Efficient AAV9 Purification Using a Single-Step AAV9 Magnetic Affinity Beads Isolation
Abstract
:1. Introduction
2. Results
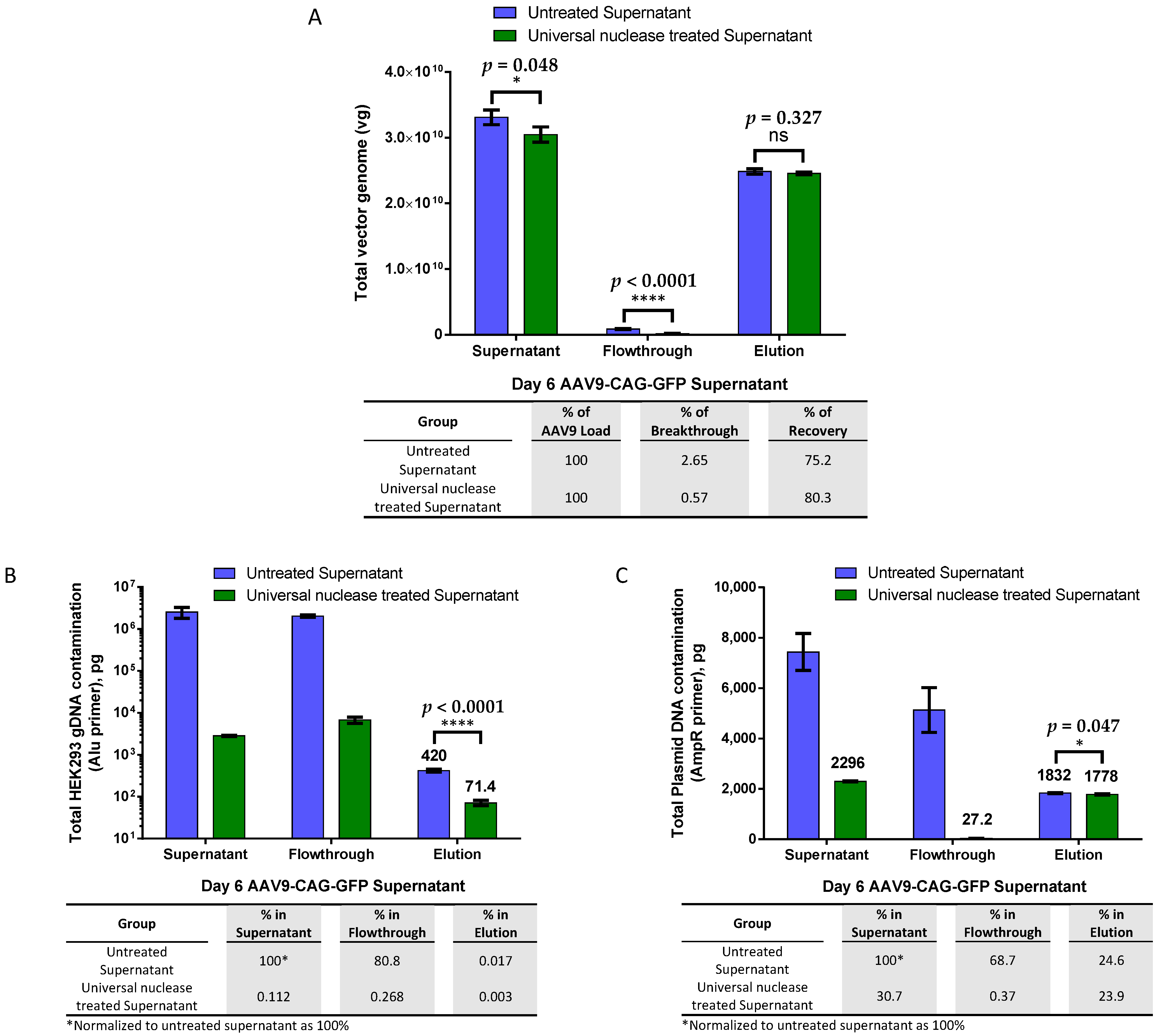
2.1. Effective AAV9 Purification from Crude Viral Supernatant and DNA Contamination Removal with Magnetic Affinity Beads, Eliminating the Need for Endonuclease Treatment
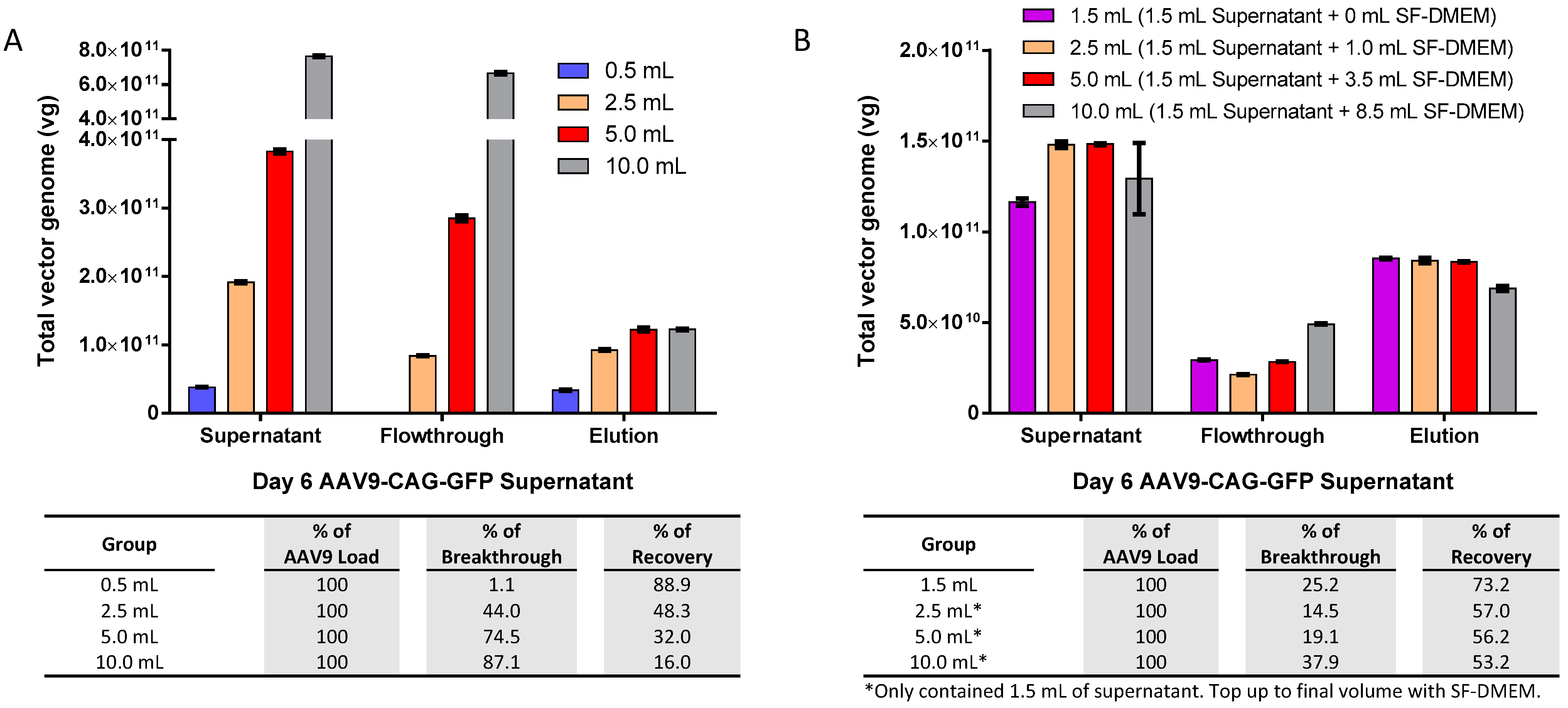
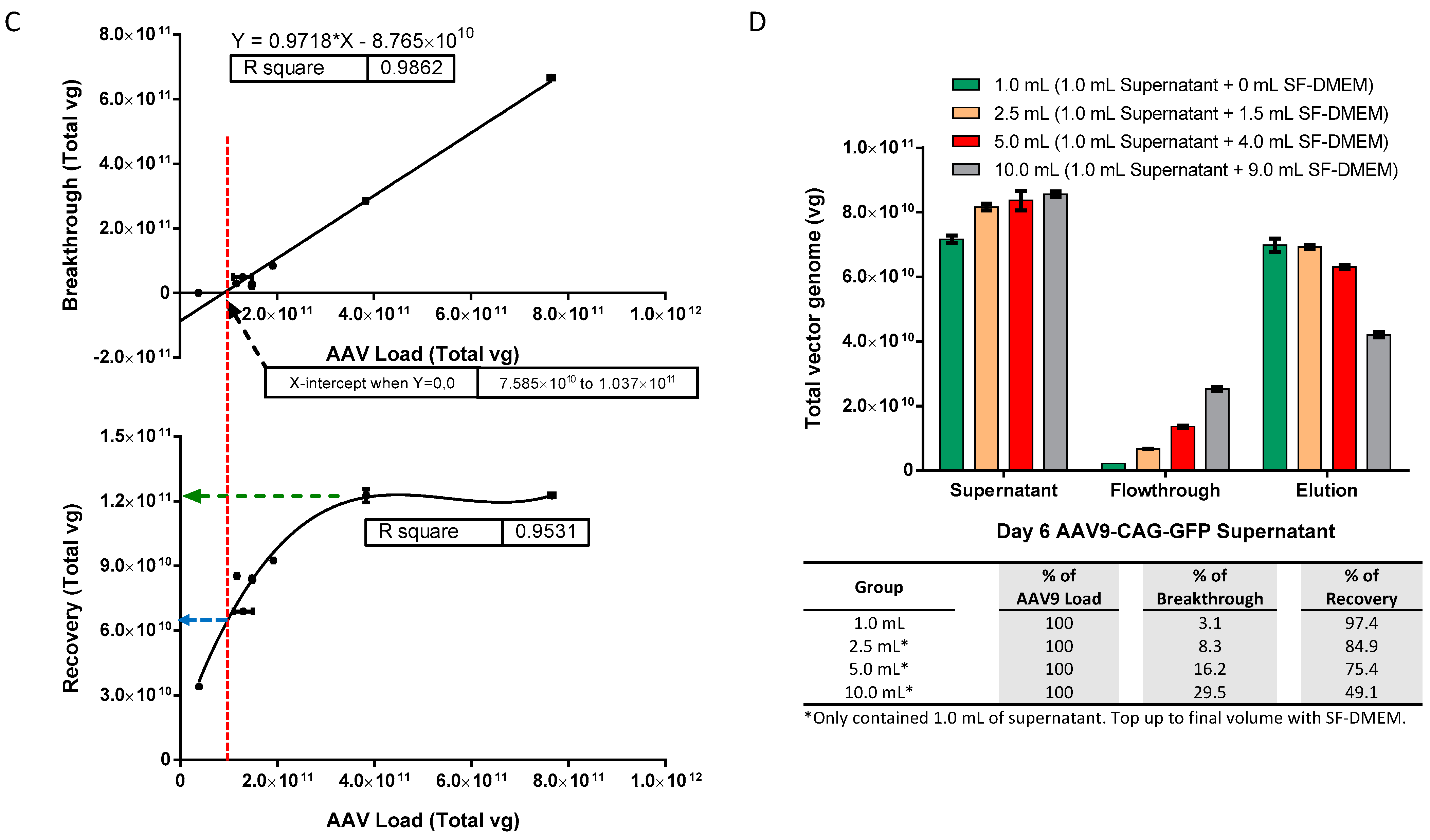
2.2. Optimal AAV9 Magnetic Affinity Beads Utilization for Maximum AAV9 Purification Yield with Minimal Loss in Flowthrough
2.3. Reusability of AAV9 Magnetic Affinity Beads
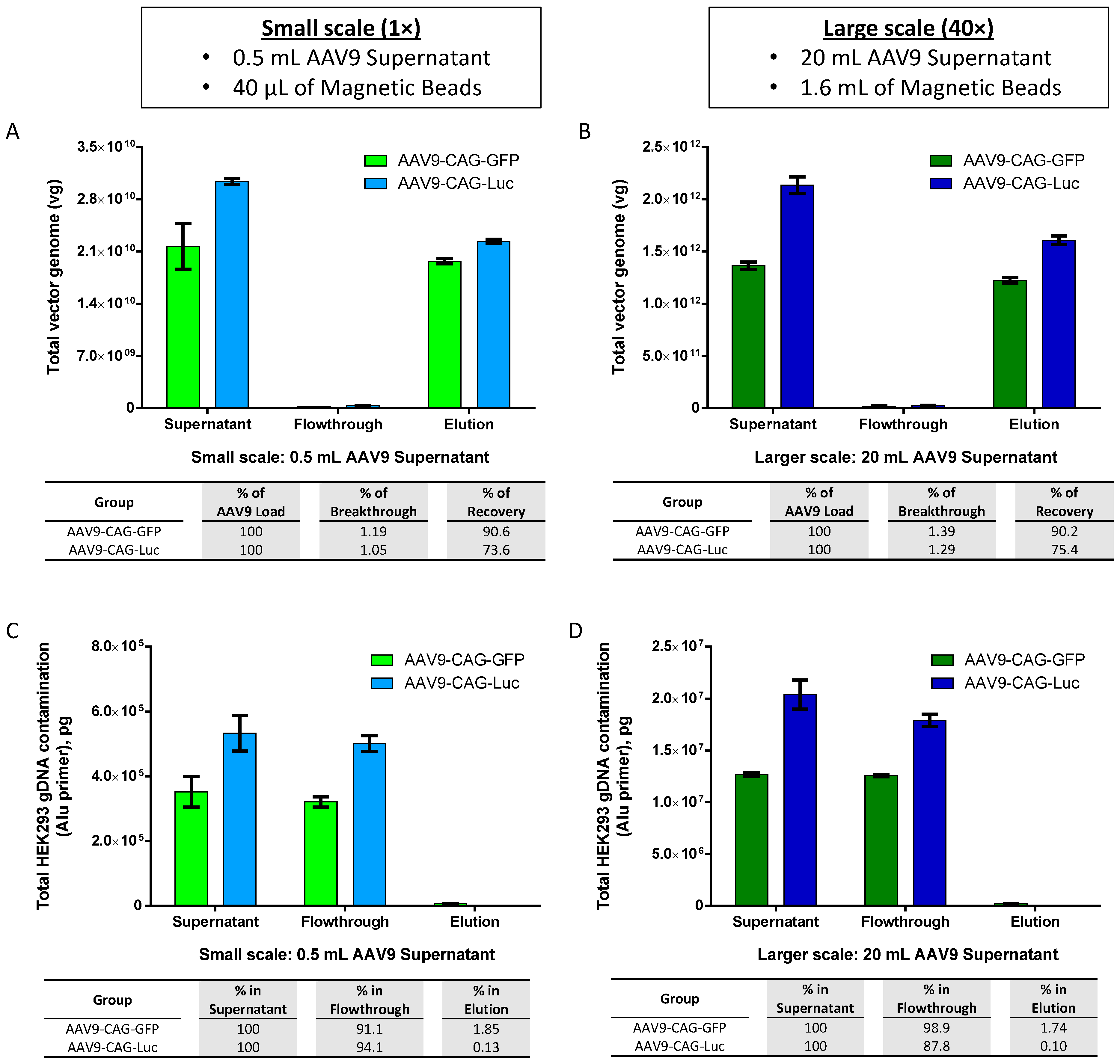
2.4. Scalability of AAV9 Purification with AAV9 Magnetic Affinity Beads
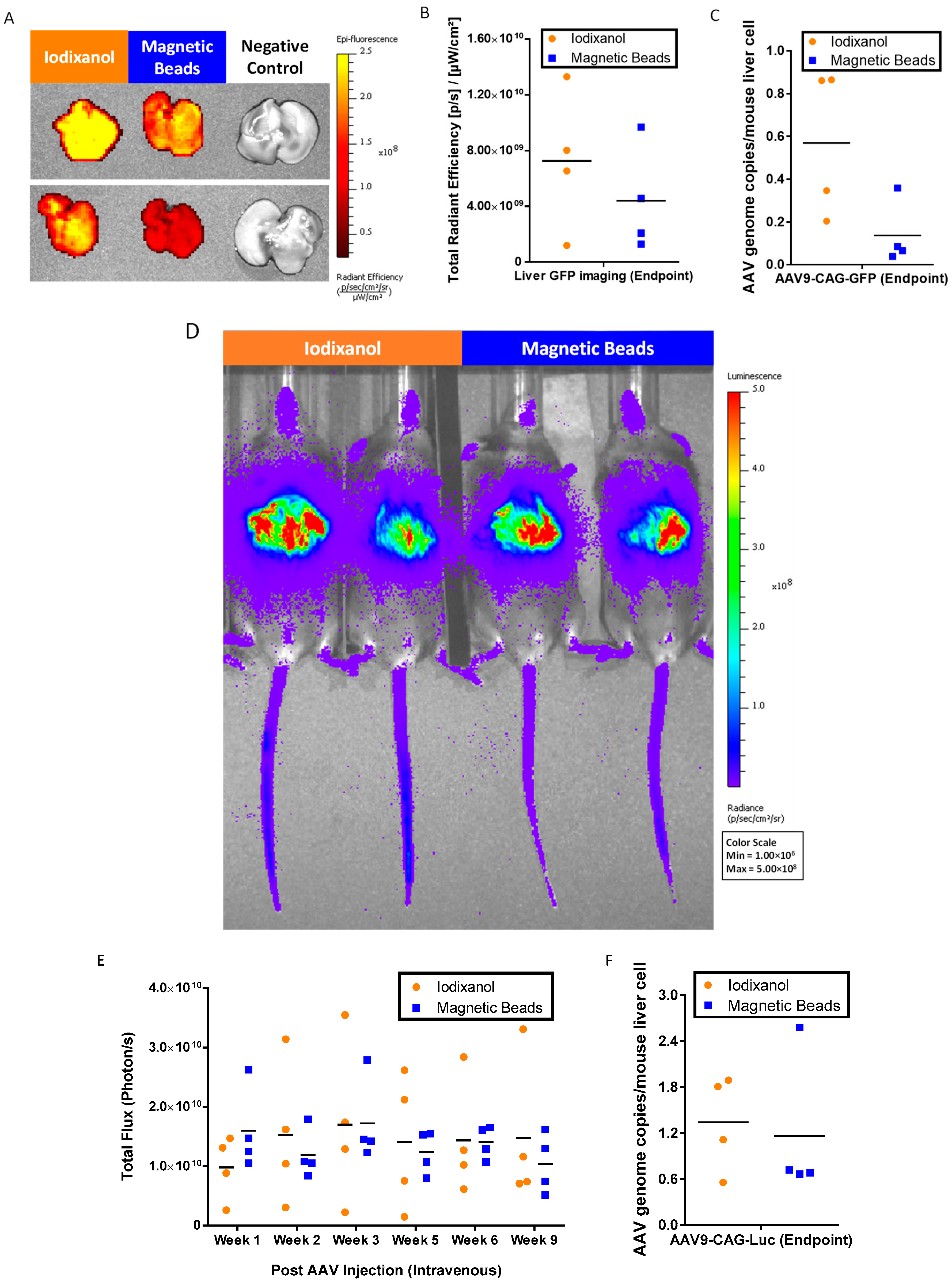
2.5. In Vivo Bioactivity of AAV9 Purification with AAV9 Magnetic Affinity Beads
3. Discussion
4. Materials and Methods
4.1. Packaging of AAV9 Viral Vector
4.2. Purification of the AAV9 Viral Vector Using the Iodixanol Density Gradient Centrifugation Method
4.3. Purification of AAV9 Using the Magnetic Affinity Beads Method
4.4. Regeneration Wash of Dynabeads CaptureSelect AAV9 Magnetic Affinity Beads
4.5. Titration of the AAV9 Viral Vector by Quantitative PCR
4.6. Estimation of Total HEK293 gDNA Contamination by Quantitative PCR
4.7. Estimation of Total Plasmid DNA Contamination by Quantitative PCR
4.8. SDS-PAGE and Silver Stain
4.9. Cryo-Electron Microscopy Grid Preparation, Data Collection, and Image Processing
4.10. UV A260/280 Ratio Determination
4.11. In Vivo Experiments
4.12. In Vivo Non-Invasive Luciferase Imaging
4.13. Ex Vivo Liver GFP Imaging
4.14. AAV Viral Genome Copies Quantification in the Liver
