Proteomic Profiling of Tears in Blau Syndrome Patients in Identification of Potential Disease Biomarkers
Abstract
1. Introduction
2. Results
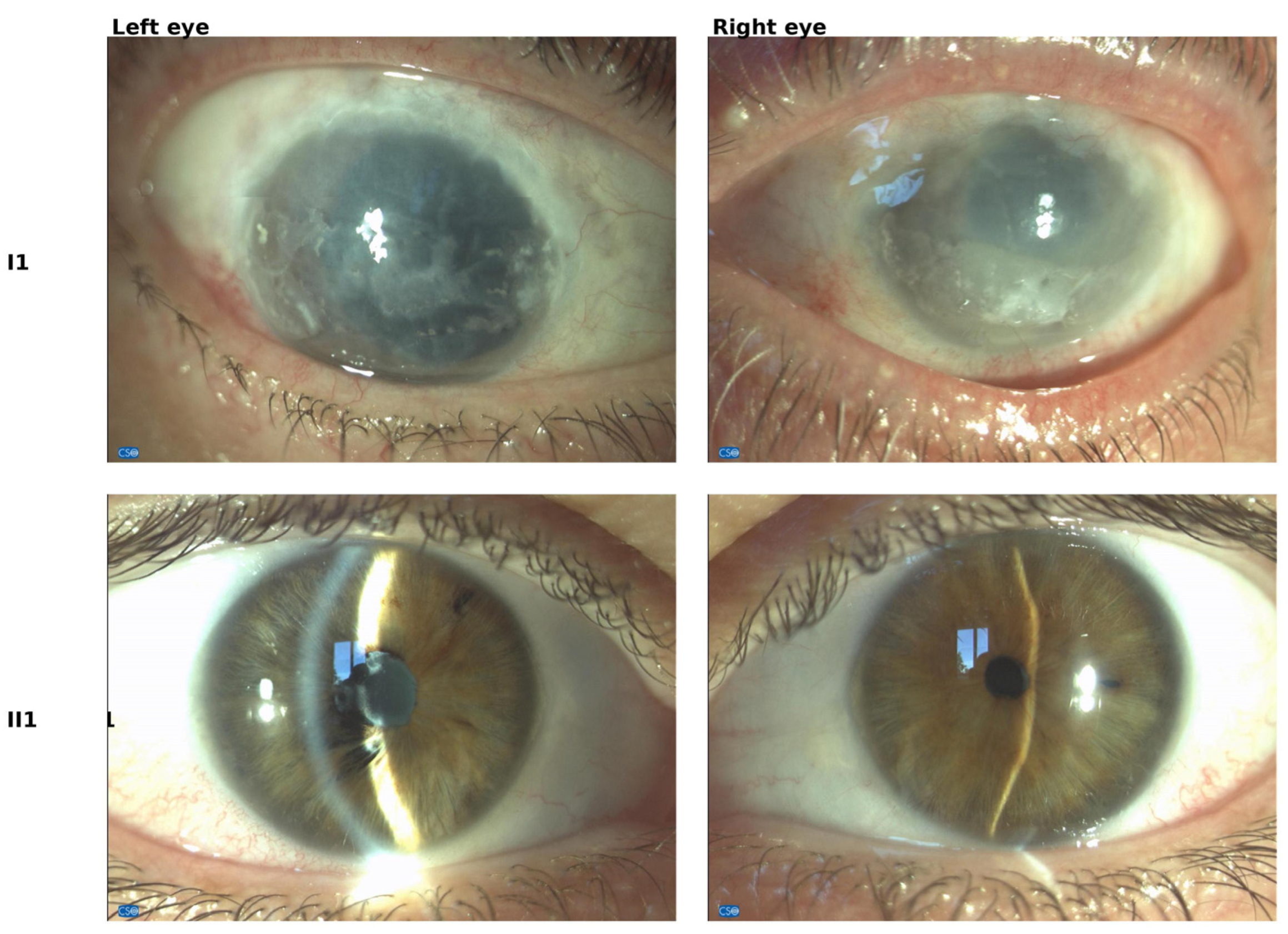
2.1. Ocular Manifestations
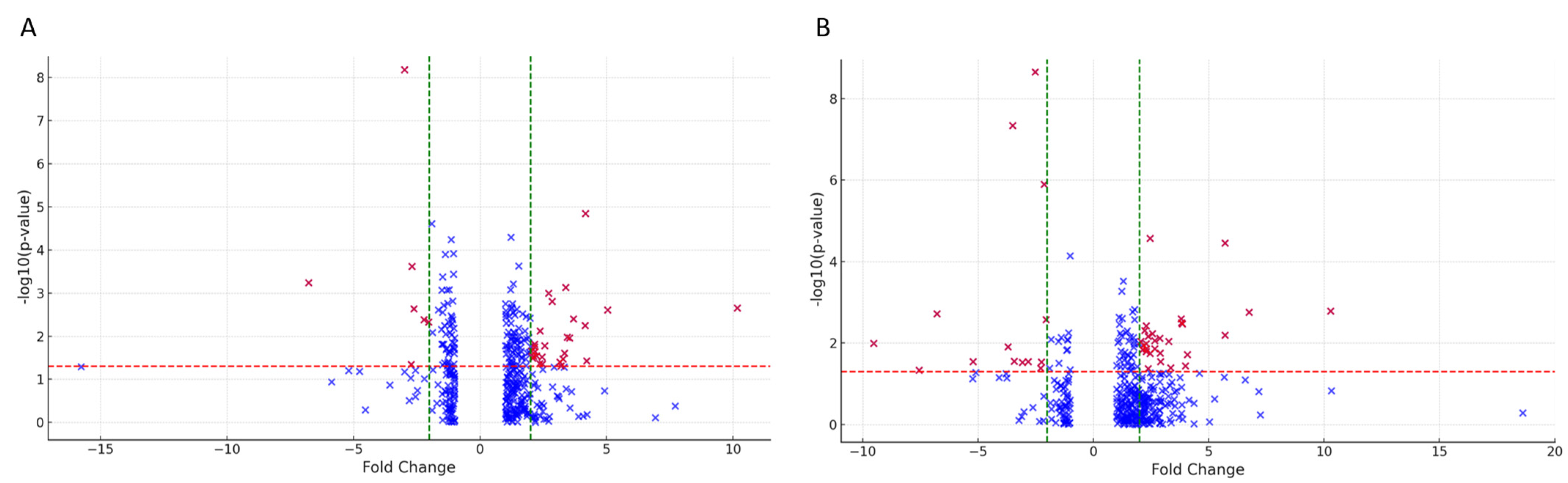
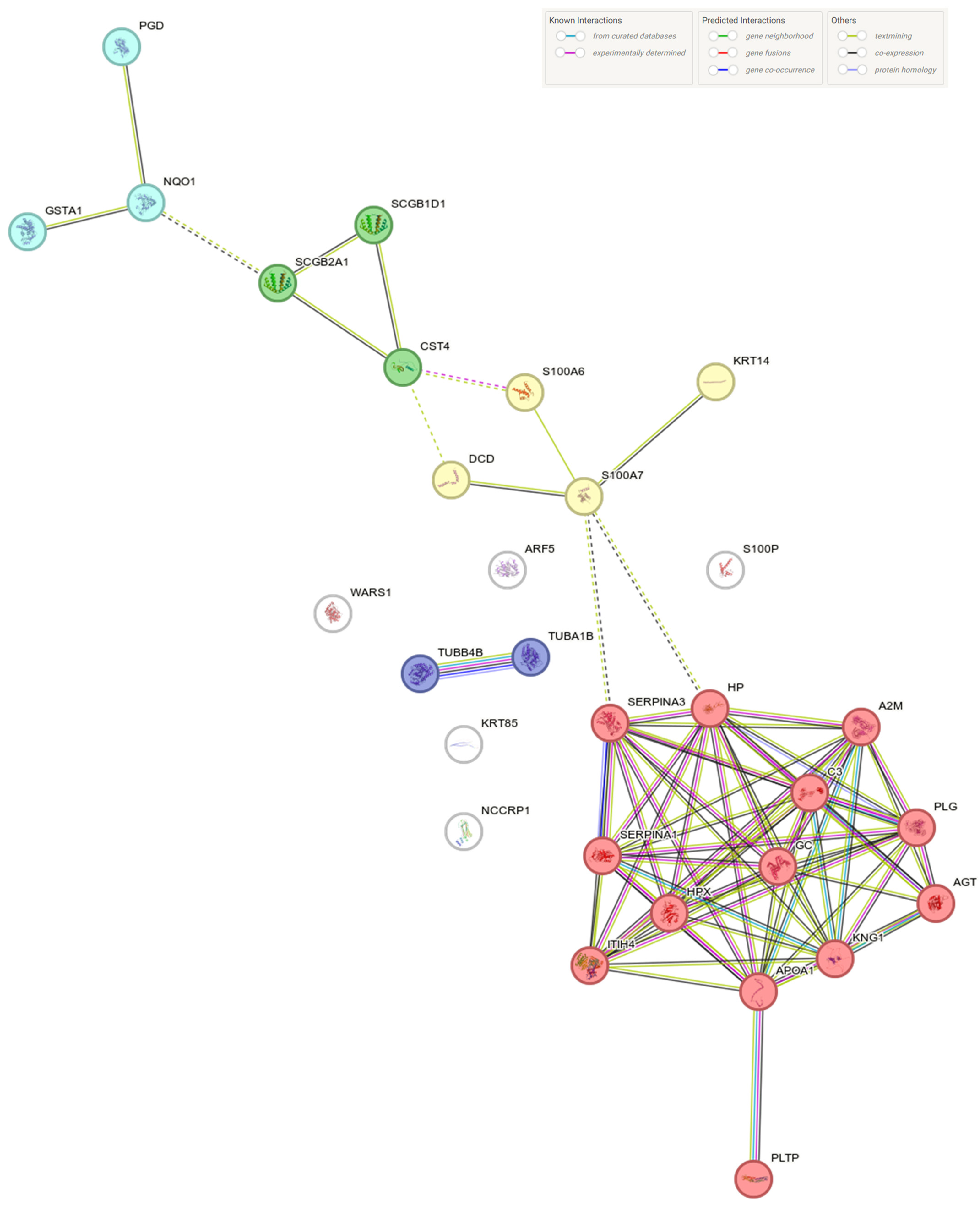
2.2. Tears Proteomic Profiles
3. Discussion
4. Material and Methods
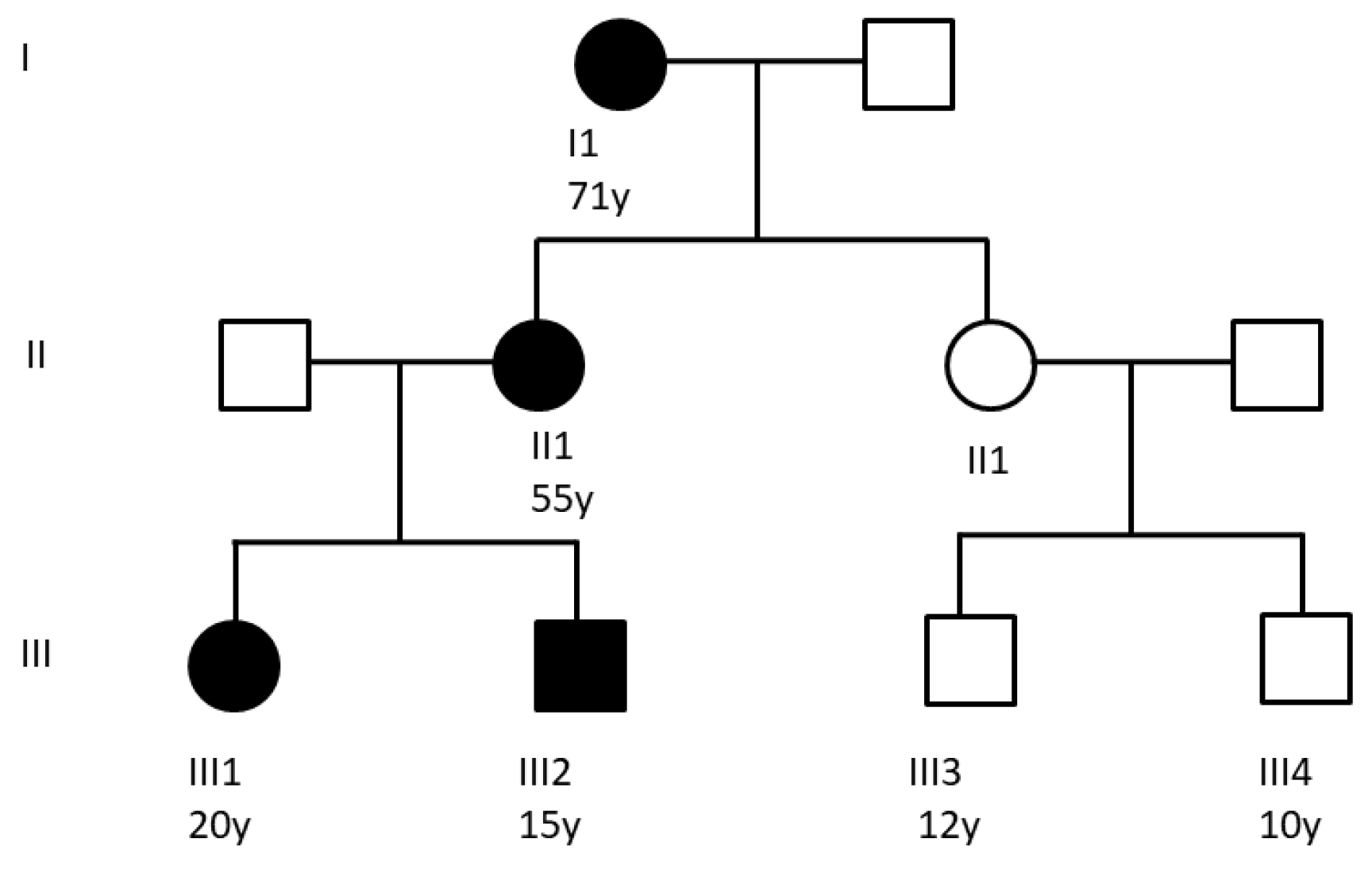
4.1. Patients
Clinical Features of NOD2 p.E383K Carriers
4.2. Tears Sampling
4.3. Protein Extraction
4.4. Protein Digestion
4.5. Mass Spectrometry and Protein Identification
4.6. Statistical and Bioinformatic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sfriso, P.; Caso, F.; Tognon, S.; Galozzi, P.; Gava, A.; Punzi, L. Blau Syndrome, Clinical and Genetic Aspects. Autoimmun. Rev. 2012, 12, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Galozzi, P.; Negm, O.; Greco, E.; Alkhattabi, N.; Gava, A.; Sfriso, P.; Fairclough, L.; Todd, I.; Tighe, P.; Punzi, L. Ex Vivo and in Vitro Production of Pro-Inflammatory Cytokines in Blau Syndrome. Reumatismo 2015, 66, 277–284. [Google Scholar] [CrossRef][Green Version]
- Mao, L.; Dhar, A.; Meng, G.; Fuss, I.; Montgomery-Recht, K.; Yang, Z.; Xu, Q.; Kitani, A.; Strober, W. Blau syndrome NOD2 mutations result in loss of NOD2 cross-regulatory function. Front. Immunol. 2022, 13, 988862. [Google Scholar] [CrossRef]
- van Duist, M.M.; Albrecht, M.; Podswiadek, M.; Giachino, D.; Lengauer, T.; Punzi, L.; De Marchi, M. A new CARD15 mutation in Blau syndrome. European journal of human genetics. Eur. J. Hum. Genet. 2005, 13, 742–747. [Google Scholar] [CrossRef]
- Saulsbury, F.T.; Wouters, C.H.; Martin, T.M.; Austin, C.R.; Doyle, T.M.; Goodwin, K.A.; Rosé, C.D. Incomplete penetrance of the NOD2 E383K substitution among members of a pediatric granulomatous arthritis pedigree. Arthritis Rheum. 2009, 60, 1804–1806. [Google Scholar] [CrossRef]
