Tick-Derived Peptide Blocks Potassium Channel TREK-1
Abstract
1. Introduction
2. Results
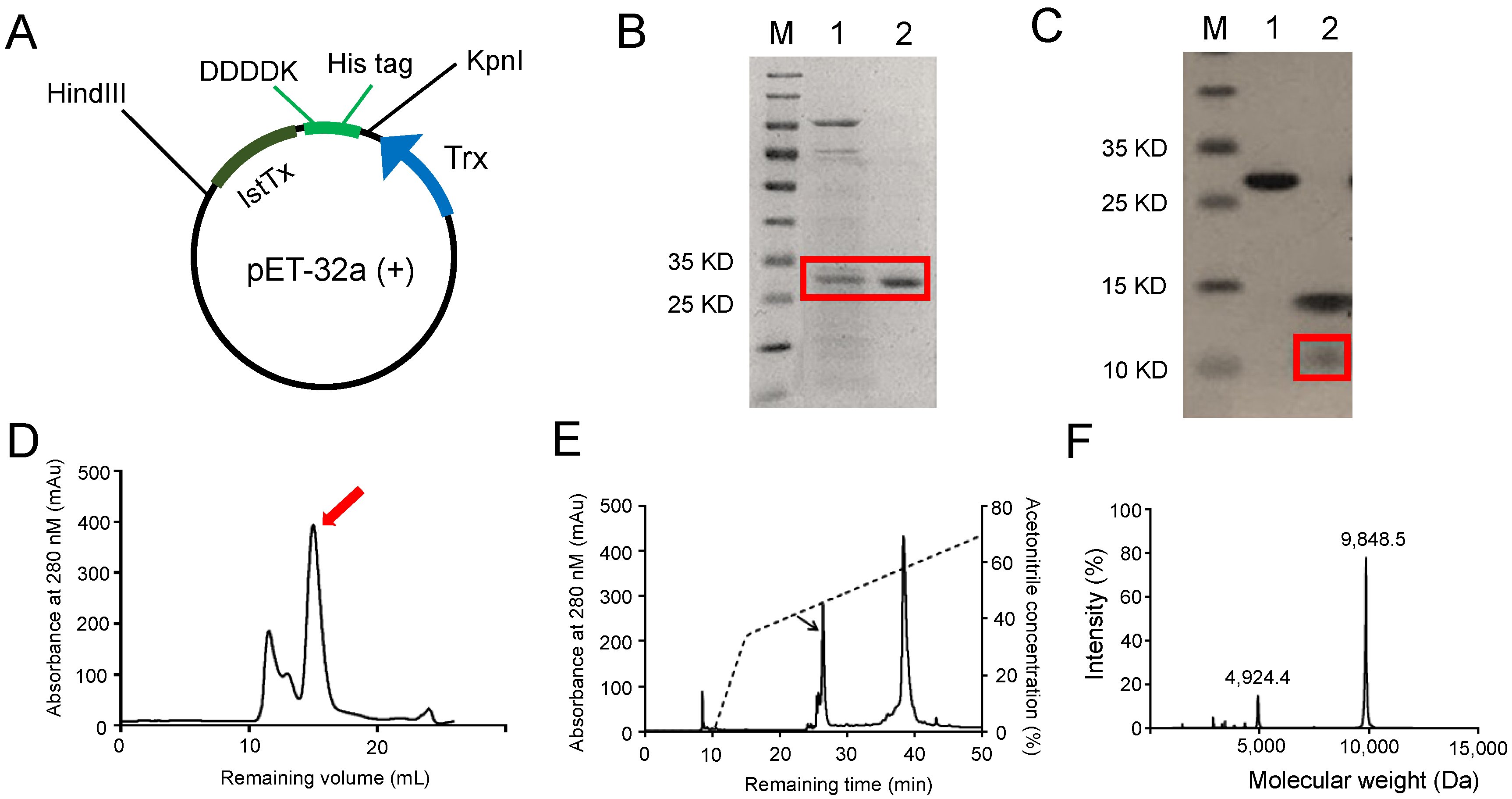
2.1. Recombinant Expression and Purification of the Peptide IstTx
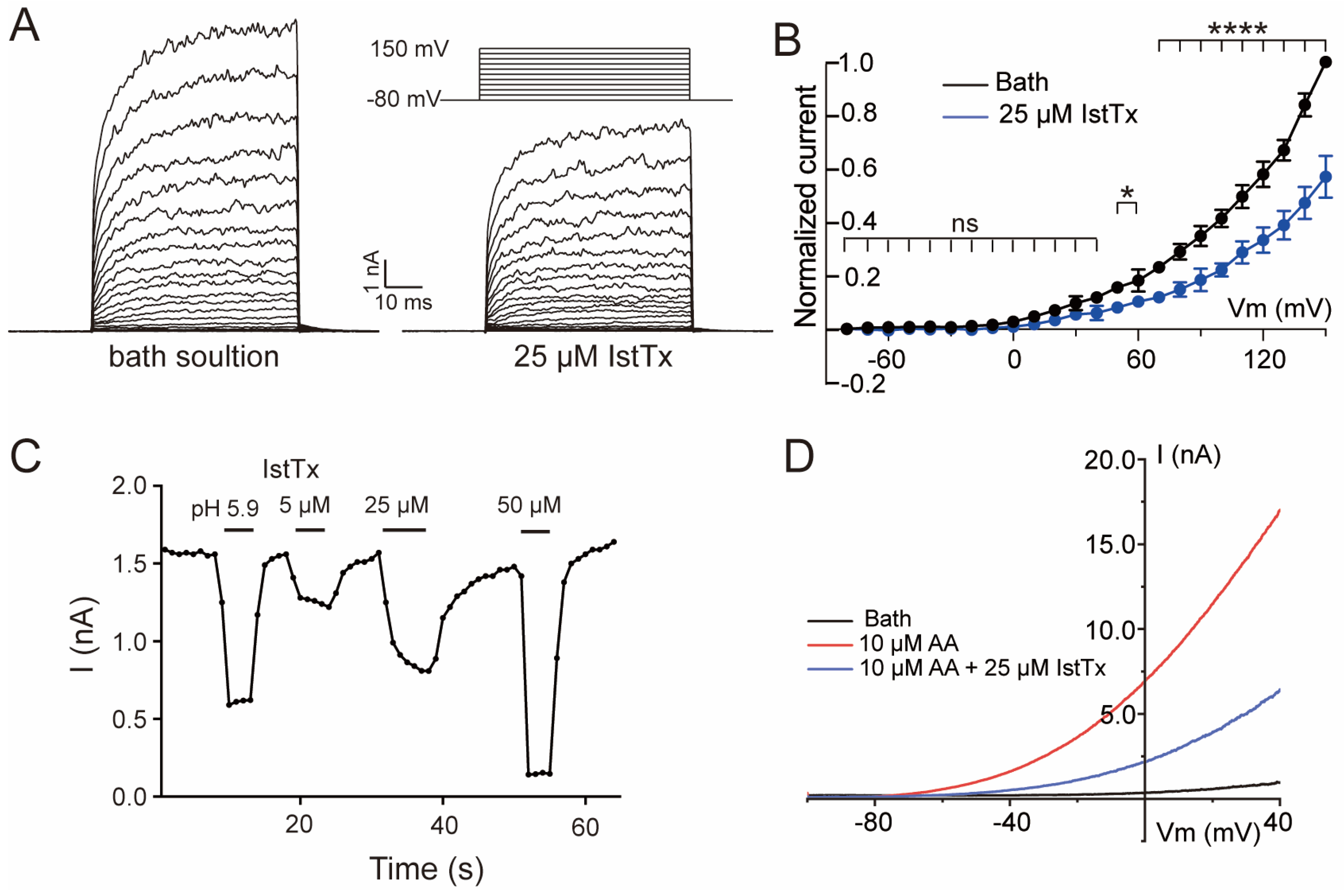
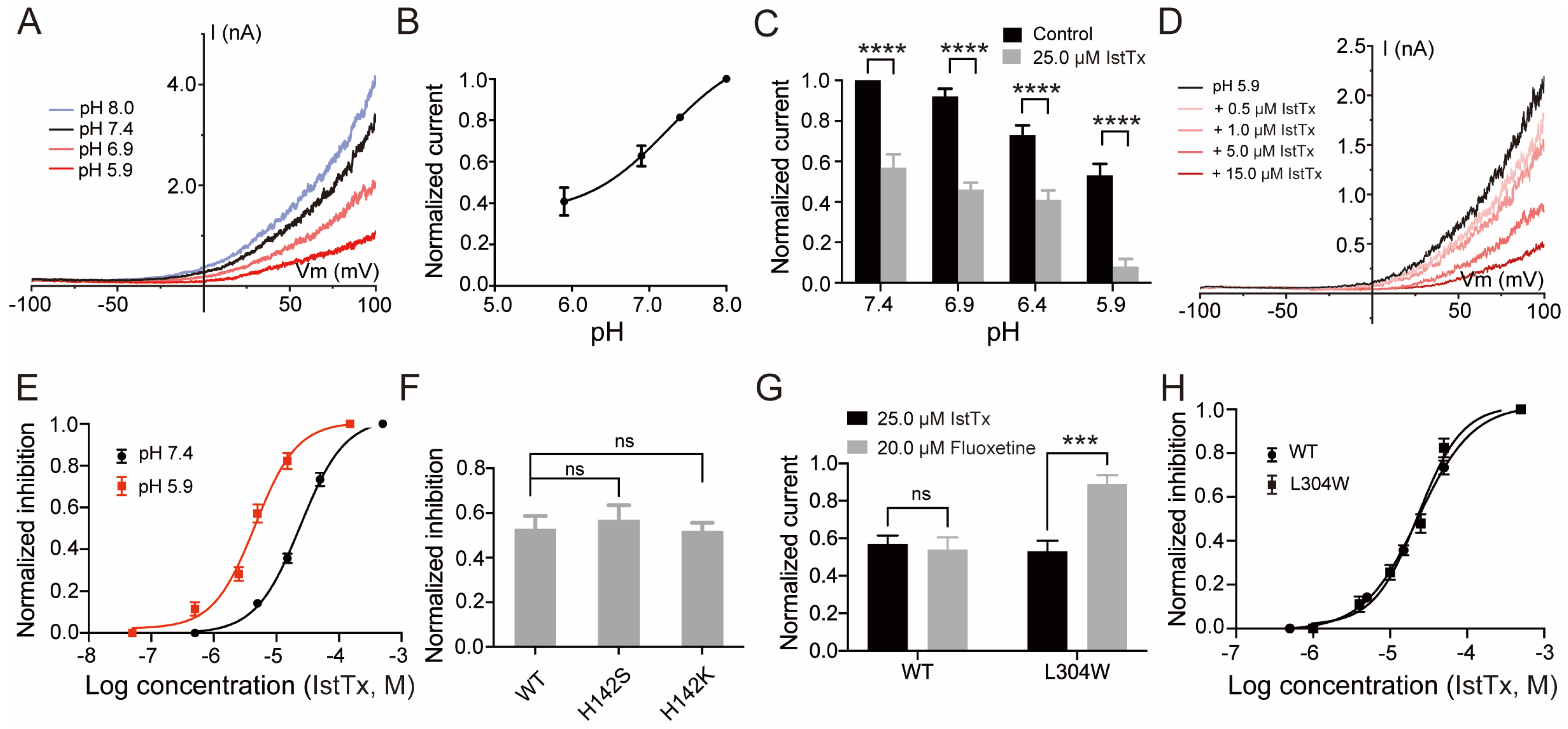
2.2. IstTx Inhibited the TREK-1 Channel
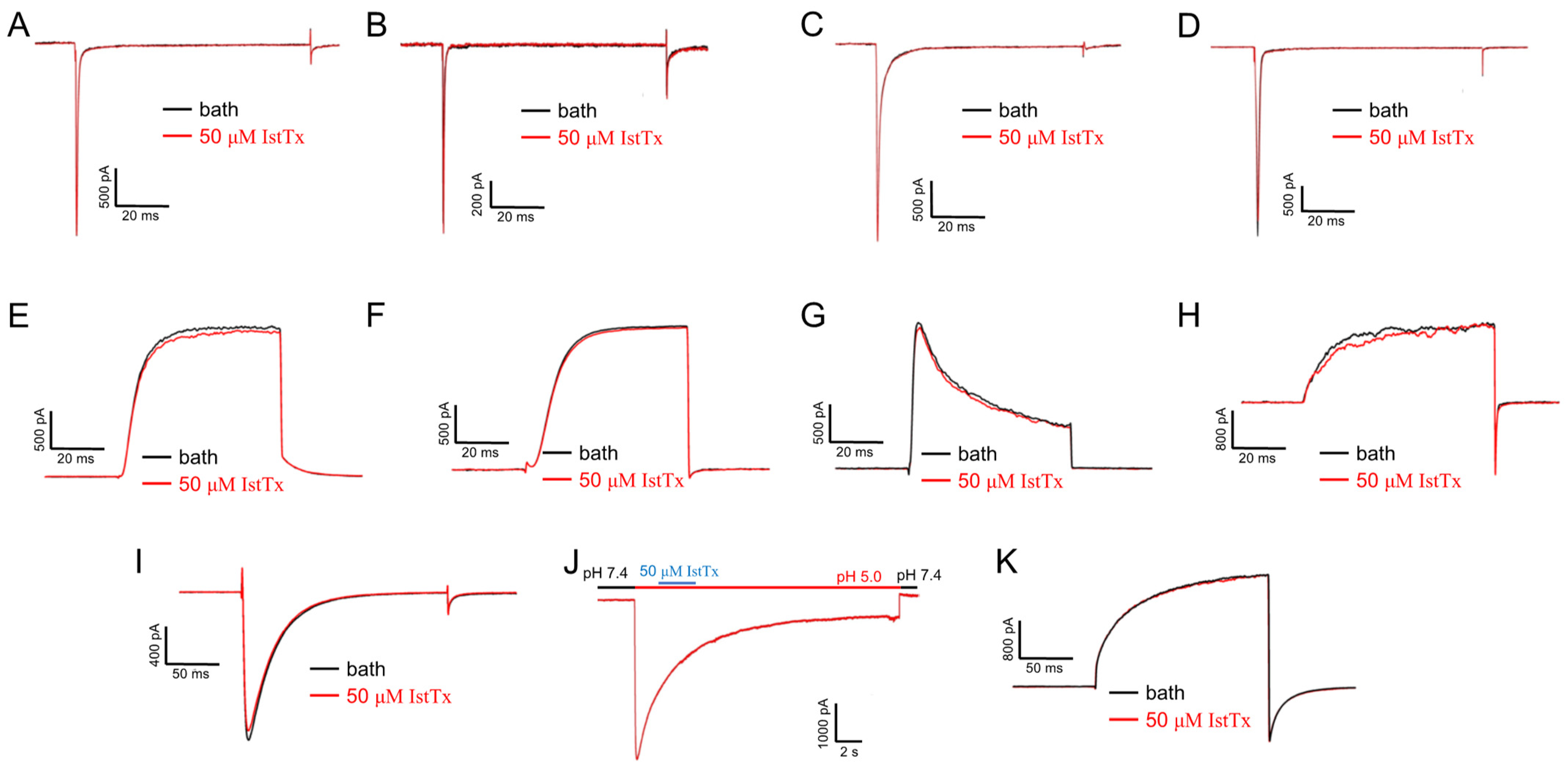
2.3. Selectivity of IstTx
2.4. Some Features of IstTx Inhibiting the TREK-1 Channel
2.5. Acidification Enhanced the Inhibition of IstTx on the TREK-1 Channel
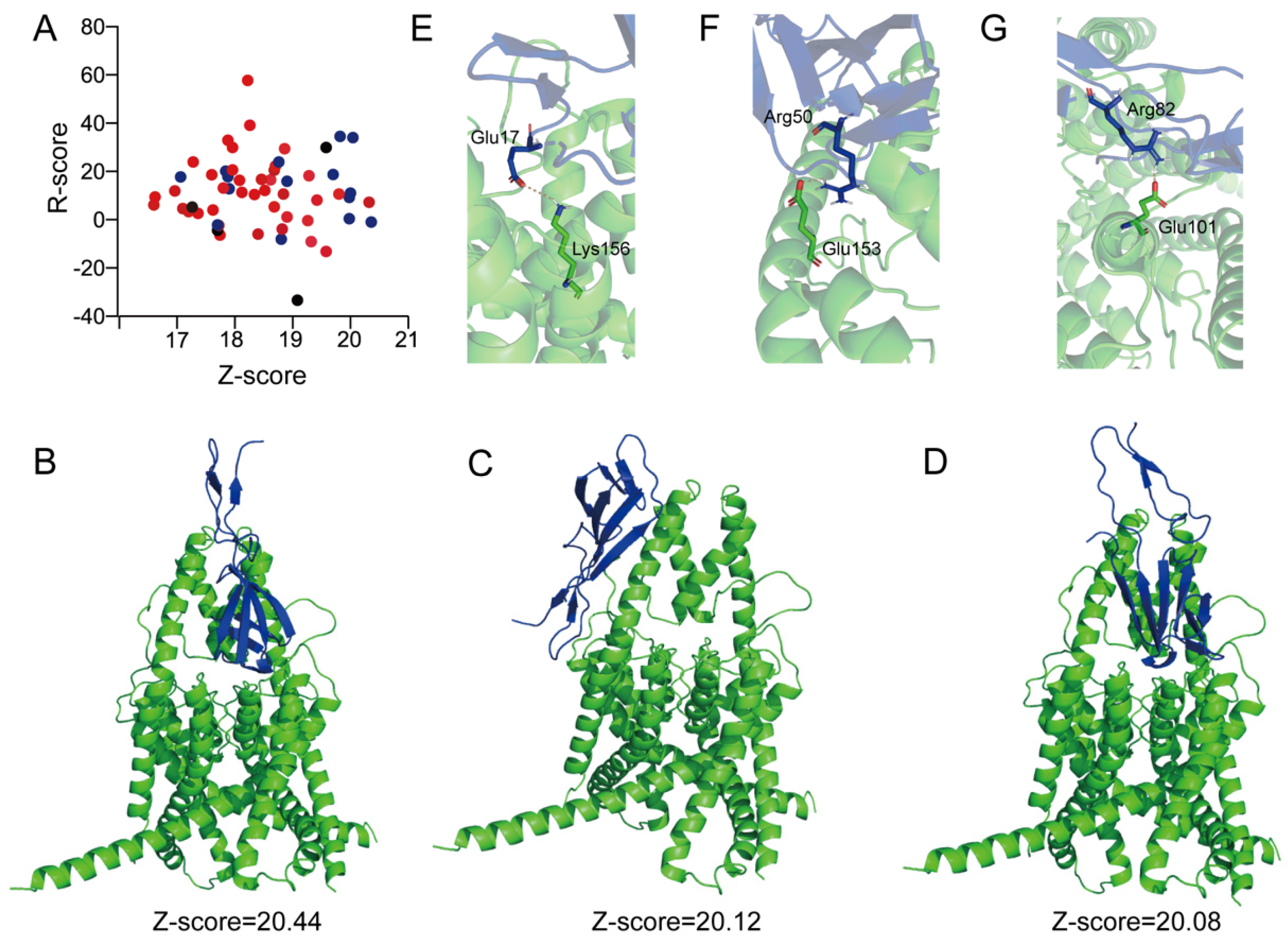
2.6. Molecular Docking of the Peptide IstTx Interacting with the TREK-1 Channel
3. Discussion
4. Materials and Methods
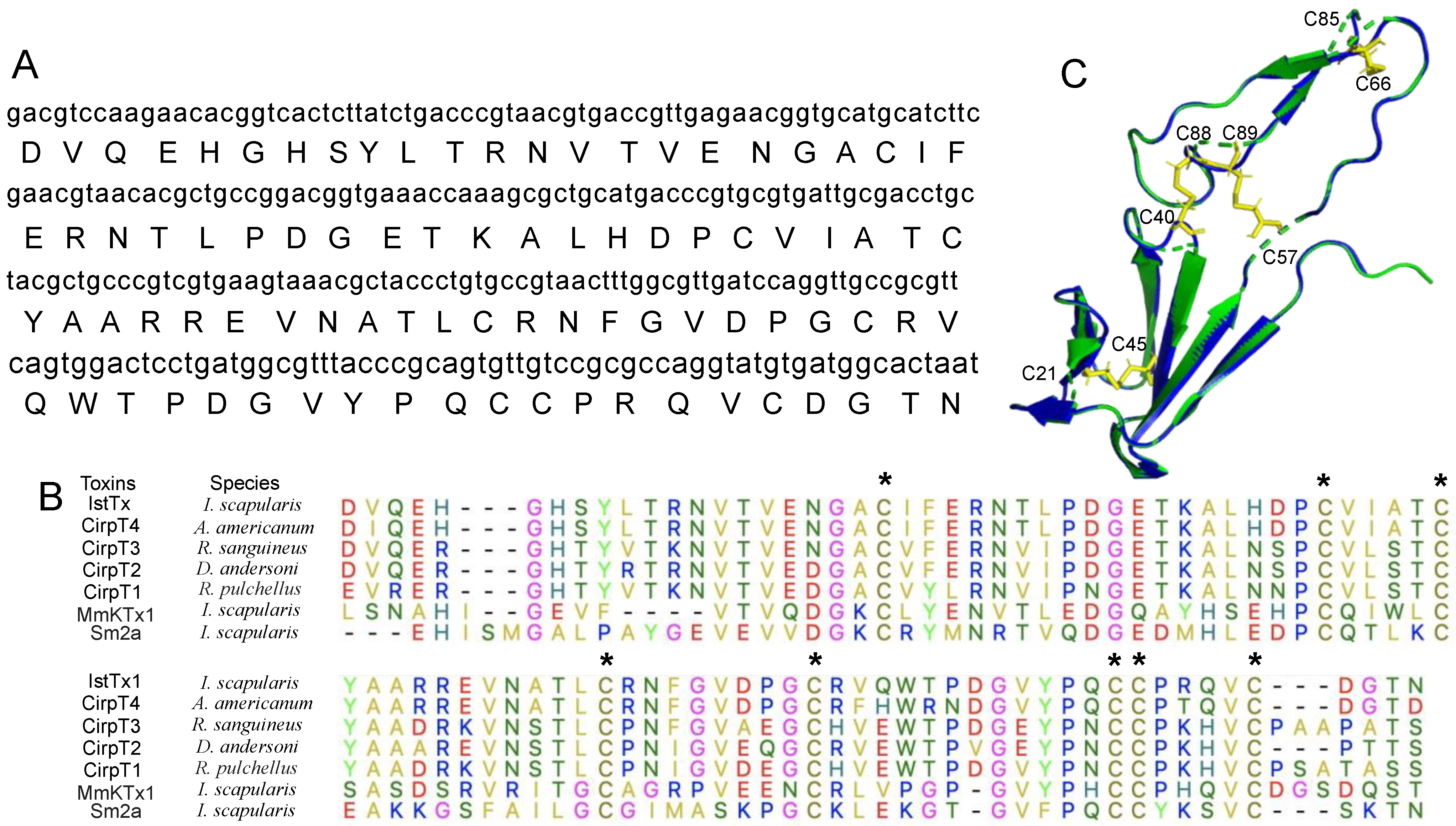
4.1. Sequence Alignment and Homologous Modeling
4.2. Recombinant Expression and Purification of IstTx
4.3. Cell Culture and Plasmids Transfection
4.4. Electrophysiology
4.5. Molecular Docking
4.6. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jongejan, F.; Uilenberg, G. The global importance of ticks. Parasitology 2004, 129, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Mans, B.J.; de Klerk, D.; Pienaar, R.; Latif, A.A. Nuttalliella namaqua: A living fossil and closest relative to the ancestral tick lineage: Implications for the evolution of blood-feeding in ticks. PLoS ONE 2011, 6, e23675. [Google Scholar] [CrossRef] [PubMed]
- Almazan, C.; Tipacamu, G.A.; Rodriguez, S.; Mosqueda, J.; Perez de Leon, A. Immunological control of ticks and tick-borne diseases that impact cattle health and production. Front. Biosci. 2018, 23, 1535–1551. [Google Scholar] [CrossRef] [PubMed]
- Mead, P.S. Epidemiology of Lyme disease. Infect. Dis. Clin. N. Am. 2015, 29, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Borchers, A.T.; Keen, C.L.; Huntley, A.C.; Gershwin, M.E. Lyme disease: A rigorous review of diagnostic criteria and treatment. J. Autoimmun. 2015, 57, 82–115. [Google Scholar] [CrossRef] [PubMed]