4.15. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zincarelli, C.; Soltys, S.; Rengo, G.; Rabinowitz, J.E. Analysis of AAV Serotypes 1–9 Mediated Gene Expression and Tropism in Mice After Systemic Injection. Mol. Ther. 2008, 16, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Pires, G.; Azevedo, M.D.; Lanzillo, M.; Roux-Petronelli, C.; Binz, P.-A.; Cudalbu, C.; Sandi, C.; Tenenbaum, L.; Braissant, O. Rescue of myocytes and locomotion through AAV2/9-2YF intracisternal gene therapy in a rat model of creatine transporter deficiency. Mol. Ther. Methods Clin. Dev. 2024, 32, 101251. [Google Scholar] [CrossRef] [PubMed]
- Pankajakshan, D.; Makinde, T.O.; Gaurav, R.; Del Core, M.; Hatzoudis, G.; Pipinos, I.; Agrawal, D.K. Successful Transfection of Genes Using AAV-2/9 Vector in Swine Coronary and Peripheral Arteries. J. Surg. Res. 2012, 175, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Kepreotis, S.V.; Oh, J.G.; Park, M.; Yoo, J.; Lee, C.; Mercola, M.; Hajjar, R.J.; Jeong, D. Inhibition of miR-25 ameliorates cardiac and skeletal muscle dysfunction in aged mdx/utrn haploinsufficient (+/−) mice. Mol. Ther. Nucleic Acids 2024, 35, 102174. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Khan, R.; Lee, J.Y.; Tassew, G.; Oskouian, B.; Allende, M.L.; Proia, R.L.; Yin, X.; Ortega, J.G.; Bhattacharya, M.; et al. Gene therapy with AAV9-SGPL1 in an animal model of lung fibrosis. J. Pathol. 2024, 263, 22–31. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhang, C.; Zhou, Y.; Li, H.; Xiao, W.; Herzog, R.W.; Xu, J.; Zhang, J.; Chen, Y.E.; Han, R. Liver-specific in vivo base editing of Angptl3 via AAV delivery efficiently lowers blood lipid levels in mice. Cell Biosci. 2023, 13, 109. [Google Scholar] [CrossRef]
- Wu, I.; Zeng, A.; Greer-Short, A.; Aycinena, J.A.; Tefera, A.E.; Shenwai, R.; Farshidfar, F.; Van Pell, M.; Xu, E.; Reid, C.; et al. AAV9:PKP2 improves heart function and survival in a Pkp2-deficient mouse model of arrhythmogenic right ventricular cardiomyopathy. Commun. Med. 2024, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, V.; Ayuso, E.; Mallol, C.; Agudo, J.; Casellas, A.; Obach, M.; Muñoz, S.; Salavert, A.; Bosch, F. In