Synthetic Amphipathic Helical Peptide L-37pA Ameliorates the Development of Acute Respiratory Distress Syndrome (ARDS) and ARDS-Induced Pulmonary Fibrosis in Mice
Abstract
1. Introduction
2. Results
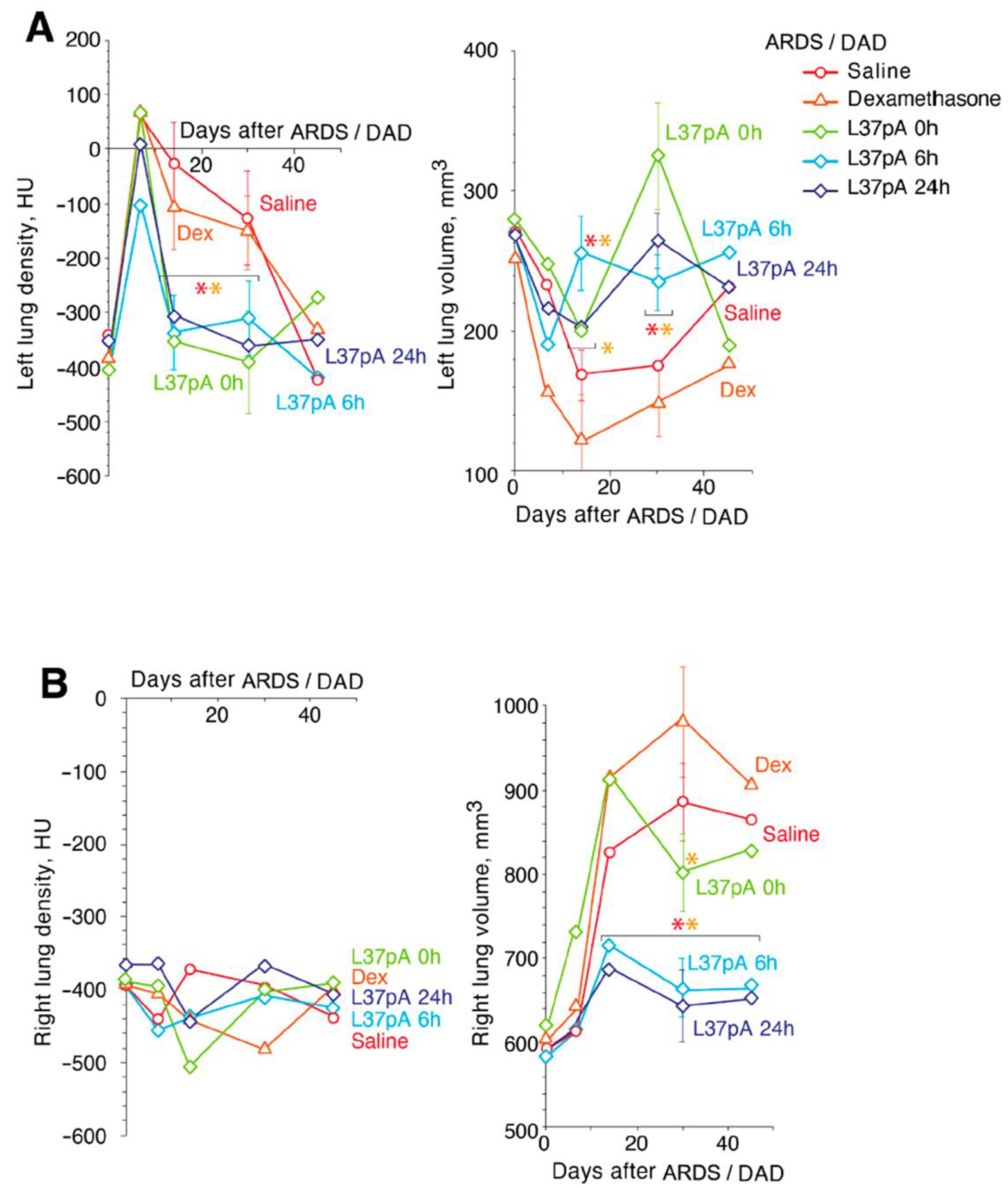
2.1. Contrast CT Bronchoscopy
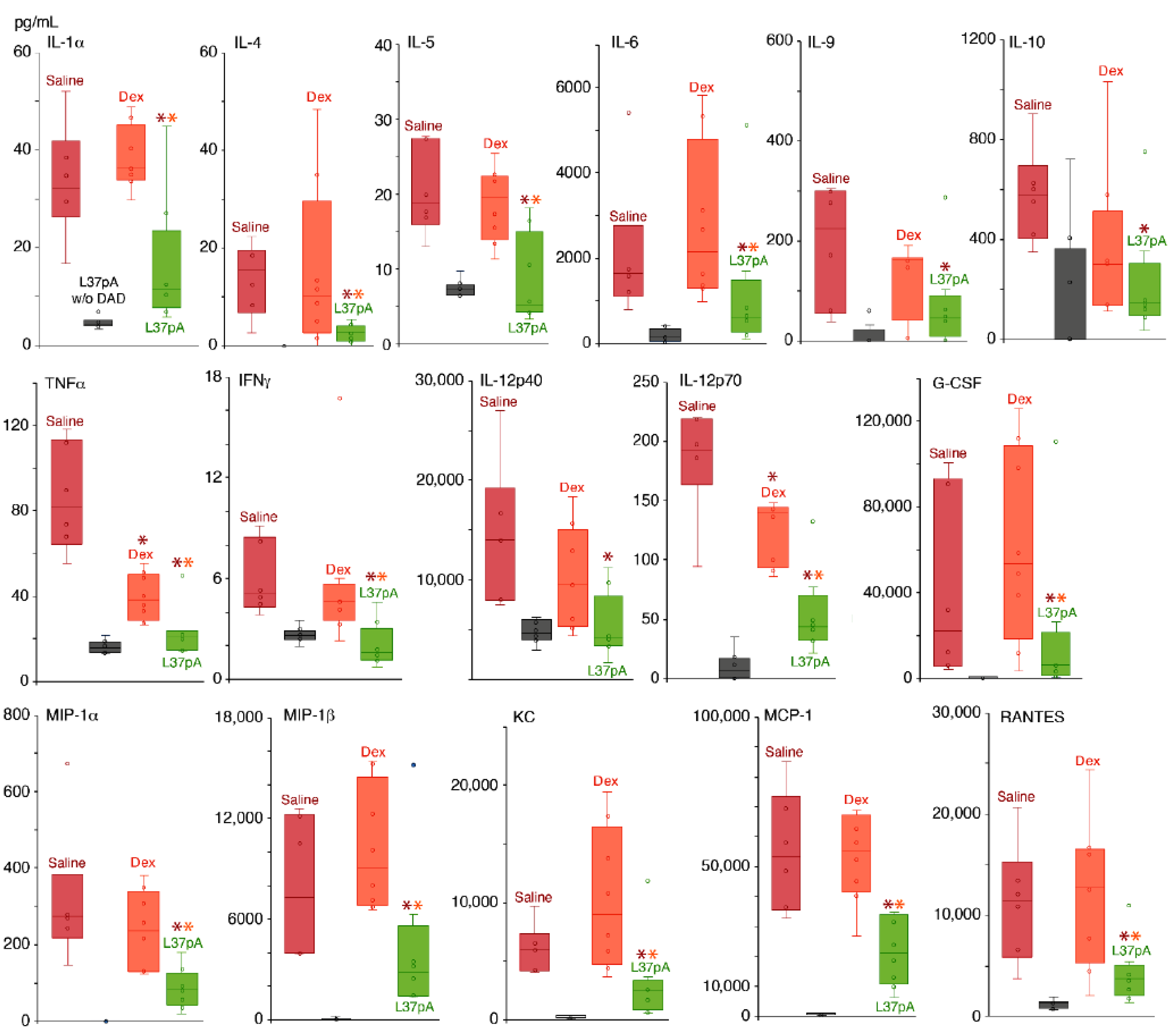
2.2. Blood Plasma Chemokine and Cytokine Levels
2.3. Measurements of Respiratory Parameters in ARDS/DAD Treated With L37pA
2.4. Histological Analyses of ARDS/DAD Lungs
2.5. Pulmonary Fibrosis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Reagents
5.2. Compounds, Targeted Delivery and Lung Damage
5.3. Experimental Groups
5.4. Conformation of Targeted Delivery of Compounds and Lung Damage Evaluation
5.5. Chemokine and Cytokine Assays
5.6. Assessment of Lung Density and Lung Volume in ARDS/DAD Mice
5.7. Measurements of Respiratory Dysfunction in ARDS/DAD Treated with L37pA
5.8. Histology Examinations
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Kerkela, E.R.; Ala-aho, J.; Lohi, R.; Grenman, M.K.; Saarialho-Kere, U. Differential patterns of stromelysin-2 (MMP-10) and MT1-MMP (MMP-14) expression in epithelial skin cancers. Br. J. Cancer 2001, 84, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Robbesyn, F.; Augé, N.; Vindis, C.; Cantero, A.V.; Barbaras, R.; Negre-Salvayre, A.; Salvayre, R. High-density lipoproteins prevent the oxidized low-density lipoprotein-induced epidermal [corrected] growth factor receptor activation and subsequent matrix metalloproteinase-2 upregulation. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1206–1212. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L.; Terasaka, N.; Pagler, T.; Wang, N. HDL, ABC transporters, and cholesterol efflux: Implications for the treatment of atherosclerosis. Cell Metab. 2008, 7, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Baranova, I.N.; Bocharov, A.V.; Vishnyakova, T.G.; Chen, Z.; Birukova, A.A.; Ke, Y.; Hu, X.; Yuen, P.S.T.; Star, R.A.; Birukov, K.G.; et al. Class B Scavenger Receptors BI and BII Protect Against LPS-induced Acute Lung Injury in Mice by Mediating LPS Clearance. Infect. Immun. 2021, 89, e0030121. [Google Scholar] [CrossRef]
- Baranova, I.N.; Kurlander, R.; Bocharov, A.V.; Vishnyakova, T.G.; Chen, Z.; Remaley, A.T.; Csako, G.; Patterson, A.P.; Eggerman, T.L. Role of human CD36 in bacterial recognition, phagocytosis, and pathogen-induced JNK-mediated signaling. J. Immunol. 2008, 181, 7147–7156. [Google Scholar] [CrossRef]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; van Gils, J.M.; Deng, J.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A.; et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010, 11, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Stuart, L.M.; Deng, J.; Silver, J.M.; Takahashi, K.; Tseng, A.A.; Hennessy, E.J.; Ezekowitz, R.A.; Moore, K.J. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J. Cell Biol. 2005, 170, 477–485. [Google Scholar] [CrossRef]
