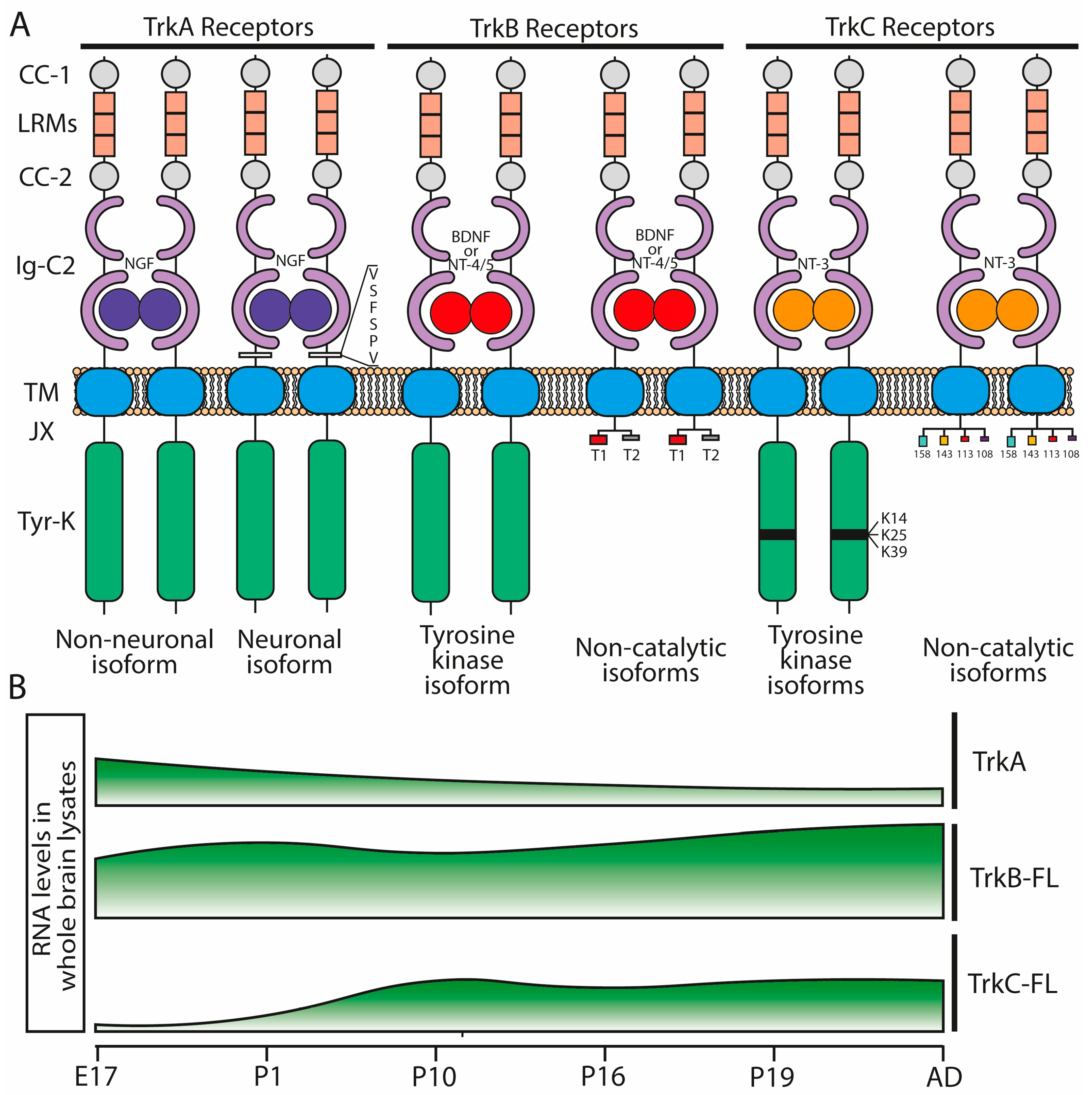
3. Neurotrophin Receptors
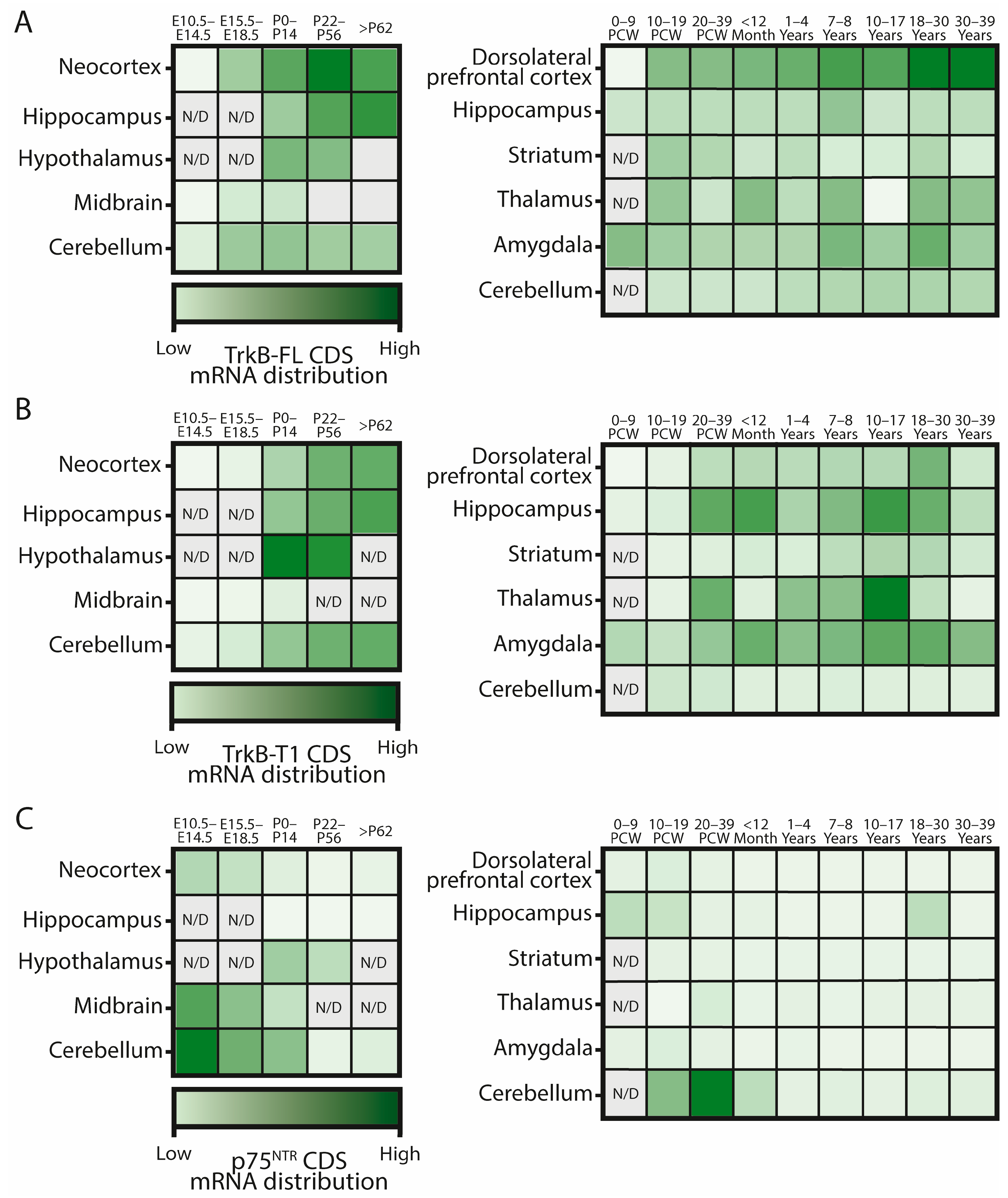
3.1. Trk Receptors
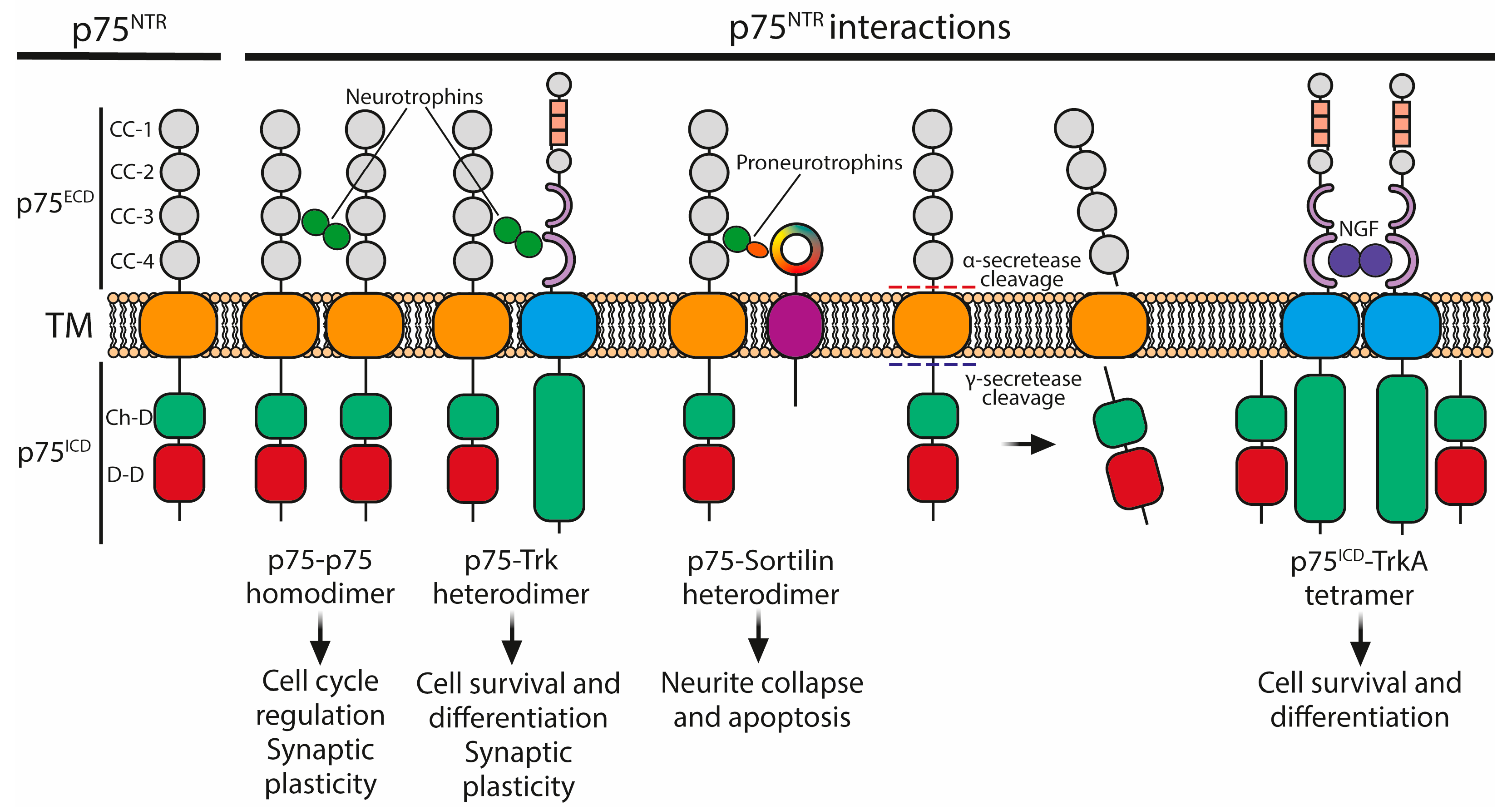
3.2. The p75 Neurotrophin Receptor
4. Role of BDNF in GABAergic Neurons
4.1. Role of BDNF in the GABAergic Cortex
4.2. Role of BDNF in the Striatum
5. BDNF on Neurodevelopmental Disorders with Impairments in GABAergic Neurons
5.1. BDNF in Autism Spectrum Disorder
5.2. BDNF in Rett Syndrome
5.3. BDNF in Schizophrenia
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Spemann, H.; Mangold, H. Induction of Embryonic Primordia by Implantation of Organizers from a Different Species. 1923. Int. J. Dev. Biol. 2001, 45, 13–38. [Google Scholar] [PubMed]
- Hamburger, V.; Levi-Montalcini, R. Proliferation, Differentiation and Degeneration in the Spinal Ganglia of the Chick Embryo under Normal and Experimental Conditions. J. Exp. Zool. 1949, 111, 457–501. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R.; Hamburger, V. Selective Growth Stimulating Effects of Mouse Sarcoma on the Sensory and Sympathetic Nervous System of the Chick Embryo. J. Exp. Zool. 1951, 116, 321–361. [Google Scholar] [CrossRef] [PubMed]
- Levi-Montalcini, R.; Hamburger, V. A Diffusible Agent of Mouse Sarcoma, Producing Hyperplasia of Sympathetic Ganglia and Hyperneurotization of Viscera in the Chick Embryo. J. Exp. Zool. 1953, 123, 233–287. [Google Scholar] [CrossRef]
- Barde, Y.A.; Edgar, D.; Thoenen, H. Purification of a New Neurotrophic Factor from Mammalian Brain. EMBO J. 1982, 1, 549–553. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.J.O.; Bromberg, E.; De Vries, E.F.J. Brain-Derived Neurotrophic Factor in Brain Disorders: Focus on Neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Lourenço, C.; Ribeiro-Rodrigues, L.; Fonseca-Gomes, J.; Tanqueiro, S.R.; Belo, R.F.; Ferreira, C.B.; Rei, N.; Ferreira-Manso, M.; De Almeida-Borlido, C.; Costa-Coelho, T.; et al. Challenges of BDNF-Based Therapies: From Common to Rare Diseases. Pharmacol. Res. 2020, 162, 105281. [Google Scholar] [CrossRef]
- Hohn, A.; Leibrock, J.; Bailey, K.; Barde, Y.-A. Identification and Characterization of a Novel Member of the Nerve Growth Factor/Brain-Derived Neurotrophic Factor Family. Nature 1990, 344, 339–341. [Google Scholar] [CrossRef]
- Jones, K.R.; Reichardt, L.F. Molecular Cloning of a Human Gene That Is a Member of the Nerve Growth Factor Family. Proc. Natl. Acad. Sci. USA 1990, 87, 8060–8064. [Google Scholar] [CrossRef]
- Maisonpierre, P.C.; Belluscio, L.; Friedman, B.; Alderson, R.F.; Wiegand, S.J.; Furth, M.E.; Lindsay, R.M.; Yancopoulos, G.D. NT-3, BDNF, and NGF in the Developing Rat Nervous System: Parallel as Well as Reciprocal Patterns of Expression. Neuron 1990, 5, 501–509. [Google Scholar] [CrossRef]
- Rosenthal, A.; Goeddel, D.V.; Nguyen, T.; Lewis, M.; Shih, A.; Laramee, G.R.; Nikolics, K.; Winslow, J.W. Primary Structure and Biological Activity of a Novel Human Neurotrophic Factor. Neuron 1990, 4, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Berkemeier, L.R.; Winslow, J.W.; Kaplan, D.R.; Nikolics, K.; Goeddel, D.V.; Rosenthal, A. Neurotrophin-5: A Novel Neurotrophic Factor That Activates Trk and trkB. Neuron 1991, 7, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Hallböök, F.; Ibáñez, C.F.; Persson, H. Evolutionary Studies of the Nerve Growth Factor Family Reveal a Novel Member Abundantly Expressed in Xenopus Ovary. Neuron 1991, 6, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Ip, N.Y.; Ibáñez, C.F.; Nye, S.H.; McClain, J.; Jones, P.F.; Gies, D.R.; Belluscio, L.; Le Beau, M.M.; Espinosa, R.; Squinto, S.P. Mammalian Neurotrophin-4: Structure, Chromosomal Localization, Tissue Distribution, and Receptor Specificity. Proc. Natl. Acad. Sci. USA 1992, 89, 3060–3064. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, T.; Belluardo, N.; Metsis, M.; Persson, H. Widespread and Developmentally Regulated Expression of Neurotrophin-4 mRNA in Rat Brain and Peripheral Tissues. Eur. J. Neurosci. 1993, 5, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Pagliusi, S.R.; Hohn, A.; Leibrock, J.; Barde, Y.A. Regional Distribution of Brain-Derived Neurotrophic Factor mRNA in the Adult Mouse Brain. EMBO J. 1990, 9, 2459–2464. [Google Scholar] [CrossRef] [PubMed]
- Esvald, E.-E.; Tuvikene, J.; Kiir, C.S.; Avarlaid, A.; Tamberg, L.; Sirp, A.; Shubina, A.; Cabrera-Cabrera, F.; Pihlak, A.; Koppel, I.; et al. Revisiting the Expression of BDNF and Its Receptors in Mammalian Development. Front. Mol. Neurosci. 2023, 16, 1182499. [Google Scholar] [CrossRef] [PubMed]
- Murase, K.; Igarashi, K.; Hayashi, K. Neurotrophin-3 (NT-3) Levels in the Developing Rat Nervous System and in Human Samples. Clin. Chim. Acta 1994, 227, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Nishio, T.; Akiguchi, I.; Furukawa, S. Detailed Distribution of Nerve Growth Factor in Rat Brain Determined by a Highly Sensitive Enzyme Immunoassay. Exp. Neurol. 1992, 116, 76–84. [Google Scholar] [CrossRef]
- Conner, J.M.; Lauterborn, J.C.; Yan, Q.; Gall, C.M.; Varon, S. Distribution of Brain-Derived Neurotrophic Factor (BDNF) Protein and mRNA in the Normal Adult Rat CNS: Evidence for Anterograde Axonal Transport. J. Neurosci. 1997, 17, 2295–2313. [Google Scholar] [CrossRef]
