Phage Therapy: An Alternative Approach to Combating Multidrug-Resistant Bacterial Infections in Cystic Fibrosis
Abstract
:1. Cystic Fibrosis and Related Bacterial Infections
2. An Overview on Main Pathogens of Patients with CF
3. Phage Therapy Overall
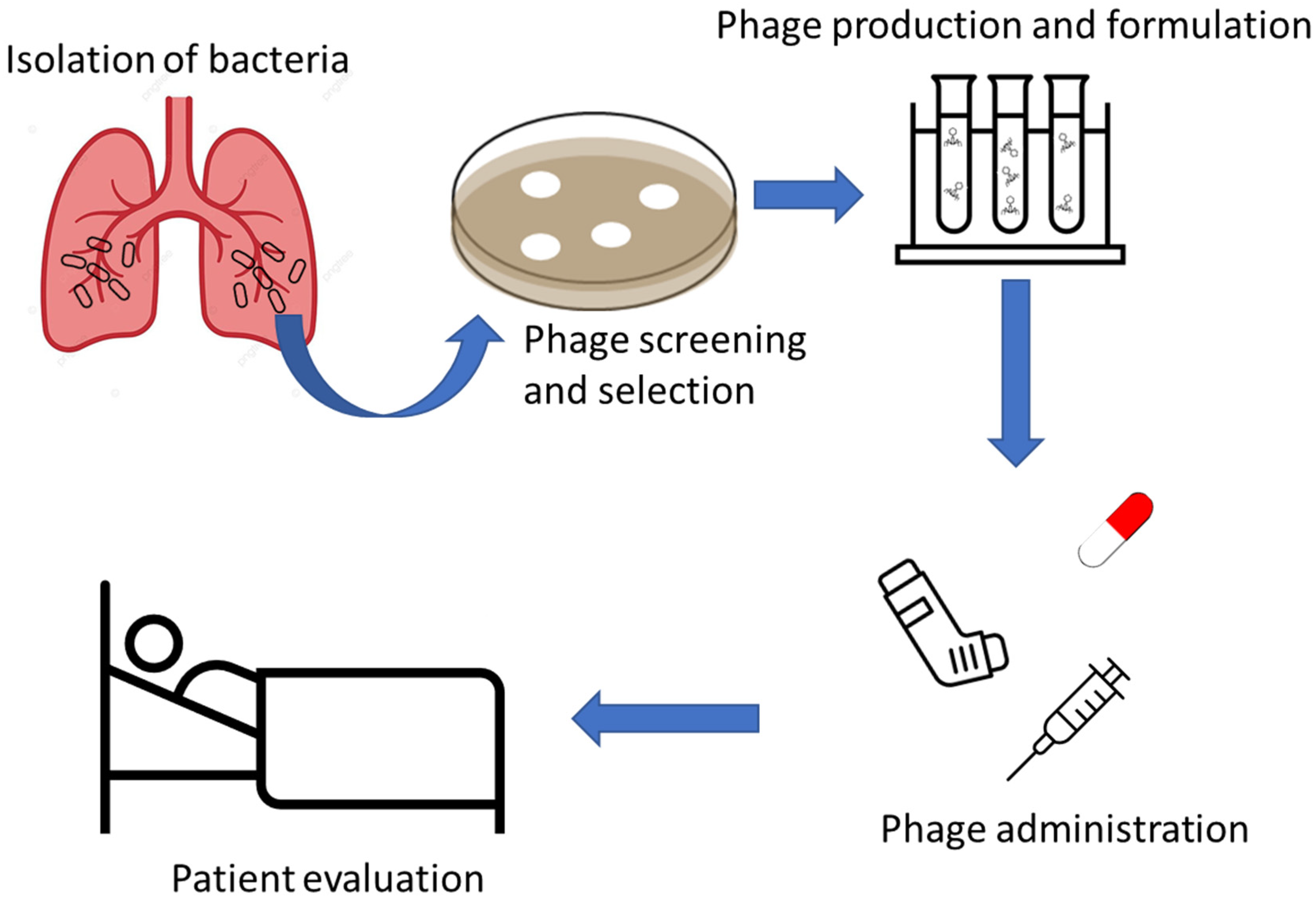
4. Phage Therapy in Patients with Cystic Fibrosis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morrison, C.B.; Markovetz, M.R.; Ehre, C. Mucus, mucins, and cystic fibrosis. Pediatr. Pulmonol. 2019, 54 (Suppl. 3), S84–S96. [Google Scholar] [CrossRef]
- Chen, Q.; Ilanga, M.; Simbassa, S.B.; Chirra, B.; Shah, K.N.; Cannon, C.L. Synergistic antimicrobial effects of ibuprofen combined with standard-of-care antibiotics against cystic fibrosis pathogens. Biomedicines 2023, 11, 2936. [Google Scholar] [CrossRef]
- Al Shakirchi, M.; Sorjonen, K.; Hjelte, L.; Klingspor, L.; Bergman, P.; Ericson, P.; Svedberg, M.; Lindberg, U.; Hansen, C.; Monestrol, I. Impact of lumacaftor/ivacaftor on the bacterial and fungal respiratory pathogens in cystic fibrosis: A prospective multicenter cohort study in Sweden. Ther. Adv. Respir. Dis. 2024, 18, 17534666241254090. [Google Scholar] [CrossRef]
- Gnaien, M.; Maufrais, C.; Rebai, Y.; Kallel, A.; Ma, L.; Hamouda, S.; Khalsi, F.; Meftah, K.; Smaoui, H.; Khemiri, M.; et al. A gain-of-function mutation in zinc cluster transcription factor Rob1 drives Candida albicans adaptive growth in the cystic fibrosis lung environment. PLoS Pathog. 2024, 20, e1012154. [Google Scholar] [CrossRef]
- Hallouch, O.; Marinos, J.; Thibault, F.; Vu, K.N.; Chalaoui, J.; Bourgouin, P.; Péloquin, L.; Freire, V.; Tremblay, F.; Chartrand-Lefebvre, C. Cystic fibrosis in the 21st century: What every radiologist should know. Clin. Imaging 2022, 84, 118–129. [Google Scholar] [CrossRef]
- Castellani, C.; Assael, B.M. Cystic fibrosis: A clinical view. Cell. Mol. Life Sci. 2017, 74, 129–140. [Google Scholar] [CrossRef]
- Available online: www.genet.sickkids.on.ca (accessed on 3 June 2024).
- Lopes-Pacheco, M. CFTR modulators: The changing face of cystic fibrosis in the era of precision medicine. Front. Pharmacol. 2019, 10, 1662. [Google Scholar] [CrossRef]
- O’Sullivan, B.P.; Freedman, S.D. Cystic fibrosis. Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef]
- Shanthikumar, S.; Neeland, M.N.; Saffery, R.; Ranganathan, S. Gene modifiers of cystic fibrosis lung disease: A systematic review. Pediatr. Pulmonol. 2019, 54, 1356–1366. [Google Scholar] [CrossRef]
- Graeber, S.Y.; Renz, D.M.; Stahl, M.; Pallenberg, S.T.; Sommerburg, O.; Naehrlich, L.; Berges, J.; Dohna, M.; Ringshausen, F.C.; Doellinger, F.; et al. Effects of Elexacaftor/Tezacaftor/Ivacaftor therapy on lung clearance index and magnetic resonance imaging in patients with cystic fibrosis and one or two F508del alleles. Am. J. Respir. Crit. Care Med. 2022, 206, 311–320. [Google Scholar] [CrossRef]
- Lopes-Pacheco, M.; Sabirzhanova, I.; Rapino, D.; Morales, M.M.; Guggino, W.B.; Cebotaru, L. Correctors rescue CFTR mutations in nucleotide-binding domain 1 (NBD1) by modulating proteostasis. Chembiochem 2016, 17, 493–505. [Google Scholar] [CrossRef]
- Heifets, L. Mycobacterial infections caused by nontuberculous mycobacteria. Semin. Respir. Crit. Care Med. 2004, 25, 283–295. [Google Scholar] [CrossRef]
- Schmalstig, A.A.; Wiggins, A.; Badillo, D.; Wetzel, K.S.; Hatfull, G.F.; Braunstein, M. Bacteriophage infection and killing of intracellular. mBio 2024, 15, e0292423. [Google Scholar] [CrossRef] [PubMed]