vivo genetic engineering of murine pancreatic beta cells mediated by single-stranded adeno-associated viral vectors of serotypes 6, 8 and 9. Diabetologia 2011, 54, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.K.; Likhite, S.; Vetter, T.A.; Baird, M.C.; McGovern, V.; Sierra Delgado, A.; Mendel, T.; Burghes, A.; Meyer, K.C. In-depth comparison of Anc80L65 and AAV9 retinal targeting and characterization of cross-reactivity to multiple AAV serotypes in humans. Mol. Ther. Methods Clin. Dev. 2023, 30, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Tao, Y.; Wang, X.; Wang, X.; Shu, Y.; Liu, Y.; Kang, W.; Chen, S.; Cheng, Z.; Yan, B.; et al. RNA base editing therapy cures hearing loss induced by OTOF gene mutation. Mol. Ther. 2023, 31, 3520–3530. [Google Scholar] [CrossRef] [PubMed]
- Gannon, A.-L.; Darbey, A.L.; Chensee, G.; Lawrence, B.M.; O’Donnell, L.; Kelso, J.; Reed, N.; Parameswaran, S.; Smith, S.; Smith, L.B.; et al. A Novel Model Using AAV9-Cre to Knockout Adult Leydig Cell Gene Expression Reveals a Physiological Role of Glucocorticoid Receptor Signalling in Leydig Cell Function. Int. J. Mol. Sci. 2022, 23, 15015. [Google Scholar] [CrossRef] [PubMed]
- Asico, L.D.; Cuevas, S.; Ma, X.; Jose, P.A.; Armando, I.; Konkalmatt, P.R. Nephron segment-specific gene expression using AAV vectors. Biochem. Biophys. Res. Commun. 2018, 497, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, V.; Muñoz, S.; Casana, E.; Mallol, C.; Elias, I.; Jambrina, C.; Ribera, A.; Ferre, T.; Franckhauser, S.; Bosch, F. In Vivo Adeno-Associated Viral Vector–Mediated Genetic Engineering of White and Brown Adipose Tissue in Adult Mice. Diabetes 2013, 62, 4012–4022. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.; Bak, H.; Tustian, A.D. Moving from the bench towards a large scale, industrial platform process for adeno-associated viral vector purification. Biotechnol. Bioeng. 