Identification and Expression Analysis of Cytochrome P450 Genes Probably Involved in Triterpenoid Saponins Biosynthesis in Astragalus mongholicus
Abstract
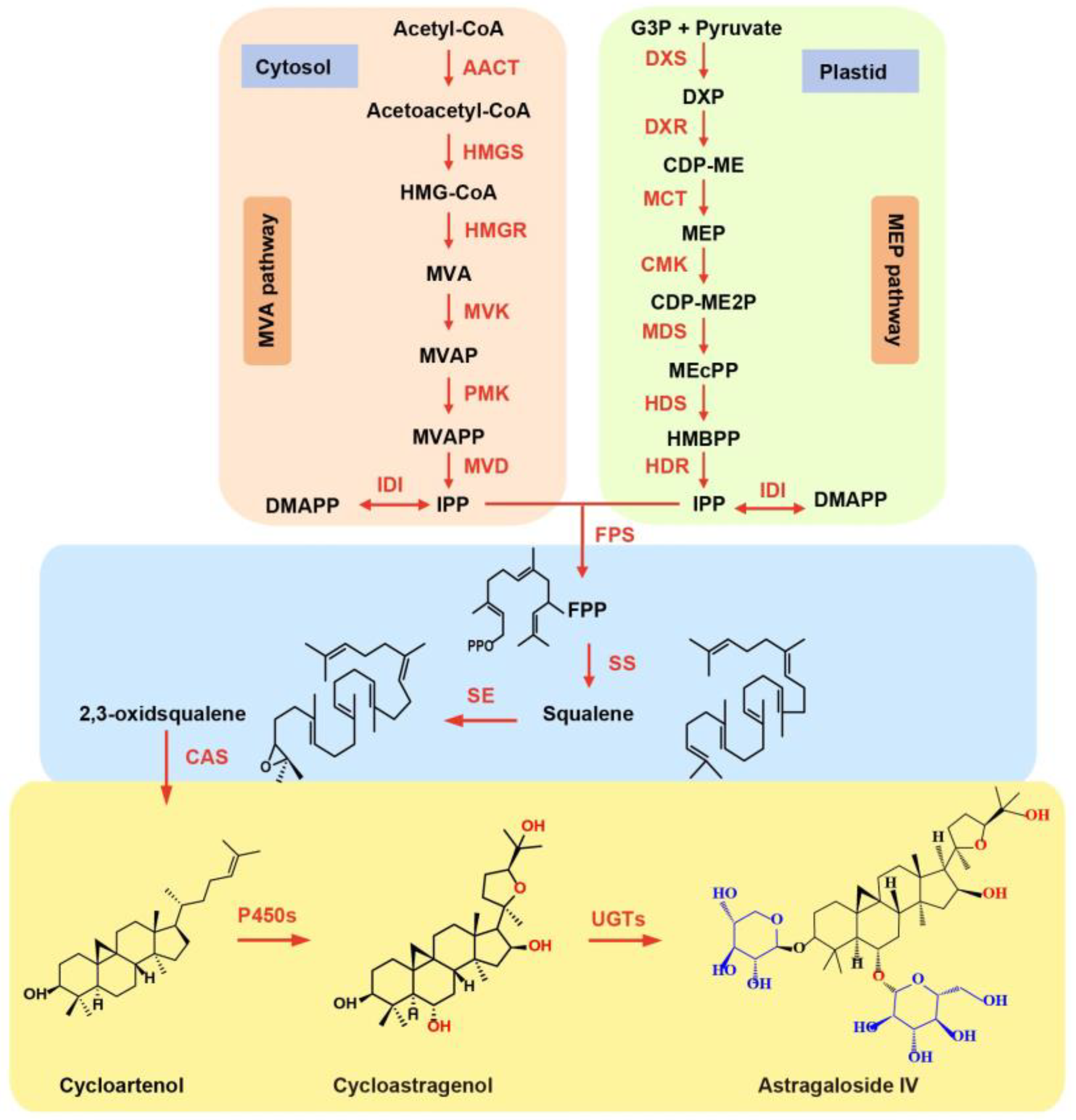
:1. Introduction
2. Results
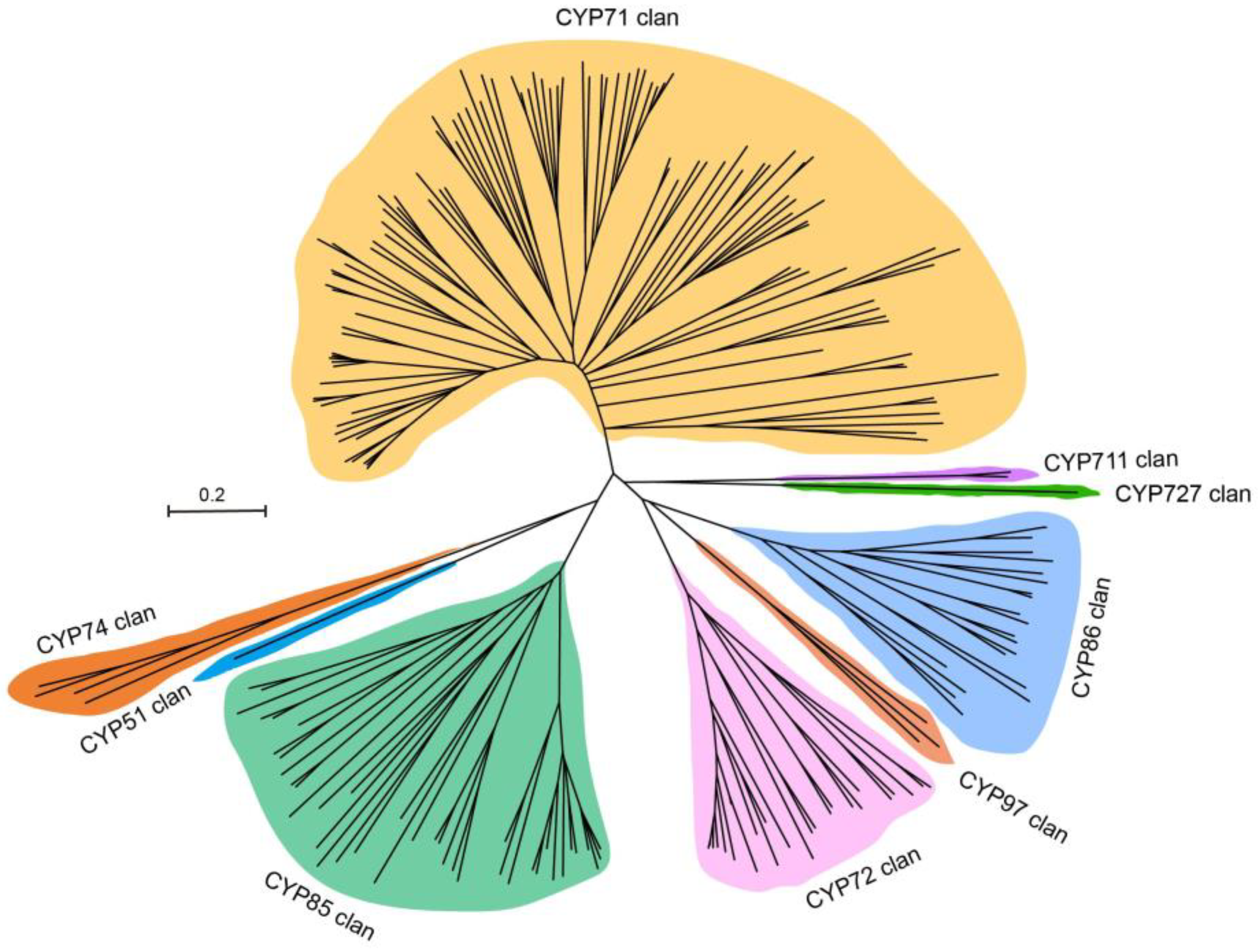
2.1. Identification of P450 Genes in A. mongholicus
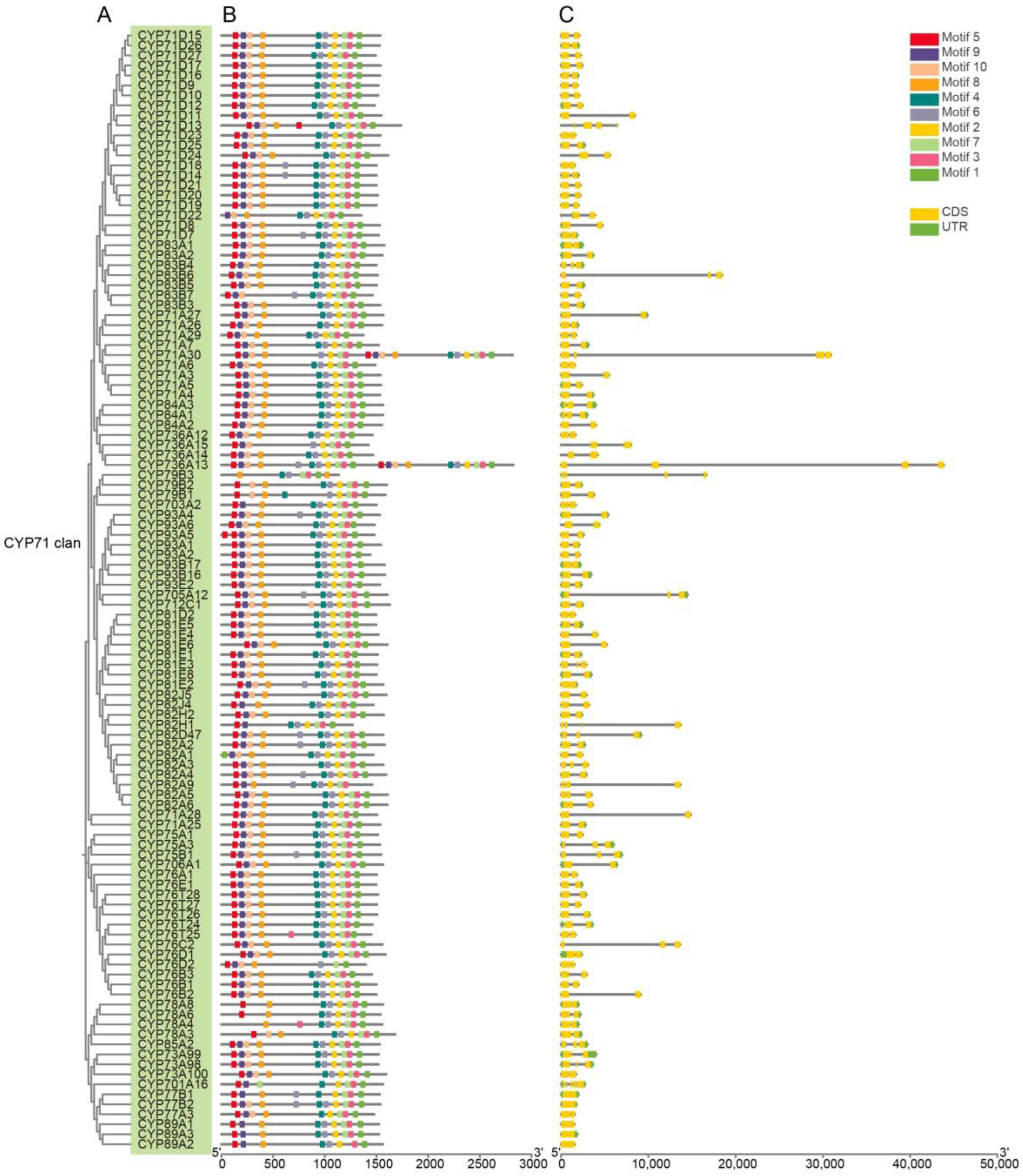
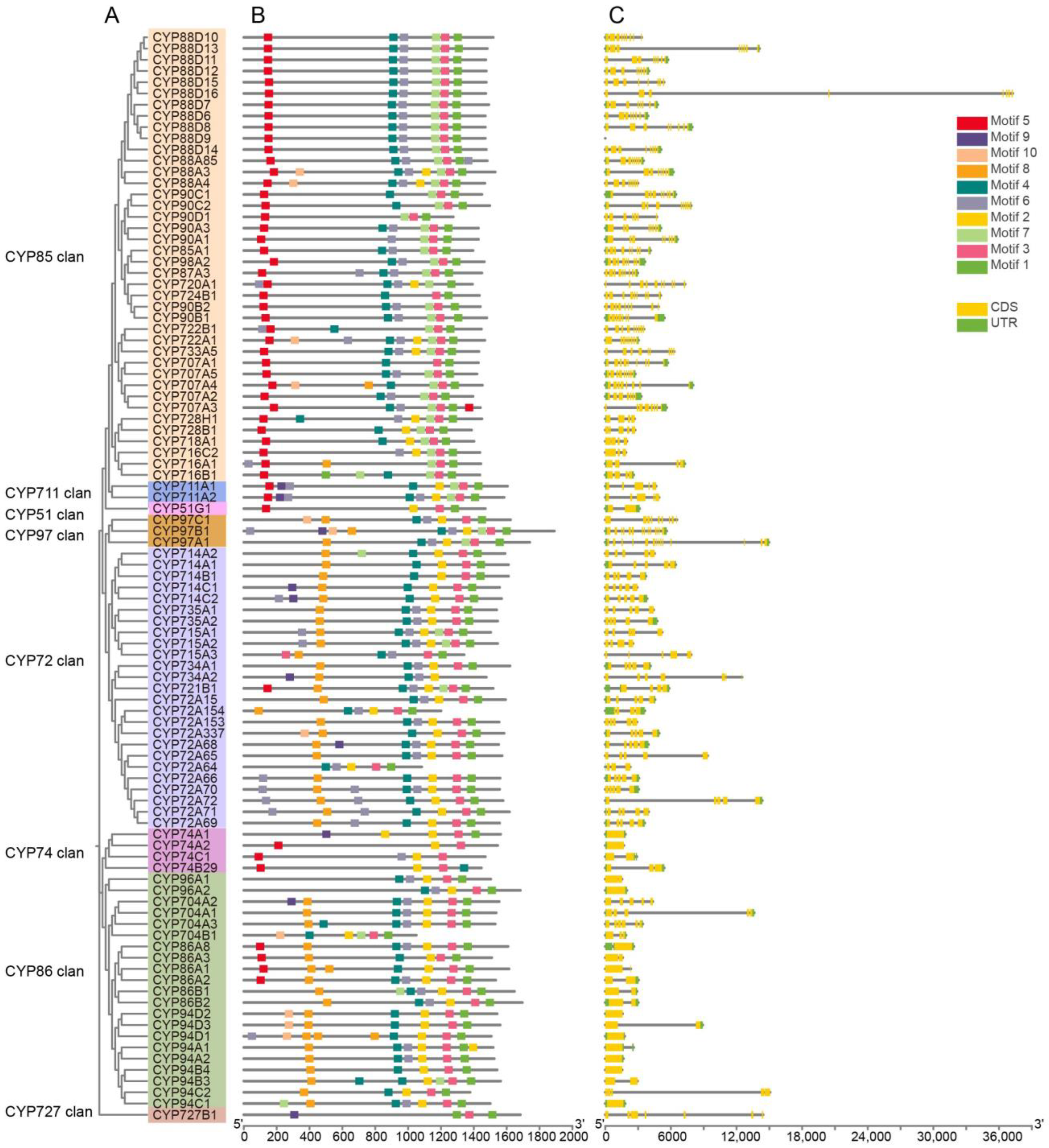
2.2. Gene Structure and Conserved Motifs Analysis of AmP450s
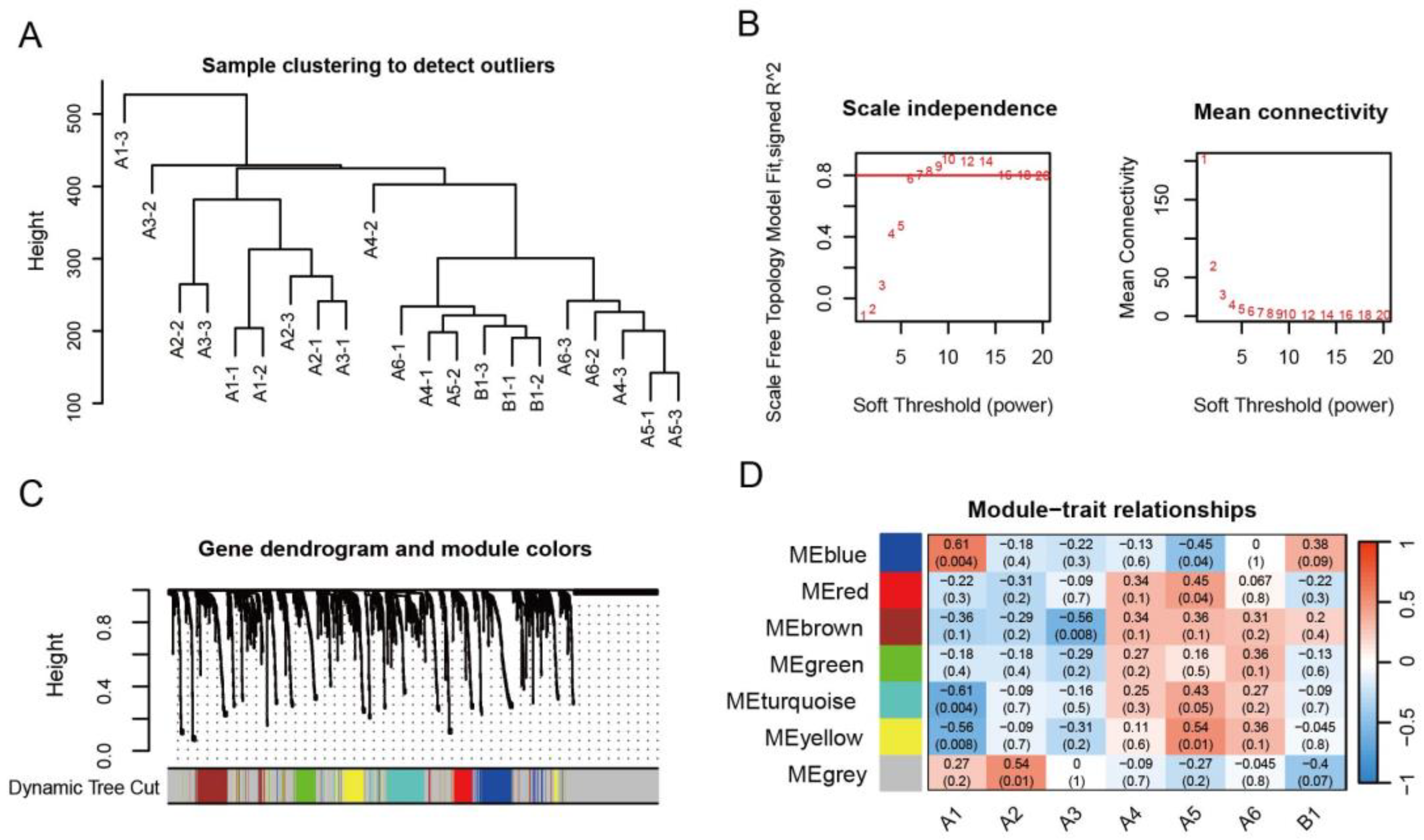
2.3. Analysis of the AmP450 Genes Involved in Astragaloside Biosynthesis by WGCNA
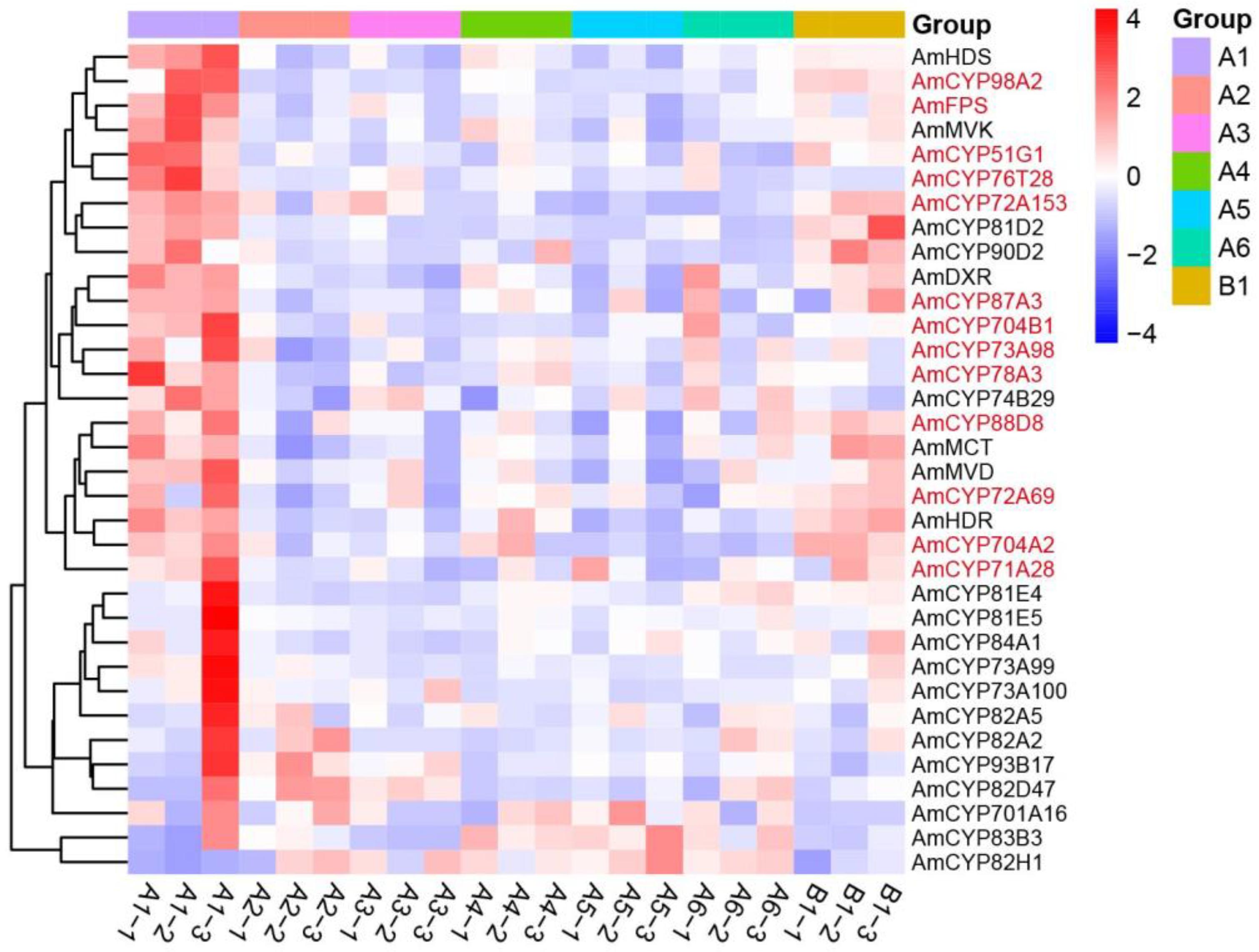
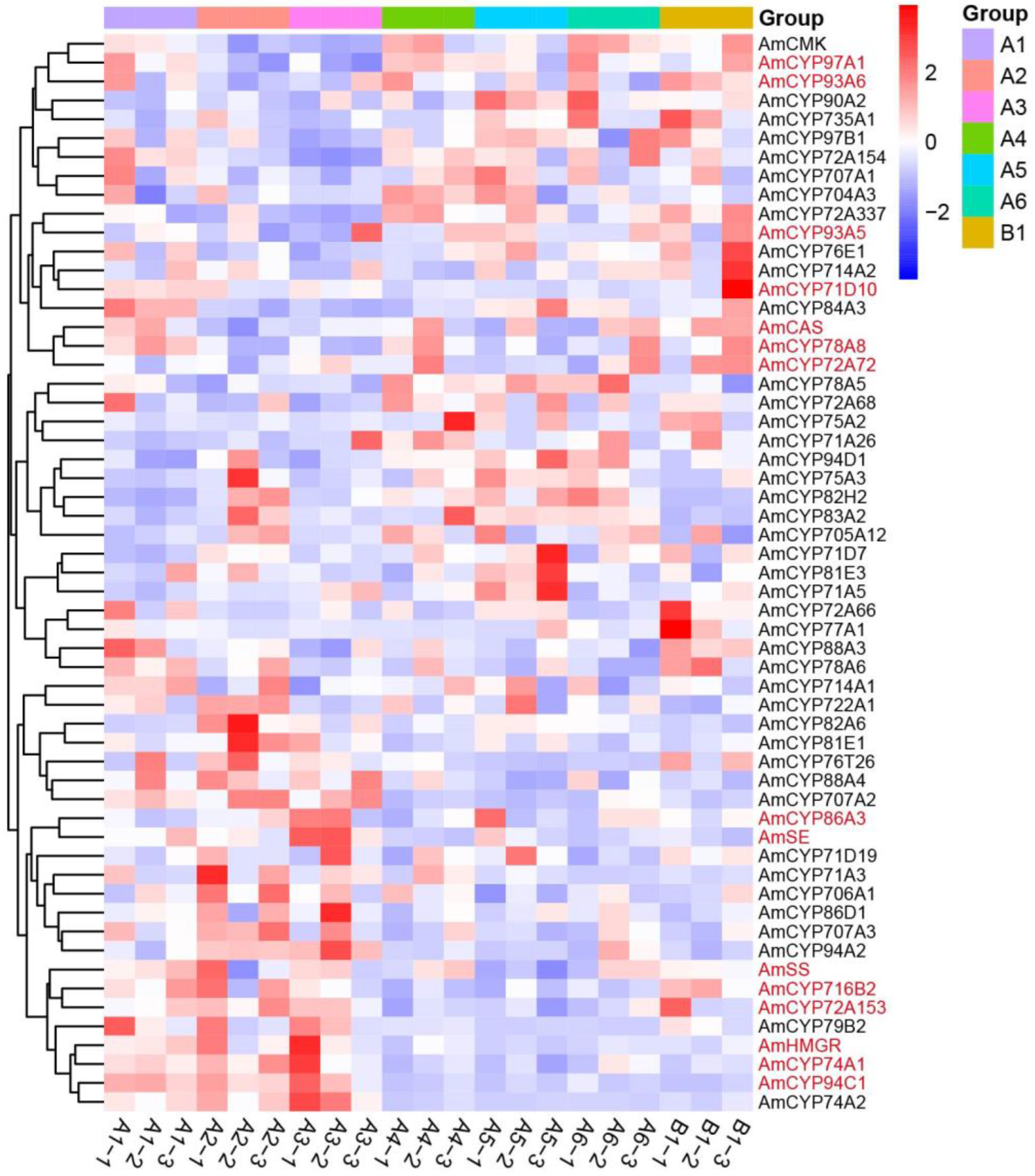
2.4. Expression Profiles of AmP450 Genes in Different Tissues of A. mongholicus
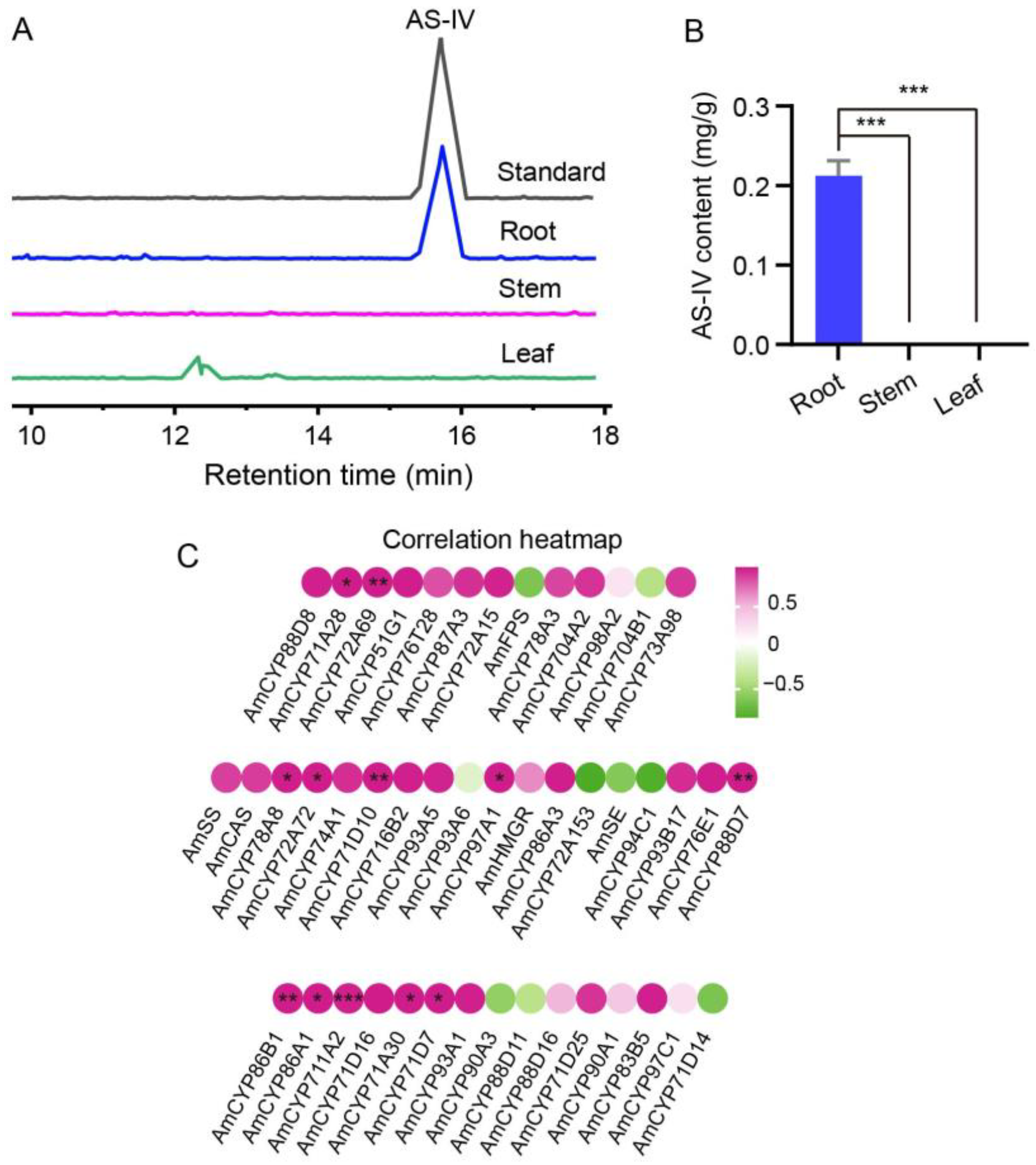
2.5. A Closely Relationship between Expression of P450s and AS-IV Biosynthesis
3. Discussion
3.1. Comprehensive Identification of P450 Genes in A. mongholicus
3.2. AmP450s Involved in Astragaloside Synthesis
4. Materials and Methods
4.1. Plant Materials
4.2. Identification of CYP450 Genes from A. mongholicus
4.3. Sequence Analyses, Structural Characterization and Phylogenetic Tree Construction
4.4. Weighted Gene Co-Expression Network Analysis
4.5. Gene Expression Analysis by qRT-PCR
4.6. HPLC-ELSD Analysis of the Content of AS-IV
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nelson, D.; Werck-Reichhart, D. A P450-centric view of plant evolution. Plant J. 2011, 66, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Mohamed, R. Plant cytochrome P450s: Nomenclature and involvement in natural product biosynthesis. Protoplasma 2016, 253, 1197–1209. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.R. Cytochrome P450 nomenclature, 2004. Methods Mol. Biol. 2006, 320, 1–10. [Google Scholar] [PubMed]