- Cocorullo, M.; Chiarelli, L.R.; Stelitano, G. Improving protection to prevent bacterial infections: Preliminary applications of reverse vaccinology against the main cystic fibrosis pathogens. Vaccines 2023, 11, 1221. [Google Scholar] [CrossRef]
- Recchia, D.; Stelitano, G.; Stamilla, A.; Gutierrez, D.L.; Degiacomi, G.; Chiarelli, L.R.; Pasca, M.R. Infections in cystic fibrosis individuals: A review on therapeutic options. Int. J. Mol. Sci. 2023, 24, 4635. [Google Scholar] [CrossRef]
- Wnorowska, U.; Łysik, D.; Piktel, E.; Zakrzewska, M.; Okła, S.; Lesiak, A.; Spałek, J.; Mystkowska, J.; Savage, P.B.; Janmey, P.; et al. Ceragenin-mediated disruption of Pseudomonas aeruginosa biofilms. PLoS ONE 2024, 19, e0298112. [Google Scholar] [CrossRef]
- Matos, G.R.; Feliciano, J.R.; Leitão, J.H. Non-coding regulatory sRNAs from bacteria of the Burkholderia cepacia complex. Appl. Microbiol. Biotechnol. 2024, 108, 280. [Google Scholar] [CrossRef]
- Lord, R.; Jones, A.M.; Horsley, A. Antibiotic treatment for Burkholderia cepacia complex in people with cystic fibrosis experiencing a pulmonary exacerbation. Cochrane Database Syst. Rev. 2020, 4, CD009529. [Google Scholar] [CrossRef]
- Slack, M.P.E.; Cripps, A.W.; Grimwood, K.; Mackenzie, G.A.; Ulanova, M. Invasive Haemophilus influenzae Infections after 3 decades of Hib protein conjugate vaccine use. Clin. Microbiol. Rev. 2021, 34, e0002821. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.D.; Herrmann, J.L.; Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat. Rev. Microbiol. 2020, 18, 392–407. [Google Scholar] [CrossRef] [PubMed]
- Catherinot, E.; Roux, A.L.; Macheras, E.; Hubert, D.; Matmar, M.; Dannhoffer, L.; Chinet, T.; Morand, P.; Poyart, C.; Heym, B.; et al. Acute respiratory failure involving an R variant of Mycobacterium abscessus. J. Clin. Microbiol. 2009, 47, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Degiacomi, G.; Sammartino, J.C.; Chiarelli, L.R.; Riabova, O.; Makarov, V.; Pasca, M.R. Mycobacterium abscessus, an emerging and worrisome pathogen among cystic fibrosis patients. Int. J. Mol. Sci. 2019, 20, 5868. [Google Scholar] [CrossRef] [PubMed]
- Gilljam, M.; Scherstén, H.; Silverborn, M.; Jönsson, B.; Ericsson Hollsing, A. Lung transplantation in patients with cystic fibrosis and Mycobacterium abscessus infection. J. Cyst. Fibros. 2010, 9, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.V.; Viljoen, A.; Ghigo, E.; Herrmann, J.L.; Kremer, L. Glycopeptidolipids, a double-edged sword of the Mycobacterium abscessus complex. Front. Microbiol. 2018, 9, 1145. [Google Scholar] [CrossRef] [PubMed]
- Howard, S.T.; Rhoades, E.; Recht, J.; Pang, X.; Alsup, A.; Kolter, R.; Lyons, C.R.; Byrd, T.F. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 2006, 152, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Catherinot, E.; Clarissou, J.; Etienne, G.; Ripoll, F.; Emile, J.F.; Daffé, M.; Perronne, C.; Soudais, C.; Gaillard, J.L.; Rottman, M. Hypervirulence of a rough variant of the Mycobacterium abscessus type strain. Infect. Immun. 2007, 75, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Cocorullo, M.; Bettoni, C.; Foiadelli, S.; Stelitano, G. Moles of Molecules against Mycobacterium abscessus. Future Pharmacol. 2023, 3, 637–663. [Google Scholar] [CrossRef]
- Floto, R.A.; Olivier, K.N.; Saiman, L.; Daley, C.L.; Herrmann, J.L.; Nick, J.A.; Noone, P.G.; Bilton, D.; Corris, P.; Gibson, R.L.; et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax 2016, 71 (Suppl. 1), i1–i22. [Google Scholar] [CrossRef] [PubMed]