- Duron, O.; Noël, V.; McCoy, K.D.; Bonazzi, M.; Sidi-Boumedine, K.; Morel, O.; Vavre, F.; Zenner, L.; Jourdain, E.; Durand, P.; et al. The Recent Evolution of a Maternally-Inherited Endosymbiont of Ticks Led to the Emergence of the Q Fever Pathogen, Coxiella burnetii. PLoS Pathog. 2015, 11, e1004892. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Valle, M.; Moolhuijzen, P.; Barrero, R.A.; Ong, C.T.; Busch, G.; Karbanowicz, T.; Booth, M.; Clark, R.; Koehbach, J.; Ijaz, H.; et al. Transcriptome and toxin family analysis of the paralysis tick, Ixodes holocyclus. Int. J. Parasitol. 2018, 48, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Kotál, J.; Langhansová, H.; Lieskovská, J.; Andersen, J.F.; Francischetti, I.M.; Chavakis, T.; Kopecký, J.; Pedra, J.H.; Kotsyfakis, M.; Chmelař, J. Modulation of host immunity by tick saliva. J. Proteom. 2015, 128, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Chmelar, J.; Kotal, J.; Langhansova, H.; Kotsyfakis, M. Protease Inhibitors in Tick Saliva: The Role of Serpins and Cystatins in Tick-host-Pathogen Interaction. Front. Cell. Infect. Microbiol. 2017, 7, 216. [Google Scholar] [CrossRef] [PubMed]
- Skallova, A.; Iezzi, G.; Ampenberger, F.; Kopf, M.; Kopecky, J. Tick saliva inhibits dendritic cell migration, maturation, and function while promoting development of Th2 responses. J. Immunol. 2008, 180, 6186–6192. [Google Scholar] [CrossRef] [PubMed]
- Juncadella, I.J.; Garg, R.; Ananthnarayanan, S.K.; Yengo, C.M.; Anguita, J. T-cell signaling pathways inhibited by the tick saliva immunosuppressor, Salp15. FEMS Immunol. Med. Microbiol. 2007, 49, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Wang, P.; Adusumilli, S.; Booth, C.J.; Narasimhan, S.; Anguita, J.; Fikrig, E. Antibodies against a tick protein, Salp15, protect mice from the Lyme disease agent. Cell Host Microbe 2009, 6, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Simo, L.; Kazimirova, M.; Richardson, J.; Bonnet, S.I. The Essential Role of Tick Salivary Glands and Saliva in Tick Feeding and Pathogen Transmission. Front. Cell. Infect. Microbiol. 2017, 7, 281. [Google Scholar] [CrossRef] [PubMed]