- Villanueva-Mendoza, C.; Arellanes-García, L.; Cubas-Lorenzo, V.; Jimenez-Martinez, M.C.; Flores-Suárez, L.F.; Zenteno, J.C. Familial case of Blau syndrome associated with a CARD15/NOD2 mutation. Ophthalmic Genet. 2010, 31, 155–158. [Google Scholar] [CrossRef]
- Harada, J.; Nakajima, T.; Kanazawa, N. A Case of Blau Syndrome with NOD2 E383K Mutation. Pediatr. Dermatol. 2016, 33, e385–e387. [Google Scholar] [CrossRef]
- Parkhouse, R.; Boyle, J.P.; Monie, T.P. Blau syndrome polymorphisms in NOD2 identify nucleotide hydrolysis and helical domain 1 as signalling regulators. FEBS Lett. 2014, 588, 3382–3389. [Google Scholar] [CrossRef]
- Caso, F.; Galozzi, P.; Costa, L.; Sfriso, P.; Cantarini, L.; Punzi, L. Review: Autoinflammatory Granulomatous Diseases: From Blau Syndrome and Early-Onset Sarcoidosis to NOD2-Mediated Disease and Crohn’s Disease. RMD Open 2015, 1, e000097. [Google Scholar] [CrossRef]
- Kurokawa, T.; Kikuchi, T.; Ohta, K.; Imai, H.; Yoshimura, N. Ocular Manifestations in Blau Syndrome Associated with a CARD15/Nod2 Mutation. Ophthalmology 2003, 110, 2040–2044. [Google Scholar] [CrossRef]
- Suresh, S.; Tsui, E. Ocular Manifestations of Blau Syndrome. Curr. Opin. Ophthalmol. 2020, 31, 532–537. [Google Scholar] [CrossRef]
- Kreps, E.O.; Al Julandani, D.; Guly, C.M.; Arostegui, J.I.; Dick, A.D.; Ramanan, A.V. Long-Term Visual Outcome of Patients with Blau Syndrome. Ocul. Immunol. Inflamm. 2024, 1–5. [Google Scholar] [CrossRef]
- Galozzi, P.; Negm, O.; Bindoli, S.; Tighe, P.; Sfriso, P.; Punzi, L. A Pro-Inflammatory Signature Constitutively Activated in Monogenic Autoinflammatory Diseases. Int. J. Mol. Sci. 2022, 23, 1828. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Kambe, N.; Ueki, Y.; Kanazawa, N.; Izawa, K.; Honda, Y.; Kawakami, A.; Takei, S.; Tonomura, K.; Inoue, M.; et al. Clinical Characteristics and Treatment of 50 Cases of Blau Syndrome in Japan Confirmed by Genetic Analysis of the NOD2 Mutation. Ann. Rheum Dis. 2020, 79, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Punzi, L.; Furlan, A.; Podswiadek, M.; Gava, A.; Valente, M.; De Marchi, M.; Peserico, A. Clinical and Genetic Aspects of Blau Syndrome: A 25-Year Follow-up of One Family and a Literature Review. Autoimmun. Rev. 2009, 8, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Hagan, S.; Martin, E.; Enríquez-De-Salamanca, A. Tear Fluid Biomarkers in Ocular and Systemic Disease: Potential Use for Predictive, Preventive and Personalised Medicine. Epma J. 2016, 7, 1–20. [Google Scholar] [CrossRef]
- Wouters, C.H.; Maes, A.; Foley, K.P.; Bertin, J.; Rose, C.D. Blau syndrome, the prototypic auto-inflammatory granulomatous disease. Pediatr. Rheumatol. Online J. 2014, 12, 33. [Google Scholar] [CrossRef]
- Sun, C.; Cao, C.; Zhao, T.; Guo, H.; Fleming, B.C.; Owens, B.; Beveridge, J.; McAllister, S.; Wei, L. A2M Inhibits Inflammatory Mediators of Chondrocytes by Blocking IL-1β/NF-ΚB Pathway. J. Orthop. Res. 2023, 41, 241–248. [Google Scholar] [CrossRef]
- Shi, Z.; Rudzinski, M.; Meerovitch, K.; Ric Lebrun-Julien, F.; Birman, E.; Di Polo, A.; Saragovi, H.U. 2-Macroglobulin Is a Mediator of Retinal Ganglion Cell Death in Glaucoma *. J. Biol. Chem. 2008, 283, 29156–29165. [Google Scholar] [CrossRef]
- Cater, J.H.; Kumita, J.R.; Abdallah, R.Z.; Zhao, G.; Bernardo-Gancedo, A.; Henry, A.; Winata, W.; Chi, M.; Grenyer, B.S.F.; Townsend, M.L.; et al. Human Pregnancy Zone Protein Stabilizes Misfolded Proteins Including Preeclampsia-and Alzheimer’s-Associated Amyloid Beta Peptide. Proc. Natl. Acad. Sci. USA 2019, 116, 6101–6110. [Google Scholar] [CrossRef]