- Vishnyakova, T.G.; Bocharov, A.V.; Baranova, I.N.; Chen, Z.; Remaley, A.T.; Csako, G.; Eggerman, T.L.; Patterson, A.P. Binding and internalization of lipopolysaccharide by Cla-1, a human orthologue of rodent scavenger receptor B1. J. Biol. Chem. 2003, 278, 22771–22780. [Google Scholar] [CrossRef]
- Baranova, I.N.; Bocharov, A.V.; Vishnyakova, T.G.; Kurlander, R.; Chen, Z.; Fu, D.; Arias, I.M.; Csako, G.; Patterson, A.P.; Eggerman, T.L. CD36 is a novel serum amyloid A (SAA) receptor mediating SAA binding and SAA-induced signaling in human and rodent cells. J. Biol. Chem. 2010, 285, 8492–8506. [Google Scholar] [CrossRef]
- Baranova, I.N.; Souza, A.C.P.; Bocharov, A.V.; Vishnyakova, T.G.; Hu, X.; Vaisman, B.L.; Amar, M.J.; Chen, Z.; Remaley, A.T.; Patterson, A.P.; et al. Human SR-BII mediates SAA uptake and contributes to SAA pro-inflammatory signaling in vitro and in vivo. PLoS ONE 2017, 12, e0175824. [Google Scholar] [CrossRef]
- Baranova, I.N.; Vishnyakova, T.G.; Bocharov, A.V.; Kurlander, R.; Chen, Z.; Kimelman, M.L.; Remaley, A.T.; Csako, G.; Thomas, F.; Eggerman, T.L.; et al. Serum amyloid A binding to CLA-1 (CD36 and LIMPII analogous-1) mediates serum amyloid A protein-induced activation of ERK1/2 and p38 mitogen-activated protein kinases. J. Biol. Chem. 2005, 280, 8031–8040. [Google Scholar] [CrossRef]
- Baranova, I.N.; Souza, A.C.; Bocharov, A.V.; Vishnyakova, T.G.; Hu, X.; Vaisman, B.L.; Amar, M.J.; Chen, Z.; Kost, Y.; Remaley, A.T.; et al. Human SR-BI and SR-BII Potentiate Lipopolysaccharide-Induced Inflammation and Acute Liver and Kidney Injury in Mice. J. Immunol. 2016, 196, 3135–3147. [Google Scholar] [CrossRef] [PubMed]
- Baranova, I.N.; Vishnyakova, T.G.; Bocharov, A.V.; Leelahavanichkul, A.; Kurlander, R.; Chen, Z.; Souza, A.C.; Yuen, P.S.; Star, R.A.; Csako, G.; et al. Class B scavenger receptor types I and II and CD36 mediate bacterial recognition and proinflammatory signaling induced by Escherichia coli, lipopolysaccharide, and cytosolic chaperonin 60. J. Immunol. 2012, 188, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ji, A.; de Beer, F.C.; Tannock, L.R.; van der Westhuyzen, D.R. SR-BI protects against endotoxemia in mice through its roles in glucocorticoid production and hepatic clearance. J. Clin. Investig. 