- Friedman, W.J.; Black, I.B.; Kaplan, D.R. Distribution of the Neurotrophins Brain-Derived Neurotrophic Factor, Neurotrophin-3, and Neurotrophin-4/5 in the Postnatal Rat Brain: An Immunocytochemical Study. Neuroscience 1998, 84, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-T.; Li, L.-Y.; Zou, X.-L.; Song, X.-B.; Hu, Y.-L.; Feng, Z.-T.; Wang, T.T.-H. Immunohistochemical Distribution of NGF, BDNF, NT-3, and NT-4 in Adult Rhesus Monkey Brains. J. Histochem. Cytochem. 2007, 55, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Arévalo, J.C.; Deogracias, R. Mechanisms Controlling the Expression and Secretion of BDNF. Biomolecules 2023, 13, 789. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, T.; Palm, K.; Metsis, M.; Reintam, T.; Paalme, V.; Saarma, M.; Persson, H. Multiple Promoters Direct Tissue-Specific Expression of the Rat BDNF Gene. Neuron 1993, 10, 475–489. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Lu, B. Diverse Functions of Multiple Bdnf Transcripts Driven by Distinct Bdnf Promoters. Biomolecules 2023, 13, 655. [Google Scholar] [CrossRef] [PubMed]
- Colliva, A.; Tongiorgi, E. Distinct Role of 5′UTR Sequences in Dendritic Trafficking of BDNF mRNA: Additional Mechanisms for the BDNF Splice Variants Spatial Code. Mol. Brain 2021, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Maynard, K.R.; Hobbs, J.W.; Sukumar, M.; Kardian, A.S.; Jimenez, D.V.; Schloesser, R.J.; Martinowich, K. Bdnf mRNA Splice Variants Differentially Impact CA1 and CA3 Dendrite Complexity and Spine Morphology in the Hippocampus. Brain Struct. Funct. 2017, 222, 3295–3307. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Chu, P.; Guo, W.; Lu, B. A Subpopulation of Bdnf-E1–Expressing Glutamatergic Neurons in the Lateral Hypothalamus Critical for Thermogenesis Control. Mol. Metab. 2020, 31, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Maynard, K.R.; Hill, J.L.; Calcaterra, N.E.; Palko, M.E.; Kardian, A.; Paredes, D.; Sukumar, M.; Adler, B.D.; Jimenez, D.V.; Schloesser, R.J.; et al. Functional Role of BDNF Production from Unique Promoters in Aggression and Serotonin Signaling. Neuropsychopharmacology 2016, 41, 1943–1955. [Google Scholar] [CrossRef]
- Maynard, K.R.; Hobbs, J.W.; Phan, B.N.; Gupta, A.; Rajpurohit, S.; Williams, C.; Rajpurohit, A.; Shin, J.H.; Jaffe, A.E.; Martinowich, K. BDNF-TrkB Signaling in Oxytocin Neurons Contributes to Maternal Behavior. eLife 2018, 7, e33676. [Google Scholar] [CrossRef]
- Hong, E.J.; McCord, A.E.; Greenberg, M.E. A Biological Function for the Neuronal Activity-Dependent Component of Bdnf Transcription in the Development of Cortical Inhibition. Neuron 2008, 60, 610–624. [Google Scholar] [CrossRef] [PubMed]
- Sakata, K.; Woo, N.H.; Martinowich, K.; Greene, J.S.; Schloesser, R.J.; Shen, L.; Lu, B. Critical Role of Promoter IV-Driven BDNF Transcription in GABAergic Transmission and Synaptic Plasticity in the Prefrontal Cortex. Proc. Natl. Acad. Sci. USA 2009, 106, 5942–5947. [Google Scholar] [CrossRef] [PubMed]
- Kolbeck, R.; Bartke, I.; Eberle, W.; Barde, Y. Brain-Derived Neurotrophic Factor Levels in the Nervous System of Wild-Type and Neurotrophin Gene Mutant Mice. J. Neurochem. 1999, 72, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Barbacid, M. The Trk Family of Neurotrophin Receptors. J. Neurobiol. 1994, 25, 1386–1403. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Trk Receptors: Roles in Neuronal Signal Transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Tebar, A.; Dechant, G.; Barde, Y.-A. Binding of Brain-Derived Neurotrophic Factor to the Nerve Growth Factor Receptor. Neuron 1990, 4, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tébar, A.; Dechant, G.; Götz, R.; Barde, Y.A. Binding of Neurotrophin-3 to Its Neuronal Receptors and Interactions with Nerve Growth Factor and Brain-Derived Neurotrophic Factor. EMBO J. 1992, 11, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Rydén, M.; Murray-Rust, J.; Glass, D.; Ilag, L.L.; Trupp, M.; Yancopoulos, G.D.; McDonald, N.Q.; Ibáñez, C.F. Functional Analysis of Mutant Neurotrophins Deficient in Low-Affinity Binding Reveals a Role for p75LNGFR in NT-4 Signalling. EMBO J. 1995, 14, 1979–1990. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.D.; Duarte, C.B. p75NTR Processing and Signaling: Functional Role. In Handbook of Neurotoxicity; Kostrzewa, R.M., Ed.; Springer: New York, NY, USA, 2014; pp. 1899–1923. ISBN 978-1-4614-5835-7. [Google Scholar]
- Conroy, J.N.; Coulson, E.J. High-Affinity TrkA and P75 Neurotrophin Receptor Complexes: A Twisted Affair. J. Biol. Chem. 2022, 298, 101568. [Google Scholar] [CrossRef]
- Valenzuela, D.M.; Maisonpierre, P.C.; Glass, D.J.; Rojas, E.; Nuñez, L.; Kong, Y.; Gies, D.R.; Stitt, T.N.; Ip, N.Y.; Yancopoulos, G.D. Alternative Forms of Rat TrkC with Different Functional Capabilities. Neuron 1993, 10, 963–974. [Google Scholar] [CrossRef]
- Lee, F.S.; Chao, M.V. Activation of Trk Neurotrophin Receptors in the Absence of Neurotrophins. Proc. Natl. Acad. Sci. USA 2001, 98, 3555–3560. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.S.; Rajagopal, R.; Kim, A.H.; Chang, P.C.; Chao, M.V. Activation of Trk Neurotrophin Receptor Signaling by Pituitary Adenylate Cyclase-Activating Polypeptides. J. Biol. Chem. 2002, 277, 9096–9102. [Google Scholar] [CrossRef]
- Rajagopal, R.; Chen, Z.-Y.; Lee, F.S.; Chao, M.V. Transactivation of Trk Neurotrophin Receptors by G-Protein-Coupled Receptor Ligands Occurs on Intracellular Membranes. J. Neurosci. 2004, 24, 6650–6658. [Google Scholar] [CrossRef] [PubMed]
- Jeanneteau, F.; Garabedian, M.J.; Chao, M.V. Activation of Trk Neurotrophin Receptors by Glucocorticoids Provides a Neuroprotective Effect. Proc. Natl. Acad. Sci. USA 2008, 105, 4862–4867. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Z.; Pan, E.; Xiong, Z.-Q.; McNamara, J.O. Zinc-Mediated Transactivation of TrkB Potentiates the Hippocampal Mossy Fiber-CA3 Pyramid Synapse. Neuron 2008, 57, 546–558. [Google Scholar] [CrossRef]