2020, 117, 3199–3211. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; El-Gohary, Y.; Prasadan, K.; Shiota, C.; Xiao, X.; Wiersch, J.; Paredes, J.; Tulachan, S.; Gittes, G.K. Rapid and simplified purification of recombinant adeno-associated virus. J. Virol. Methods 2012, 183, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Ferran, B.; Tsukahara, Y.; Shang, Q.; Desai, S.; Fedoce, A.; Pimentel, D.R.; Luptak, I.; Adachi, T.; Ido, Y.; et al. Production of adeno-associated virus vectors for in vitro and in vivo applications. Sci. Rep. 2019, 9, 13601. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lock, M.; Prongay, A.J.; Alvira, M.R.; Petkov, B.; Wilson, J.M. Identification of an adeno-associated virus binding epitope for AVB sepharose affinity resin. Mol. Ther. Methods Clin. Dev. 2015, 2, 15040. [Google Scholar] [CrossRef] [PubMed]
- Florea, M.; Nicolaou, F.; Pacouret, S.; Zinn, E.M.; Sanmiguel, J.; Andres-Mateos, E.; Unzu, C.; Wagers, A.J.; Vandenberghe, L.H. High-efficiency purification of divergent AAV serotypes using AAVX affinity chromatography. Mol. Ther. Methods Clin. Dev. 2023, 28, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Werle, A.K.; Powers, T.W.; Zobel, J.F.; Wappelhorst, C.N.; Jarrold, M.F.; Lyktey, N.A.; Sloan, C.D.K.; Wolf, A.J.; Adams-Hall, S.; Baldus, P.; et al. Comparison of analytical techniques to quantitate the capsid content of adeno-associated viral vectors. Mol. Ther. Methods Clin. Dev. 2021, 23, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Sia, K.C.; Gan, S.U.; Mohd Rodhi, S.H.; Fu, Z.Y.; Kopchick, J.J.; Waters, M.J.; Lee, K.O. First use of gene therapy to treat growth hormone resistant dwarfism in a mouse model. Gene Ther. 2022, 29, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Sia, K.C.; Fu, Z.Y.; Calne, R.Y.; Nathwani, A.C.; Lee, K.O.; Gan, S.U. Modification of a Constitutive to Glucose-Responsive Liver-Specific Promoter Resulted in Increased Efficacy of Adeno-Associated Virus Serotype 8-Insulin Gene Therapy of Diabetic Mice. Cells 2020, 9, 2474. [Google Scholar] [CrossRef] [PubMed]