- Nelson, D.R.; Schuler, M.A.; Paquette, S.M.; Werck-Reichhart, D.; Bak, S. Comparative genomics of rice and Arabidopsis. Analysis of 727 cytochrome P450 genes and pseudogenes from a monocot and a dicot. Plant Physiol. 2004, 135, 756–772. [Google Scholar] [CrossRef] [PubMed]
- Bak, S.; Beisson, F.; Bishop, G.; Hamberger, B.; Höfer, R.; Paquette, S.; Werck-Reichhart, D. Cytochromes p450. Arab. Book/Am. Soc. Plant Biol. 2002, 9, e0144. [Google Scholar] [CrossRef] [PubMed]
- Hasemann, C.A.; Kurumbail, R.G.; Boddupalli, S.S.; Peterson, J.A.; Deisenhofer, J. Structure and function of cytochromes P450: A comparative analysis of three crystal structures. Structure 1995, 3, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Panwar, R.; Mittal, P.; Hassan, M.I.; Singh, I.K. Plant cytochrome P450s: Role in stress tolerance and potential applications for human welfare. Int. J. Biol. Macromol. 2021, 184, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Yang, J.; Ma, L.; Yan, S.; Pang, Y. Genome-Wide Identification and Analyses of Drought/Salt-Responsive Cytochrome P450 Genes in Medicago truncatula. Int. J. Mol. Sci. 2021, 22, 9957. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhu, X.; Wang, H.; Liu, T.; Cheng, J.; Jiang, H. Discovery and modification of cytochrome P450 for plant natural products biosynthesis. Synth. Syst. Biotechnol. 2020, 5, 187–199. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Liu, Y.; Liao, X.; Chu, H.; Chang, H.; Cao, Y.; Li, Z.; Zhang, T.; Cheng, J.; et al. PCPD: Plant cytochrome P450 database and web-based tools for structural construction and ligand docking. Synth. Syst. Biotechnol. 2021, 6, 102–109. [Google Scholar] [CrossRef]