- Papadea, C.; Check, I.J. Human Immunoglobulin G and Immunoglobulin G Subclasses: Biochemical, Genetic, and Clinical Aspects. Crit. Rev. Clin. Lab Sci. 1989, 27, 27–58. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.K.; Liao, X.; Mohamed Ali Abdulla Aljufairi, F.; Man Wong, Y.; Chiu, J.T.; Mak, H.; Cheng, A.C.; Chin, J.K.; Chu, B.C.; Kwong, C.H.; et al. Ocular Surface Evaluation in Immunoglobulin G4–Related Ophthalmic Disease. Am. J. Ophthalmol. 2023, 256, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.X.; Yang, Y.; Hou, J.C.; Shu, Q.; Yin, Y.X.; Fu, W.T.; Han, F.; Hou, T.J.; Zeng, C.L.; Nemeth, E.; et al. Increased Gene Copy Number of DEFA1/DEFA3 Worsens Sepsis by Inducing Endothelial Pyroptosis. Proc. Natl. Acad. Sci. USA 2019, 116, 3161–3170. [Google Scholar] [CrossRef] [PubMed]
- Siraki, A.G. NC-ND License The Many Roles of Myeloperoxidase: From Inflammation and Immunity to Biomarkers, Drug Metabolism and Drug Discovery. Redox Biol. 2021, 46, 102109. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.P.; Vanet, A.; Witko-Sarsat, V.; Melchior, M.; Mccabe, D.; Gabay, J.E. Azurocidin, a Natural Antibiotic from Human Neutrophils: Expression, Antimicrobial Activity, and Secretion. Protein Expr. Purif. 1996, 7, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Kai-Larsen, Y.; Frithiof, R.; Sorensen, O.E.; Kenne, E.; Scharffetter-Kochanek, K.; Eriksson, E.E.; Herwald, H.; Agerberth, B.; Lindbom, L. Neutrophil Primary Granule Proteins HBP and HNP1-3 Boost Bacterial Phagocytosis by Human and Murine Macrophages. J. Clin. Investig. 2008, 118, 3491–3502. [Google Scholar] [CrossRef] [PubMed]
- Oleg Chertov, B.; Ueda, H.; Ling Xu, L.; Tani, K.; Murphy, W.J.; Ming Wang, J.; Zack Howard, O.; Sayers, T.J.; Oppenheim, J.J. Identification of Human Neutrophil-Derived Cathepsin G and Azurocidin/CAP37 as Chemoattractants for Mononuclear Cells and Neutrophils. J. Exp. Med. 1997, 186, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Mun, Y.; Hwang, J.S.; Shin, Y.J. Role of Neutrophils on the Ocular Surface. Int. J. Mol. Sci. 2021, 22, 10386. [Google Scholar] [CrossRef] [PubMed]