- Goettsch, C.; Hutcheson, J.D.; Hagita, S.; Rogers, M.A.; Creager, M.D.; Pham, T.; Choi, J.; Mlynarchik, A.K.; Pieper, B.; Kjolby, M.; et al. A single injection of gain-of-function mutant PCSK9 adeno-associated virus vector induces cardiovascular calcification in mice with no genetic modification. Atherosclerosis 2016, 251, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lunev, E.; Karan, A.; Egorova, T.; Bardina, M. Adeno-Associated Viruses for Modeling Neurological Diseases in Animals: Achievements and Prospects. Biomedicines 2022, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Krooss, S.A.; Dai, Z.; Schmidt, F.; Rovai, A.; Fakhiri, J.; Dhingra, A.; Yuan, Q.; Yang, T.; Balakrishnan, A.; Steinbrück, L.; et al. Ex Vivo/In vivo Gene Editing in Hepatocytes Using “All-in-One” CRISPR-Adeno-Associated Virus Vectors with a Self-Linearizing Repair Template. iScience 2020, 23, 100764. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Wang, J.H.; Liu, G.S. Methods for Ex Vivo AAV Transduction in Rodent and Human Retinal Explants. Methods Mol. Biol. 2023, 2678, 169–175. [Google Scholar] [PubMed]
- Wang, D.; Zhou, Q.; Qiu, X.; Liu, X.; Zhang, C. Optimizing rAAV6 transduction of primary T cells for the generation of anti-CD19 AAV-CAR-T cells. Biomed. Pharmacother. 2022, 150, 113027. [Google Scholar] [CrossRef] [PubMed]
- Chu, W.; Shastry, S.; Barbieri, E.; Prodromou, R.; Greback-Clarke, P.; Smith, W.; Moore, B.; Kilgore, R.; Cummings, C.; Pancorbo, J.; et al. Peptide ligands for the affinity purification of adeno-associated viruses from HEK 293 cell lysates. Biotechnol. Bioeng. 2023, 120, 2283–2300. [Google Scholar] [CrossRef]
- Challener, C.A. Determining and Optimizing Dynamic Binding Capacity. BioPharm Int. 2023, 36, 10–14, 34. [Google Scholar]