- Neff, S.L.; Doing, G.; Reiter, T.; Hampton, T.H.; Greene, C.S.; Hogan, D. Pseudomonas aeruginosa transcriptome analysis of metal restriction in ex vivo cystic fibrosis sputum. Microbiol. Spectr. 2024, 12, e0315723. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Zhu, J.; Xiao, Y. Antibacterial peptide Reg4 ameliorates Pseudomonas aeruginosa-induced pulmonary inflammation and fibrosis. Microbiol. Spectr. 2024, 12, e0390523. [Google Scholar] [CrossRef]
- Botelho, J.; Grosso, F.; Peixe, L. Antibiotic resistance in Pseudomonas aeruginosa–Mechanisms, epidemiology and evolution. Drug Resist. Updates 2019, 44, 100640. [Google Scholar] [CrossRef] [PubMed]
- Tigabu, A.; Getaneh, A. Staphylococcus aureus, ESKAPE bacteria challenging current health care and community settings: A literature review. Clin. Lab. 2021, 67, 1539–1549. [Google Scholar] [CrossRef] [PubMed]
- Naorem, R.S.; Pangabam, B.D.; Bora, S.S.; Goswami, G.; Barooah, M.; Hazarika, D.J.; Fekete, C. Identification of putative vaccine and drug targets against the methicillin-resistant Staphylococcus aureus by reverse vaccinology and subtractive genomics Approaches. Molecules 2022, 27, 2083. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.J.; Lowy, F.D. Pathogenesis of methicillin-resistant Staphylococcus aureus infection. Clin. Infect. Dis. 2008, 46 (Suppl. 5), S350–S359. [Google Scholar] [CrossRef] [PubMed]
- Green, S.I.; Clark, J.R.; Santos, H.H.; Weesner, K.E.; Salazar, K.C.; Aslam, S.; Campbell, J.W.; Doernberg, S.B.; Blodget, E.; Morris, M.I.; et al. A retrospective, observational study of 12 cases of expanded-access customized phage therapy: Production, characteristics, and clinical outcomes. Clin. Infect. Dis. 2023, 77, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Dedrick, R.M.; Smith, B.E.; Cristinziano, M.; Freeman, K.G.; Jacobs-Sera, D.; Belessis, Y.; Whitney Brown, A.; Cohen, K.A.; Davidson, R.M.; van Duin, D.; et al. Phage therapy of mycobacterium infections: Compassionate use of phages in 20 patients with drug-resistant mycobacterial disease. Clin. Infect. Dis. 2023, 76, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Yeoh, E.; Fitzgerald, D.A.; Selvadurai, H. A systematic review on the use of bacteriophage in treating Staphylococcus aureus and Pseudomonas aeruginosa infections in cystic fibrosis. Paediatr. Respir. Rev. 2023, 48, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef] [PubMed]
- Moreau-Marquis, S.; O’Toole, G.A.; Stanton, B.A. Tobramycin and FDA-approved iron chelators eliminate Pseudomonas aeruginosa biofilms on cystic fibrosis cells. Am. J. Respir. Cell Mol. Biol. 2009, 41, 305–313. [Google Scholar] [CrossRef]
- Nikolich, M.P.; Filippov, A.A. Bacteriophage therapy: Developments and directions. Antibiotics 2020, 9, 135. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Koutsokera, A.; Csajka, C.; Blanchon, S.; Sauty, A.; Brunet, J.F.; von Garnier, C.; Resch, G.; Guery, B. Phage therapy for pulmonary infections: Lessons from clinical experiences and key considerations. Eur. Respir. Rev. 2022, 31, 220121. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Souli, M.; Billard, M.; Campbell, J.; Conrad, D.; Ellison, D.W.; Evans, B.; Evans, S.R.; Greenwood-Quaintance, K.E.; Filippov, A.A.; et al. Safety and microbiological activity of phage therapy in persons with cystic fibrosis colonized with Pseudomonas aeruginosa: Study protocol for a phase 1b/2, multicenter, randomized, double-blind, placebo-controlled trial. Trials 2022, 23, 1057. [Google Scholar] [CrossRef]
- Hatfull, G.F. Phage Therapy for Nontuberculous Mycobacteria: Challenges and Opportunities. Pulm. Ther. 2023, 9, 91–107. [Google Scholar] [CrossRef] [PubMed]