- Angeles-Han, S.T.; Yeh, S.; Patel, P.; Duong, D.; Jenkins, K.; Rouster-Stevens, K.A.; Altaye, M.; Fall, N.; Thornton, S.; Prahalad, S.; et al. Discovery of Tear Biomarkers in Children with Chronic Non-Infectious Anterior Uveitis: A Pilot Study. J. Ophthalmic Inflamm. Infect. 2018, 8, 17. [Google Scholar] [CrossRef]
- Eidet, J.R.; Jørstad, Ø.K.; Fostad, I.G.; Olstad, O.K.; Sørland, R.; Moe, M.C.; Petrovski, G.; Pepaj, M. Unilateral Acute Anterior Uveitis Is Associated with Ipsilateral Changes in the Tear Fluid Proteome That Involves the LXR/RXR Pathway. J. Ophthalmic Inflamm. Infect. 2020, 10, 13. [Google Scholar] [CrossRef]
- Jang, J.H.; Bruse, S.; Liu, Y.; Duffy, V.; Zhang, C.; Oyamada, N.; Randell, S.; Matsumoto, A.; Thompson, D.C.; Lin, Y.; et al. Aldehyde Dehydrogenase 3A1 Protects Airway Epithelial Cells from Cigarette Smoke-Induced DNA Damage and Cytotoxicity. Free Radic. Biol. Med. 2014, 68, 80. [Google Scholar] [CrossRef]
- Guntermann, A.; Fatoba, O.; Kronenberg, M.; Reinehr, S.; Grotegut, P.; Schargus, M.; Tsai, T.; Ivanova, S.; Serschnitzki, B.; Kumowski, N.; et al. Investigation of Inter- and Intra-Day Variability of Tear Fluid Regarding Flow Rate, Protein Concentration as Well as Protein Composition. Investig. Ophthalmol. Vis. Sci. 2023, 64, 13. [Google Scholar] [CrossRef]
- Scalcon, V.; Folda, A.; Lupo, M.G.; Tonolo, F.; Pei, N.; Battisti, I.; Ferri, N.; Arrigoni, G.; Bindoli, A.; Holmgren, A.; et al. Mitochondrial Depletion of Glutaredoxin 2 Induces Metabolic Dysfunction-Associated Fatty Liver Disease in Mice. Redox Biol. 2022, 51, 102277. [Google Scholar] [CrossRef]
- STRING Website. Available online: https://www.string-db.org (accessed on 4 March 2024).