- Mizutani, M.; Sato, F. Unusual P450 reactions in plant secondary metabolism. Arch. Biochem. Biophys. 2011, 507, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Triterpene Structural Diversification by Plant Cytochrome P450 Enzymes. Front. Plant Sci. 2017, 8, 1886. [Google Scholar] [CrossRef]
- Hansen, C.C.; Nelson, D.R.; Møller, B.L.; Werck-Reichhart, D. Plant cytochrome P450 plasticity and evolution. Mol. Plant. 2021, 14, 1244–1265. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, X.; Guo, W. The cytochrome P450 superfamily: Key players in plant development and defense. J. Integr. Agric. 2015, 14, 1673–1686. [Google Scholar] [CrossRef]
- Babu, P.R.; Rao, K.V.; Reddy, V.D. Structural organization and classification of cytochrome P450 genes in flax (Linum usitatissimum L.). Gene 2013, 513, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Guttikonda, S.K.; Trupti, J.; Bisht, N.C.; Chen, H.; An, Y.Q.; Pandey, S.; Yu, O. Whole genome co-expression analysis of soybean cytochrome P450 genes identifies nodulation-specific P450 monooxygenases. BMC Plant Biol. 2010, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, H.; Ma, R.; Chang, Y.; Qin, X.; Xu, J.; Fu, Y. Genome-wide transcriptome analysis and characterization of the cytochrome P450 flavonoid biosynthesis genes in pigeon pea (Cajanus cajan). Planta 2022, 255, 120. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Gao, L.; Du, G.; Qin, X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv. Pharmacol. 2020, 87, 89–112. [Google Scholar]
