Antioxidant Defense in the Toughest Animals on the Earth: Its Contribution to the Extreme Resistance of Tardigrades
Abstract
:1. Introduction: Tardigrades, the Heroes
2. Resistance of Tardigrades to UV and Ionizing Radiation
3. Tardigrades in Space
4. What Makes Tardigrades So Tough?
4.1. Trehalose
4.2. Heat Shock Proteins
4.3. Late Embryogenesis-Abundant (LEA) Proteins
4.4. Tardigrade-Unique Proteins
4.5. DNA Repair Proteins
4.6. Damage Suppressor Protein (Dsup)
4.7. TDR1 Protein
4.8. Stress-Signaling Pathways
4.9. Other Mechanisms
4.10. Horizontal Gene Transfer
5. Role of Oxidative Stress and Antioxidants
5.1. Oxidative Stress Accompanies Environmental Stress
5.2. Oxidative Stress Can Be Necessary for Cryptobiotic Survival
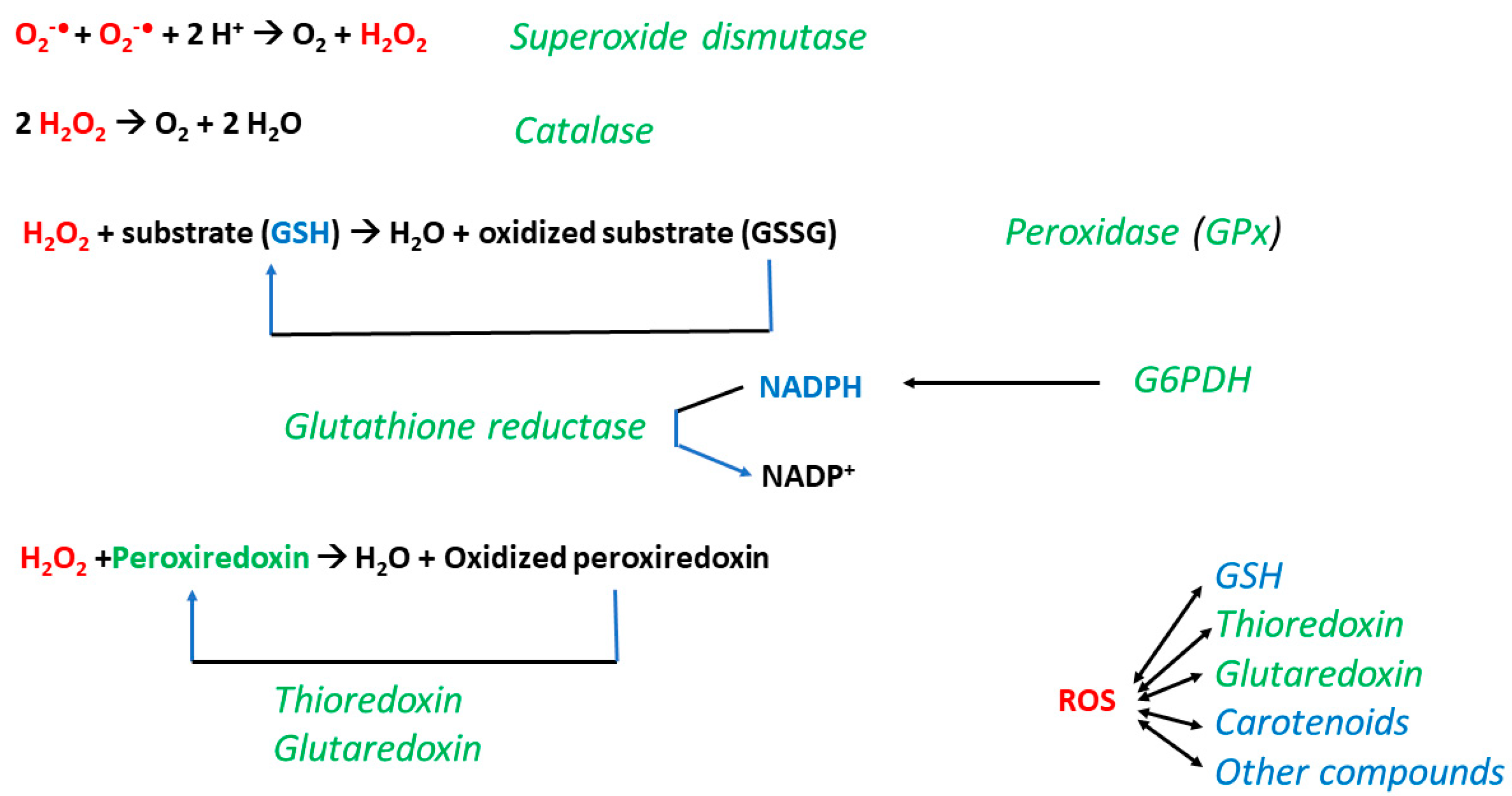
5.3. Antioxidant Defense of Tardigrades
5.4. Induction of Antioxidant Defense by Environmental Factors
6. Discussion: What Can We Learn from Tardigrades?
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hashimoto, T.; Kunieda, T. DNA protection protein, a novel mechanism of radiation tolerance: Lessons from tardigrades. Life 2017, 7, 26. [Google Scholar] [CrossRef]
- Erdmann, W.; Kaczmarek, Ł. Tardigrades in space research-past and future. Orig. Life Evol. Biosph. 2017, 47, 545–553. [Google Scholar]
- Jezierska, M.; Miernik, A.; Sojka, J.; Student, S.; Śliwińska, M.A.; Gross, V.; Poprawa, I. Oogenesis in the tardigrade Hypsibius exemplaris Gąsiorek, Stec, Morek & Michalczyk, 2018 (Eutardigrada, Hypsibiidae). Micron 2021, 150, 103126. [Google Scholar]
- Guidetti, R.; Rizzo, A.M.; Altiero, T.; Rebecchi, L. What can we learn from the toughest animals of the Earth? Water bears (tardigrades) as multicellular model organisms in order to perform scientific preparations for lunar exploration. Planet. Space Sci. 2012, 74, 97–102. [Google Scholar] [CrossRef]
- Jørgensen, A.; Kristensen, R.M.; Møbjerg, N. Phylogeny and integrative taxonomy of Tardigrada. In Water Bears: The Biology of Tardigrades; Schill, R.O., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 95–114. [Google Scholar]
- Schmidt-Rhaesa, A. Tardigrades—Are they really miniaturized dwarfs? Zool. Anz. 2001, 240, 549–555. [Google Scholar] [CrossRef]
- Gross, V.; Treffkorn, S.; Reichelt, J.; Epple, L.; Lüter, C.; Mayer, G. Miniaturization of tardigrades (water bears): Morphological and genomic perspectives. Arthropod Struct. Dev. 2019, 48, 12–19. [Google Scholar] [CrossRef]
- Cooper, K.W. The first fossil tardigrade: Beorn leggi Cooper, from Cretaceous amber. Psyche J. Entomol. 1964, 71, 41–48. [Google Scholar] [CrossRef]
- Guidetti, R.; Bertolani, R. Paleontology and molecular dating. In Water Bears: The Biology of Tardigrades; Schill, R.O., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 131–143. [Google Scholar]