- Kazimirova, M.; Stibraniova, I. Tick salivary compounds: Their role in modulation of host defences and pathogen transmission. Front. Cell. Infect. Microbiol. 2013, 3, 43. [Google Scholar] [CrossRef] [PubMed]
- Aounallah, H.; Bensaoud, C.; M’ghirbi, Y.; Faria, F.; Chmelař, J.; Kotsyfakis, M. Tick Salivary Compounds for Targeted Immunomodulatory Therapy. Front. Immunol. 2020, 11, 583845. [Google Scholar] [CrossRef] [PubMed]
- Enyedi, P.; Czirjak, G. Molecular background of leak K+ currents: Two-pore domain potassium channels. Physiol. Rev. 2010, 90, 559–605. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.N.; Long, S.B. Crystal structure of the human two-pore domain potassium channel K2P1. Science 2012, 335, 432–436. [Google Scholar] [CrossRef]
- Xiantaoli; Dyachenko, V.; Zuzarte, M.; Putzke, C.; Preisigmuller, R.; Isenberg, G.; Daut, J. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc. Res. 2006, 69, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Schewe, M.; Nematian-Ardestani, E.; Sun, H.; Musinszki, M.; Cordeiro, S.; Bucci, G.; de Groot, B.L.; Tucker, S.J.; Rapedius, M.; Baukrowitz, T. A Non-canonical Voltage-Sensing Mechanism Controls Gating in K2P K(+) Channels. Cell 2016, 164, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Maingret, F.; Honore, E.; Lazdunski, M.; Patel, A.J. Molecular basis of the voltage-dependent gating of TREK-1, a mechano-sensitive K(+) channel. Biochem. Biophys Res. Commun. 2002, 292, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Mathie, A.; Veale, E.L.; Cunningham, K.P.; Holden, R.G.; Wright, P.D. Two-Pore Domain Potassium Channels as Drug Targets: Anesthesia and Beyond. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Csáki, R.; Nagaraj, C.; Almássy, J.; Khozeimeh, M.A.; Jeremic, D.; Olschewski, H.; Dobolyi, A.; Hoetzenecker, K.; Olschewski, A.; Enyedi, P.; et al. The TREK-1 potassium channel is a potential pharmacological target for vasorelaxation in pulmonary hypertension. Br. J. Pharmacol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.J.; Honoré, E.; Lesage, F.; Fink, M.; Romey, G.; Lazdunski, M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat. Neurosci. 