2008, 118, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Song, Z.; Li, M.; Wu, Q.; Wang, D.; Feng, H.; Bernard, P.; Daugherty, A.; Huang, B.; Li, X.A. Scavenger Receptor BI Protects against Septic Death through Its Role in Modulating Inflammatory Response. J. Biol. Chem. 2009, 284, 19826–19834. [Google Scholar] [CrossRef] [PubMed]
- Leelahavanichkul, A.; Bocharov, A.V.; Kurlander, R.; Baranova, I.N.; Vishnyakova, T.G.; Souza, A.C.; Hu, X.; Doi, K.; Vaisman, B.; Amar, M.; et al. Class B scavenger receptor types I and II and CD36 targeting improves sepsis survival and acute outcomes in mice. J. Immunol. 2012, 188, 2749–2758. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; Fogelman, A.M. Apolipoprotein A-I mimetic peptides and their role in atherosclerosis prevention. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Neyen, C.; Plüddemann, A.; Roversi, P.; Thomas, B.; Cai, L.; van der Westhuyzen, D.R.; Sim, R.B.; Gordon, S. Macrophage scavenger receptor A mediates adhesion to apolipoproteins A-I and E. Biochemistry 2009, 48, 11858–11871. [Google Scholar] [CrossRef]
- Madenspacher, J.H.; Azzam, K.M.; Gong, W.; Gowdy, K.M.; Vitek, M.P.; Laskowitz, D.T.; Remaley, A.T.; Wang, J.M.; Fessler, M.B. Apolipoproteins and apolipoprotein mimetic peptides modulate phagocyte trafficking through chemotactic activity. J. Biol. Chem. 2012, 287, 43730–43740. [Google Scholar] [CrossRef]
- Buga, G.M.; Frank, J.S.; Mottino, G.A.; Hakhamian, A.; Narasimha, A.; Watson, A.D.; Yekta, B.; Navab, M.; Reddy, S.T.; Anantharamaiah, G.M.; et al. D-4F reduces EO6 immunoreactivity, SREBP-1c mRNA levels, and renal inflammation in LDL receptor-null mice fed a Western diet. J. Lipid Res. 2008, 49, 192–205. [Google Scholar] [CrossRef]
- Buga, G.M.; Frank, J.S.; Mottino, G.A.; Hendizadeh, M.; Hakhamian, A.; Tillisch, J.H.; Reddy, S.T.; Navab, M.; Anantharamaiah, G.M.; Ignarro, L.J.; et al. D-4F decreases brain arteriole inflammation and improves cognitive performance in LDL receptor-null mice on a Western diet. J. Lipid Res. 2006, 47, 2148–2160. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Banquerigo, M.L.; Hama, S.; Navab, M.; Park, G.S.; Van Lenten, B.J.; Wagner, A.C.; Fogelman, A.M.; Brahn, E. Treatment with an apolipoprotein A-1 mimetic peptide in combination with pravastatin inhibits collagen-induced arthritis. Clin. Immunol. 2008, 127, 234–244. [Google Scholar] [CrossRef]