- Yoshida, Y.; Koutsovoulos, G.; Laetsch, D.R.; Stevens, L.; Kumar, S.; Horikawa, D.D.; Ishino, K.; Komine, S.; Kunieda, T.; Tomita, M.; et al. Comparative genomics of the tardigrades Hypsibius dujardini and Ramazzottius varieornatus. PLoS Biol. 2017, 15, e2002266. [Google Scholar] [CrossRef]
- Fleming, J.F.; Arakawa, K. Systematics of tardigrada: A reanalysis of tardigrade taxonomy with specific reference to Guil et al. (2019). Zool. Scr. 2021, 50, 376–382. [Google Scholar] [CrossRef]
- Grothman, G.T.; Johansson, C.; Chilton, G.; Kagoshima, H.; Tsujimoto, M.; Suzuki, A.C. Gilbert Rahm and the status of Mesotardigrada Rahm, 1937. Zool. Sci. 2017, 34, 5–10. [Google Scholar] [CrossRef]
- Degma, P.; Gábrišová, N. Limno-terrestrial Tardigrada of Sub-antarctic islands—An annotated review. Diversity 2023, 15, 1109. [Google Scholar] [CrossRef]
- Guil, N.; Machordom, A.; Guidetti, R. High level of phenotypic homoplasy amongst eutardigrades (Tardigrada) based on morphological and total evidence phylogenetic analyses. Zool. J. Linn. Soc. 2013, 169, 1–26. [Google Scholar] [CrossRef]
- Kamilari, M.; Jørgensen, A.; Schiøtt, M.; Møbjerg, N. Comparative transcriptomics suggest unique molecular adaptations within tardigrade lineages. BMC Genom. 2019, 20, 607. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, R.; Bertolani, R. Tardigrade taxonomy: An updated check list of the taxa and a list of characters for their identification. Zootaxa 2005, 845, 1–46. [Google Scholar] [CrossRef]
- Degma, P.; Guidetti, R. Notes to the current checklist of Tardigrada. Zootaxa 2007, 1579, 41–53. [Google Scholar] [CrossRef]
- Vicente, F.; Bertolani, R. Considerations on the taxonomy of the Phylum Tardigrada. Zootaxa 2013, 3626, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Rebecchi, L.; Altiero, T.; Guidetti, R. Anhydrobiosis: The extreme limit of desiccation tolerance. Invertebr. Surviv. J. 2007, 4, 65–81. [Google Scholar]
- Ito, M.; Saigo, T.; Abe, W.; Kubo, T.; Kunieda, T. Establishment of an isogenic strain of the desiccation-sensitive tardigrade Isohypsibius myrops (Parachela, Eutardigrada) and its life history traits. Zool. J. Linn. Soc. 2016, 178, 863–870. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Imura, S.; Kanda, H. Recovery and reproduction of an Antarctic tardigrade retrieved from a moss sample frozen for over 30 years. Cryobiology 2016, 72, 78–81. [Google Scholar] [CrossRef]
- Alpert, P. Constraints of tolerance: Why are desiccation-tolerant organisms so small or rare? J. Exp. Biol. 2006, 209, 1575–1584. [Google Scholar] [CrossRef]
- Farrant, J.M.; Cooper, K.; Nell, H. Desiccation tolerance. Plant Stress Physiol. 2012, 238, 265. [Google Scholar]
- Pigon, A.; Weglarska, B. Rate of metabolism in tardigrades during active life and anabiosis. Nature 1955, 176, 121–122. [Google Scholar] [CrossRef]
- Keilin, D. The problem of anabiosis or latent life: History and current concept. Proc. R. Soc. Lond. 1959, B150, 149–191. [Google Scholar]
