Epigenetic Landscapes of Pain: DNA Methylation Dynamics in Chronic Pain
Abstract
:1. Introduction
2. Search Strategy and Selection Criteria
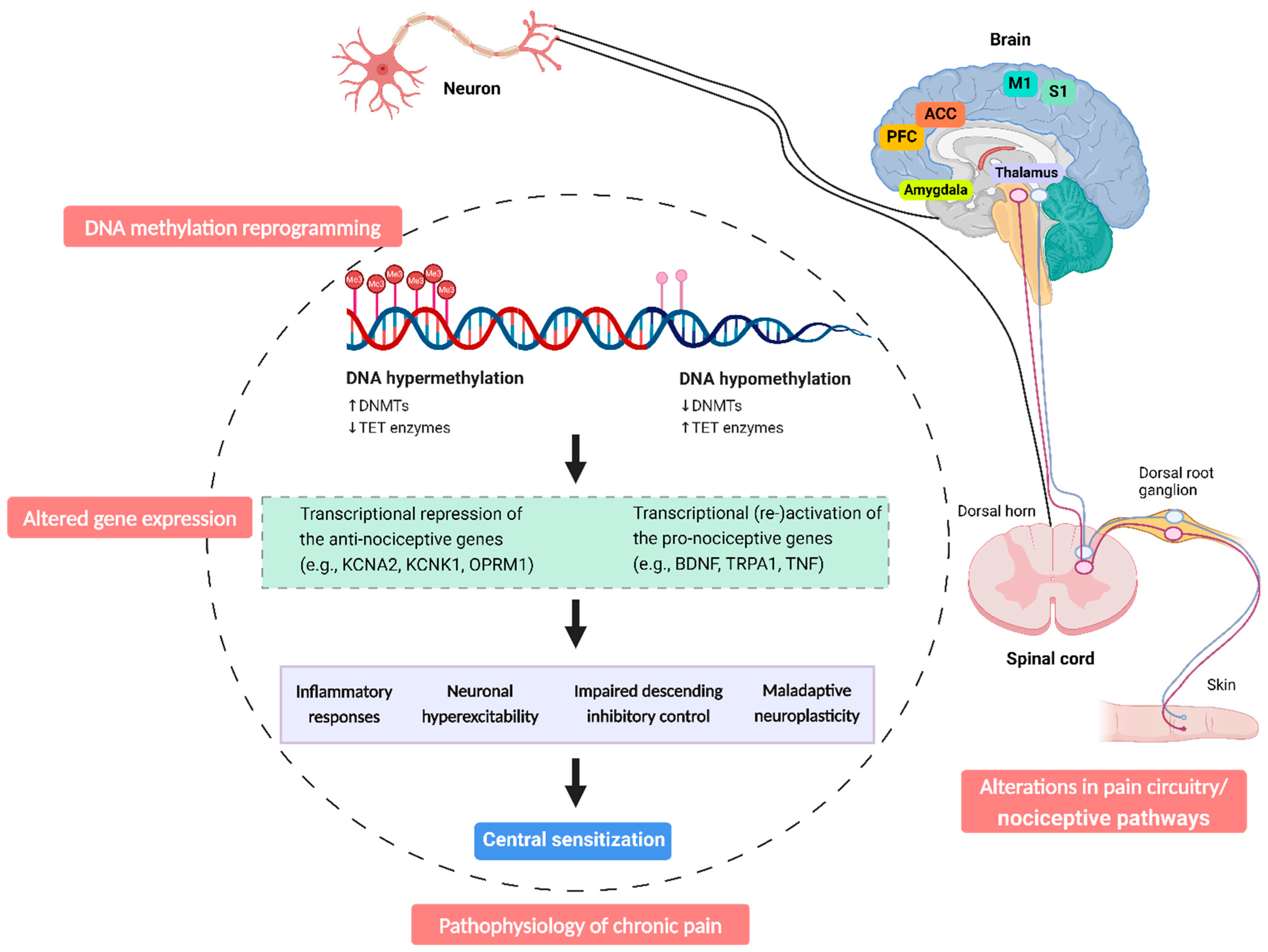
3. DNA Methylation Dynamics in Chronic Pain
3.1. DNMTs: Writers of DNA Methylation in Pain Chronicity
3.2. MBD: Readers of DNA Methylation in Pain Modulation
3.3. TET: Erasers of DNA Methylation in Chronic Pain
4. Altered DNA Methylation Patterns in Patients with Chronic Pain: DNA Hypermethylation and Hypomethylation
| Pain Conditions | Study Design | Tissue | Global DNA Methylation | Specific Genes | Functional Genomic Analyses Methods | Functional Enrichment Analysis | Reference |
|---|---|---|---|---|---|---|---|
| Acute low back pain (n = 14), Chronic low back pain (n = 15), Healthy controls (n = 16) | Cross-sectional | Blood tissue | DNA hypomethylation (genome-wide) | BDNF, CX3CR1, TNF, and many others | \ | \ | [106] |
| Non-specific chronic low back pain (n = 50), Healthy controls (n = 48) | Cross-sectional | Blood tissue | DNA hypomethylation (genome-wide) | CELSR1, KIF11, NAV1, and many others | Gene Ontology (GO) pathway enrichment analyses | Immune signalling, endochondral ossification, and G-protein-coupled transmissions | [27] |
| Early-stage disc degeneration (n = 8), Advanced-stage disc degeneration (n = 8) | Cohort study | Nucleus pulpous tissues from intervertebral disc | DNA hypermethylation (genome-wide) | CARD14, GNL3, MAPKAPK5, and many others | Gene Ontology (GO) pathway enrichment analyses | Hemophilic cell adhesion and cell–cell adhesion | [107] |
| Fibromyalgia (n = 42), Healthy controls (n = 42) | Cross-sectional | Blood tissue | DNA hypermethylation (genome-wide) | GCSAML, GRM2, TRPA1, and many others | \ | \ | [105] |
| Fibromyalgia (n = 24), Healthy controls (n = 24) | Cross-sectional | Blood tissue | DNA hypomethylation (genome-wide) | \ | MetaCore network analysis | MAPK signalling pathway and actin cytoskeleton regulation | [23] |
| Fibromyalgia (n = 10), Healthy controls (n = 42) | Cross-sectional | Blood tissue | DNA hypermethylation (genome-wide) | BDNF, NAT15, HDAC4, and many others | Gene Ontology (GO) pathway enrichment analyses | Neuron differentiation and nervous system development | [22] |
| Chronic widespread pain (n = 50), Twins without chronic widespread pain (n = 50), Healthy controls (n = 1608) | Cohort study | Blood tissue | \ | \ | Gene Ontology (GO) pathway enrichment analyses | Neurological pathways | [24] |
| Chronic fatigue syndrome and comorbid fibromyalgia (n = 28), Healthy controls (n = 26) | Cross-sectional | Blood tissue | DNA hypomethylation (gene-specific) | BDNF | \ | \ | [20] |
| Chronic fatigue syndrome and comorbid fibromyalgia (n = 28), Healthy controls (n = 26) | Cross-sectional | Blood tissue | DNA hypermethylation (gene-specific) | COMT | \ | \ | [31] |
| Chronic nociceptive pain (n = 18), Chronic neuropathic pain (n = 19), Healthy controls (n = 20) | Cohort study | Blood tissue | \ | RAB10, RBFOX-1/CGRP, MAGI2, and many others | STRING (https://string-db.org/) | Neuro-musculoskeletal system, immune response, and inflammation | [118] |
| Knee osteoarthritis pain (n = 182), Healthy controls (n = 31) | Cross-sectional | Blood tissue | DNA hypermethylation (genome-wide) | RNF39, KCNC1, ZFP57, and many others | Ingenuity Pathway Analysis | Immune response and inflammation | [119] |
| Musculoskeletal pain (n = 20), Healthy controls (n = 9) | Cross-sectional | Blood tissue | DNA hypomethylation (genome-wide) | PM20D1, LCLAT1, GNE, and many others | Ingenuity Pathway Analysis | Immune response and GABA receptor signalling | [120] |
| Complex regional pain syndrome (n = 8), Neuropathic pain (n = 38) | Cross-sectional | Blood tissue | DNA hypomethylation (genome-wide) | COL11A1, GPR75, GPR75, and many others | Gene Ontology (GO) pathway enrichment analyses | Immune function | [108] |
| Multisomatoform disorder (n = 136), Healthy controls (n = 145) | Cohort study | Blood tissue | DNA hypomethylation (gene-specific) | Leptin promoter | \ | \ | [121] |
| Chronic back pain or postherpetic neuralgia (n = 12) | Observational study | Blood tissue | DNA hypermethylation (gene-specific) | TRPA1 | \ | \ | [26] |