- Yao, Z.; Mates, J.M.; Cheplowitz, A.M.; Hammer, L.P.; Maiseyeu, A.; Phillips, G.S.; Wewers, M.D.; Rajaram, M.V.; Robinson, J.M.; Anderson, C.L.; et al. Blood-Borne Lipopolysaccharide Is Rapidly Eliminated by Liver Sinusoidal Endothelial Cells via High-Density Lipoprotein. J. Immunol. 2016, 197, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Matthay, M.A. Acute lung injury: Epidemiology, pathogenesis, and treatment. J. Aerosol. Med. Pulm. Drug Deliv. 2010, 23, 243–252. [Google Scholar] [CrossRef]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Ragaller, M.; Richter, T. Acute lung injury and acute respiratory distress syndrome. J. Emergencies Trauma Shock. 2010, 3, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bocharov, A.V.; Wu, T.; Baranova, I.N.; Birukova, A.A.; Sviridov, D.; Vishnyakova, T.G.; Remaley, A.T.; Eggerman, T.L.; Patterson, A.P.; Birukov, K.G. Synthetic Amphipathic Helical Peptides Targeting CD36 Attenuate Lipopolysaccharide-Induced Inflammation and Acute Lung Injury. J. Immunol. 2016, 197, 611–619. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Roger, T.; Calandra, T. TLR2-mediated neutrophil depletion exacerbates bacterial sepsis. Proc. Natl. Acad. Sci. USA 2009, 106, 6889–6890. [Google Scholar] [CrossRef] [PubMed]
- Roger, T.; Froidevaux, C.; Le Roy, D.; Reymond, M.K.; Chanson, A.L.; Mauri, D.; Burns, K.; Riederer, B.M.; Akira, S.; Calandra, T. Protection from lethal gram-negative bacterial sepsis by targeting Toll-like receptor 4. Proc. Natl. Acad. Sci. USA 2009, 106, 2348–2352. [Google Scholar] [CrossRef]
- Chen, X.; Wang, T.; Song, L.; Liu, X. Activation of multiple Toll-like receptors serves different roles in sepsis-induced acute lung injury. Exp. Ther. Med. 2019, 18, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Mallampalli, R.K. The acute respiratory distress syndrome: From mechanism to translation. J. Immunol. 2015, 194, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Fan, K.; Wang, K.; Bian, C. Atractylodin attenuates lipopolysaccharide-induced acute lung injury by inhibiting NLRP3 inflammasome and TLR4 pathways. J. Pharmacol. Sci. 2018, 136, 203–211. [Google Scholar] [CrossRef]
- Tang, F.; Fan, K.; Wang, K.; Bian, C. Amygdalin attenuates acute liver injury induced by D-galactosamine and lipopolysaccharide by regulating the NLRP3, NF-kappaB and Nrf2/NQO1 signalling pathways. Biomed. Pharmacother. 2019, 111, 527–536. [Google Scholar] [CrossRef]
- Souza, A.C.; Bocharov, A.V.; Baranova, I.N.; Vishnyakova, T.G.; Huang, Y.G.; Wilkins, K.J.; Hu, X.; Street, J.M.; Alvarez-Prats, A.; Mullick, A.E.; et al. Antagonism of scavenger receptor CD36 by 5A peptide prevents chronic kidney disease progression in mice independent of blood pressure regulation. Kidney Int. 2016, 89, 809–822. [Google Scholar] [CrossRef]