- Ramløv, H.; Westh, P. Cryptobiosis in the eutardigrade Adorybiotus (Richtersius) coronifer: Tolerance to alcohols, temperature and de novo protein synthesis. Zool. Anz. 2001, 240, 517–523. [Google Scholar] [CrossRef]
- Wilanowska, P.A.; Rzymski, P.; Kaczmarek, Ł. Long-term survivability of tardigrade Paramacrobiotus experimentalis (Eutardigrada) at increased magnesium perchlorate levels: Implications for astrobiological research. Life 2024, 14, 335. [Google Scholar] [CrossRef]
- Kinchin, I. Tardigrades and anhydrobiosis: Water bears and water loss. Biochemist 2008, 30, 18–20. [Google Scholar] [CrossRef]
- Wełnicz, W.; Grohme, M.A.; Kaczmarek, L.; Schill, R.O.; Frohme, M. Anhydrobiosis in tardigrades--the last decade. J. Insect Physiol. 2011, 57, 577–583. [Google Scholar] [CrossRef]
- Guidetti, R.; Altiero, T.; Rebecchi, L. On dormancy strategies in tardigrades. J. Insect Physiol. 2011, 57, 567–576. [Google Scholar] [CrossRef]
- Richaud, M.; Le Goff, E.; Cazevielle, C.; Ono, F.; Mori, Y.; Saini, N.L.; Cuq, P.; Baghdiguian, S.; Godefroy, N.; Galas, S. Ultrastructural analysis of the dehydrated tardigrade Hypsibius exemplaris unveils an anhydrobiotic-specific architecture. Sci. Rep. 2020, 10, 4324. [Google Scholar] [CrossRef]
- Møbjerg, N.; Neves, R.C. New insights into survival strategies of tardigrades. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 254, 110890. [Google Scholar] [CrossRef]
- Smythers, A.L.; Joseph, K.M.; O’Dell, H.M.; Clark, T.A.; Crislip, J.R.; Flinn, B.B.; Daughtridge, M.H.; Stair, E.R.; Mubarek, S.N.; Lewis, H.C.; et al. Chemobiosis reveals tardigrade tun formation is dependent on reversible cysteine oxidation. PLoS ONE 2024, 19, e0295062. [Google Scholar] [CrossRef]
- Møbjerg, N.; Halberg, K.A.; Jørgensen, A.; Persson, D.; Bjørn, M.; Ramløv, H.; Kristensen, R.M. Survival in extreme environments–on the current knowledge of adaptations in tardigrades. Acta Physiol. 2011, 202, 409–420. [Google Scholar] [CrossRef]
- Kaczmarek, Ł.; Roszkowska, M.; Fontaneto, D.; Jezierska, M.; Pietrzak, B.; Wieczorek, R.; Poprawa, I.; Kosicki, J.Z.; Karachitos, A.; Kmita, H. Staying young and fit? Ontogenetic and phylogenetic consequences of animal anhydrobiosis. J. Zool. 2019, 309, 1–11. [Google Scholar] [CrossRef]
- Hengherr, S.; Brümmer, F.; Schill, R.O. Anhydrobiosis in tardigrades and its effects on longevity traits. J. Zool. 2008, 275, 216–220. [Google Scholar] [CrossRef]
- Roszkowska, M.; Gołdyn, B.; Wojciechowska, D.; Księżkiewicz, Z.; Fiałkowska, E.; Pluskota, M.; Kmita, H.; Kaczmarek, Ł. How long can tardigrades survive in the anhydrobiotic state? A search for tardigrade anhydrobiosis patterns. PLoS ONE 2023, 18, e0270386. [Google Scholar] [CrossRef]