- Li, L.; Hou, X.; Xu, R.; Liu, C.; Tu, M. Research review on the pharmacological effects of astragaloside IV. Fundam. Clin. Pharmacol. 2017, 31, 17–36. [Google Scholar] [CrossRef]
- Tuan, P.A.; Chung, E.; Thwe, A.A.; Li, X.; Kim, Y.B.; Mariadhas, V.A.; Al-Dhabi, N.A.; Lee, J.-H.; Park, S.U. Transcriptional Profiling and Molecular Characterization of Astragalosides, Calycosin, and Calycosin-7-O-beta-D-glucoside Biosynthesis in the Hairy Roots of Astragalus membranaceus in Response to Methyl Jasmonate. J. Agric. Food Chem. 2015, 63, 6231–6240. [Google Scholar] [CrossRef]
- Chen, Y.; Fang, T.; Su, H.; Duan, S.; Ma, R.; Wang, P.; Wu, L.; Sun, W.; Hu, Q.; Zhao, M.; et al. A reference-grade genome assembly for Astragalus mongholicus and insights into the biosynthesis and high accumulation of triterpenoids and flavonoids in its roots. Plant Commun. 2022, 13, 100469. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Zhang, M.; Xu, L.; Yi, Y.; Wang, L.; Wang, H.; Wang, Z.; Xing, J.; Li, P.; Zhang, X.; et al. Identification of oxidosqualene cyclases associated with saponin biosynthesis from Astragalus membranaceus reveals a conserved motif important for catalytic function. J. Adv. Res. 2023, 43, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yi, Y.; Gao, B.H.; Su, H.F.; Bao, Y.O.; Shi, X.M.; Wang, H.D.; Li, F.D.; Ye, M.; Qiao, X. Functional Characterization and Protein Engineering of a Triterpene 3-/6-/2′-O-Glycosyltransferase Reveal a Conserved Residue Critical for the Regiospecificity. Angew. Chem. Int. Ed. Engl. 2022, 61, e202113587. [Google Scholar] [CrossRef]