- Mu, W.; Sharma, M.; Heymans, R.; Ritou, E.; Rezek, V.; Hamid, P.; Kossyvakis, A.; Sen Roy, S.; Grijalva, V.; Chattopadhyay, A.; et al. Apolipoprotein A-I mimetics attenuate macrophage activation in chronic treated HIV. AIDS 2021, 35, 543–553. [Google Scholar] [CrossRef]
- Navab, M.; Shechter, I.; Anantharamaiah, G.M.; Reddy, S.T.; Van Lenten, B.J.; Fogelman, A.M. Structure and function of HDL mimetics. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 164–168. [Google Scholar] [CrossRef]
- Chernov, A.S.; Minakov, A.A.; Kazakov, V.A.; Rodionov, M.V.; Rybalkin, I.N.; Vlasik, T.N.; Yashin, D.V.; Saschenko, L.P.; Kudriaeva, A.A.; Belogurov, A.A.; et al. A new mouse unilateral model of diffuse alveolar damage of the lung. Inflamm. Res. 2022, 71, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Navab, M.; Anantharamaiah, G.M.; Reddy, S.T.; Van Lenten, B.J.; Datta, G.; Garber, D.; Fogelman, A.M. Potential clinical utility of high-density lipoprotein-mimetic peptides. Curr. Opin. Lipidol. 2006, 17, 440–444. [Google Scholar] [CrossRef]
- Bocharov, A.V.; Baranova, I.N.; Vishnyakova, T.G.; Remaley, A.T.; Csako, G.; Thomas, F.; Patterson, A.P.; Eggerman, T.L. Targeting of scavenger receptor class B type I by synthetic amphipathic alpha-helical-containing peptides blocks lipopolysaccharide (LPS) uptake and LPS-induced pro-inflammatory cytokine responses in THP-1 monocyte cells. J. Biol. Chem. 2004, 279, 36072–36082. [Google Scholar] [CrossRef]
- Meriwether, D.; Sulaiman, D.; Volpe, C.; Dorfman, A.; Grijalva, V.; Dorreh, N.; Solorzano-Vargas, R.S.; Wang, J.; O’Connor, E.; Papesh, J. Apolipoprotein A-I mimetics mitigate intestinal inflammation in COX2-dependent inflammatory bowel disease model. J. Clin. Investig. 2019, 129, 3670–3685. [Google Scholar] [CrossRef]
- Yao, X.; Dai, C.; Fredriksson, K.; Dagur, P.K.; McCoy, J.P.; Qu, X.; Yu, Z.X.; Keeran, K.J.; Zywicke, G.J.; Amar, M.J.; et al. 5A, an apolipoprotein A-I mimetic peptide, attenuates the induction of house dust mite-induced asthma. J. Immunol. 2011, 186, 576–583. [Google Scholar] [CrossRef]
- Yang, N.; Tian, H.; Zhan, E.; Zhai, L.; Jiao, P.; Yao, S.; Lu, G.; Mu, Q.; Wang, J.; Zhao, A.; et al. Reverse-D-4F improves endothelial progenitor cell function and attenuates LPS-induced acute lung injury. Respir. Res. 2019, 20, 131. [Google Scholar] [CrossRef]