5. Long-Lasting Dynamic DNA Methylation Reprogramming: The Key to the Transition from Acute to Chronic Pain States?
| Pain Models | Methylation Marker/Enzyme | When and Time Course | Tissue | DNA Methylation Regulation | Gene-Specific Expression Regulation | Inhibition (Inhibitors) | Nociceptive Behaviour Response to Inhibitors | Reference |
|---|---|---|---|---|---|---|---|---|
| SNI-induced neuropathic pain model | \ | Starting on day 1, lasting for 1 year post-SNI | Prefrontal cortex | \ | MAPKBP1, Icos, Unc5cl, and many others | \ | \ | [49] |
| Partial SNL-induced neuropathic pain model | DNMT3a ↓ | 30 days post-SNI | Amygdala | \ | \ | \ | \ | [48] |
| SNI-induced neuropathic pain model | \ | 9 months post-SNI | Prefrontal cortex, T cells | \ | KCNAB3, KCNC4, DNMT1, and many others | \ | \ | [103] |
| SNI-induced neuropathic pain model | \ | 6 months post-SNI | Prefrontal cortex, amygdala | \ | \ | Environmental manipulation | Nociceptive hypersensitivity ↓ | [123] |
| SNL-induced neuropathic pain model | DNMT3b ↓ | Starting on day 1, lasting for 14 days post-SNL | Spinal dorsal horn | DNA hypomethylation (gene-specific) | GPR151 ↑ CXCR3 ↑ | SiRNA-DNMT3b | Nociceptive hypersensitivity ↓ | [63,64] |
| SNL-induced neuropathic pain model | DNMT3a ↑ | 7 days post-SNL | Spinal dorsal horn | DNA hypermethylation (gene-specific) | Kcna2 (Kv1.2) ↓ | AAV5-Dnmt3a shRNA | Nociceptive hypersensitivity ↓ | [51] |
| Bone cancer pain model | DNMT3a ↑ | Starting on day 3, lasting for 12 days post | Spinal dorsal horn | DNA hypermethylation (gene-specific) | Kcna2 (Kv1.2) ↓ | Decitabine | Nociceptive hypersensitivity ↓ | [54] |
| SNL-induced neuropathic pain model | TET1 ↑ 5-hmC ↑ | Starting on day 3, lasting for 21 days post-SNL | Spinal dorsal horn | DNA hypomethylation (gene-specific) | BDNF ↑ | SiRNA-TET1 | Nociceptive hypersensitivity ↓ | [97] |
| CFA-induced inflammatory pain model | TET1 ↑ TET3 ↑ 5-hmC ↑ | Starting on day 3, lasting for 14 days post-CFA | Spinal cord, Blood tissue | DNA hypomethylation (gene-specific) | STAT3 ↑ | Lenti-T1-siRNA, Lenti-T3-siRNA | Nociceptive hypersensitivity ↓ | [92] |
| Paclitaxel-induced neuropathic pain model | DNMT3a ↑ | Starting on day 7, lasting for 21 days post-paclitaxel | DRG | DNA hypermethylation (gene-specific) | K2P.1.1 ↓ | RG108 | Nociceptive hypersensitivity ↓ | [55] |
| SNL-induced neuropathic pain model | DNMT1 ↑ | Starting on day 3, lasting for 14 days post-SNL | DRG | DNA hypermethylation (gene-specific) | Kcna2 (Kv1.2) ↓ | RG108 | Nociceptive hypersensitivity ↓ | [50] |
| SNL-induced neuropathic pain model | DNMT3a ↑ MBD1 ↑ | 7 days post-SNL | DRG | DNA hypermethylation (gene-specific) | Oprm1 (MOR) ↓ | ShRNA-DNMT3a | Nociceptive hypersensitivity ↓, Analgesic effects of morphine ↑, Analgesic tolerance of morphine ↓ | [59] |
| CFA-induced inflammatory pain model | TET1 ↑ | Starting on day 3, lasting for 14 days post-CFA | DRG | DNA hypomethylation (gene-specific) | TRPV1 ↑ | Bobcat339 hydrochloride | Nociceptive hypersensitivity ↓ | [91] |
| Bone cancer pain model | TET1 ↑ | Starting on day 3, lasting for 21 days post-tumour cell inoculation | DRG | DNA hypomethylation (gene-specific) | TRPV4 ↑ | Bobcat339 hydrochloride | Nociceptive hypersensitivity ↓ | [133] |
| Diabetic neuropathic pain model | TET2 ↑ | 28 days post | DRG | DNA hypomethylation (gene-specific) | TXNIP ↑ NLRP3 ↑ | SiRNA-TET2 | Nociceptive hypersensitivity ↓ | [95] |
| SNL-induced neuropathic pain model | MBD1 ↑ | \ | DRG | DNA Hypermethylation (gene-specific) | MOR and KV.1.2 ↓ | SiRNA-MBD1 | Nociceptive hypersensitivity ↓ | [70] |
| SNI-induced neuropathic pain model | MeCP2 ↑ | 28 days post-SNI | DRG | DNA hypomethylation (gene-specific) | BDNF ↑ | MeCP2-null | Nociceptive hypersensitivity ↓ | [70] |
| Oral cancer pain model | \ | \ | DRG | DNA hypermethylation (gene-specific) | Oprm1 (MOR) ↓ | Decitabine | Nociceptive hypersensitivity ↓ | [134] |
6. DNA Methylation Modification in Patients with Chronic Pain: Links to Central Sensitization
7. DNA Methylation Patterns in Chronic Pain: Epigenetic Interventions in a Broader Picture
8. Conclusions and Future Direction
- (i)
- Causal Relationship: Is there a causal relationship between DNA methylation and the occurrence of chronic pain in humans? Do DNA methylation changes result from chronic pain or precede its emergence? Are DNA methylation alterations merely stochastic footprints downstream of underlying pain neuropathology in patients with chronic pain conditions?
- (ii)
- Temporal Dynamics: How rapidly do DNA methylation changes occur, and are they persistent during chronic pain? How does epigenetic dysregulation at the onset of acute pain progress as pain transitions from acute to chronic in humans?
- (iii)
- Gene-Specific vs. Domain-Wide Changes: What is the extent of DNA methylation alterations in the brain after chronic pain induction, and are these changes specific to pain-related genes or broader domains and/or gene families?
- (iv)
- Translation to Clinical Practise: How can we translate epidemiological findings to the clinic? How can findings at the population level be applied to individual patients? What is the potential impact on pain perception, and to what extent can we influence this through therapy?
- (v)
- Therapeutic Potential: is DNA methylation reversible and targetable by existing or proposed treatments? Which of these processes should be targeted for therapeutic intervention?