- DAVID Website. Available online: https://david.ncifcrf.gov/summary.jsp (accessed on 4 March 2024).


| A vs. B | |||
| Gene Name | Protein Description | Fold Change | p Value * |
| S100A7 | S100 calcium binding protein A7 | −6.78 | 0.0006 |
| A2M | Alpha-2 macroglobulin | 5.03 | 0.0025 |
| IGHG4 | Immunoglobulin heavy constant gamma 4 | 10.17 | 0.0022 |
| A vs. C | |||
| Gene Name | Protein Description | Fold Change | p Value * |
| CAMP | Cathelicidin antimicrobial peptide | −9.53 | 0.0050 |
| PRTN3 | Proteinase 3 | −7.56 | 0.0260 |
| DEFA3 | Defensin alpha 3 | −6.78 | 0.0010 |
| MPO | Myeloperoxidase | −5.21 | 0.0218 |
| SERPINA3 | Serpin family A member 3 | 5.705312 | 0.0030 |
| HP | Haptoglobin | 5.706 | 2.47 × 10−5 |
| A2M | Alpha-2 macroglobulin | 6.746384 | 0.0007 |
| IGHG4 | Immunoglobulin heavy constant gamma 4 | 10.27741 | 0.0010 |
| KRT31 | Keratin 31 | 60.95701 | 0.0190 |
| I1 vs. II1 | |||
| Gene Name | Protein Description | Fold Change | pValue * |
| KRT4 | Keratin 4 | 5.25 | 0.0162 |
| ALDH3A1 | Aldehyde dehydrogenase 3 family member A1 | 5.90 | 0.0065 |
| DEFA3 | Defensin alpha 3 | 9.93 | 0.0168 |
| MPO | Myeloperoxidase | 16.50 | 0.0030 |
| AZU1 | Azurocidin | 24.84 | 0.0164 |
| I1 vs. III1-2 | |||
| Gene Name | Protein Description | Fold Change | pValue * |
| PRR27 | Proline-rich 27 | −10.47 | 0.0209 |
| IGLV3-21 | Immunoglobulin lambda variable 3–21 | −8.39 | 8.76 × 10−6 |
| ALDH1A1 | Aldehyde dehydrogenase 1 family member A1 | 5.00 | 0.0055 |
| APOA4 | Apolipoprotein A4 | 5.05 | 0.0024 |
| AKR1C1 | Aldo-keto reductase family 1 member C1 | 5.17 | 0.0406 |
| ITIH2 | Inter-alpha-trypsin inhibitor heavy chain 2 | 5.18 | 0.0192 |
| CRYZ | Crystallin zeta | 5.20 | 0.0037 |
| AK1 | Adenylate kinase 1 | 5.35 | 0.0283 |
| AKR1C2 | Aldo-keto reductase family 1 member C2 | 5.39 | 0.0049 |
| MYH14 | Myosin heavy chain 14 | 5.40 | 0.0013 |
| CRYAB | Crystallin alpha B | 5.42 | 0.0113 |
| TALDO1 | Transaldolase 1 | 5.60 | 0.0119 |
| DEFA3 | Defensin alpha 3 | 5.64 | 0.0127 |
| KRT4 | Keratin 4 | 5.69 | 0.0075 |
| KRT13 | Keratin 13 | 5.81 | 0.0201 |
| NQO1 | NAD(P)H quinone dehydrogenase 1 | 5.89 | 0.0054 |
| GNB2 | G protein subunit beta 2 | 5.92 | 7.29 × 10−6 |
| LCN2 | Lipocalin 2 | 6.03 | 0.0120 |
| F2 | Coagulation factor II, thrombin | 6.28 | 0.0068 |
| SERPINA3 | Serpin family A member 3 | 6.35 | 0.0145 |
| HPX | Hemopexin | 6.90 | 0.0434 |
| MPO | Myeloperoxidase | 6.94 | 0.0028 |
| AZU1 | Azurocidin | 7.07 | 0.0076 |
| KRT7 | Keratin 7 | 7.28 | 5.16 × 10−5 |
| AGT | Angiotensinogen | 7.97 | 0.0031 |
| GLUL | Glutamate–ammonia ligase | 8.70 | 0.0213 |
| SPARCL1 | SPARC-like 1 | 10.98 | 0.0010 |
| APOC3 | Apolipoprotein C3 | 11.36 | 0.0184 |
| SERPING1 | Serpin family G member 1 | 12.32 | 0.0201 |
| ALDH3A1 | Aldehyde dehydrogenase 3 family member A1 | 16.87 | 0.0055 |
| II1 vs. III1-2 | |||
| Gene Name | Protein Description | Fold Change | pValue * |
| PRR27 | Proline-rich 27 | −14.66 | 0.0189 |
| CXCL17 | C–X–C motif chemokine ligand 17 | −5.36 | 0.0001 |
| ITIH1 | Inter-alpha-trypsin inhibitor heavy chain 1 | 5.10 | 0.0364 |
| SERPINA3 | Serpin family A member 3 | 5.32 | 0.0272 |
| AHSG | Alpha 2-HS glycoprotein | 5.61 | 0.0303 |
| IGHG2 | Immunoglobulin heavy constant gamma 2 | 5.62 | 0.0242 |
| KNG1 | Kininogen 1 | 5.73 | 0.0104 |
| SERPINB1 | Serpin family B member 1 | 6.09 | 0.0360 |
| GLUL | Glutamate–ammonia ligase | 7.30 | 0.0151 |
| F2 | Coagulation factor II, thrombin | 7.59 | 0.0186 |