1999, 2, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Heurteaux, C.; Guy, N.; Laigle, C.; Blondeau, N.; Duprat, F.; Mazzuca, M.; Lang-Lazdunski, L.; Widmann, C.; Zanzouri, M.; Romey, G.; et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004, 23, 2684–2695. [Google Scholar] [CrossRef] [PubMed]
- Sabbadini, M.; Yost, C.S. Molecular biology of background K channels: Insights from K(2P) knockout mice. J. Mol. Biol. 2009, 385, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Heurteaux, C.; Lucas, G.; Guy, N.; El Yacoubi, M.; Thümmler, S.; Peng, X.-D.; Noble, F.; Blondeau, N.; Widmann, C.; Borsotto, M.; et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat. Neurosci. 2006, 9, 1134–1141. [Google Scholar] [CrossRef]
- Deng, P.Y.; Poudel, S.K.; Rojanathammanee, L.; Porter, J.E.; Lei, S. Serotonin inhibits neuronal excitability by activating two-pore domain k+ channels in the entorhinal cortex. Mol. Pharmacol. 2007, 72, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Zhang, X.; Zhang, L.; Sui, Y.; Hui, J.; Liu, S.; Wang, Y.; Li, L.; Zhang, Z. Fluoxetine attenuates the inhibitory effect of glucocorticoid hormones on neurogenesis in vitro via a two-pore domain potassium channel, TREK-1. Psychopharmacology 2011, 214, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Kennard, L.E.; Chumbley, J.R.; Ranatunga, K.M.; Armstrong, S.J.; Veale, E.L.; Mathie, A. Inhibition of the human two-pore domain potassium channel, TREK-1, by fluoxetine and its metabolite norfluoxetine. Br. J. Pharmacol. 2005, 144, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Rong, M.; Liu, J.; Zhang, M.; Wang, G.; Zhao, G.; Wang, G.; Zhang, Y.; Hu, K.; Lai, R. A sodium channel inhibitor ISTX-I with a novel structure provides a new hint at the evolutionary link between two toxin folds. Sci. Rep. 2016, 6, 29691. [Google Scholar] [CrossRef] [PubMed]