- Wright, J.F.; Le, T.; Prado, J.; Bahr-Davidson, J.; Smith, P.H.; Zhen, Z.; Sommer, J.M.; Pierce, G.F.; Qu, G. Identification of factors that contribute to recombinant AAV2 particle aggregation and methods to prevent its occurrence during vector purification and formulation. Mol. Ther. 2005, 12, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.H.; Wright, J.F.; Qu, G.; Patarroyo-White, S.; Parker, A.; Sommer, J.M. 900. Packaging of Host Cell and Plasmid DNA into Recombinant Adeno-Associated Virus Particles Produced by Triple Transfection. Mol. Ther. 2003, 7, S348. [Google Scholar]
- Brimble, M.A.; Winston, S.M.; Davidoff, A.M. Stowaways in the cargo: Contaminating nucleic acids in rAAV preparations for gene therapy. Mol. Ther. 2023, 31, 2826–2838. [Google Scholar] [CrossRef] [PubMed]
- Ravi, N.; Huerta, J.; Ferreira, G. Evaluating multiproduct chromatography Protein A resin reuse for monoclonal antibodies in biopharmaceutical manufacturing. Biotechnol. Prog. 2023, 39, e3333. [Google Scholar] [CrossRef] [PubMed]
- Ertl, H.C.J. Immunogenicity and toxicity of AAV gene therapy. Front. Immunol. 2022, 13, 975803. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.K.; Samulski, R.J. Addressing high dose AAV toxicity—‘one and done’ or ‘slower and lower’? Expert Opin. Biol. Ther. 2022, 22, 1067–1071. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Li, M.; Zhong, L.; Su, Q.; Li, J.; Li, S.; He, R.; Zhang, Y.; Hendricks, G.; Wang, J.; et al. Empty virions in AAV8 vector preparations reduce transduction efficiency and may cause total viral particle dose-limiting side effects. Mol. Ther. Methods Clin. Dev. 2014, 1, 20139. [Google Scholar] [CrossRef] [PubMed]