- Puehringer, D.; Orel, N.; Lüningschrör, P.; Subramanian, N.; Herrmann, T.; Chao, M.V.; Sendtner, M. EGF Transactivation of Trk Receptors Regulates the Migration of Newborn Cortical Neurons. Nat. Neurosci. 2013, 16, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Mitre, M.; Saadipour, K.; Williams, K.; Khatri, L.; Froemke, R.C.; Chao, M.V. Transactivation of TrkB Receptors by Oxytocin and Its G Protein-Coupled Receptor. Front. Mol. Neurosci. 2022, 15, 891537. [Google Scholar] [CrossRef] [PubMed]
- Snider, W.D. Functions of the Neurotrophins during Nervous System Development: What the Knockouts Are Teaching Us. Cell 1994, 77, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Ernfors, P.; Lee, K.-F.; Jaenisch, R. Mice Lacking Brain-Derived Neurotrophic Factor Develop with Sensory Deficits. Nature 1994, 368, 147–150. [Google Scholar] [CrossRef]
- Liu, X.; Ernfors, P.; Wu, H.; Jaenisch, R. Sensory but Not Motor Neuron Deficits in Mice Lacking NT4 and BDNF. Nature 1995, 375, 238–241. [Google Scholar] [CrossRef]
- Chen, K.S.; Nishimura, M.C.; Armanini, M.P.; Crowley, C.; Spencer, S.D.; Phillips, H.S. Disruption of a Single Allele of the Nerve Growth Factor Gene Results in Atrophy of Basal Forebrain Cholinergic Neurons and Memory Deficits. J. Neurosci. 1997, 17, 7288–7296. [Google Scholar] [CrossRef]
- Rauskolb, S.; Zagrebelsky, M.; Dreznjak, A.; Deogracias, R.; Matsumoto, T.; Wiese, S.; Erne, B.; Sendtner, M.; Schaeren-Wiemers, N.; Korte, M.; et al. Global Deprivation of Brain-Derived Neurotrophic Factor in the CNS Reveals an Area-Specific Requirement for Dendritic Growth. J. Neurosci. 2010, 30, 1739–1749. [Google Scholar] [CrossRef]
- Reichardt, L.F. Neurotrophin-Regulated Signalling Pathways. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef]
- Park, H.; Poo, M. Neurotrophin Regulation of Neural Circuit Development and Function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Cao, T.; Matyas, J.J.; Renn, C.L.; Faden, A.I.; Dorsey, S.G.; Wu, J. Function and Mechanisms of Truncated BDNF Receptor TrkB.T1 in Neuropathic Pain. Cells 2020, 9, 1194. [Google Scholar] [CrossRef]
- Biffo, S.; Offenhäuser, N.; Carter, B.D.; Barde, Y.-A. Selective Binding and Internalisation by Truncated Receptors Restrict the Availability of BDNF during Development. Development 1995, 121, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Holt, L.M.; Hernandez, R.D.; Pacheco, N.L.; Torres Ceja, B.; Hossain, M.; Olsen, M.L. Astrocyte Morphogenesis Is Dependent on BDNF Signaling via Astrocytic TrkB.T1. eLife 2019, 8, e44667. [Google Scholar] [CrossRef]
- Aroeira, R.I.; Sebastião, A.M.; Valente, C.A. BDNF, via Truncated TrkB Receptor, Modulates GlyT1 and GlyT2 in Astrocytes: Modulation of GlyT by BDNF. Glia 2015, 63, 2181–2197. [Google Scholar] [CrossRef] [PubMed]
- Dorsey, S.G.; Renn, C.L.; Carim-Todd, L.; Barrick, C.A.; Bambrick, L.; Krueger, B.K.; Ward, C.W.; Tessarollo, L. In Vivo Restoration of Physiological Levels of Truncated TrkB.T1 Receptor Rescues Neuronal Cell Death in a Trisomic Mouse Model. Neuron 2006, 51, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Saba, J.; Turati, J.; Ramírez, D.; Carniglia, L.; Durand, D.; Lasaga, M.; Caruso, C. Astrocyte Truncated Tropomyosin Receptor Kinase B Mediates Brain-derived Neurotrophic Factor Anti-apoptotic Effect Leading to Neuroprotection. J. Neurochem. 2018, 146, 686–702. [Google Scholar] [CrossRef]
- Michaelsen, K.; Zagrebelsky, M.; Berndt-Huch, J.; Polack, M.; Buschler, A.; Sendtner, M.; Korte, M. Neurotrophin Receptors TrkB.T1 and P75 NTR Cooperate in Modulating Both Functional and Structural Plasticity in Mature Hippocampal Neurons. Eur. J. Neurosci. 2010, 32, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Underwood, C.K.; Coulson, E.J. The P75 Neurotrophin Receptor. Int. J. Biochem. Cell Biol. 2008, 40, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Fahnestock, M.; Michalski, B.; Xu, B.; Coughlin, M.D. The Precursor Pro-Nerve Growth Factor Is the Predominant Form of Nerve Growth Factor in Brain and Is Increased in Alzheimer’s Disease. Mol. Cell. Neurosci. 2001, 18, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.A.; Leon, W.C.; Fragoso, G.; Mushynski, W.E.; Almazan, G.; Cuello, A.C. Amyloid β-Induced Nerve Growth Factor Dysmetabolism in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2009, 68, 857–869. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Harte-Hargrove, L.C.; Siao, C.-J.; Marinic, T.; Clarke, R.; Ma, Q.; Jing, D.; LaFrancois, J.J.; Bath, K.G.; Mark, W.; et al. proBDNF Negatively Regulates Neuronal Remodeling, Synaptic Transmission, and Synaptic Plasticity in Hippocampus. Cell Rep. 2014, 7, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Aloyz, R.S.; Bamji, S.X.; Pozniak, C.D.; Toma, J.G.; Atwal, J.; Kaplan, D.R.; Miller, F.D. P53 Is Essential for Developmental Neuron Death as Regulated by the TrkA and P75 Neurotrophin Receptors. J. Cell Biol. 1998, 143, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Linggi, M.S.; Burke, T.L.; Williams, B.B.; Harrington, A.; Kraemer, R.; Hempstead, B.L.; Yoon, S.O.; Carter, B.D. Neurotrophin Receptor Interacting Factor (NRIF) Is an Essential Mediator of Apoptotic Signaling by the P75 Neurotrophin Receptor. J. Biol. Chem. 2005, 280, 13801–13808. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.B.E.; Howell, J.; Kodama, Y.; Barker, P.A.; Bonni, A. Characterization of the C-Jun N-Terminal Kinase-Bim EL Signaling Pathway in Neuronal Apoptosis. J. Neurosci. 2004, 24, 8762–8770. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bhakar, A.L.; Howell, J.L.; Paul, C.E.; Salehi, A.H.; Becker, E.B.E.; Said, F.; Bonni, A.; Barker, P.A. Apoptosis Induced by p75NTR Overexpression Requires Jun Kinase-Dependent Phosphorylation of Bad. J. Neurosci. 2003, 23, 11373–11381. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Tucker, K.L.; Barde, Y.-A. Neurotrophin Binding to the P75 Receptor Modulates Rho Activity and Axonal Outgrowth. Neuron 1999, 24, 585–593. [Google Scholar] [CrossRef]