- Reichhardt, M.P.; Johnson, S.; Tang, T.; Morgan, T.; Tebeka, N.; Popitsch, N.; Deme, J.C.; Jore, M.M.; Lea, S.M. An inhibitor of complement C5 provides structural insights into activation. Proc. Natl. Acad. Sci. USA 2020, 117, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.; Lesage, F.; Duprat, F.; Heurteaux, C.; Reyes, R.; Fosset, M.; Lazdunski, M. A neuronal two P domain K+ channel stimulated by arachidonic acid and polyunsaturated fatty acids. EMBO J. 1998, 17, 3297–3308. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Lewis, A. Spadin Selectively Antagonizes Arachidonic Acid Activation of TREK-1 Channels. Front. Pharmacol. 2020, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Ben-Abu, Y.; Hen, S.; Zilberberg, N. A novel mechanism for human K2P2.1 channel gating. Facilitation of C-type gating by protonation of extracellular histidine residues. J. Biol. Chem. 2008, 283, 19448–19455. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, F.; Zhang, B.; Lee, B.H.; Li, B.; Luo, L.; Zheng, J.; Lai, R. A bimodal activation mechanism underlies scorpion toxin-induced pain. Sci. Adv. 2017, 3, e1700810. [Google Scholar] [CrossRef] [PubMed]
- Lolicato, M.; Arrigoni, C.; Mori, T.; Sekioka, Y.; Bryant, C.; Clark, K.A.; Minor, D.L. K2P2.1 (TREK-1)-activator complexes reveal a cryptic selectivity filter binding site. Nature 2017, 547, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Silva, J.R.; Lu, X.; Luo, L.; Wang, Y.; Xu, L.; Aierken, A.; Shynykul, Z.; Kamau, P.M.; Luo, A.; et al. Molecular game theory for a toxin-dominant food chain model. Natl. Sci. Rev. 2019, 6, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, F.; Wei, N.; Hong, J.; Li, B.; Luo, L.; Rong, M.; Yarov-Yarovoy, V.; Zheng, J.; Wang, K.; et al. A pain-inducing centipede toxin targets the heat activation machinery of nociceptor TRPV1. Nat. Commun. 2015, 6, 8297. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.-C.; Sun, F.-D.; Zhang, L.; Huang, B.; An, H.-L.; Rong, M.-Q.; Du, C.-W. General mechanism of spider toxin family I acting on sodium channel Nav1.7. Zool. Res. 2022, 43, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Li, S.; Huang, B.; Hu, Y.; Zeng, X.; Peng, Y.; Du, C.; Rong, M. Molecular mechanism by which spider-driving peptide potentiates coagulation factors. Biomed. Pharmacother. 2023, 166, 115421. [Google Scholar] [CrossRef]