- Duan, Y.; Du, W.; Song, Z.; Chen, R.; Xie, K.; Liu, J.; Chen, D.; Dai, J. Functional characterization of a cycloartenol synthase and four glycosyltransferases in the biosynthesis of cycloastragenol-type astragalosides from Astragalus membranaceus. Acta Pharm. Sin. B 2023, 13, 271–283. [Google Scholar] [CrossRef]
- Luo, Y.Y.; Zhang, X.; Zhang, F.S.; Li, H.J.; Zhang, J.Q.; Du, G.H.; Qin, X.M. Molecular mechanism underlying difference of astragaloside IV content in imitating wild and cultivated Astragalus mongholicus. Zhongguo Zhong yao za zhi 2022, 47, 3463–3474. [Google Scholar]
- Wei, K.; Chen, H. Global identification, structural analysis and expression characterization of cytochrome P450 monooxygenase superfamily in rice. BMC Genom. 2018, 19, 35. [Google Scholar] [CrossRef]
- Li, Y.; Wei, K. Comparative functional genomics analysis of cytochrome P450 gene superfamily in wheat and maize. BMC Plant Biol. 2020, 20, 93. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, X.; Xu, Y.; Zhong, Y.; Li, Y.; Chen, J.; Li, X.; Nan, P. Global transcriptome analysis profiles metabolic pathways in traditional herb Astragalus membranaceus Bge. var. mongolicus (Bge.) Hsiao. BMC Genomics 2015, 16, S15. [Google Scholar]
- Karunanithi, P.S.; Dhanota, P.; Addison, J.B.; Tong, S.; Fiehn, O.; Zerbe, P. Functional characterization of the cytochrome P450 monooxygenase CYP71AU87 indicates a role in marrubiin biosynthesis in the medicinal plant Marrubium vulgare. BMC Plant Biol. 2019, 19, 114. [Google Scholar] [CrossRef]
- Yu, F.; Okamoto, S.; Harada, H.; Yamasaki, K.; Misawa, N.; Utsumi, R. Zingiber zerumbet CYP71BA1 catalyzes the conversion of α-humulene to 8-hydroxy-α-humulene in zerumbone biosynthesis. Cell. Mol. Life Sci. 2011, 68, 1033–1040. [Google Scholar] [CrossRef]