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Atkinson, C.; Bhalla, K.; Birbeck, G.; Burstein, R.; Chou, D.; Dellavalle, R.; Danaei, G.; Ezzati, M.; Fahimi, A.; et al. The state of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA 2013, 310, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, Z.; Fraser, K.; Grol-Prokopczyk, H.; Zajacova, A. A global study of pain prevalence across 52 countries: Examining the role of country-level contextual factors. Pain 2022, 163, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.; Thomas, S.; Stabile, V.; Han, X.; Shotwell, M.; McQueen, K. Prevalence of chronic pain in low-income and middle-income countries: A systematic review and meta-analysis. Lancet 2015, 385 (Suppl. S2), S10. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, D.J.; Richard, P. The economic costs of pain in the United States. J. Pain. 2012, 13, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.H.; Fors, E.A.; Korwisi, B.; Barke, A.; Cameron, P.; Colvin, L.; Richardson, C.; Rief, W.; Treede, R.D.; The IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Applicability in primary care. Pain 2019, 160, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Deyo, R.; Friedly, J.; Skelly, A.; Weimer, M.; Fu, R.; Dana, T.; Kraegel, P.; Griffin, J.; Grusing, S. Systemic Pharmacologic Therapies for Low Back Pain: A Systematic Review for an American College of Physicians Clinical Practice Guideline. Ann. Intern. Med. 2017, 166, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; George, S.Z.; Clauw, D.J.; Fernández-de-las-Peñas, C.; Kosek, E.; Ickmans, K.; Fernández-Carnero, J.; Polli, A.; Kapreli, E.; Huysmans, E.; et al. Central sensitisation in chronic pain conditions: Latest discoveries and their potential for precision medicine. Lancet Rheumatol. 2021, 3, e383–e392. [Google Scholar] [CrossRef] [PubMed]
- Kregel, J.; Meeus, M.; Malfliet, A.; Dolphens, M.; Danneels, L.; Nijs, J.; Cagnie, B. Structural and functional brain abnormalities in chronic low back pain: A systematic review. Semin. Arthritis Rheum. 2015, 45, 229–237. [Google Scholar] [CrossRef]
- Albrecht, D.S.; Forsberg, A.; Sandstrom, A.; Bergan, C.; Kadetoff, D.; Protsenko, E.; Lampa, J.; Lee, Y.C.; Hoglund, C.O.; Catana, C.; et al. Brain glial activation in fibromyalgia—A multi-site positron emission tomography investigation. Brain Behav. Immun. 2019, 75, 72–83. [Google Scholar] [CrossRef]
- Ji, R.R.; Xu, Z.Z.; Gao, Y.J. Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Knotkova, H.; Hamani, C.; Sivanesan, E.; Le Beuffe, M.F.E.; Moon, J.Y.; Cohen, S.P.; Huntoon, M.A. Neuromodulation for chronic pain. Lancet 2021, 397, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. Gene therapy for chronic pain. Nat. Rev. Drug Discov. 2021, 20, 344. [Google Scholar] [CrossRef] [PubMed]
- Descalzi, G.; Ikegami, D.; Ushijima, T.; Nestler, E.J.; Zachariou, V.; Narita, M. Epigenetic mechanisms of chronic pain. Trends Neurosci. 2015, 38, 237–246. [Google Scholar] [CrossRef]
- Gershman, A.; Sauria, M.E.G.; Guitart, X.; Vollger, M.R.; Hook, P.W.; Hoyt, S.J.; Jain, M.; Shumate, A.; Razaghi, R.; Koren, S.; et al. Epigenetic patterns in a complete human genome. Science 2022, 376, eabj5089. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Tamura, G.; Waki, T.; Kawata, S.; Terashima, M.; Nishizuka, S.; Motoyama, T. Demethylation of MAGE promoters during gastric cancer progression. Br. J. Cancer 2004, 90, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M. DNA hypermethylation in disease: Mechanisms and clinical relevance. Epigenetics 2019, 14, 1141–1163. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Hajkova, P.; Ecker, J.R. Dynamic DNA methylation: In the right place at the right time. Science 2018, 361, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Moller Johansen, L.; Gerra, M.C.; Arendt-Nielsen, L. Time course of DNA methylation in pain conditions: From experimental models to humans. Eur. J. Pain. 2021, 25, 296–312. [Google Scholar] [CrossRef]
- Polli, A.; Ghosh, M.; Bakusic, J.; Ickmans, K.; Monteyne, D.; Velkeniers, B.; Bekaert, B.; Godderis, L.; Nijs, J. DNA Methylation and Brain-Derived Neurotrophic Factor Expression Account for Symptoms and Widespread Hyperalgesia in Patients with Chronic Fatigue Syndrome and Comorbid Fibromyalgia. Arthritis Rheumatol. 2020, 72, 1936–1944. [Google Scholar] [CrossRef]
- Paoloni-Giacobino, A.; Luthi, F.; Stenz, L.; Le Carre, J.; Vuistiner, P.; Leger, B. Altered BDNF Methylation in Patients with Chronic Musculoskeletal Pain and High Biopsychosocial Complexity. J. Pain. Res. 2020, 13, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Menzies, V.; Lyon, D.E.; Archer, K.J.; Zhou, Q.; Brumelle, J.; Jones, K.H.; Gao, G.; York, T.P.; Jackson-Cook, C. Epigenetic alterations and an increased frequency of micronuclei in women with fibromyalgia. Nurs. Res. Pract. 2013, 2013, 795784. [Google Scholar] [CrossRef] [PubMed]
- Ciampi de Andrade, D.; Maschietto, M.; Galhardoni, R.; Gouveia, G.; Chile, T.; Victorino Krepischi, A.C.; Dale, C.S.; Brunoni, A.R.; Parravano, D.C.; Cueva Moscoso, A.S.; et al. Epigenetics insights into chronic pain: DNA hypomethylation in fibromyalgia-a controlled pilot-study. Pain 2017, 158, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Livshits, G.; Malkin, I.; Freidin, M.B.; Xia, Y.; Gao, F.; Wang, J.; Spector, T.D.; MacGregor, A.; Bell, J.T.; Williams, F.M.K. Genome-wide methylation analysis of a large population sample shows neurological pathways involvement in chronic widespread musculoskeletal pain. Pain 2017, 158, 1053–1062. [Google Scholar] [CrossRef]
- Burri, A.; Marinova, Z.; Robinson, M.D.; Kuhnel, B.; Waldenberger, M.; Wahl, S.; Kunze, S.; Gieger, C.; Livshits, G.; Williams, F. Are Epigenetic Factors Implicated in Chronic Widespread Pain? PLoS ONE 2016, 11, e0165548. [Google Scholar] [CrossRef] [PubMed]
- Sukenaga, N.; Ikeda-Miyagawa, Y.; Tanada, D.; Tunetoh, T.; Nakano, S.; Inui, T.; Satoh, K.; Okutani, H.; Noguchi, K.; Hirose, M. Correlation between DNA Methylation of TRPA1 and Chronic Pain States in Human Whole Blood Cells. Pain. Med. 2016, 17, 1906–1910. [Google Scholar] [CrossRef] [PubMed]
- Aroke, E.N.; Overstreet, D.S.; Penn, T.M.; Crossman, D.K.; Jackson, P.; Tollefsbol, T.O.; Quinn, T.L.; Yi, N.; Goodin, B.R. Identification of DNA methylation associated enrichment pathways in adults with non-specific chronic low back pain. Mol. Pain. 2020, 16. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; de Boer, I.; Sutherland, H.G.; Pijpers, J.A.; Bron, C.; Bainomugisa, C.; Haupt, L.M.; van den Maagdenberg, A.; Griffiths, L.R.; Nyholt, D.R.; et al. Alterations in DNA methylation associate with reduced migraine and headache days after medication withdrawal treatment in chronic migraine patients: A longitudinal study. Clin. Epigenetics 2023, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Stephens, K.E.; Levine, J.D.; Aouizerat, B.E.; Paul, S.M.; Abrams, G.; Conley, Y.P.; Miaskowski, C. Associations between genetic and epigenetic variations in cytokine genes and mild persistent breast pain in women following breast cancer surgery. Cytokine 2017, 99, 203–213. [Google Scholar] [CrossRef]