- Shonukan, T.; Bagayogo, I.; McCrea, P.; Chao, M.; Hempstead, B. Neurotrophin-Induced Melanoma Cell Migration Is Mediated through the Actin-Bundling Protein Fascin. Oncogene 2003, 22, 3616–3623. [Google Scholar] [CrossRef]
- Zagrebelsky, M.; Holz, A.; Dechant, G.; Barde, Y.-A.; Bonhoeffer, T.; Korte, M. The P75 Neurotrophin Receptor Negatively Modulates Dendrite Complexity and Spine Density in Hippocampal Neurons. J. Neurosci. 2005, 25, 9989–9999. [Google Scholar] [CrossRef]
- Singh, K.K.; Park, K.J.; Hong, E.J.; Kramer, B.M.; Greenberg, M.E.; Kaplan, D.R.; Miller, F.D. Developmental Axon Pruning Mediated by BDNF-p75NTR–Dependent Axon Degeneration. Nat. Neurosci. 2008, 11, 649–658. [Google Scholar] [CrossRef]
- Deinhardt, K.; Kim, T.; Spellman, D.S.; Mains, R.E.; Eipper, B.A.; Neubert, T.A.; Chao, M.V.; Hempstead, B.L. Neuronal Growth Cone Retraction Relies on Proneurotrophin Receptor Signaling Through Rac. Sci. Signal. 2011, 4, ra82. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Rauskolb, S.; Polack, M.; Klose, J.; Kolbeck, R.; Korte, M.; Barde, Y.-A. Biosynthesis and Processing of Endogenous BDNF: CNS Neurons Store and Secrete BDNF, not pro-BDNF. Nat. Neurosci. 2008, 11, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, G.; Zaitsev, E.; Senatorov, V.V.; Yang, J.; Hempstead, B.L.; Lu, B. Control of Extracellular Cleavage of ProBDNF by High Frequency Neuronal Activity. Proc. Natl. Acad. Sci. USA 2009, 106, 1267–1272. [Google Scholar] [CrossRef]
- Woo, N.H.; Teng, H.K.; Siao, C.-J.; Chiaruttini, C.; Pang, P.T.; Milner, T.A.; Hempstead, B.L.; Lu, B. Activation of p75NTR by proBDNF Facilitates Hippocampal Long-Term Depression. Nat. Neurosci. 2005, 8, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Frade, J.M.; López-Sánchez, N. A Novel Hypothesis for Alzheimer Disease Based on Neuronal Tetraploidy Induced by P75 NTR. Cell Cycle 2010, 9, 1934–1941. [Google Scholar] [CrossRef]
- Frade, J.M.; Ovejero-Benito, M.C. Neuronal Cell Cycle: The Neuron Itself and Its Circumstances. Cell Cycle 2015, 14, 712–720. [Google Scholar] [CrossRef]
- Camuso, S.; La Rosa, P.; Fiorenza, M.T.; Canterini, S. Pleiotropic Effects of BDNF on the Cerebellum and Hippocampus: Implications for Neurodevelopmental Disorders. Neurobiol. Dis. 2022, 163, 105606. [Google Scholar] [CrossRef]
- Vicario-Abejón, C.; Collin, C.; McKay, R.D.G.; Segal, M. Neurotrophins Induce Formation of Functional Excitatory and Inhibitory Synapses between Cultured Hippocampal Neurons. J. Neurosci. 1998, 18, 7256–7271. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, E.G.; An, J.J.; Orefice, L.L.; Baydyuk, M.; Liao, G.-Y.; Zheng, K.; Lu, B.; Xu, B. BDNF Promotes Differentiation and Maturation of Adult-Born Neurons through GABAergic Transmission. J. Neurosci. 2012, 32, 14318–14330. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Sun, X.; Yang, Y.; Du, Y.; Lin, Y.; Xiang, J.; Zhou, N. Reduction of BDNF Results in GABAergic Neuroplasticity Dysfunction and Contributes to Late-Life Anxiety Disorder. Behav. Neurosci. 2019, 133, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Götz, M.; Huttner, W.B. The Cell Biology of Neurogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Ortega, J.A.; Alcántara, S. BDNF/MAPK/ERK–Induced BMP7 Expression in the Developing Cerebral Cortex Induces Premature Radial Glia Differentiation and Impairs Neuronal Migration. Cereb. Cortex 2010, 20, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.; Mi, D.; Llorca, A.; Marín, O. Development and Functional Diversification of Cortical Interneurons. Neuron 2018, 100, 294–313. [Google Scholar] [CrossRef] [PubMed]
- Llorca, A.; Deogracias, R. Origin, Development, and Synaptogenesis of Cortical Interneurons. Front. Neurosci. 2022, 16, 929469. [Google Scholar] [CrossRef] [PubMed]
- Kohara, K.; Kitamura, A.; Adachi, N.; Nishida, M.; Itami, C.; Nakamura, S.; Tsumoto, T. Inhibitory but Not Excitatory Cortical Neurons Require Presynaptic Brain-Derived Neurotrophic Factor for Dendritic Development, as Revealed by Chimera Cell Culture. J. Neurosci. 2003, 23, 6123–6131. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.A.; Zeiler, S.R.; Tamowski, S.; Jones, K.R. Brain-Derived Neurotrophic Factor Is Required for the Maintenance of Cortical Dendrites. J. Neurosci. 2003, 23, 6856–6865. [Google Scholar] [CrossRef]
- Patz, S. Parvalbumin Expression in Visual Cortical Interneurons Depends on Neuronal Activity and TrkB Ligands during an Early Period of Postnatal Development. Cereb. Cortex 2004, 14, 342–351. [Google Scholar] [CrossRef]