- Pan, Z.; Baerson, S.R.; Wang, M.; Bajsa-Hirschel, J.; Rimando, A.M.; Wang, X.; Dhammika Nanayakkara, N.P.; Noonan, B.P.; Fromm, M.E.; Dayan, F.E.; et al. A cytochrome P450 CYP71 enzyme expressed in Sorghum bicolor root hair cells participates in the biosynthesis of the benzoquinone allelochemical sorgoleone. New Phytol. 2018, 218, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Snyder, J.H.; Yang, D.S.; Huhman, D.V.; Watson, B.S.; Allen, S.N.; Tang, Y.; Miettinen, K.; Arendt, P.; Pollier, J.; et al. Integrated metabolomics identifies CYP72A67 and CYP72A68 oxidases in the biosynthesis of Medicago truncatula oleanate sapogenins. Metabolomics 2019, 15, 85. [Google Scholar] [CrossRef]
- Takase, S.; Kera, K.; Nagashima, Y.; Mannen, K.; Hosouchi, T.; Shinpo, S.; Kawashima, M.; Kotake, Y.; Yamada, H.; Saga, Y.; et al. Allylic hydroxylation of triterpenoids by a plant cytochrome P450 triggers key chemical transformations that produce a variety of bitter compounds. J. Biol. Chem. 2019, 294, 18662–18673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Z.; Xiao, G.; Zhai, M.; Pan, X.; Huang, R.; Zhang, H. CYP71D8L is a key regulator involved in growth and stress responses by mediating gibberellin homeostasis in rice. J. Exp. Bot. 2020, 71, 1160–1170. [Google Scholar] [PubMed]
- Xiao, F.; Goodwin, S.M.; Xiao, Y.; Sun, Z.; Baker, D.; Tang, X.; Jenks, M.A.; Zhou, J.-M. Arabidopsis CYP86A2 represses Pseudomonas syringae type III genes and is required for cuticle development. EMBO J. 2004, 23, 2903–2913. [Google Scholar] [CrossRef]