- Pomytkin, I.A.; Karkischenko, V.N.; Fokin, Y.V.; Nesterov, M.S.; Petrova, N.V. A Model of Fatal Acute Lung Injury and Acute Respiratory Distress Syndrome. J. Biomed. 2020, 16, 24–33. [Google Scholar] [CrossRef]
- Chimenti, L.; Morales-Quinteros, L.; Puig, F.; Camprubi-Rimblas, M.; Guillamat-Prats, R.; Gómez, M.N.; Tijero, J.; Blanch, L.; Matute-Bello, G.; Artigas, A. Comparison of direct and indirect models of early induced acute lung injury. ICMx 2020, 8 (Suppl. 1), 62. [Google Scholar] [CrossRef]
- Domscheit, H.; Hegeman, M.A.; Carvalho, N.; Spieth, P.M. Dynamics of Lipopolysaccharide-Induced Lung Injury in Rodents. Front. Physiol. 2020, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Yang, J.; Bi, Z.; He, C.; Lei, H.; Yu, W.; Yang, Y.; Fan, C.; Lu, S.; Peng, X.; et al. A mouse model for SARS-CoV-2-induced acute respiratory distress syndrome. Signal Transduct. Target. Ther. 2021, 6, 1. [Google Scholar] [CrossRef]
- Bi, Z.; Hong, W.; Que, H.; He, C.; Ren, W.; Yang, J.; Lu, T.; Chen, L.; Lu, S.; Peng, X.; et al. Inactivated SARS-CoV-2 induces acute respiratory distress syndrome in human ACE2-transgenic mice. Signal Transduct. Target. Ther. 2021, 6, 439. [Google Scholar] [CrossRef] [PubMed]
- Kwak, D.; Bradley, P.B.; Subbotina, N.; Ling, S.; Teitz-Tennenbaum, S.; Osterholzer, J.J.; Sisson, T.H.; Kim, K.K. CD36/Lyn kinase interactions within macrophages promotes pulmonary fibrosis in response to oxidized phospholipid. Respir. Res. 2023, 24, 314. [Google Scholar] [CrossRef]
- Stella, G.M.; D’Agnano, V.; Piloni, D.; Saracino, L.; Lettieri, S.; Mariani, F.; Lancia, A.; Bortolotto, C.; Rinaldi, P.; Falanga, F.; et al. The oncogenic landscape of the idiopathic pulmonary fibrosis: A narrative review. Transl. Lung Cancer Res. 2022, 11, 472–496. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Batiha, G.E.; Faidah, H.; Al-Gareeb, A.I.; Saad, H.M.; Simal-Gandara, J. Pirfenidone and post-COVID-19 pulmonary fibrosis: Invoked again for realistic goals. Inflammopharmacology 2022, 30, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Hama Amin, B.J.; Kakamad, F.H.; Ahmed, G.S.; Ahmed, S.F.; Abdulla, B.A.; Mohammed, S.H.; Mikael, T.M.; Salih, R.Q.; Ali, R.K.; Salh, A.M.; et al. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann. Med. Surg. 2022, 77, 103590. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Balan, I.; Yadav, S.; Matos, W.F.; Kharawala, A.; Gaddam, M.; Sarabia, N.; Koneru, S.C.; Suddapalli, S.K.; Marzban, S. Post-COVID-19 Pulmonary Fibrosis. Cureus 2022, 14, e22770. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.J.; Kunjathoor, V.V.; Koehn, S.L.; Manning, J.J.; Tseng, A.A.; Silver, J.M.; McKee, M.; Freeman, M.W. Loss of receptor-mediated lipid uptake via scavenger receptor A or CD36 pathways does not ameliorate atherosclerosis in hyperlipidemic mice. J. Clin. Investig. 2005, 115, 2192–2201. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, W.; Stonik, J.A.; Murphy, A.; Demosky, S.J.; Sethi, A.A.; Moore, X.L.; Chin-Dusting, J.; Remaley, A.T.; Sviridov, D. Structure/function relationships of apolipoprotein a-I mimetic peptides: Implications for antiatherogenic activities of high-density lipoprotein. Circ. Res. 2010, 107, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Sayre, L.M.; Podrez, E.A. Analysis of relationship between oxidized phospholipid structure and interaction with the class B scavenger receptors. Methods Mol. Biol. 2015, 1208, 29–48. [Google Scholar] [PubMed]
- Puranik, R.; Bao, S.; Nobecourt, E.; Nicholls, S.J.; Dusting, G.J.; Barter, P.J.; Celermajer, D.S.; Rye, K.A. Low dose apolipoprotein A-I rescues carotid arteries from inflammation in vivo. Atherosclerosis 2008, 196, 240–247. [Google Scholar] [CrossRef]
- Sharifov, O.F.; Xu, X.; Gaggar, A.; Grizzle, W.E.; Mishra, V.K.; Honavar, J.; Litovsky, S.H.; Palgunachari, M.N.; White, C.R.; Anantharamaiah, G.M.; et al. Anti-inflammatory mechanisms of apolipoprotein A-I mimetic peptide in acute respiratory distress syndrome secondary to sepsis. PLoS ONE 2013, 8, e64486. [Google Scholar] [CrossRef] [PubMed]
- Chernov, A.S.; Rodionov, M.V.; Kazakov, V.A.; Ivanova, K.A.; Meshcheryakov, F.A.; Kudriaeva, A.A.; Gabibov, A.G.; Telegin, G.B.; Belogurov, A.A., Jr. CCR5/CXCR3 antagonist TAK-779 prevents diffuse alveolar damage of the lung in the murine model of the acute respiratory distress syndrome. Front. Pharmacol. 2024, 15, 1351655. [Google Scholar] [CrossRef]
- Sharapova, T.N.; Romanova, E.A.; Chernov, A.S.; Minakov, A.N.; Kazakov, V.A.; Kudriaeva, A.A.; Belogurov, A.A., Jr.; Ivanova, O.K.; Gabibov, A.G.; Telegin, G.B.; et al. Protein PGLYRP1/Tag7 Peptides Decrease the Proinflammatory Response in Human Blood Cells and Mouse Model of Diffuse Alveolar Damage of Lung through Blockage of the TREM-1 and TNFR1 Receptors. Int. J. Mol. Sci. 2021, 22, 11213. [Google Scholar] [CrossRef]


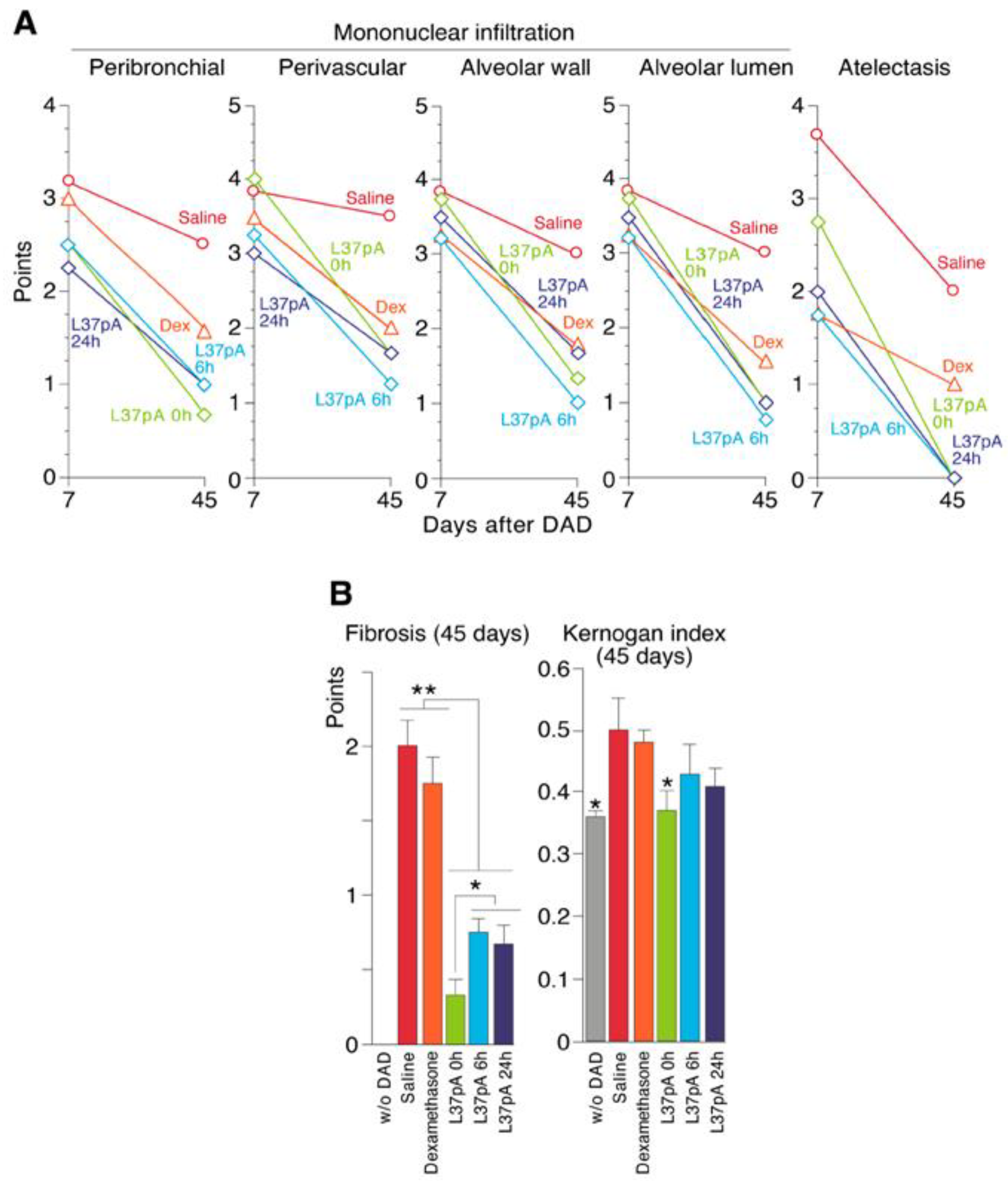
| Days after ARDS/DAD | Groups | PB Infiltration | PV Infiltration | Mononuclear Infiltration AW | Mononuclear Infiltration AL | DA |
|---|---|---|---|---|---|---|
| 7 | ARDS + saline (n = 6) | 3.17 | 3.84 | 3.84 | 3.84 | 3.67 |
| ARDS + IV DEX 0 h (n = 6) | 3 | 3.5 | 3.25 | 3.25 | 1.75 | |