- Chidambaran, V.; Zhang, X.; Martin, L.J.; Ding, L.; Weirauch, M.T.; Geisler, K.; Stubbeman, B.L.; Sadhasivam, S.; Ji, H. DNA methylation at the mu-1 opioid receptor gene (OPRM1) promoter predicts preoperative, acute, and chronic postsurgical pain after spine fusion. Pharmgenomics Pers. Med. 2017, 10, 157–168. [Google Scholar] [CrossRef]
- Polli, A.; Hendrix, J.; Ickmans, K.; Bakusic, J.; Ghosh, M.; Monteyne, D.; Velkeniers, B.; Bekaert, B.; Nijs, J.; Godderis, L. Genetic and epigenetic regulation of Catechol-O-methyltransferase in relation to inflammation in chronic fatigue syndrome and Fibromyalgia. J. Transl. Med. 2022, 20, 487. [Google Scholar] [CrossRef] [PubMed]
- Bates, S.E. Epigenetic Therapies for Cancer. N. Engl. J. Med. 2020, 383, 650–663. [Google Scholar] [CrossRef] [PubMed]
- Topper, M.J.; Vaz, M.; Marrone, K.A.; Brahmer, J.R.; Baylin, S.B. The emerging role of epigenetic therapeutics in immuno-oncology. Nat. Rev. Clin. Oncol. 2020, 17, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.A.; Cohen, S.P.; Clauw, D.J.; Littlejohn, G.; Usui, C.; Hauser, W. Nociplastic pain: Towards an understanding of prevalent pain conditions. Lancet 2021, 397, 2098–2110. [Google Scholar] [CrossRef] [PubMed]
- Denk, F.; McMahon, S.B. Chronic pain: Emerging evidence for the involvement of epigenetics. Neuron 2012, 73, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Spector, T.D.; Deloukas, P.; Bell, J.T.; Engelhardt, B.E. Predicting genome-wide DNA methylation using methylation marks, genomic position, and DNA regulatory elements. Genome Biol. 2015, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Fatemi, M.; Pao, M.M.; Jeong, S.; Gal-Yam, E.N.; Egger, G.; Weisenberger, D.J.; Jones, P.A. Footprinting of mammalian promoters: Use of a CpG DNA methyltransferase revealing nucleosome positions at a single molecule level. Nucleic Acids Res. 2005, 33, e176. [Google Scholar] [CrossRef]
- Greenberg, M.V.C.; Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar] [CrossRef]
- Michalak, E.M.; Burr, M.L.; Bannister, A.J.; Dawson, M.A. The roles of DNA, RNA and histone methylation in ageing and cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 573–589. [Google Scholar] [CrossRef]
- Goll, M.G.; Bestor, T.H. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 2005, 74, 481–514. [Google Scholar] [CrossRef] [PubMed]
- Mortusewicz, O.; Schermelleh, L.; Walter, J.; Cardoso, M.C.; Leonhardt, H. Recruitment of DNA methyltransferase I to DNA repair sites. Proc. Natl. Acad. Sci. USA 2005, 102, 8905–8909. [Google Scholar] [CrossRef] [PubMed]
- Klose, R.J.; Bird, A.P. Genomic DNA methylation: The mark and its mediators. Trends Biochem. Sci. 2006, 31, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Garriga, J.; Laumet, G.; Chen, S.R.; Zhang, Y.; Madzo, J.; Issa, J.J.; Pan, H.L.; Jelinek, J. Nerve Injury-Induced Chronic Pain Is Associated with Persistent DNA Methylation Reprogramming in Dorsal Root Ganglion. J. Neurosci. 2018, 38, 6090–6101. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xu, D.; Wang, T.; Zhang, Y.; Yang, X.; Wang, X.; Tang, Y. Epigenetic reduction of miR-214-3p upregulates astrocytic colony-stimulating factor-1 and contributes to neuropathic pain induced by nerve injury. Pain 2020, 161, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Z.P.; Zheng, H.Z.; Zhang, S.; Zhang, Z.L.; Chen, Y.; You, Y.S.; Yang, M.H. Abnormal DNA methylation in the lumbar spinal cord following chronic constriction injury in rats. Neurosci. Lett. 2016, 610, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Gao, Y.; Jin, D.; Xu, X.; Tan, S.; Yu, H.; Zhao, Q.; Zhao, L.; Wang, W.; Wang, D. DNMT3a methylation in neuropathic pain. J. Pain. Res. 2017, 10, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Cai, Y.; Pan, Z.Z. Pain vulnerability and DNA methyltransferase 3a involved in the affective dimension of chronic pain. Mol. Pain. 2017, 13. [Google Scholar] [CrossRef]
- Topham, L.; Gregoire, S.; Kang, H.; Salmon-Divon, M.; Lax, E.; Millecamps, M.; Szyf, M.; Stone, L.S. The transition from acute to chronic pain: Dynamic epigenetic reprogramming of the mouse prefrontal cortex up to 1 year after nerve injury. Pain 2020, 161, 2394–2409. [Google Scholar] [CrossRef]
- Sun, L.; Gu, X.; Pan, Z.; Guo, X.; Liu, J.; Atianjoh, F.E.; Wu, S.; Mo, K.; Xu, B.; Liang, L.; et al. Contribution of DNMT1 to Neuropathic Pain Genesis Partially through Epigenetically Repressing Kcna2 in Primary Afferent Neurons. J. Neurosci. 2019, 39, 6595–6607. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Liang, L.; Gu, X.; Li, Z.; Wu, S.; Sun, L.; Atianjoh, F.E.; Feng, J.; Mo, K.; Jia, S.; et al. DNA methyltransferase DNMT3a contributes to neuropathic pain by repressing Kcna2 in primary afferent neurons. Nat. Commun. 2017, 8, 14712. [Google Scholar] [CrossRef]
- Fan, L.; Guan, X.; Wang, W.; Zhao, J.Y.; Zhang, H.; Tiwari, V.; Hoffman, P.N.; Li, M.; Tao, Y.X. Impaired neuropathic pain and preserved acute pain in rats overexpressing voltage-gated potassium channel subunit Kv1.2 in primary afferent neurons. Mol. Pain. 2014, 10, 8. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, Z.; Zhang, H.; Atianjoh, F.E.; Zhao, J.Y.; Liang, L.; Wang, W.; Guan, X.; Kao, S.C.; Tiwari, V.; et al. A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat. Neurosci. 2013, 16, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.R.; Fan, L.C.; Wu, S.; Mao, Q.; Li, Z.; Lutz, B.; Xu, J.T.; Lu, Z.; Tao, Y.X. DNMT3a contributes to the development and maintenance of bone cancer pain by silencing Kv1.2 expression in spinal cord dorsal horn. Mol. Pain. 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Wu, S.; Gu, X.; Du, S.; Mo, K.; Sun, L.; Cao, J.; Bekker, A.; Chen, L.; Tao, Y.X. DNMT3a-triggered downregulation of K(2p) 1.1 gene in primary sensory neurons contributes to paclitaxel-induced neuropathic pain. Int. J. Cancer 2019, 145, 2122–2134. [Google Scholar] [CrossRef]
- Li, Y.; Tatsui, C.E.; Rhines, L.D.; North, R.Y.; Harrison, D.S.; Cassidy, R.M.; Johansson, C.A.; Kosturakis, A.K.; Edwards, D.D.; Zhang, H.; et al. Dorsal root ganglion neurons become hyperexcitable and increase expression of voltage-gated T-type calcium channels (Cav3.2) in paclitaxel-induced peripheral neuropathy. Pain 2017, 158, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; North, R.Y.; Rhines, L.D.; Tatsui, C.E.; Rao, G.; Edwards, D.D.; Cassidy, R.M.; Harrison, D.S.; Johansson, C.A.; Zhang, H.; et al. DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain. J. Neurosci. 2018, 38, 1124–1136. [Google Scholar] [CrossRef]
- Kindler, C.H.; Yost, C.S. Two-pore domain potassium channels: New sites of local anesthetic action and toxicity. Reg. Anesth. Pain. Med. 2005, 30, 260–274. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, J.Y.; Gu, X.; Liang, L.; Wu, S.; Mo, K.; Feng, J.; Guo, W.; Zhang, J.; Bekker, A.; et al. Nerve injury-induced epigenetic silencing of opioid receptors controlled by DNMT3a in primary afferent neurons. Pain 2017, 158, 1153–1165. [Google Scholar] [CrossRef]