- Lusiak-Szelachowska, M.; Miedzybrodzki, R.; Drulis-Kawa, Z.; Cater, K.; Knezevic, P.; Winogradow, C.; Amaro, K.; Jonczyk-Matysiak, E.; Weber-Dabrowska, B.; Rekas, J.; et al. Bacteriophages and antibiotic interactions in clinical practice: What we have learned so far. J. Biomed. Sci. 2022, 29, 23. [Google Scholar] [CrossRef] [PubMed]
- Zaldastanishvili, E.; Leshkasheli, L.; Dadiani, M.; Nadareishvili, L.; Askilashvili, L.; Kvatadze, N.; Goderdzishvili, M.; Kutateladze, M.; Balarjishvili, N. Phage therapy experience at the Eliava Phage Therapy Center: Three cases of bacterial persistence. Viruses 2021, 13, 1901. [Google Scholar] [CrossRef]
- Ling, K.M.; Stick, S.M.; Kicic, A. Pulmonary bacteriophage and cystic fibrosis airway mucus: Friends or foes? Front. Med. 2023, 10, 1088494. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Le, T.; Korn, A.M.; Peterson, H.N.; Liu, M.; Gonzalez, C.F.; Gill, J.J. Phage Milagro: A platform for engineering a broad host range virulent phage for Burkholderia. J. Virol. 2023, 97, e0085023. [Google Scholar] [CrossRef]
- Hesse, S.; Rajaure, M.; Wall, E.; Johnson, J.; Bliskovsky, V.; Gottesman, S.; Adhya, S. Phage resistance in multidrug-resistant Klebsiella pneumoniae ST258 evolves via diverse mutations that culminate in impaired adsorption. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F. Resistance development to bacteriophages occurring during bacteriophage therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef]
- Zhvania, P.; Hoyle, N.S.; Nadareishvili, L.; Nizharadze, D.; Kutateladze, M. Phage therapy in a 16-year-old boy with Netherton syndrome. Front. Med. 2017, 4, 94. [Google Scholar] [CrossRef]
- Gorski, A.; Miedzybrodzki, R.; Weber-Dabrowska, B.; Fortuna, W.; Letkiewicz, S.; Rogoz, P.; Jonczyk-Matysiak, E.; Dabrowska, K.; Majewska, J.; Borysowski, J. Phage therapy: Combating infections with potential for evolving from merely a treatment for complications to targeting diseases. Front. Microbiol. 2016, 7, 1515. [Google Scholar] [CrossRef]
- Martínez-Gallardo, M.J.; Villicaña, C.; Yocupicio-Monroy, M.; Alcaraz-Estrada, S.L.; León-Félix, J. Current knowledge in the use of bacteriophages to combat infections caused by Pseudomonas aeruginosa in cystic fibrosis. Folia Microbiol. 2023, 68, 1–16. [Google Scholar] [CrossRef]
- Bradley, J.S.; Hajama, H.; Akong, K.; Jordan, M.; Stout, D.; Rowe, R.S.; Conrad, D.J.; Hingtgen, S.; Segall, A.M. Bacteriophage therapy of multidrug-resistant achromobacter in an 11-year-old boy with cystic fibrosis assessed by metagenome analysis. Pediatr. Infect. Dis. J. 2023, 42, 754–759. [Google Scholar] [CrossRef]
- Chung, K.M.; Liau, X.L.; Tang, S.S. Bacteriophages and their host range in multidrug-resistant bacterial disease treatment. Pharmaceuticals 2023, 16, 1467. [Google Scholar] [CrossRef]
- Ashworth, E.A.; Wright, R.C.T.; Shears, R.K.; Wong, J.K.L.; Hassan, A.; Hall, J.P.J.; Kadioglu, A.; Fothergill, J.L. Exploiting lung adaptation and phage steering to clear pan-resistant Pseudomonas aeruginosa infections in vivo. Nat. Commun. 2024, 15, 1547. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage therapy for antibiotic-resistant bacterial infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef]
- Lauman, P.; Dennis, J.J. Advances in phage therapy: Targeting the Burkholderia cepacia complex. Viruses 2021, 13, 1331. [Google Scholar] [CrossRef]
- Howard-Varona, C.; Hargreaves, K.R.; Abedon, S.T.; Sullivan, M.B. Lysogeny in nature: Mechanisms, impact and ecology of temperate phages. ISME J. 2017, 11, 1511–1520. [Google Scholar] [CrossRef]