| ARDS + IV 10 mg/mL L37pA at 6 h (n = 6) | 2.5 | 3.25 | 3.25 | 3.25 | 1.75 | |
| ARDS + IV 10 mg/mL L37pA at 24 h (n = 6) | 2.25 | 3 | 3.5 | 3.5 | 2 | |
| ARDS + IV 10 mg/mL L37pA at 0 h (n = 6) | 2.5 | 4 | 3.75 | 3.75 | 2.75 | |
| 45 | ARDS + saline (n = 6) | 2.5 | 3.5 | 3 | 3 | 2 |
| ARDS + IV DEX 0 h (n = 6) | 1 | 2 | 1.75 | 1.5 | 1 | |
| ARDS + IV 10 mg/mL L37pA at 6 h (n = 6) | 1 | 1.25 | 1 | 0.75 | 0 | |
| ARDS + IV 10 mg/mL L37pA at 24 h (n = 6) | 1 | 1.67 | 1.67 | 1 | 0 | |
| ARDS + IV 10 mg/mL L37pA at 0 h (n = 6) | 0.67 | 1.67 | 1.33 | 1 | 0 |
| Group | ARDS by LPS + GC | Treatment | Group Description |
|---|---|---|---|
| 1 | ARDS | IV Saline | ARDS + Saline |
| 2 | ARDS | IV 5 mg/mL Dexamethasone | ARDS + DEX |
| 3 | ARDS | IV 10 mg/mL L37pA at 0 h | ARDS + L37pA at 0 h |
| 4 | ARDS | IV 10 mg/mL L37pA at 6 h | ARDS + L37pA at 6 h |
| 5 | ARDS | IV 10 mg/mL L37pA at 24 h | ARDS + L37pA at 24 h |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernov, A.S.; Telegin, G.B.; Minakov, A.N.; Kazakov, V.A.; Rodionov, M.V.; Palikov, V.A.; Kudriaeva, A.A.; Belogurov, A.A., Jr. Synthetic Amphipathic Helical Peptide L-37pA Ameliorates the Development of Acute Respiratory Distress Syndrome (ARDS) and ARDS-Induced Pulmonary Fibrosis in Mice. Int. J. Mol. Sci. 2024, 25, 8384. https://doi.org/10.3390/ijms25158384
Chernov AS, Telegin GB, Minakov AN, Kazakov VA, Rodionov MV, Palikov VA, Kudriaeva AA, Belogurov AA Jr. Synthetic Amphipathic Helical Peptide L-37pA Ameliorates the Development of Acute Respiratory Distress Syndrome (ARDS) and ARDS-Induced Pulmonary Fibrosis in Mice. International Journal of Molecular Sciences. 2024; 25(15):8384. https://doi.org/10.3390/ijms25158384
Chicago/Turabian StyleChernov, Aleksandr S., Georgii B. Telegin, Alexey N. Minakov, Vitaly A. Kazakov, Maksim V. Rodionov, Viktor A. Palikov, Anna A. Kudriaeva, and Alexey A. Belogurov, Jr. 2024. "Synthetic Amphipathic Helical Peptide L-37pA Ameliorates the Development of Acute Respiratory Distress Syndrome (ARDS) and ARDS-Induced Pulmonary Fibrosis in Mice" International Journal of Molecular Sciences 25, no. 15: 8384. https://doi.org/10.3390/ijms25158384
APA StyleChernov, A. S., Telegin, G. B., Minakov, A. N., Kazakov, V. A., Rodionov, M. V., Palikov, V. A., Kudriaeva, A. A., & Belogurov, A. A., Jr. (2024). Synthetic Amphipathic Helical Peptide L-37pA Ameliorates the Development of Acute Respiratory Distress Syndrome (ARDS) and ARDS-Induced Pulmonary Fibrosis in Mice. International Journal of Molecular Sciences, 25(15), 8384. https://doi.org/10.3390/ijms25158384