- Woolf, C.J. Mu and delta opioid receptors diverge. Cell 2009, 137, 987–988. [Google Scholar] [CrossRef]
- Obara, I.; Parkitna, J.R.; Korostynski, M.; Makuch, W.; Kaminska, D.; Przewlocka, B.; Przewlocki, R. Local peripheral opioid effects and expression of opioid genes in the spinal cord and dorsal root ganglia in neuropathic and inflammatory pain. Pain 2009, 141, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Back, S.K.; Lee, J.; Hong, S.K.; Na, H.S. Loss of spinal mu-opioid receptor is associated with mechanical allodynia in a rat model of peripheral neuropathy. Pain 2006, 123, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.C.; Zhang, W.W.; Yang, T.; Guo, C.Y.; Cao, D.L.; Zhang, Z.J.; Gao, Y.J. Demethylation of G-Protein-Coupled Receptor 151 Promoter Facilitates the Binding of Kruppel-Like Factor 5 and Enhances Neuropathic Pain after Nerve Injury in Mice. J. Neurosci. 2018, 38, 10535–10551. [Google Scholar] [CrossRef]
- Jiang, B.C.; He, L.N.; Wu, X.B.; Shi, H.; Zhang, W.W.; Zhang, Z.J.; Cao, D.L.; Li, C.H.; Gu, J.; Gao, Y.J. Promoted Interaction of C/EBPalpha with Demethylated Cxcr3 Gene Promoter Contributes to Neuropathic Pain in Mice. J. Neurosci. 2017, 37, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Noguchi, K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004, 74, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xue, Z.Y.; Yuan, Y.; Huang, S.S.; Fan, Y.H.; Zhu, X.; Wei, L. Upregulation of CXCR4 through promoter demethylation contributes to inflammatory hyperalgesia in rats. CNS Neurosci. Ther. 2018, 24, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Chahrour, M.; Jung, S.Y.; Shaw, C.; Zhou, X.; Wong, S.T.; Qin, J.; Zoghbi, H.Y. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 2008, 320, 1224–1229. [Google Scholar] [CrossRef]
- Nan, X.; Ng, H.H.; Johnson, C.A.; Laherty, C.D.; Turner, B.M.; Eisenman, R.N.; Bird, A. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 1998, 393, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Clouaire, T.; Stancheva, I. Methyl-CpG binding proteins: Specialized transcriptional repressors or structural components of chromatin? Cell Mol. Life Sci. 2008, 65, 1509–1522. [Google Scholar] [CrossRef]
- Mo, K.; Wu, S.; Gu, X.; Xiong, M.; Cai, W.; Atianjoh, F.E.; Jobe, E.E.; Zhao, X.; Tu, W.F.; Tao, Y.X. MBD1 Contributes to the Genesis of Acute Pain and Neuropathic Pain by Epigenetic Silencing of Oprm1 and Kcna2 Genes in Primary Sensory Neurons. J. Neurosci. 2018, 38, 9883–9899. [Google Scholar] [CrossRef]
- Manners, M.T.; Tian, Y.; Zhou, Z.; Ajit, S.K. MicroRNAs downregulated in neuropathic pain regulate MeCP2 and BDNF related to pain sensitivity. FEBS Open Bio 2015, 5, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Samaco, R.C.; Neul, J.L. Complexities of Rett syndrome and MeCP2. J. Neurosci. 2011, 31, 7951–7959. [Google Scholar] [CrossRef] [PubMed]
- Downs, J.; Geranton, S.M.; Bebbington, A.; Jacoby, P.; Bahi-Buisson, N.; Ravine, D.; Leonard, H. Linking MECP2 and pain sensitivity: The example of Rett syndrome. Am. J. Med. Genet. A 2010, 152A, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.U.; Hundley, R.J.; Wilson, A.K.; Warren, Z.; Vehorn, A.; Carvalho, C.M.; Lupski, J.R.; Ramocki, M.B. The behavioral phenotype in MECP2 duplication syndrome: A comparison with idiopathic autism. Autism Res. 2013, 6, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, C.; Guo, Q.L.; Yan, J.Q.; Zhu, X.Y.; Huang, C.S.; Zou, W.Y. Intrathecal 5-azacytidine inhibits global DNA methylation and methyl- CpG-binding protein 2 expression and alleviates neuropathic pain in rats following chronic constriction injury. Brain Res. 2011, 1418, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, D.; Dai, Z.; You, Y.; Chen, Y.; Lei, C.; Lv, Y.; Wang, Y. Intraperitoneal 5-Azacytidine Alleviates Nerve Injury-Induced Pain in Rats by Modulating DNA Methylation. Mol. Neurobiol. 2023, 60, 2186–2199. [Google Scholar] [CrossRef] [PubMed]
- Orefice, L.L.; Zimmerman, A.L.; Chirila, A.M.; Sleboda, S.J.; Head, J.P.; Ginty, D.D. Peripheral Mechanosensory Neuron Dysfunction Underlies Tactile and Behavioral Deficits in Mouse Models of ASDs. Cell 2016, 166, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Tochiki, K.K.; Cunningham, J.; Hunt, S.P.; Geranton, S.M. The expression of spinal methyl-CpG-binding protein 2, DNA methyltransferases and histone deacetylases is modulated in persistent pain states. Mol. Pain. 2012, 8, 14. [Google Scholar] [CrossRef]
- Ou, M.; Chen, Y.; Liu, J.; Zhang, D.; Yang, Y.; Shen, J.; Miao, C.; Tang, S.J.; Liu, X.; Mulkey, D.K.; et al. Spinal astrocytic MeCP2 regulates Kir4.1 for the maintenance of chronic hyperalgesia in neuropathic pain. Prog. Neurobiol. 2023, 224, 102436. [Google Scholar] [CrossRef]
- Bhattacherjee, A.; Mu, Y.; Winter, M.K.; Knapp, J.R.; Eggimann, L.S.; Gunewardena, S.S.; Kobayashi, K.; Kato, S.; Krizsan-Agbas, D.; Smith, P.G. Neuronal cytoskeletal gene dysregulation and mechanical hypersensitivity in a rat model of Rett syndrome. Proc. Natl. Acad. Sci. USA 2017, 114, E6952–E6961. [Google Scholar] [CrossRef]
- Zhang, R.; Huang, M.; Cao, Z.; Qi, J.; Qiu, Z.; Chiang, L.Y. MeCP2 plays an analgesic role in pain transmission through regulating CREB / miR-132 pathway. Mol. Pain. 2015, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Franchini, D.M.; Schmitz, K.M.; Petersen-Mahrt, S.K. 5-Methylcytosine DNA demethylation: More than losing a methyl group. Annu. Rev. Genet. 2012, 46, 419–441. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y. Reversing DNA methylation: Mechanisms, genomics, and biological functions. Cell 2014, 156, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Ooi, S.K.; Bestor, T.H. The colorful history of active DNA demethylation. Cell 2008, 133, 1145–1148. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef]
- Kaas, G.A.; Zhong, C.; Eason, D.E.; Ross, D.L.; Vachhani, R.V.; Ming, G.L.; King, J.R.; Song, H.; Sweatt, J.D. TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation. Neuron 2013, 79, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Pastor, W.A.; Aravind, L.; Rao, A. TETonic shift: Biological roles of TET proteins in DNA demethylation and transcription. Nat. Rev. Mol. Cell Biol. 2013, 14, 341–356. [Google Scholar] [CrossRef]
- Kriaucionis, S.; Heintz, N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 2009, 324, 929–930. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, P.; Grandi, F.C.; Davala, S.; Masarapu, V.; Indelli, P.F.; Goodman, S.B.; Bhutani, N. Inhibition of TET1 prevents the development of osteoarthritis and reveals the 5hmC landscape that orchestrates pathogenesis. Sci. Transl. Med. 2020, 12, eaax2332. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Zhang, H.L.; Lu, X.; Du, H.; Li, Y.C.; Zhang, P.A.; Xu, G.Y. Targeting GATA1 and p2x7r Locus Binding in Spinal Astrocytes Suppresses Chronic Visceral Pain by Promoting DNA Demethylation. Neurosci. Bull. 2022, 38, 359–372. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Xu, Z.H.; Zhu, J.; Ma, J.L.; Gao, Y.P.; Xu, G.Y. Increased ten-eleven translocation methylcytosine dioxygenase one in dorsal root ganglion contributes to inflammatory pain in CFA rats. Mol. Pain. 2022, 18. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Xue, Z.Y.; Li, G.F.; Sun, M.L.; Zhang, M.; Hao, L.Y.; Tang, Q.Q.; Zhu, L.J.; Cao, J.L. DNA Hydroxymethylation by Ten-eleven Translocation Methylcytosine Dioxygenase 1 and 3 Regulates Nociceptive Sensitization in a Chronic Inflammatory Pain Model. Anesthesiology 2017, 127, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhang, M.; Ma, T.; Xue, Z.Y.; Li, G.F.; Hao, L.Y.; Zhu, L.J.; Li, Y.Q.; Ding, H.L.; Cao, J.L. Hydroxymethylation of microRNA-365-3p Regulates Nociceptive Behaviors via Kcnh2. J. Neurosci. 2016, 36, 2769–2781. [Google Scholar] [CrossRef] [PubMed]