- Wei, X.-S.; Liu, L.-Z.; Bian, X.-L.; Zeng, L.; Lee, W.-H.; Lin, L.; Zhang, Y.; Wang, Q.-Q. Protein composition of extracellular vesicles from skin secretions of the amphibian Bombina maxima. Zool. Res. 2022, 43, 687–690. [Google Scholar] [CrossRef] [PubMed]
- DU, C.; Yuan, F.; Duan, X.; Rong, M.; Meng, E.; Liu, C. Isolation and structural identification of a potassium ion channel Kv4.1 inhibitor SsTx-P2 from centipede venom. J. Zhejiang Univ. Med. Sci. 2024, 53, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.-G.; Wei, D.-B.; Zhang, X.-J.; Xu, T.-S.; Li, X.-Y.; Zhang, H.-Y.; An, Z.-F.; Storey, K.B.; Chen, Q. Surviving winter on the Qinghai-Xizang Plateau: Extensive reversible protein phosphorylation plays a dominant role in regulating hypometabolism in hibernating Nanorana parkeri. Zool. Res. 2024, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, R. The chemistry and biological activities of peptides from amphibian skin secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.; Kamau, P.M.; Thuku, R.C.; Lai, R. Design methods for antimicrobial peptides with improved performance. Zool. Res. 2023, 44, 1095–1114. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Chai, J.; Xu, Z.; Wu, J.; He, S.; Liao, H.; Huang, P.; Huang, X.; Chen, X.; Jiang, H.; et al. Cath-KP, a novel peptide derived from frog skin, prevents oxidative stress damage in a Parkinson’s disease model. Zool. Res. 2024, 45, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Andersen, J.F.; Wang, Y.; Tokumasu, F.; Ribeiro, J.M.C.; Ma, D.; Xu, X.; An, S.; Francischetti, I.M.B.; et al. A novel family of RGD-containing disintegrins (Tablysin-15) from the salivary gland of the horsefly Tabanus yao targets alphaIIbbeta3 or alphaVbeta3 and inhibits platelet aggregation and angiogenesis. Thromb. Haemost. 2011, 105, 1032–1045. [Google Scholar] [CrossRef]