| HPX | Hemopexin | 7.77 | 0.0391 |
| VTN | Vitronectin | 9.08 | 0.0163 |
| AGT | Angiotensinogen | 10.29 | 0.0143 |
| SERPING1 | Serpin family G member 1 | 10.41 | 0.0248 |
| KRT83 | Keratin 83 | 78.90 | 0.0420 |
| KRT33B | Keratin 33B | 192.48 | 0.0294 |
| A vs. B | |||
| Category | Term | % | pValue |
| GOTERM_CC_DIRECT | GO:0070062~extracellular exosome | 81.82 | 1 × 10−26 |
| GOTERM_MF_DIRECT | GO:0005515~protein binding | 79.55 | 0.02 |
| GOTERM_CC_DIRECT | GO:0005615~extracellular space | 75.00 | 2.23 × 10−24 |
| GOTERM_CC_DIRECT | GO:0005576~extracellular region | 70.45 | 3.99 × 10−20 |
| GOTERM_CC_DIRECT | GO:0072562~blood microparticle | 38.64 | 4.62 × 10−24 |
| GOTERM_CC_DIRECT | GO:0035578~azurophil granule lumen | 18.18 | 7.55 × 10−10 |
| GOTERM_BP_DIRECT | GO:0006953~acute-phase response | 13.64 | 2.12 × 10−8 |
| GOTERM_BP_DIRECT | GO:0019731~antibacterial humoral response | 13.64 | 2.98 × 10−7 |
| GOTERM_CC_DIRECT | GO:0031093~platelet alpha granule lumen | 13.64 | 3 × 10−7 |
| GOTERM_MF_DIRECT | GO:0042803~protein homodimerization activity | 13.64 | 0.02 |
| GOTERM_BP_DIRECT | GO:0006958~complement activation, classical pathway | 11.36 | 2.26 × 10−6 |
| GOTERM_BP_DIRECT | GO:0042742~defense response to bacterium | 11.36 | 4.30 × 10−4 |
| GOTERM_BP_DIRECT | GO:0006508~proteolysis | 11.36 | 0.04 |
| GOTERM_CC_DIRECT | GO:0034774~secretory granule lumen | 11.36 | 1.01 × 10−4 |
| GOTERM_MF_DIRECT | GO:0004867~serine-type endopeptidase inhibitor activity | 11.36 | 8.71 × 10−5 |
| GOTERM_MF_DIRECT | GO:0005200~structural constituent of cytoskeleton | 11.36 | 9.35 × 10−5 |
| GOTERM_MF_DIRECT | GO:0005102~receptor binding | 11.36 | 0.01 |
| A vs. C | |||
| Category | Term | % | pValue |
| GOTERM_MF_DIRECT | GO:0005515~protein binding | 82.35 | 0.05 |
| GOTERM_CC_DIRECT | GO:0070062~extracellular exosome | 79.41 | 1.66 × 10−19 |
| GOTERM_CC_DIRECT | GO:0005576~extracellular region | 64.71 | 3.02 × 10−13 |
| GOTERM_CC_DIRECT | GO:0005615~extracellular space | 61.76 | 4.64 × 10−13 |
| GOTERM_CC_DIRECT | GO:0072562~blood microparticle | 44.12 | 3.59 × 10−22 |
| GOTERM_BP_DIRECT | GO:0006953~acute-phase response | 14.71 | 5.50 × 10−7 |
| GOTERM_BP_DIRECT | GO:0006958~complement activation, classical pathway | 14.71 | 8.13 × 10−7 |
| GOTERM_CC_DIRECT | GO:0031093~platelet alpha granule lumen | 14.71 | 4.20 × 10−6 |
| GOTERM_MF_DIRECT | GO:0004867~serine-type endopeptidase inhibitor activity | 14.71 | 3.64 × 10−5 |
| GOTERM_MF_DIRECT | GO:0005102~receptor binding | 14.71 | 4.16 × 10−3 |
| GOTERM_BP_DIRECT | GO:0050853~B cell receptor signaling pathway | 11.76 | 1.27 × 10−4 |
| GOTERM_BP_DIRECT | GO:0019731~antibacterial humoral response | 11.76 | 1.86 × 10−4 |
| GOTERM_BP_DIRECT | GO:0002250~adaptive immune response | 11.76 | 0.04 |
| GOTERM_CC_DIRECT | GO:0042571~immunoglobulin complex, circulating | 11.76 | 3.16 × 10−7 |
| GOTERM_CC_DIRECT | GO:0071735~IgG immunoglobulin complex | 11.76 | 1.07 × 10−6 |
| GOTERM_CC_DIRECT | GO:0035578~azurophil granule lumen | 11.76 | 4.18 × 10−4 |
| GOTERM_CC_DIRECT | GO:0034774~secretory granule lumen | 11.76 | 8.49 × 10−4 |
| GOTERM_CC_DIRECT | GO:0005788~endoplasmic reticulum lumen | 11.76 | 0.01 |
| GOTERM_MF_DIRECT | GO:0034987~immunoglobulin receptor binding | 11.76 | 2.18 × 10−6 |
| GOTERM_MF_DIRECT | GO:0048306~calcium-dependent protein binding | 11.76 | 3.92 × 10−4 |
| GOTERM_MF_DIRECT | GO:0003823~antigen binding | 11.76 | 1.89 × 10−3 |