- Chamessian, A.G.; Qadri, Y.J.; Cummins, M.; Hendrickson, M.; Berta, T.; Buchheit, T.; Van de Ven, T. 5-Hydroxymethylcytosine (5hmC) and Ten-eleven translocation 1-3 (TET1-3) proteins in the dorsal root ganglia of mouse: Expression and dynamic regulation in neuropathic pain. Somatosens. Mot. Res. 2017, 34, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, X.; Sun, Q.; Zhang, Y.; Liu, J.; Hu, T.; Wu, W.; Wei, C.; Liu, M.; Ding, Y.; et al. The upregulation of NLRP3 inflammasome in dorsal root ganglion by ten-eleven translocation methylcytosine dioxygenase 2 (TET2) contributed to diabetic neuropathic pain in mice. J. Neuroinflammation 2022, 19, 302. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.C.; Ho, Y.C.; Lai, C.Y.; Chou, D.; Wang, H.H.; Chen, G.D.; Lin, T.B.; Peng, H.Y. Melatonin impedes Tet1-dependent mGluR5 promoter demethylation to relieve pain. J. Pineal Res. 2017, 63, e12436. [Google Scholar] [CrossRef]
- Hsieh, M.C.; Lai, C.Y.; Ho, Y.C.; Wang, H.H.; Cheng, J.K.; Chau, Y.P.; Peng, H.Y. Tet1-dependent epigenetic modification of BDNF expression in dorsal horn neurons mediates neuropathic pain in rats. Sci. Rep. 2016, 6, 37411. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wei, G.; Ji, F.; Jia, S.; Wu, S.; Guo, X.; He, L.; Pan, Z.; Miao, X.; Mao, Q.; et al. TET1 Overexpression Mitigates Neuropathic Pain through Rescuing the Expression of mu-Opioid Receptor and Kv1.2 in the Primary Sensory Neurons. Neurotherapeutics 2019, 16, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Stenz, L.; Zewdie, S.; Laforge-Escarra, T.; Prados, J.; La Harpe, R.; Dayer, A.; Paoloni-Giacobino, A.; Perroud, N.; Aubry, J.M. BDNF promoter I methylation correlates between post-mortem human peripheral and brain tissues. Neurosci. Res. 2015, 91, 1–7. [Google Scholar] [CrossRef]
- Nohesara, S.; Ghadirivasfi, M.; Mostafavi, S.; Eskandari, M.R.; Ahmadkhaniha, H.; Thiagalingam, S.; Abdolmaleky, H.M. DNA hypomethylation of MB-COMT promoter in the DNA derived from saliva in schizophrenia and bipolar disorder. J. Psychiatr. Res. 2011, 45, 1432–1438. [Google Scholar] [CrossRef]
- Davies, M.N.; Volta, M.; Pidsley, R.; Lunnon, K.; Dixit, A.; Lovestone, S.; Coarfa, C.; Harris, R.A.; Milosavljevic, A.; Troakes, C.; et al. Functional annotation of the human brain methylome identifies tissue-specific epigenetic variation across brain and blood. Genome Biol. 2012, 13, R43. [Google Scholar] [CrossRef] [PubMed]
- Shumay, E.; Logan, J.; Volkow, N.D.; Fowler, J.S. Evidence that the methylation state of the monoamine oxidase A (MAOA) gene predicts brain activity of MAO A enzyme in healthy men. Epigenetics 2012, 7, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Massart, R.; Dymov, S.; Millecamps, M.; Suderman, M.; Gregoire, S.; Koenigs, K.; Alvarado, S.; Tajerian, M.; Stone, L.S.; Szyf, M. Overlapping signatures of chronic pain in the DNA methylation landscape of prefrontal cortex and peripheral T cells. Sci. Rep. 2016, 6, 19615. [Google Scholar] [CrossRef] [PubMed]
- Gerra, M.C.; Carnevali, D.; Pedersen, I.S.; Donnini, C.; Manfredini, M.; Gonzalez-Villar, A.; Trinanes, Y.; Pidal-Miranda, M.; Arendt-Nielsen, L.; Carrillo-de-la-Pena, M.T. DNA methylation changes in genes involved in inflammation and depression in fibromyalgia: A pilot study. Scand. J. Pain. 2021, 21, 372–383. [Google Scholar] [CrossRef]
- Gerra, M.C.; Carnevali, D.; Ossola, P.; Gonzalez-Villar, A.; Pedersen, I.S.; Trinanes, Y.; Donnini, C.; Manfredini, M.; Arendt-Nielsen, L.; Carrillo-de-la-Pena, M.T. DNA Methylation Changes in Fibromyalgia Suggest the Role of the Immune-Inflammatory Response and Central Sensitization. J. Clin. Med. 2021, 10, 4992. [Google Scholar] [CrossRef] [PubMed]
- Eller, O.C.; Glidden, N.; Knight, B.; McKearney, N.; Perry, M.; Bernier Carney, K.M.; Starkweather, A.; Young, E.E.; Baumbauer, K.M. A Role for Global DNA Methylation Level and IL2 Expression in the Transition from Acute to Chronic Low Back Pain. Front. Pain Res. 2021, 2, 744148. [Google Scholar] [CrossRef] [PubMed]
- Ikuno, A.; Akeda, K.; Takebayashi, S.I.; Shimaoka, M.; Okumura, K.; Sudo, A. Genome-wide analysis of DNA methylation profile identifies differentially methylated loci associated with human intervertebral disc degeneration. PLoS ONE 2019, 14, e0222188. [Google Scholar] [CrossRef] [PubMed]
- Bruehl, S.; Gamazon, E.R.; Van de Ven, T.; Buchheit, T.; Walsh, C.G.; Mishra, P.; Ramanujan, K.; Shaw, A. DNA methylation profiles are associated with complex regional pain syndrome after traumatic injury. Pain 2019, 160, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Grevet, E.H.; da Silva, B.S.; Charao, M.F.; Rovaris, D.L.; Bau, C.H.D. The neuroendocrine modulation of global DNA methylation in neuropsychiatric disorders. Mol. Psychiatry 2021, 26, 66–69. [Google Scholar] [CrossRef]
- Stefanska, B.; Huang, J.; Bhattacharyya, B.; Suderman, M.; Hallett, M.; Han, Z.G.; Szyf, M. Definition of the landscape of promoter DNA hypomethylation in liver cancer. Cancer Res. 2011, 71, 5891–5903. [Google Scholar] [CrossRef]
- Hansen, K.D.; Timp, W.; Bravo, H.C.; Sabunciyan, S.; Langmead, B.; McDonald, O.G.; Wen, B.; Wu, H.; Liu, Y.; Diep, D.; et al. Increased methylation variation in epigenetic domains across cancer types. Nat. Genet. 2011, 43, 768–775. [Google Scholar] [CrossRef]
- Gombert, S.; Rhein, M.; Winterpacht, A.; Munster, T.; Hillemacher, T.; Leffler, A.; Frieling, H. Transient receptor potential ankyrin 1 promoter methylation and peripheral pain sensitivity in Crohn’s disease. Clin. Epigenetics 2019, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Sukenaga, N.; Ohmuraya, M.; Matsuki, Y.; Maeda, L.; Takao, Y.; Hirose, M. Association between neuropathic pain characteristics and DNA methylation of transient receptor potential ankyrin 1 in human peripheral blood. Medicine 2020, 99, e19325. [Google Scholar] [CrossRef]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef]
- Khan, S.; Patra, P.H.; Somerfield, H.; Benya-Aphikul, H.; Upadhya, M.; Zhang, X. IQGAP1 promotes chronic pain by regulating the trafficking and sensitization of TRPA1 channels. Brain 2023, 146, 2595–2611. [Google Scholar] [CrossRef]
- Tajerian, M.; Alvarado, S.; Millecamps, M.; Dashwood, T.; Anderson, K.M.; Haglund, L.; Ouellet, J.; Szyf, M.; Stone, L.S. DNA methylation of SPARC and chronic low back pain. Mol. Pain. 2011, 7, 65. [Google Scholar] [CrossRef]