- Beeton, C.; Pennington, M.W.; Norton, R.S. Analogs of the sea anemone potassium channel blocker ShK for the treatment of autoimmune diseases. Inflamm. Allergy Drug Targets 2011, 10, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Bohlen, C.J.; Chesler, A.T.; Sharif-Naeini, R.; Medzihradszky, K.F.; Zhou, S.; King, D.; Sánchez, E.E.; Burlingame, A.L.; Basbaum, A.I.; Julius, D. A heteromeric Texas coral snake toxin targets acid-sensing ion channels to produce pain. Nature 2011, 479, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Chemin, J.; Patel, A.J.; Delmas, P.; Sachs, F.; Lazdunski, M.; Honore, E. Regulation of the Mechano-Gated K2P Channel TREK-1 by Membrane Phospholipids. Curr. Top. Membr. 2007, 59, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Pope, L.; Lolicato, M.; Minor, D.L., Jr. Polynuclear Ruthenium Amines Inhibit K(2P) Channels via a “Finger in the Dam” Mechanism. Cell Chem. Biol. 2020, 27, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Chen, L.; Cheng, X.; Ma, Y.; Li, X.; Zhang, B.; Li, L.; Zhang, S.; Guo, F.; Li, Y.; et al. An allosteric ligand-binding site in the extracellular cap of K2P channels. Nat. Commun. 2017, 8, 378. [Google Scholar] [CrossRef]
- Djillani, A.; Pietri, M.; Mazella, J.; Heurteaux, C.; Borsotto, M. Fighting against depression with TREK-1 blockers: Past and future. A focus on spadin. Pharmacol. Ther. 2019, 194, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Mazella, J.; Pétrault, O.; Lucas, G.; Deval, E.; Béraud-Dufour, S.; Gandin, C.; El-Yacoubi, M.; Widmann, C.; Guyon, A.; Chevet, E.; et al. Spadin, a sortilin-derived peptide, targeting rodent TREK-1 channels: A new concept in the antidepressant drug design. PLoS Biol. 2010, 8, e1000355. [Google Scholar] [CrossRef] [PubMed]
- Alloui, A.; Zimmermann, K.; Mamet, J.; Duprat, F.; Noël, J.; Chemin, J.; Guy, N.; Blondeau, N.; Voilley, N.; Rubat-Coudert, C.; et al. TREK-1, a K+ channel involved in polymodal pain perception. EMBO J. 2006, 25, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Royal, P.; Andres-Bilbe, A.; Prado, P.