- Du, Z.; Tertrais, M.; Courtand, G.; Leste-Lasserre, T.; Cardoit, L.; Masmejean, F.; Halgand, C.; Cho, Y.H.; Garret, M. Differential Alteration in Expression of Striatal GABAAR Subunits in Mouse Models of Huntington’s Disease. Front. Mol. Neurosci. 2017, 10, 198. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Huertas, C.; Rico, B. CREB-Dependent Regulation of GAD65 Transcription by BDNF/TrkB in Cortical Interneurons. Cereb. Cortex 2011, 21, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Holter, M.C.; Hewitt, L.T.; Nishimura, K.J.; Knowles, S.J.; Bjorklund, G.R.; Shah, S.; Fry, N.R.; Rees, K.P.; Gupta, T.A.; Daniels, C.W.; et al. Hyperactive MEK1 Signaling in Cortical GABAergic Neurons Promotes Embryonic Parvalbumin Neuron Loss and Defects in Behavioral Inhibition. Cereb. Cortex 2021, 31, 3064–3081. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.G.; Zhang, H.; Murthy, V.N. Deletion of TrkB in Parvalbumin Interneurons Alters Cortical Neural Dynamics. J. Cell. Physiol. 2022, 237, 949–964. [Google Scholar] [CrossRef]
- Grabert, J.; Wahle, P. Neuronal Activity and TrkB Ligands Influence Kv3.1b and Kv3.2 Expression in Developing Cortical Interneurons. Neuroscience 2008, 156, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, J.W.; Oohashi, T.; Pizzorusso, T. The Roles of Perineuronal Nets and the Perinodal Extracellular Matrix in Neuronal Function. Nat. Rev. Neurosci. 2019, 20, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Willis, A.; Pratt, J.A.; Morris, B.J. BDNF and JNK Signaling Modulate Cortical Interneuron and Perineuronal Net Development: Implications for Schizophrenia-Linked 16p11.2 Duplication Syndrome. Schizophr. Bull. 2021, 47, 812–826. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.; Alfonsi, F.; Kurniawan, N.D.; Milne, M.R.; Kasherman, M.A.; Delogu, A.; Piper, M.; Coulson, E.J. The P75 Neurotrophin Receptor Is Required for the Survival of Neuronal Progenitors and Normal Formation of the Basal Forebrain, Striatum, Thalamus and Neocortex. Development 2019, 146, dev181933. [Google Scholar] [CrossRef]
- Baho, E.; Chattopadhyaya, B.; Lavertu-Jolin, M.; Mazziotti, R.; Awad, P.N.; Chehrazi, P.; Groleau, M.; Jahannault-Talignani, C.; Vaucher, E.; Ango, F.; et al. P75 Neurotrophin Receptor Activation Regulates the Timing of the Maturation of Cortical Parvalbumin Interneuron Connectivity and Promotes Juvenile-like Plasticity in Adult Visual Cortex. J. Neurosci. 2019, 39, 4489–4510. [Google Scholar] [CrossRef]
- Chehrazi, P.; Lee, K.K.Y.; Lavertu-Jolin, M.; Abbasnejad, Z.; Carreño-Muñoz, M.I.; Chattopadhyaya, B.; Di Cristo, G. The P75 Neurotrophin Receptor in Preadolescent Prefrontal Parvalbumin Interneurons Promotes Cognitive Flexibility in Adult Mice. Biol. Psychiatry 2023, 94, 310–321. [Google Scholar] [CrossRef]
- Knowles, R.; Dehorter, N.; Ellender, T. From Progenitors to Progeny: Shaping Striatal Circuit Development and Function. J. Neurosci. 2021, 41, 9483–9502. [Google Scholar] [CrossRef] [PubMed]
- Dudman, J.T.; Gerfen, C.R. The Basal Ganglia. In The Rat Nervous System; Elsevier: Amsterdam, The Netherlands, 2015; pp. 391–440. ISBN 978-0-12-374245-2. [Google Scholar]
- Chuhma, N.; Tanaka, K.F.; Hen, R.; Rayport, S. Functional Connectome of the Striatal Medium Spiny Neuron. J. Neurosci. 2011, 31, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, D.; Petryszyn, S.; Sanchez, M.G.; Bories, C.; Beaulieu, J.M.; De Koninck, Y.; Parent, A.; Parent, M. Striatal Neurons Expressing D1 and D2 Receptors Are Morphologically Distinct and Differently Affected by Dopamine Denervation in Mice. Sci. Rep. 2017, 7, 41432. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R. Segregation of D1 and D2 Dopamine Receptors in the Striatal Direct and Indirect Pathways: An Historical Perspective. Front. Synaptic Neurosci. 2023, 14, 1002960. [Google Scholar] [CrossRef] [PubMed]
- Florio, T.M.; Scarnati, E.; Rosa, I.; Di Censo, D.; Ranieri, B.; Cimini, A.; Galante, A.; Alecci, M. The Basal Ganglia: More than Just a Switching Device. CNS Neurosci. Ther. 2018, 24, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Eisinger, R.S.; Urdaneta, M.E.; Foote, K.D.; Okun, M.S.; Gunduz, A. Non-Motor Characterization of the Basal Ganglia: Evidence from Human and Non-Human Primate Electrophysiology. Front. Neurosci. 2018, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Xu, B. BDNF Signaling and Survival of Striatal Neurons. Front. Cell. Neurosci. 2014, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Ehinger, Y.; Soneja, D.; Phamluong, K.; Salvi, A.; Ron, D. Identification of Novel BDNF-Specific Corticostriatal Circuitries. Eneuro 2023, 10, ENEURO.0238-21.2023. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Abi-Dargham, A.; Howes, O.D. Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 2019, 42, 205–220. [Google Scholar] [CrossRef]
- Ayon-Olivas, M.; Wolf, D.; Andreska, T.; Granado, N.; Lüningschrör, P.; Ip, C.W.; Moratalla, R.; Sendtner, M. Dopaminergic Input Regulates the Sensitivity of Indirect Pathway Striatal Spiny Neurons to Brain-Derived Neurotrophic Factor. Biology 2023, 12, 1360. [Google Scholar] [CrossRef]
- Andreska, T.; Lüningschrör, P.; Wolf, D.; McFleder, R.L.; Ayon-Olivas, M.; Rattka, M.; Drechsler, C.; Perschin, V.; Blum, R.; Aufmkolk, S.; et al. DRD1 Signaling Modulates TrkB Turnover and BDNF Sensitivity in Direct Pathway Striatal Medium Spiny Neurons. Cell Rep. 2023, 42, 112575. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yui, D.; Luikart, B.W.; McKay, R.M.; Li, Y.; Rubenstein, J.L.; Parada, L.F. Conditional Ablation of Brain-Derived Neurotrophic Factor-TrkB Signaling Impairs Striatal Neuron Development. Proc. Natl. Acad. Sci. USA 2012, 109, 15491–15496. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Russell, T.; Liao, G.-Y.; Zang, K.; An, J.J.; Reichardt, L.F.; Xu, B. TrkB Receptor Controls Striatal Formation by Regulating the Number of Newborn Striatal Neurons. Proc. Natl. Acad. Sci. USA 2011, 108, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Baydyuk, M.; Xie, Y.; Tessarollo, L.; Xu, B. Midbrain-Derived Neurotrophins Support Survival of Immature Striatal Projection Neurons. J. Neurosci. 2013, 33, 3363–3369. [Google Scholar] [CrossRef] [PubMed]