- Bradshaw, A.D.; Sage, E.H. SPARC, a matricellular protein that functions in cellular differentiation and tissue response to injury. J. Clin. Investig. 2001, 107, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Stenz, L.; Carre, J.L.; Luthi, F.; Vuistiner, P.; Burrus, C.; Paoloni-Giacobino, A.; Leger, B. Genome-Wide Epigenomic Analyses in Patients with Nociceptive and Neuropathic Chronic Pain Subtypes Reveals Alterations in Methylation of Genes Involved in the Neuro-Musculoskeletal System. J. Pain. 2022, 23, 326–336. [Google Scholar] [CrossRef]
- Montesino-Goicolea, S.; Meng, L.; Rani, A.; Huo, Z.; Foster, T.C.; Fillingim, R.B.; Cruz-Almeida, Y. Enrichment of genomic pathways based on differential DNA methylation profiles associated with knee osteoarthritis pain. Neurobiol. Pain. 2022, 12, 100107. [Google Scholar] [CrossRef] [PubMed]
- Montesino-Goicolea, S.; Sinha, P.; Huo, Z.; Rani, A.; Foster, T.C.; Cruz-Almeida, Y. Enrichment of genomic pathways based on differential DNA methylation profiles associated with chronic musculoskeletal pain in older adults: An exploratory study. Mol. Pain. 2020, 16. [Google Scholar] [CrossRef]
- Achenbach, J.; Rhein, M.; Glahn, A.; Frieling, H.; Karst, M. Leptin promoter methylation in female patients with painful multisomatoform disorder and chronic widespread pain. Clin. Epigenetics 2022, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, S.; Millecamps, M.; Naso, L.; Do Carmo, S.; Cuello, A.C.; Szyf, M.; Stone, L.S. Therapeutic benefits of the methyl donor S-adenosylmethionine on nerve injury-induced mechanical hypersensitivity and cognitive impairment in mice. Pain 2017, 158, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Tajerian, M.; Alvarado, S.; Millecamps, M.; Vachon, P.; Crosby, C.; Bushnell, M.C.; Szyf, M.; Stone, L.S. Peripheral nerve injury is associated with chronic, reversible changes in global DNA methylation in the mouse prefrontal cortex. PLoS ONE 2013, 8, e55259. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Sun, H.; Fu, Y.; Li, Z.; Pais-Vieira, M.; Galhardo, V.; Neugebauer, V. Cognitive impairment in pain through amygdala-driven prefrontal cortical deactivation. J. Neurosci. 2010, 30, 5451–5464. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, S.; Tajerian, M.; Millecamps, M.; Suderman, M.; Stone, L.S.; Szyf, M. Peripheral nerve injury is accompanied by chronic transcriptome-wide changes in the mouse prefrontal cortex. Mol. Pain. 2013, 9, 21. [Google Scholar] [CrossRef] [PubMed]
- Descalzi, G.; Mitsi, V.; Purushothaman, I.; Gaspari, S.; Avrampou, K.; Loh, Y.E.; Shen, L.; Zachariou, V. Neuropathic pain promotes adaptive changes in gene expression in brain networks involved in stress and depression. Sci. Signal 2017, 10, eaaj1549. [Google Scholar] [CrossRef] [PubMed]
- Metz, A.E.; Yau, H.J.; Centeno, M.V.; Apkarian, A.V.; Martina, M. Morphological and functional reorganization of rat medial prefrontal cortex in neuropathic pain. Proc. Natl. Acad. Sci. USA 2009, 106, 2423–2428. [Google Scholar] [CrossRef] [PubMed]
- Shiers, S.; Price, T.J. Molecular, circuit, and anatomical changes in the prefrontal cortex in chronic pain. Pain 2020, 161, 1726–1729. [Google Scholar] [CrossRef] [PubMed]
- Parisien, M.; Samoshkin, A.; Tansley, S.N.; Piltonen, M.H.; Martin, L.J.; El-Hachem, N.; Dagostino, C.; Allegri, M.; Mogil, J.S.; Khoutorsky, A.; et al. Genetic pathway analysis reveals a major role for extracellular matrix organization in inflammatory and neuropathic pain. Pain 2019, 160, 932–944. [Google Scholar] [CrossRef]
- Baliki, M.N.; Chialvo, D.R.; Geha, P.Y.; Levy, R.M.; Harden, R.N.; Parrish, T.B.; Apkarian, A.V. Chronic pain and the emotional brain: Specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J. Neurosci. 2006, 26, 12165–12173. [Google Scholar] [CrossRef]
- Alvarado, S.; Tajerian, M.; Suderman, M.; Machnes, Z.; Pierfelice, S.; Millecamps, M.; Stone, L.S.; Szyf, M. An epigenetic hypothesis for the genomic memory of pain. Front. Cell. Neurosci. 2015, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Monteggia, L.M. Role of DNA methylation and the DNA methyltransferases in learning and memory. Dialogues Clin. Neurosci. 2014, 16, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.H.; Niu, Z.; Liu, Y.; Liu, P.L.; Lin, X.L.; Zhang, L.; Chen, L.; Song, Y.; Sun, R.; Zhang, H.L. TET1-TRPV4 Signaling Contributes to Bone Cancer Pain in Rats. Brain Sci. 2023, 13, 644. [Google Scholar] [CrossRef] [PubMed]
- Viet, C.T.; Dang, D.; Ye, Y.; Ono, K.; Campbell, R.R.; Schmidt, B.L. Demethylating drugs as novel analgesics for cancer pain. Clin. Cancer Res. 2014, 20, 4882–4893. [Google Scholar] [CrossRef] [PubMed]
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2016, 18, 20–30. [Google Scholar] [CrossRef]
- Xiong, H.Y.; Hendrix, J.; Schabrun, S.; Wyns, A.; Campenhout, J.V.; Nijs, J.; Polli, A. The Role of the Brain-Derived Neurotrophic Factor in Chronic Pain: Links to Central Sensitization and Neuroinflammation. Biomolecules 2024, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, X.; Wang, W.; Lu, Y.G.; Pan, Z.Z. Brain-derived neurotrophic factor-mediated downregulation of brainstem K+-Cl- cotransporter and cell-type-specific GABA impairment for activation of descending pain facilitation. Mol. Pharmacol. 2013, 84, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Franco-Enzastiga, U.; Garcia, G.; Murbartian, J.; Gonzalez-Barrios, R.; Salinas-Abarca, A.B.; Sanchez-Hernandez, B.; Tavares-Ferreira, D.; Herrera, L.A.; Barragan-Iglesias, P.; Delgado-Lezama, R.; et al. Sex-dependent pronociceptive role of spinal α5-GABAA receptor and its epigenetic regulation in neuropathic rodents. J. Neurochem. 2021, 156, 897–916. [Google Scholar] [CrossRef] [PubMed]
- Sikandar, S.; Minett, M.S.; Millet, Q.; Santana-Varela, S.; Lau, J.; Wood, J.N.; Zhao, J. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain 2018, 141, 1028–1039. [Google Scholar] [CrossRef]
- Sarchielli, P.; Mancini, M.L.; Floridi, A.; Coppola, F.; Rossi, C.; Nardi, K.; Acciarresi, M.; Pini, L.A.; Calabresi, P. Increased levels of neurotrophins are not specific for chronic migraine: Evidence from primary fibromyalgia syndrome. J. Pain. 2007, 8, 737–745. [Google Scholar] [CrossRef]
- Jablochkova, A.; Backryd, E.; Kosek, E.; Mannerkorpi, K.; Ernberg, M.; Gerdle, B.; Ghafouri, B. Unaltered low nerve growth factor and high brain-derived neurotrophic factor levels in plasma from patients with fibromyalgia after a 15-week progressive resistance exercise. J. Rehabil. Med. 2019, 51, 779–787. [Google Scholar] [CrossRef]
- Haas, L.; Portela, L.V.; Bohmer, A.E.; Oses, J.P.; Lara, D.R. Increased plasma levels of brain derived neurotrophic factor (BDNF) in patients with fibromyalgia. Neurochem. Res. 2010, 35, 830–834. [Google Scholar] [CrossRef]
- Simao, A.P.; Mendonca, V.A.; de Oliveira Almeida, T.M.; Santos, S.A.; Gomes, W.F.; Coimbra, C.C.; Lacerda, A.C. Involvement of BDNF in knee osteoarthritis: The relationship with inflammation and clinical parameters. Rheumatol. Int. 2014, 34, 1153–1157. [Google Scholar] [CrossRef]