- Tao, X.; Wang, M.X.; Dai, Y.; Wang, Y.; Fan, Y.F.; Mao, P.; Ma, X.R. Identification and Expression Profile of CYPome in Perennial Ryegrass and Tall Fescue in Response to Temperature Stress. Front. Plant Sci. 2017, 8, 1519. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, J.; Qin, L.; Shi, W.; Xia, G.; Liu, S. TaCYP81D5, one member in a wheat cytochrome P450 gene cluster, confers salinity tolerance via reactive oxygen species scavenging. Plant Biotechnol. 2020, 18, 791–804. [Google Scholar] [CrossRef]
- Guo, P.; Bai, G.; Carver, B.; Li, R.; Bernardo, A.; Baum, M. Transcriptional analysis between two wheat near-isogenic lines contrasting in aluminum tolerance under aluminum stress. Mol. Genet. 2007, 277, 1–12. [Google Scholar] [CrossRef]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.V.; Jugulam, M. Role of Cytochrome P450 Enzymes in Plant Stress Response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef]
- Dobritsa, A.A.; Shrestha, J.; Morant, M.; Pinot, F.; Matsuno, M.; Swanson, R.; Møller, B.P.; Preuss, D. CYP704B1 is a long-chain fatty acid omega-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol. 2009, 151, 574–589. [Google Scholar] [CrossRef]
- Xia, Y.; Su, Q.; Li, X.; Yan, S.; Liu, J.; He, C.; Huang, H.; Jiang, W.; Pang, Y. Two CYP93A enzymes play a dual role in isoflavonoid biosynthesis in Glycine max L. Plant Physiol. Bioch. 2023, 203, 108073. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Lu, M.; Luo, Q.; Tan, F.; Zhao, Z.; Liu, M.; He, Y. OsCPD1 and OsCPD2 are functional brassinosteroid biosynthesis genes in rice. Plant Sci. 2022, 325, 111482. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 1994, 2, 28–36. [Google Scholar] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant. 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree of Life (iTOL) v6: Recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 2024, 52, W78–W82. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC bioinformatics 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, X.; Luo, Y.; Li, H.; Qin, X. Biosynthetic mechanisms of isoflavone accumulation affected by different growth patterns in Astragalus mongholicus products. BMC Plant Biol. 2022, 22, 410. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yang, B.; Zhang, F.; Wang, J.; Xue, K.; Hussain Chang, B.; Zhang, J.; Qin, X. Identification and Expression Analysis of Cytochrome P450 Genes Probably Involved in Triterpenoid Saponins Biosynthesis in Astragalus mongholicus. Int. J. Mol. Sci. 2024, 25, 8333. https://doi.org/10.3390/ijms25158333
Wang J, Yang B, Zhang F, Wang J, Xue K, Hussain Chang B, Zhang J, Qin X. Identification and Expression Analysis of Cytochrome P450 Genes Probably Involved in Triterpenoid Saponins Biosynthesis in Astragalus mongholicus. International Journal of Molecular Sciences. 2024; 25(15):8333. https://doi.org/10.3390/ijms25158333
Chicago/Turabian StyleWang, Junxiu, Baoping Yang, Fusheng Zhang, Jiaorui Wang, Kunlun Xue, Babar Hussain Chang, Jianqin Zhang, and Xuemei Qin. 2024. "Identification and Expression Analysis of Cytochrome P450 Genes Probably Involved in Triterpenoid Saponins Biosynthesis in Astragalus mongholicus" International Journal of Molecular Sciences 25, no. 15: 8333. https://doi.org/10.3390/ijms25158333