- Lauman, P.; Dennis, J.J. Synergistic interactions among Burkholderia cepacia complex-targeting phages reveal a novel therapeutic role for lysogenization-capable phages. Microbiol. Spectr. 2023, 11, e0443022. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, H.R.; Griffith, M.P.; Rangachar Srinivasa, V.; Wallace, N.R.; Li, A.; Cooper, V.S.; Shields, R.K.; Van Tyne, D. Harnessing the Diversity of Burkholderia spp. Prophages for Therapeutic Potential. Cells 2024, 13, 428. [Google Scholar] [CrossRef] [PubMed]
- Ipoutcha, T.; Racharaks, R.; Huttelmaier, S.; Wilson, C.J.; Ozer, E.A.; Hartmann, E.M. A synthetic biology approach to assemble and reboot clinically relevant Pseudomonas aeruginosa tailed phages. Microbiol. Spectr. 2024, 12, e0289723. [Google Scholar] [CrossRef]
- Tortuel, D.; Tahrioui, A.; David, A.; Cambronel, M.; Nilly, F.; Clamens, T.; Maillot, O.; Barreau, M.; Feuilloley, M.G.J.; Lesouhaitier, O.; et al. Pf4 Phage Variant Infection Reduces Virulence-Associated Traits in Pseudomonas aeruginosa. Microbiol. Spectr. 2022, 10, e0154822. [Google Scholar] [CrossRef] [PubMed]
- Prokopczuk, F.I.; Im, H.; Campos-Gomez, J.; Orihuela, C.J.; Martínez, E. Engineered Superinfective Pf Phage Prevents Dissemination of Pseudomonas aeruginosa in a Mouse Burn Model. mBio 2023, 14, e0047223. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 2019, 32, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Johansen, M.D.; Alcaraz, M.; Dedrick, R.M.; Roquet-Baneres, F.; Hamela, C.; Hatfull, G.F.; Kremer, L. Mycobacteriophage-antibiotic therapy promotes enhanced clearance of drug-resistant Mycobacterium abscessus. Dis. Model. Mech. 2021, 14, dmm049159. [Google Scholar] [CrossRef]
- Martin, I.; Morales, S.; Alton, E.; Davies, J.C. Lytic bacteriophage Is a promising adjunct to common antibiotics across cystic fibrosis clinical strains and culture models of Pseudomonas aeruginosa infection. Antibiotics 2023, 12, 593. [Google Scholar] [CrossRef]
- Namonyo, S.; Carvalho, G.; Guo, J.; Weynberg, K.D. Novel bacteriophages show activity against selected Australian clinical strains of Pseudomonas aeruginosa. Microorganisms 2022, 10, 210. [Google Scholar] [CrossRef]
- Hibstu, Z.; Belew, H.; Akelew, Y.; Mengist, H.M. Phage therapy: A different approach to fight bacterial infections. Biologics 2022, 16, 173–186. [Google Scholar] [CrossRef]
- Holm, A.E.; Schultz, H.H.L.; Johansen, H.K.; Pressler, T.; Lund, T.K.; Iversen, M.; Perch, M. Bacterial re-colonization occurs early after lung transplantation in cystic fibrosis patients. J. Clin. Med. 2021, 10, 1275. [Google Scholar] [CrossRef]
- Petrovic Fabijan, A.; Lin, R.C.Y.; Ho, J.; Maddocks, S.; Ben Zakour, N.L.; Iredell, J.R.; Team, W.B.T. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 465–472. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Abad, L.; Storey, N.; Kaganovsky, A.M.; Smith, B.E.; Aull, H.A.; Cristinziano, M.; Morkowska, A.; Murthy, S.; Loebinger, M.R.; et al. The problem of Mycobacterium abscessus complex: Multi-drug resistance, bacteriophage susceptibility and potential healthcare transmission. Clin. Microbiol. Infect. 2023, 29, 1335.e9–1335.e16. [Google Scholar] [CrossRef]
- Międzybrodzki, R.; Borysowski, J.; Weber-Dąbrowska, B.; Fortuna, W.; Letkiewicz, S.; Szufnarowski, K.; Pawełczyk, Z.; Rogóż, P.; Kłak, M.; Wojtasik, E.; et al. Clinical aspects of phage therapy. Adv. Virus Res. 2012, 83, 73–121. [Google Scholar] [CrossRef]