- Strobel, B.; Miller, F.D.; Rist, W.; Lamla, T. Comparative Analysis of Cesium Chloride- and Iodixanol-Based Purification of Recombinant Adeno-Associated Viral Vectors for Preclinical Applications. Hum. Gene Ther. Methods 2015, 26, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.R.H.; Bernier, A.; Moço, P.D.; Schrag, J.; Chahal, P.S.; Kamen, A. Development of a scalable and robust AEX method for enriched rAAV preparations in genome-containing VCs of serotypes 5, 6, 8, and 9. Mol. Ther. Methods Clin. Dev. 2021, 21, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.; Argento, C.; Pieracci, J.; Bakhshayeshi, M. Separating Empty and Full Recombinant Adeno-Associated Virus Particles Using Isocratic Anion Exchange Chromatography. Biotechnol. J. 2020, 16, e2000015. [Google Scholar] [CrossRef] [PubMed]
- Turco, F.; Wegelius, A.; Lind, O.; Norrman, N.; Magnusson, A.-C.; Sund-Lundström, C.; Norén, B.; Hedberg, J.; Palmgren, R. Combined clarification and affinity capture using magnetic resin enables efficient separation of rAAV5 from cell lysate. Mol. Ther. Methods Clin. Dev. 2023, 30, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Zolotukhin, S.; Byrne, B.J.; Mason, E.; Zolotukhin, I.; Potter, M.; Chesnut, K.; Summerford, C.; Samulski, R.J.; Muzyczka, N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999, 6, 973–985. [Google Scholar] [CrossRef]
- Funakoshi, K.; Bagheri, M.; Zhou, M.; Suzuki, R.; Abe, H.; Akashi, H. Highly sensitive and specific Alu-based quantification of human cells among rodent cells. Sci. Rep. 2017, 7, 13202. [Google Scholar] [CrossRef] [PubMed]
- Shu Uin, G.; Maria, N.; Zhen Ying, F.; Kok Onn, L.; Kian Chuan, S.; Amit Chunilal, N.; Peruta Marco, D.; Roy Yorke, C. Correction of Murine Diabetic Hyperglycaemia with A Single Systemic Administration of an AAV2/8 Vector Containing A Novel Codon Optimized Human Insulin Gene. Curr. Gene Ther. 2016, 16, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Mastronarde, D.N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 2005, 152, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Zivanov, J.; Nakane, T.; Scheres, S.H.W. Estimation of high-order aberrations and anisotropic magnification from cryo-EM data sets in RELION-3.1. IUCrJ 2020, 7, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Q.; Palovcak, E.; Armache, J.