- Koshimizu, H.; Matsuoka, H.; Nakajima, Y.; Kawai, A.; Ono, J.; Ohta, K.; Miki, T.; Sunagawa, M.; Adachi, N.; Suzuki, S. Brain-derived Neurotrophic Factor Predominantly Regulates the Expression of Synapse-related Genes in the Striatum: Insights from in Vitro Transcriptomics. Neuropsychopharmacol. Rep. 2021, 41, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Goggi, J.; Pullar, I.A.; Carney, S.L.; Bradford, H.F. Signalling Pathways Involved in the Short-Term Potentiation of Dopamine Release by BDNF. Brain Res. 2003, 968, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.-S.; Kim, I.S.; Baik, J.-H.; Kim, D.; Cocco, L.; Suh, P.-G. The Function of PLCγ1 in Developing Mouse mDA System. Adv. Biol. Regul. 2020, 75, 100654. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, J.; Kim, H.-J.; Lee, B.E.; Jeong, J.; Cho, E.J.; Jang, H.-J.; Shin, K.J.; Kim, M.J.; Chae, Y.C.; et al. PLCγ1 in Dopamine Neurons Critically Regulates Striatal Dopamine Release via VMAT2 and Synapsin III. Exp. Mol. Med. 2023, 55, 2357–2375. [Google Scholar] [CrossRef] [PubMed]
- Hourez, R.; Azdad, K.; Vanwalleghem, G.; Roussel, C.; Gall, D.; Schiffmann, S.N. Activation of Protein Kinase C and Inositol 1,4,5-Triphosphate Receptors Antagonistically Modulate Voltage-Gated Sodium Channels in Striatal Neurons. Brain Res. 2005, 1059, 189–196. [Google Scholar] [CrossRef]
- Clements, M.A.; Swapna, I.; Morikawa, H. Inositol 1,4,5-Triphosphate Drives Glutamatergic and Cholinergic Inhibition Selectively in Spiny Projection Neurons in the Striatum. J. Neurosci. 2013, 33, 2697–2708. [Google Scholar] [CrossRef]
- Kärkkäinen, E.; Yavich, L.; Miettinen, P.O.; Tanila, H. Opposing Effects of APP/PS1 and TrkB.T1 Genotypes on Midbrain Dopamine Neurons and Stimulated Dopamine Release in Vivo. Brain Res. 2015, 1622, 452–465. [Google Scholar] [CrossRef] [PubMed]
- Torres-Cruz, F.M.; César Vivar-Cortés, I.; Moran, I.; Mendoza, E.; Gómez-Pineda, V.; García-Sierra, F.; Hernández-Echeagaray, E. NT-4/5 Antagonizes the BDNF Modulation of Corticostriatal Transmission: Role of the TrkB.T1 Receptor. CNS Neurosci. Ther. 2019, 25, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.R.; Costa, J.T.; Melo, C.V.; Felizzi, F.; Monteiro, P.; Pinto, M.J.; Inácio, A.R.; Wieloch, T.; Almeida, R.D.; Grãos, M.; et al. Excitotoxicity Downregulates TrkB.FL Signaling and Upregulates the Neuroprotective Truncated TrkB Receptors in Cultured Hippocampal and Striatal Neurons. J. Neurosci. 2012, 32, 4610–4622. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, J.L.; Surmeier, D.J. Impaired Striatal Function in Huntington’s Disease Is Due to Aberrant p75NTR Signaling. Rare Dis. 2014, 2, e968482. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wehner, A.B.; Milen, A.M.; Albin, R.L.; Pierchala, B.A. The P75 Neurotrophin Receptor Augments Survival Signaling in the Striatum of Pre-Symptomatic Q175 Mice. Neuroscience 2016, 324, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yan, A.; Zhao, J.; Yang, S.; Song, L.; Liu, Z. The P75 Neurotrophin Receptor as a Novel Intermediate in L-Dopa-Induced Dyskinesia in Experimental Parkinson’s Disease. Exp. Neurol. 2021, 342, 113740. [Google Scholar] [CrossRef] [PubMed]
- Darcq, E.; Morisot, N.; Phamluong, K.; Warnault, V.; Jeanblanc, J.; Longo, F.M.; Massa, S.M.; Ron, D. The Neurotrophic Factor Receptor P75 in the Rat Dorsolateral Striatum Drives Excessive Alcohol Drinking. J. Neurosci. 2016, 36, 10116–10127. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthi, K.; Lin, Y. The Contribution of GABAergic Dysfunction to Neurodevelopmental Disorders. Trends Mol. Med. 2011, 17, 452–462. [Google Scholar] [CrossRef]
- Jiang, C.-C.; Lin, L.-S.; Long, S.; Ke, X.-Y.; Fukunaga, K.; Lu, Y.-M.; Han, F. Signalling Pathways in Autism Spectrum Disorder: Mechanisms and Therapeutic Implications. Signal Transduct. Target. Ther. 2022, 7, 229. [Google Scholar] [CrossRef]
- Bicks, L.K.; Geschwind, D.H. Functional Neurogenomics in Autism Spectrum Disorders: A Decade of Progress. Curr. Opin. Neurobiol. 2024, 86, 102858. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Varela-Nallar, L. Wnt Signalling in Neuronal Differentiation and Development. Cell Tissue Res. 2015, 359, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, W.-C.; Sejourne, J.; Clipperton-Allen, A.E.; Page, D.T. Pten Mutations Alter Brain Growth Trajectory and Allocation of Cell Types through Elevated -Catenin Signaling. J. Neurosci. 2015, 35, 10252–10267. [Google Scholar] [CrossRef] [PubMed]
- Lu, B. BDNF and Activity-Dependent Synaptic Modulation: Figure 1. Learn. Mem. 2003, 10, 86–98. [Google Scholar] [CrossRef] [PubMed]
- West, A.E.; Chen, W.G.; Dalva, M.B.; Dolmetsch, R.E.; Kornhauser, J.M.; Shaywitz, A.J.; Takasu, M.A.; Tao, X.; Greenberg, M.E. Calcium Regulation of Neuronal Gene Expression. Proc. Natl. Acad. Sci. USA 2001, 98, 11024–11031. [Google Scholar] [CrossRef] [PubMed]
- Ilchibaeva, T.; Tsybko, A.; Lipnitskaya, M.; Eremin, D.; Milutinovich, K.; Naumenko, V.; Popova, N. Brain-Derived Neurotrophic Factor (BDNF) in Mechanisms of Autistic-like Behavior in BTBR Mice: Crosstalk with the Dopaminergic Brain System. Biomedicines 2023, 11, 1482. [Google Scholar] [CrossRef] [PubMed]
- Sgritta, M.; Vignoli, B.; Pimpinella, D.; Griguoli, M.; Santi, S.; Bialowas, A.; Wiera, G.; Zacchi, P.; Malerba, F.; Marchetti, C.; et al. Impaired Synaptic Plasticity in an Animal Model of Autism Exhibiting Early Hippocampal GABAergic-BDNF/TrkB Signaling Alterations. iScience 2023, 26, 105728. [Google Scholar] [CrossRef] [PubMed]