- Laske, C.; Stransky, E.; Eschweiler, G.W.; Klein, R.; Wittorf, A.; Leyhe, T.; Richartz, E.; Kohler, N.; Bartels, M.; Buchkremer, G.; et al. Increased BDNF serum concentration in fibromyalgia with or without depression or antidepressants. J. Psychiatr. Res. 2007, 41, 600–605. [Google Scholar] [CrossRef]
- Stefani, L.C.; Leite, F.M.; da Graca, L.T.M.; Zanette, S.A.; de Souza, A.; Castro, S.M.; Caumo, W. BDNF and serum S100B levels according the spectrum of structural pathology in chronic pain patients. Neurosci. Lett. 2019, 706, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, B.; Korallus, C.; Gutenbrunner, C. Serum level of brain-derived neurotrophic factor in fibromyalgia syndrome correlates with depression but not anxiety. Neurochem. Int. 2013, 62, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Zanette, S.A.; Dussan-Sarria, J.A.; Souza, A.; Deitos, A.; Torres, I.L.; Caumo, W. Higher serum S100B and BDNF levels are correlated with a lower pressure-pain threshold in fibromyalgia. Mol. Pain. 2014, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Kaminskas, E.; Farrell, A.T.; Wang, Y.C.; Sridhara, R.; Pazdur, R. FDA drug approval summary: Azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist 2005, 10, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Gore, S.D.; Jones, C.; Kirkpatrick, P. Decitabine. Nat. Rev. Drug Discov. 2006, 5, 891–892. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, F.; Lu, K.; Wei, A.; Jiang, Q.; Cao, W. PPARgamma preservation via promoter demethylation alleviates osteoarthritis in mice. Ann. Rheum. Dis. 2019, 78, 1420–1429. [Google Scholar] [CrossRef]
- Appel, C.K.; Scheff, N.N.; Viet, C.T.; Schmidt, B.L.; Heegaard, A.M. Decitabine attenuates nociceptive behavior in a murine model of bone cancer pain. Pain 2019, 160, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sahbaie, P.; Liang, D.; Li, W.; Shi, X.; Kingery, P.; Clark, J.D. DNA Methylation Modulates Nociceptive Sensitization after Incision. PLoS ONE 2015, 10, e0142046. [Google Scholar] [CrossRef]
- Liu, R.; Wu, X.M.; He, X.; Wang, R.Z.; Yin, X.Y.; Zhou, F.; Ji, M.H.; Shen, J.C. Contribution of DNA methyltransferases to spared nerve injury induced depression partially through epigenetically repressing Bdnf in hippocampus: Reversal by ketamine. Pharmacol. Biochem. Behav. 2021, 200, 173079. [Google Scholar] [CrossRef] [PubMed]
- Topham, L.; Gregoire, S.; Kang, H.; Salmon-Divon, M.; Lax, E.; Millecamps, M.; Szyf, M.; Stone, L. The methyl donor S-adenosyl methionine reverses the DNA methylation signature of chronic neuropathic pain in mouse frontal cortex. Pain Rep. 2021, 6, e944. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Nuesch, E.; Reichenbach, S.; Juni, P. S-Adenosylmethionine for osteoarthritis of the knee or hip. Cochrane Database Syst. Rev. 2009, 2009, CD007321. [Google Scholar] [CrossRef] [PubMed]
- Velickovic, Z.; Pavlov Dolijanovic, S.; Stojanovic, N.; Janjic, S.; Kovacevic, L.; Soldatovic, I.; Radunovic, G. The short-term effect of glucosamine-sulfate, nonanimal chondroitin-sulfate, and S-adenosylmethionine combination on ultrasonography findings, inflammation, pain, and functionality in patients with knee osteoarthritis: A pilot, double-blind, randomized, placebo-controlled clinical trial. Arch. Rheumatol. 2023, 38, 521–541. [Google Scholar] [CrossRef] [PubMed]
- Choi, L.J.; Huang, J.S. A pilot study of S-adenosylmethionine in treatment of functional abdominal pain in children. Altern. Ther. Health Med. 2013, 19, 61–64. [Google Scholar] [PubMed]
- Vinkers, C.H.; Geuze, E.; van Rooij, S.J.H.; Kennis, M.; Schur, R.R.; Nispeling, D.M.; Smith, A.K.; Nievergelt, C.M.; Uddin, M.; Rutten, B.P.F.; et al. Successful treatment of post-traumatic stress disorder reverses DNA methylation marks. Mol. Psychiatry 2021, 26, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Weaver, I.C.; Champagne, F.A.; Brown, S.E.; Dymov, S.; Sharma, S.; Meaney, M.J.; Szyf, M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: Altering epigenetic marking later in life. J. Neurosci. 2005, 25, 11045–11054. [Google Scholar] [CrossRef]
- Lax, E.; Warhaftig, G.; Ohana, D.; Maayan, R.; Delayahu, Y.; Roska, P.; Ponizovsky, A.M.; Weizman, A.; Yadid, G.; Szyf, M. A DNA Methylation Signature of Addiction in T Cells and Its Reversal with DHEA Intervention. Front. Mol. Neurosci. 2018, 11, 322. [Google Scholar] [CrossRef]
- Brueckner, B.; Lyko, F. DNA methyltransferase inhibitors: Old and new drugs for an epigenetic cancer therapy. Trends Pharmacol. Sci. 2004, 25, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L. The complex language of chromatin regulation during transcription. Nature 2007, 447, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, J.D. The emerging field of neuroepigenetics. Neuron 2013, 80, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.M.; Hemstedt, T.J.; Bading, H. Rescue of aging-associated decline in Dnmt3a2 expression restores cognitive abilities. Nat. Neurosci. 2012, 15, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Pan, H.L. Epigenetic Mechanisms of Neural Plasticity in Chronic Neuropathic Pain. ACS Chem. Neurosci. 2022, 13, 432–441. [Google Scholar] [CrossRef]
- Halder, R.; Hennion, M.; Vidal, R.O.; Shomroni, O.; Rahman, R.U.; Rajput, A.; Centeno, T.P.; van Bebber, F.; Capece, V.; Garcia Vizcaino, J.C.; et al. DNA methylation changes in plasticity genes accompany the formation and maintenance of memory. Nat. Neurosci. 2016, 19, 102–110. [Google Scholar] [CrossRef]
- Santiago, M.; Antunes, C.; Guedes, M.; Sousa, N.; Marques, C.J. TET enzymes and DNA hydroxymethylation in neural development and function—How critical are they? Genomics 2014, 104, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.U.; Ma, D.K.; Mo, H.; Ball, M.P.; Jang, M.H.; Bonaguidi, M.A.; Balazer, J.A.; Eaves, H.L.; Xie, B.; Ford, E.; et al. Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat. Neurosci. 2011, 14, 1345–1351. [Google Scholar] [CrossRef]
- Golzenleuchter, M.; Kanwar, R.; Zaibak, M.; Al Saiegh, F.; Hartung, T.; Klukas, J.; Smalley, R.L.; Cunningham, J.M.; Figueroa, M.E.; Schroth, G.P.; et al. Plasticity of DNA methylation in a nerve injury model of pain. Epigenetics 2015, 10, 200–212. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, H.-Y.; Wyns, A.; Campenhout, J.V.; Hendrix, J.; De Bruyne, E.; Godderis, L.; Schabrun, S.; Nijs, J.; Polli, A. Epigenetic Landscapes of Pain: DNA Methylation Dynamics in Chronic Pain. Int. J. Mol. Sci. 2024, 25, 8324. https://doi.org/10.3390/ijms25158324
Xiong H-Y, Wyns A, Campenhout JV, Hendrix J, De Bruyne E, Godderis L, Schabrun S, Nijs J, Polli A. Epigenetic Landscapes of Pain: DNA Methylation Dynamics in Chronic Pain. International Journal of Molecular Sciences. 2024; 25(15):8324. https://doi.org/10.3390/ijms25158324
Chicago/Turabian StyleXiong, Huan-Yu, Arne Wyns, Jente Van Campenhout, Jolien Hendrix, Elke De Bruyne, Lode Godderis, Siobhan Schabrun, Jo Nijs, and Andrea Polli. 2024. "Epigenetic Landscapes of Pain: DNA Methylation Dynamics in Chronic Pain" International Journal of Molecular Sciences 25, no. 15: 8324. https://doi.org/10.3390/ijms25158324