- Maddocks, S.; Fabijan, A.P.; Ho, J.; Lin, R.C.Y.; Ben Zakour, N.L.; Dugan, C.; Kliman, I.; Branston, S.; Morales, S.; Iredell, J.R. Bacteriophage therapy of ventilator-associated pneumonia and empyema caused by. Am. J. Respir. Crit. Care Med. 2019, 200, 1179–1181. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, A.; Lood, C.; Wubbolts, J.; Hites, M.; Balarjishvili, N.; Leshkasheli, L.; Askilashvili, L.; Kvachadze, L.; van Noort, V.; Wagemans, J.; et al. Combination of pre-adapted bacteriophage therapy and antibiotics for treatment of fracture-related infection due to pandrug-resistant Klebsiella pneumoniae. Nat. Commun. 2022, 13, 302. [Google Scholar] [CrossRef]
- LaVergne, S.; Hamilton, T.; Biswas, B.; Kumaraswamy, M.; Schooley, R.T.; Wooten, D. Phage therapy for a multidrug-resistant acinetobacter baumannii craniectomy site infection. Open Forum Infect. Dis. 2018, 5, ofy064. [Google Scholar] [CrossRef] [PubMed]
- Law, N.; Logan, C.; Yung, G.; Furr, C.L.; Lehman, S.M.; Morales, S.; Rosas, F.; Gaidamaka, A.; Bilinsky, I.; Grint, P.; et al. Successful adjunctive use of bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa infection in a cystic fibrosis patient. Infection 2019, 47, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Merabishvili, M.; Caudron, E.; Lannoy, D.; Van Simaey, L.; Duyvejonck, H.; Guillemain, R.; Thumerelle, C.; Podglajen, I.; Compain, F.; et al. A case of phage therapy against pandrug-resistant Achromobacter xylosoxidans in a 12-year-old lung-transplanted cystic fibrosis patient. Viruses 2021, 13, 60. [Google Scholar] [CrossRef]
- Gainey, A.B.; Burch, A.K.; Brownstein, M.J.; Brown, D.E.; Fackler, J.; Horne, B.; Biswas, B.; Bivens, B.N.; Malagon, F.; Daniels, R. Combining bacteriophages with cefiderocol and meropenem/vaborbactam to treat a pan-drug resistant Achromobacter species infection in a pediatric cystic fibrosis patient. Pediatr. Pulmonol. 2020, 55, 2990–2994. [Google Scholar] [CrossRef]
- Hoyle, N.; Zhvaniya, P.; Balarjishvili, N.; Bolkvadze, D.; Nadareishvili, L.; Nizharadze, D.; Wittmann, J.; Rohde, C.; Kutateladze, M. Phage therapy against Achromobacter xylosoxidans lung infection in a patient with cystic fibrosis: A case report. Res. Microbiol. 2018, 169, 540–542. [Google Scholar] [CrossRef]
- Winzig, F.; Gandhi, S.; Lee, A.; Würstle, S.; Stanley, G.L.; Capuano, I.; Neuringer, I.; Koff, J.L.; Turner, P.E.; Chan, B.K. Inhaled bacteriophage therapy for multi-drug resistant Achromobacter. Yale J. Biol. Med. 2022, 95, 413–427. [Google Scholar]
- Haidar, G.; Chan, B.K.; Cho, S.T.; Hughes Kramer, K.; Nordstrom, H.R.; Wallace, N.R.; Stellfox, M.E.; Holland, M.; Kline, E.G.; Kozar, J.M.; et al. Phage therapy in a lung transplant recipient with cystic fibrosis infected with multidrug-resistant Burkholderia multivorans. Transpl. Infect. Dis. 2023, 25, e14041. [Google Scholar] [CrossRef]
- Aslam, S.; Courtwright, A.M.; Koval, C.; Lehman, S.M.; Morales, S.; Furr, C.L.; Rosas, F.; Brownstein, M.J.; Fackler, J.R.; Sisson, B.M.; et al. Early clinical experience of bacteriophage therapy in 3 lung transplant recipients. Am. J. Transplant. 2019, 19, 2631–2639. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- Nick, J.A.; Dedrick, R.M.; Gray, A.L.; Vladar, E.K.; Smith, B.E.; Freeman, K.G.; Malcolm, K.C.; Epperson, L.E.; Hasan, N.A.; Hendrix, J.; et al. Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell 2022, 185, 1860–1874.e12. [Google Scholar] [CrossRef]
- Rossi, E.; La Rosa, R.; Bartell, J.A.; Marvig, R.L.; Haagensen, J.A.; Sommer, L.M.; Johansen, H.K. Pseudomonas aeruginosa adaptation and evolution in patients with cystic fibrosis. Nat. Rev. Microbiol. 2021, 19, 331–342. [Google Scholar] [CrossRef]
- Menon, N.D.; Penziner, S.; Montaño, E.T.; Zurich, R.; Pride, D.T.; Nair, B.G.; Nizet, V. Increased innate immune susceptibility in hyperpigmented bacteriophage-resistant mutants of Pseudomonas aeruginosa. Antimicrob. Agents. Chemother. 2022, 66, e0023922. [Google Scholar] [CrossRef]