-P.; Verba, K.A.; Cheng, Y.; Agard, D.A. MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 2017, 14, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Rohou, A.; Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 2015, 192, 216–221. [Google Scholar] [CrossRef] [PubMed]



| Group | Cryo-EM (Full) | Cryo-EM (Empty) | UV A260/280 (Full) | UV A260/280 (Empty) |
|---|---|---|---|---|
| AAV9-CAG-GFP (Iodixanol) | 91.1% | 8.9% | 80–91% (A260/280 ratio = 1.348) | 9–20% (A260/280 ratio = 1.348) |
| AAV9-CAG-GFP (Magnetic Beads) | 54.8% | 45.2% | 50–67% (A260/280 ratio = 1.164) | 33–50% (A260/280 ratio = 1.164) |
| AAV9-CAG-Luc (Iodixanol) | N.D. | N.D. | 91–100% (A260/280 ratio = 1.381) | 0-9% (A260/280 ratio = 1.381) |
| AAV9-CAG-Luc (Magnetic Beads) | N.D. | N.D. | 67–80% (A260/280 ratio = 1.287) | 20–33% (A260/280 ratio = 1.287) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sia, K.C.; Fu, Z.Y.; Mohd Rodhi, S.H.; Yee, J.H.Y.; Qu, K.; Gan, S.U. Efficient AAV9 Purification Using a Single-Step AAV9 Magnetic Affinity Beads Isolation. Int. J. Mol. Sci. 2024, 25, 8342. https://doi.org/10.3390/ijms25158342
Sia KC, Fu ZY, Mohd Rodhi SH, Yee JHY, Qu K, Gan SU. Efficient AAV9 Purification Using a Single-Step AAV9 Magnetic Affinity Beads Isolation. International Journal of Molecular Sciences. 2024; 25(15):8342. https://doi.org/10.3390/ijms25158342
Chicago/Turabian StyleSia, Kian Chuan, Zhen Ying Fu, Siti Humairah Mohd Rodhi, Joan Hua Yi Yee, Kun Qu, and Shu Uin Gan. 2024. "Efficient AAV9 Purification Using a Single-Step AAV9 Magnetic Affinity Beads Isolation" International Journal of Molecular Sciences 25, no. 15: 8342. https://doi.org/10.3390/ijms25158342