- Bludau, A.; Royer, M.; Meister, G.; Neumann, I.D.; Menon, R. Epigenetic Regulation of the Social Brain. Trends Neurosci. 2019, 42, 471–484. [Google Scholar] [CrossRef]
- Zhubi, A.; Chen, Y.; Dong, E.; Cook, E.H.; Guidotti, A.; Grayson, D.R. Increased Binding of MeCP2 to the GAD1 and RELN Promoters May Be Mediated by an Enrichment of 5-hmC in Autism Spectrum Disorder (ASD) Cerebellum. Transl. Psychiatry 2014, 4, e349. [Google Scholar] [CrossRef]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF Val66met Polymorphism Affects Activity-Dependent Secretion of BDNF and Human Memory and Hippocampal Function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Uegaki, K.; Kumanogoh, H.; Mizui, T.; Hirokawa, T.; Ishikawa, Y.; Kojima, M. BDNF Binds Its Pro-Peptide with High Affinity and the Common Val66Met Polymorphism Attenuates the Interaction. Int. J. Mol. Sci. 2017, 18, 1042. [Google Scholar] [CrossRef]
- Ma, K.; Taylor, C.; Williamson, M.; Newton, S.S.; Qin, L. Diminished Activity-Dependent BDNF Signaling Differentially Causes Autism-like Behavioral Deficits in Male and Female Mice. Front. Psychiatry 2023, 14, 1182472. [Google Scholar] [CrossRef] [PubMed]
- Neul, J.L.; Kaufmann, W.E.; Glaze, D.G.; Christodoulou, J.; Clarke, A.J.; Bahi-Buisson, N.; Leonard, H.; Bailey, M.E.S.; Schanen, N.C.; Zappella, M.; et al. Rett Syndrome: Revised Diagnostic Criteria and Nomenclature. Ann. Neurol. 2010, 68, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Pozzo-Miller, L. BDNF Deregulation in Rett Syndrome. Neuropharmacology 2014, 76, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Ure, K.; Lu, H.; Wang, W.; Ito-Ishida, A.; Wu, Z.; He, L.; Sztainberg, Y.; Chen, W.; Tang, J.; Zoghbi, H.Y. Restoration of Mecp2 Expression in GABAergic Neurons Is Sufficient to Rescue Multiple Disease Features in a Mouse Model of Rett Syndrome. eLife 2016, 5, e14198. [Google Scholar] [CrossRef] [PubMed]
- Pejhan, S.; Rastegar, M. Role of DNA Methyl-CpG-Binding Protein MeCP2 in Rett Syndrome Pathobiology and Mechanism of Disease. Biomolecules 2021, 11, 75. [Google Scholar] [CrossRef] [PubMed]
- Ebert, D.H.; Gabel, H.W.; Robinson, N.D.; Kastan, N.R.; Hu, L.S.; Cohen, S.; Navarro, A.J.; Lyst, M.J.; Ekiert, R.; Bird, A.P.; et al. Activity-Dependent Phosphorylation of MeCP2 Threonine 308 Regulates Interaction with NCoR. Nature 2013, 499, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Tillotson, R.; Bird, A. The Molecular Basis of MeCP2 Function in the Brain. J. Mol. Biol. 2020, 432, 1602–1623. [Google Scholar] [CrossRef] [PubMed]
- Chahrour, M.; Jung, S.Y.; Shaw, C.; Zhou, X.; Wong, S.T.C.; Qin, J.; Zoghbi, H.Y. MeCP2, a Key Contributor to Neurological Disease, Activates and Represses Transcription. Science 2008, 320, 1224–1229. [Google Scholar] [CrossRef] [PubMed]
- Vuu, Y.M.; Roberts, C.-T.; Rastegar, M. MeCP2 Is an Epigenetic Factor That Links DNA Methylation with Brain Metabolism. Int. J. Mol. Sci. 2023, 24, 4218. [Google Scholar] [CrossRef]
- Tzeng, C.P.; Whitwam, T.; Boxer, L.D.; Li, E.; Silberfeld, A.; Trowbridge, S.; Mei, K.; Lin, C.; Shamah, R.; Griffith, E.C.; et al. Activity-Induced MeCP2 Phosphorylation Regulates Retinogeniculate Synapse Refinement. Proc. Natl. Acad. Sci. USA 2023, 120, e2310344120. [Google Scholar] [CrossRef]
- Cohen, S.; Gabel, H.W.; Hemberg, M.; Hutchinson, A.N.; Sadacca, L.A.; Ebert, D.H.; Harmin, D.A.; Greenberg, R.S.; Verdine, V.K.; Zhou, Z.; et al. Genome-Wide Activity-Dependent MeCP2 Phosphorylation Regulates Nervous System Development and Function. Neuron 2011, 72, 72–85. [Google Scholar] [CrossRef]
- Kim, J.-W.; Autry, A.E.; Na, E.S.; Adachi, M.; Björkholm, C.; Kavalali, E.T.; Monteggia, L.M. Sustained Effects of Rapidly Acting Antidepressants Require BDNF-Dependent MeCP2 Phosphorylation. Nat. Neurosci. 2021, 24, 1100–1109. [Google Scholar] [CrossRef]
- Lyst, M.J.; Ekiert, R.; Ebert, D.H.; Merusi, C.; Nowak, J.; Selfridge, J.; Guy, J.; Kastan, N.R.; Robinson, N.D.; De Lima Alves, F.; et al. Rett Syndrome Mutations Abolish the Interaction of MeCP2 with the NCoR/SMRT Co-Repressor. Nat. Neurosci. 2013, 16, 898–902. [Google Scholar] [CrossRef]
- Li, W. Excitation and Inhibition Imbalance in Rett Syndrome. Front. Neurosci. 2022, 16, 825063. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Khare, G.; Dani, V.; Nelson, S.; Jaenisch, R. The Disease Progression of Mecp2 Mutant Mice Is Affected by the Level of BDNF Expression. Neuron 2006, 49, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Larimore, J.L.; Chapleau, C.A.; Kudo, S.; Theibert, A.; Percy, A.K.; Pozzo-Miller, L. Bdnf Overexpression in Hippocampal Neurons Prevents Dendritic Atrophy Caused by Rett-Associated MECP2 Mutations. Neurobiol. Dis. 2009, 34, 199–211. [Google Scholar] [CrossRef]
- Massa, S.M.; Yang, T.; Xie, Y.; Shi, J.; Bilgen, M.; Joyce, J.N.; Nehama, D.; Rajadas, J.; Longo, F.M. Small Molecule BDNF Mimetics Activate TrkB Signaling and Prevent Neuronal Degeneration in Rodents. J. Clin. Investig. 2010, 120, 1774–1785. [Google Scholar] [CrossRef]
- Johnson, R.A.; Lam, M.; Punzo, A.M.; Li, H.; Lin, B.R.; Ye, K.; Mitchell, G.S.; Chang, Q. 7,8-Dihydroxyflavone Exhibits Therapeutic Efficacy in a Mouse Model of Rett Syndrome. J. Appl. Physiol. 2012, 112, 704–710. [Google Scholar] [CrossRef]
- Li, W.; Bellot-Saez, A.; Phillips, M.L.; Yang, T.; Longo, F.M.; Pozzo-Miller, L. A Small-Molecule TrkB Ligand Restores Hippocampal Synaptic Plasticity and Object Location Memory in Rett Syndrome Mice. Dis. Model. Mech. 2017, 10, 837–845. [Google Scholar] [CrossRef]