Á.; Verkest, C.; Wdziekonski, B.; Schaub, S.; Baron, A.; Lesage, F.; Gasull, X.; Levitz, J.; et al. Migraine-Associated TRESK Mutations Increase Neuronal Excitability through Alternative Translation Initiation and Inhibition of TREK. Neuron 2019, 101, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Levitz, J.; Royal, P.; Comoglio, Y.; Wdziekonski, B.; Schaub, S.; Clemens, D.M.; Isacoff, E.Y.; Sandoz, G. Heterodimerization within the TREK channel subfamily produces a diverse family of highly regulated potassium channels. Proc. Natl. Acad. Sci. USA 2016, 113, 4194–4199. [Google Scholar] [CrossRef]
- Schreiber, J.A.; Dufer, M.; Seebohm, G. The Special One: Architecture, Physiology and Pharmacology of the TRESK Channel. Cell Physiol. Biochem. 2022, 56, 663–684. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Li, J.; Shao, Z.; Mwangi, J.; Xu, R.; Tian, H.; Mo, G.; Lai, R.; Yang, S. Centipede KCNQ Inhibitor SsTx Also Targets KV1.3. Toxins 2019, 11, 76. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Guan, X.; Yan, J. Two-pore channel blockade by phosphoinositide kinase inhibitors YM201636 and PI-103 determined by a histidine residue near pore-entrance. Commun. Biol. 2022, 5, 738. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, C.; Chen, L.; Liu, G.; Yuan, F.; Zhang, Z.; Rong, M.; Mo, G.; Liu, C. Tick-Derived Peptide Blocks Potassium Channel TREK-1. Int. J. Mol. Sci. 2024, 25, 8377. https://doi.org/10.3390/ijms25158377
Du C, Chen L, Liu G, Yuan F, Zhang Z, Rong M, Mo G, Liu C. Tick-Derived Peptide Blocks Potassium Channel TREK-1. International Journal of Molecular Sciences. 2024; 25(15):8377. https://doi.org/10.3390/ijms25158377
Chicago/Turabian StyleDu, Canwei, Linyan Chen, Guohao Liu, Fuchu Yuan, Zheyang Zhang, Mingqiang Rong, Guoxiang Mo, and Changjun Liu. 2024. "Tick-Derived Peptide Blocks Potassium Channel TREK-1" International Journal of Molecular Sciences 25, no. 15: 8377. https://doi.org/10.3390/ijms25158377
APA StyleDu, C., Chen, L., Liu, G., Yuan, F., Zhang, Z., Rong, M., Mo, G., & Liu, C. (2024). Tick-Derived Peptide Blocks Potassium Channel TREK-1. International Journal of Molecular Sciences, 25(15), 8377. https://doi.org/10.3390/ijms25158377