- Hahn, A.; Sami, I.; Chaney, H.; Koumbourlis, A.C.; Del Valle Mojica, C.; Cochrane, C.; Chan, B.K.; Koff, J.L. Bacteriophage therapy for pan-drug-resistant Pseudomonas aeruginosa in two persons with cystic fibrosis. J. Investig. Med. High. Impact Case Rep. 2023, 11, 23247096231188243. [Google Scholar] [CrossRef]
- Khosravi, A.; Chen, Q.; Echterhof, A.; Koff, J.L.; Bollyky, P.L. Phage therapy for respiratory infections: Opportunities and challenges. Lung 2024, 202, 223–232. [Google Scholar] [CrossRef]
- Alipour, M.; Suntres, Z.E.; Omri, A. Importance of DNase and alginate lyase for enhancing free and liposome encapsulated aminoglycoside activity against Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2009, 64, 317–325. [Google Scholar] [CrossRef]
- Leal, J.; Smyth, H.D.C.; Ghosh, D. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int. J. Pharm. 2017, 532, 555–572. [Google Scholar] [CrossRef]
- Pires, D.P.; Oliveira, H.; Melo, L.D.; Sillankorva, S.; Azeredo, J. Bacteriophage-encoded depolymerases: Their diversity and biotechnological applications. Appl. Microbiol. Biotechnol. 2016, 100, 2141–2151. [Google Scholar] [CrossRef]
- Kunz Coyne, A.J.; Stamper, K.; Bleick, C.; Kebriaei, R.; Lehman, S.M.; Rybak, M.J. Synergistic bactericidal effects of phage-enhanced antibiotic therapy against MRSA biofilms. Microbiol. Spectr. 2024, 12, e0321223. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, A.; Bozdogan, B.; Manohar, P.; Nachimuthu, R. Phage-antibiotic combinations in various treatment modalities to manage MRSA infections. Front. Pharmacol. 2024, 15, 1356179. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.H.; Sato, K.; Miyanaga, K.; Nakamura, T.; Ojima, S.; Kondo, K.; Tamura, A.; Yamashita, W.; Tanji, Y.; Kiga, K. Selective bacteriophages reduce the emergence of resistant bacteria in bacteriophage-antibiotic combination therapy. Microbiol. Spectr. 2024, 12, e0042723. [Google Scholar] [CrossRef]
- Luo, J.; Liu, M.; Ai, W.; Zheng, X.; Liu, S.; Huang, K.; Zhang, C.; Li, Q.; Luo, C. Synergy of lytic phage pB23 and meropenem combination against carbapenem-resistant. Antimicrob. Agents Chemother. 2024, 68, e0044824. [Google Scholar] [CrossRef]
- Wang, W.X.; Wu, J.Z.; Zhang, B.L.; Yu, J.Y.; Han, L.M.; Lu, X.L.; Li, H.; Fu, S.Y.; Ren, Y.Y.; Dong, H.; et al. Phage therapy combats pandrug-resistant Acinetobacter baumannii infection safely and efficiently. Int. J. Antimicrob. Agents 2024, 64, 107220. [Google Scholar] [CrossRef] [PubMed]
- Krut, O.; Bekeredjian-Ding, I. Contribution of the immune response to phage therapy. J. Immunol. 2018, 200, 3037–3044. [Google Scholar] [CrossRef]
- Bichet, M.C.; Chin, W.H.; Richards, W.; Lin, Y.W.; Avellaneda-Franco, L.; Hernandez, C.A.; Oddo, A.; Chernyavskiy, O.; Hilsenstein, V.; Neild, A.; et al. Bacteriophage uptake by mammalian cell layers represents a potential sink that may impact phage therapy. iScience 2021, 24, 102287. [Google Scholar] [CrossRef]
- Nguyen, S.; Baker, K.; Padman, B.S.; Patwa, R.; Dunstan, R.A.; Weston, T.A.; Schlosser, K.; Bailey, B.; Lithgow, T.; Lazarou, M.; et al. Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. mBio 2017, 8, 10–1128. [Google Scholar] [CrossRef]
- Lin, J.; Du, F.; Long, M.; Li, P. Limitations of phage therapy and corresponding optimization strategies: A review. Molecules 2022, 27, 1857. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.clinicaltrials.gov/study/NCT04596319?cond=Cystic%20Fibrosis&aggFilters=status:com&term=AP-PA02&rank=1&tab=results (accessed on 3 June 2024).