| I1 vs. II1 | |||
| Category | Term | % | pValue |
| GOTERM_MF_DIRECT | GO:0005515~protein binding | 85.71 | 3.8 × 10−3 |
| GOTERM_CC_DIRECT | GO:0005829~cytosol | 69.39 | 1.86 × 10−9 |
| GOTERM_CC_DIRECT | GO:0070062~extracellular exosome | 65.31 | 7.76 × 10−19 |
| GOTERM_CC_DIRECT | GO:0005737~cytoplasm | 44.9 | 0.01 |
| GOTERM_CC_DIRECT | GO:0005634~nucleus | 42.86 | 0.04 |
| GOTERM_CC_DIRECT | GO:0005615~extracellular space | 34.69 | 4.31 × 10−6 |
| GOTERM_CC_DIRECT | GO:0005576~extracellular region | 22.45 | 0.03 |
| GOTERM_MF_DIRECT | GO:0042802~identical protein binding | 20.41 | 0.03 |
| I1 vs. III1-2 | |||
| Category | Term | % | pValue |
| GOTERM_CC_DIRECT | GO:0070062~extracellular exosome | 84.14 | 3.53 × 10−147 |
| GOTERM_MF_DIRECT | GO:0005515~protein binding | 81.06 | 5 × 10−7 |
| GOTERM_CC_DIRECT | GO:0005829~cytosol | 62.11 | 8.42 × 10−29 |
| GOTERM_CC_DIRECT | GO:0005737~cytoplasm | 52.42 | 1.62 × 10−15 |
| GOTERM_CC_DIRECT | GO:0005576~extracellular region | 48.02 | 3.61 × 10−46 |
| GOTERM_CC_DIRECT | GO:0005615~extracellular space | 42.29 | 3.19 × 10−39 |
| GOTERM_CC_DIRECT | GO:0005634~nucleus | 40.53 | 2.25 × 10−4 |
| GOTERM_CC_DIRECT | GO:0005886~plasma membrane | 33.92 | 7.41 × 10−3 |
| GOTERM_MF_DIRECT | GO:0042802~identical protein binding | 22.47 | 2.08 × 10−9 |
| GOTERM_CC_DIRECT | GO:0072562~blood microparticle | 19.82 | 1.74 × 10−51 |
| GOTERM_MF_DIRECT | GO:0003723~RNA binding | 16.74 | 1 × 10−5 |
| GOTERM_MF_DIRECT | GO:0005509~calcium ion binding | 14.10 | 1.11 × 10−9 |
| GOTERM_CC_DIRECT | GO:0005925~focal adhesion | 13.66 | 6.46 × 10−16 |
| GOTERM_CC_DIRECT | GO:0034774~secretory granule lumen | 12.78 | 5.95 × 10−30 |
| GOTERM_CC_DIRECT | GO:0005788~endoplasmic reticulum lumen | 11.45 | 7.87 × 10−15 |
| GOTERM_CC_DIRECT | GO:0005739~mitochondrion | 11.45 | 0.01 |
| GOTERM_CC_DIRECT | GO:1904813~ficolin-1-rich granule lumen | 10.57 | 7.23 × 10−22 |
| GOTERM_BP_DIRECT | GO:0007165~signal transduction | 10.13 | 0.03 |
| GOTERM_CC_DIRECT | GO:0009986~cell surface | 10.13 | 2.62 × 10−6 |
| GOTERM_MF_DIRECT | GO:0045296~cadherin binding | 10.13 | 3.46 × 10−11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galozzi, P.; Bindoli, S.; Baggio, C.; Battisti, I.; Leonardi, A.; Basso, D.; Arrigoni, G.; Sfriso, P. Proteomic Profiling of Tears in Blau Syndrome Patients in Identification of Potential Disease Biomarkers. Int. J. Mol. Sci. 2024, 25, 8387. https://doi.org/10.3390/ijms25158387
Galozzi P, Bindoli S, Baggio C, Battisti I, Leonardi A, Basso D, Arrigoni G, Sfriso P. Proteomic Profiling of Tears in Blau Syndrome Patients in Identification of Potential Disease Biomarkers. International Journal of Molecular Sciences. 2024; 25(15):8387. https://doi.org/10.3390/ijms25158387
Chicago/Turabian StyleGalozzi, Paola, Sara Bindoli, Chiara Baggio, Ilaria Battisti, Andrea Leonardi, Daniela Basso, Giorgio Arrigoni, and Paolo Sfriso. 2024. "Proteomic Profiling of Tears in Blau Syndrome Patients in Identification of Potential Disease Biomarkers" International Journal of Molecular Sciences 25, no. 15: 8387. https://doi.org/10.3390/ijms25158387
APA StyleGalozzi, P., Bindoli, S., Baggio, C., Battisti, I., Leonardi, A., Basso, D., Arrigoni, G., & Sfriso, P. (2024). Proteomic Profiling of Tears in Blau Syndrome Patients in Identification of Potential Disease Biomarkers. International Journal of Molecular Sciences, 25(15), 8387. https://doi.org/10.3390/ijms25158387