- Deogracias, R.; Yazdani, M.; Dekkers, M.P.J.; Guy, J.; Ionescu, M.C.S.; Vogt, K.E.; Barde, Y.-A. Fingolimod, a Sphingosine-1 Phosphate Receptor Modulator, Increases BDNF Levels and Improves Symptoms of a Mouse Model of Rett Syndrome. Proc. Natl. Acad. Sci. USA 2012, 109, 14230–14235. [Google Scholar] [CrossRef]
- Naegelin, Y.; Kuhle, J.; Schädelin, S.; Datta, A.N.; Magon, S.; Amann, M.; Barro, C.; Ramelli, G.P.; Heesom, K.; Barde, Y.-A.; et al. Fingolimod in Children with Rett Syndrome: The FINGORETT Study. Orphanet J. Rare Dis. 2021, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Keam, S.J. Trofinetide: First Approval. Drugs 2023, 83, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, P.; Punzi, G.; Ursini, G. Brain-Derived Neurotrophic Factor and Schizophrenia. Psychiatr. Genet. 2019, 29, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Jauhar, S.; Johnstone, M.; McKenna, P.J. Schizophrenia. Lancet 2022, 399, 473–486. [Google Scholar] [CrossRef] [PubMed]
- del Pino, I.; García-Frigola, C.; Dehorter, N.; Brotons-Mas, J.R.; Alvarez-Salvado, E.; Martínez de Lagrán, M.; Ciceri, G.; Gabaldón, M.V.; Moratal, D.; Dierssen, M.; et al. Erbb4 Deletion from Fast-Spiking Interneurons Causes Schizophrenia-like Phenotypes. Neuron 2013, 79, 1152–1168. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-M.; Shen, C.-J.; Chen, X.-J.; Kong, Y.; Liu, Y.-S.; Li, X.-W.; Chen, Z.; Gao, T.-M.; Li, X.-M. Erbb4 Deficits in Chandelier Cells of the Medial Prefrontal Cortex Confer Cognitive Dysfunctions: Implications for Schizophrenia. Cereb. Cortex 2019, 29, 4334–4346. [Google Scholar] [CrossRef] [PubMed]
- Abi-Dargham, A.; Mawlawi, O.; Lombardo, I.; Gil, R.; Martinez, D.; Huang, Y.; Hwang, D.-R.; Keilp, J.; Kochan, L.; Van Heertum, R.; et al. Prefrontal Dopamine D 1 Receptors and Working Memory in Schizophrenia. J. Neurosci. 2002, 22, 3708–3719. [Google Scholar] [CrossRef]
- Xing, B.; Guo, J.; Meng, X.; Wei, S.; Li, S. The Dopamine D1 but Not D3 Receptor Plays a Fundamental Role in Spatial Working Memory and BDNF Expression in Prefrontal Cortex of Mice. Behav. Brain Res. 2012, 235, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Leriche, L.; Bezard, E.; Gross, C.; Guillin, O.; Foll, B.; Diaz, J.; Sokoloff, P. The Dopamine D3 Receptor: A Therapeutic Target for the Treatment of Neuropsychiatric Disorders. CNS Neurol. Disord.-Drug Targets 2006, 5, 25–43. [Google Scholar] [CrossRef]
- Guillin, O.; Diaz, J.; Carroll, P.; Griffon, N.; Schwartz, J.-C.; Sokoloff, P. BDNF Controls Dopamine D3 Receptor Expression and Triggers Behavioural Sensitization. Nature 2001, 411, 86–89. [Google Scholar] [CrossRef]
- Harb, M.; Jagusch, J.; Durairaja, A.; Endres, T.; Leßmann, V.; Fendt, M. BDNF Haploinsufficiency Induces Behavioral Endophenotypes of Schizophrenia in Male Mice That Are Rescued by Enriched Environment. Transl. Psychiatry 2021, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Nieto, R.R.; Carrasco, A.; Corral, S.; Castillo, R.; Gaspar, P.A.; Bustamante, M.L.; Silva, H. BDNF as a Biomarker of Cognition in Schizophrenia/Psychosis: An Updated Review. Front. Psychiatry 2021, 12, 662407. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Angelucci, F.; Aloe, L.; Iannitelli, A.; Korf, J. Nerve Growth Factor and Brain-Derived Neurotrophic Factor in Schizophrenia and Depression: Findings in Humans, and Animal Models. Curr. Neuropharmacol. 2003, 1, 109–123. [Google Scholar] [CrossRef]
- Angelucci, F.; Brenè, S.; Mathé, A.A. BDNF in Schizophrenia, Depression and Corresponding Animal Models. Mol. Psychiatry 2005, 10, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Want, A.; Morgan, J.E.; Barde, Y.-A. Brain-Derived Neurotrophic Factor Measurements in Mouse Serum and Plasma Using a Sensitive and Specific Enzyme-Linked Immunosorbent Assay. Sci. Rep. 2023, 13, 7740. [Google Scholar] [CrossRef] [PubMed]
- Want, A.; Nan, X.; Kokkali, E.; Barde, Y.-A.; Morgan, J.E. Brain-Derived Neurotrophic Factor Released from Blood Platelets Prevents Dendritic Atrophy of Lesioned Adult Central Nervous System Neurons. Brain Commun. 2023, 5, fcad046. [Google Scholar] [CrossRef]
- Klein, A.B.; Williamson, R.; Santini, M.A.; Clemmensen, C.; Ettrup, A.; Rios, M.; Knudsen, G.M.; Aznar, S. Blood BDNF Concentrations Reflect Brain-Tissue BDNF Levels across Species. Int. J. Neuropsychopharmacol. 2011, 14, 347–353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-del Caño, C.; Varela-Andrés, N.; Cebrián-León, A.; Deogracias, R. Neurotrophins and Their Receptors: BDNF’s Role in GABAergic Neurodevelopment and Disease. Int. J. Mol. Sci. 2024, 25, 8312. https://doi.org/10.3390/ijms25158312
Hernández-del Caño C, Varela-Andrés N, Cebrián-León A, Deogracias R. Neurotrophins and Their Receptors: BDNF’s Role in GABAergic Neurodevelopment and Disease. International Journal of Molecular Sciences. 2024; 25(15):8312. https://doi.org/10.3390/ijms25158312
Chicago/Turabian StyleHernández-del Caño, Carlos, Natalia Varela-Andrés, Alejandro Cebrián-León, and Rubén Deogracias. 2024. "Neurotrophins and Their Receptors: BDNF’s Role in GABAergic Neurodevelopment and Disease" International Journal of Molecular Sciences 25, no. 15: 8312. https://doi.org/10.3390/ijms25158312
APA StyleHernández-del Caño, C., Varela-Andrés, N., Cebrián-León, A., & Deogracias, R. (2024). Neurotrophins and Their Receptors: BDNF’s Role in GABAergic Neurodevelopment and Disease. International Journal of Molecular Sciences, 25(15), 8312. https://doi.org/10.3390/ijms25158312