- Stanley, G.L.; Cochrane, C.; Chan, B.; Kortright, K.; Rahman, B.; Lee, A.; Vill, A.; Sun, Y.; Stewart, J.; Britto-Leon, C.J.; et al. Cystic Fibrosis Bacteriophage Study at Yale (CYPHY) (abstract). Am. J. Respir. Crit. Care Med. 2024, 209, A6808. [Google Scholar] [CrossRef]
- Available online: www.clinicaltrials.gov/search?cond=Cystic%20Fibrosis&term=Bacteriophage%20Therapy&limit=25&page=1 (accessed on 3 June 2024).
| ClinicalTrials.gov ID | Official Title | Pathogen | Type of Phage(s) | Current Status | Last Update Posted |
|---|---|---|---|---|---|
| NCT04684641 | CYstic Fibrosis bacterioPHage Study at Yale (CYPHY): A Single-site, Randomized, Double-blind, Placebo-controlled Study of Bacteriophage Therapy YPT-01 for Pseudomonas Aeruginosa Infections in Adults with Cystic Fibrosis | P. aeruginosa | Single phage | Completed | 18 November 2023 |
| NCT01818206 | Bacteriophages Effects on Pseudomonas Aeruginosa Presents in Sputum of Cystic Fibrosis (CF) Patients | P. aeruginosa | Cocktail of 10 phages | Completed | 5 September 2013 |
| NCT05453578 | A Phase 1b/2, Multi-Centered, Randomized, Double-Blind, Placebo-Controlled Trial of the Safety and Microbiological Activity of a Single Dose of Bacteriophage Therapy in Cystic Fibrosis Subjects Colonized with Pseudomonas Aeruginosa | P. aeruginosa | Cocktail of 4 phages | Recruiting | 3 June 2024 |
| NCT05010577 | A Phase 1b/2a, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate Nebulized Bacteriophage Treatment in Outpatient Adult Cystic Fibrosis (CF) Subjects with Chronic Pseudomonas Aeruginosa (PsA) Pulmonary Infection | P. Aeruginosa | Single phage | Active, not recruiting | 18 October 2023 |
| NCT06262282 | A Prospective Standardized Assessment of People with Cystic Fibrosis and Non-tuberculosis Mycobacteria Pulmonary Disease Undergoing Treatment with Mycobacteriophage (POSTSTAMP) | Nontuberculous mycobacteria (NTM) | Enrolling by invitation | 16 February 2024 | |
| NCT04596319 | A Phase 1b/2a, Multi-Center, Double-Blind, Randomized, Placebo-Controlled, Single and Multiple Ascending Dose Study to Evaluate the Safety and Tolerability of AP-PA02 Multi-Phage Therapeutic Candidate for Inhalation in Subjects with Cystic Fibrosis and Chronic Pulmonary Pseudomonas Aeruginosa (Pa) Infection | P. Aeruginosa | Multi-phage cocktail | Completed | 31 January 2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocorullo, M.; Stelitano, G.; Chiarelli, L.R. Phage Therapy: An Alternative Approach to Combating Multidrug-Resistant Bacterial Infections in Cystic Fibrosis. Int. J. Mol. Sci. 2024, 25, 8321. https://doi.org/10.3390/ijms25158321
Cocorullo M, Stelitano G, Chiarelli LR. Phage Therapy: An Alternative Approach to Combating Multidrug-Resistant Bacterial Infections in Cystic Fibrosis. International Journal of Molecular Sciences. 2024; 25(15):8321. https://doi.org/10.3390/ijms25158321
Chicago/Turabian StyleCocorullo, Mario, Giovanni Stelitano, and Laurent Robert Chiarelli. 2024. "Phage Therapy: An Alternative Approach to Combating Multidrug-Resistant Bacterial Infections in Cystic Fibrosis" International Journal of Molecular Sciences 25, no. 15: 8321. https://doi.org/10.3390/ijms25158321