- Watanabe, M.; Nakahara, Y.; Sakashita, T.; Kikawada, T.; Fujita, A.; Hamada, N.; Horikawa, D.D.; Wada, S.; Kobayashi, Y.; Okuda, T. Physiological changes leading to anhydrobiosis improve radiation tolerance in Polypedilum vanderplanki larvae. J. Insect Physiol. 2007, 53, 573–579. [Google Scholar] [CrossRef]
- Billi, D.; Potts, M. Life and death of dried prokaryotes. Res. Microbiol. 2002, 153, 7–12. [Google Scholar] [CrossRef]
- Guidetti, R.; Jönsson, K.I. Long-term anhydrobiotic survival in semi-terrestrial micrometazoans. J. Zool. 2002, 257, 181–187. [Google Scholar] [CrossRef]
- Copley, J. Indestructible. New Sci. 1999, 164, 45–46. [Google Scholar]
- Franceschi, T. Anabiosi nei tardigradi. Boll. Mus. Ist. Biol. Univ. Genova 1948, 22, 47–49. [Google Scholar]
- Doyère, P.L.N. Memoires sur les tardigrade. Sur le facilité possedent les tardigrades, les rotifers, les anguillules des toit et quelques autres animacules, de revenir à la vie après été completement déssechées. Ann. Sci. Nat. 1842, 18, 5. [Google Scholar]
- Rahm, P.G. Biologische und physiologische Beiträge zur Kenntnis der Moosfauna. Z. Allgem. Physiol. 1921, 20, 1–35. [Google Scholar]
- Neves, R.C.; Hvidepil, L.K.; Sørensen-Hygum, T.L.; Stuart, R.M.; Møbjerg, N. Thermotolerance experiments on active and desiccated states of Ramazzottius varieornatus emphasize that tardigrades are sensitive to high temperatures. Sci. Rep. 2020, 10, 94. [Google Scholar] [CrossRef]
- Horikawa, D.D.; Kunieda, T.; Abe, W.; Watanabe, M.; Nakahara, Y.; Yukuhiro, F.; Sakashita, T.; Hamada, N. Establishment of a rearing system of the extremotolerant tardigrade Ramazzottius varieornatus: A new model animal for astrobiology. Astrobiology 2008, 8, 549–556. [Google Scholar] [CrossRef]
- Horikawa, D.D. Survival of tardigrades in extreme environments: A model animal for astrobiology. In Anoxia: Evidence for Eukaryote Survival and Paleontological Strategies; Altenbach, A.V., Bernhard, J.M., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 205–217. [Google Scholar]
- Guidetti, R.; Altiero, T.; Bertolani, R.; Grazioso, P.; Rebecchi, L. Survival of freezing by hydrated tardigrades inhabiting terrestrial and freshwater habitats. Zoology 2011, 114, 123–128. [Google Scholar] [CrossRef]
- Rahm, P.G. Weitere physiologische Versuche mit niederen Temperaturen. Ein Beitrag zur Lösung des Kalteproblems. Verh. Dtsch. Zool. Ges. 1924, 29, 106–111. [Google Scholar]
- Sømme, L.; Meier, T. Cold tolerance in Tardigrada from Dronning Maud Land, Antarctica. Polar Biol. 1995, 15, 221–224. [Google Scholar] [CrossRef]
- Becquerel, P. La suspension de la vie au dessous de 1/20 K absolu par demagnetization adiabatique de l’alun de fer dans le vide les plus eléve. CR Hebd. SÉAnce. Acad. Sci. Paris. 1950, 231, 26. [Google Scholar]
- Jönsson, K.I.; Guidetti, R. Effects of methyl bromide fumigation on anhydrobiotic micrometazoans. Ecotox. Environ. Saf. 2001, 50, 72–75. [Google Scholar] [CrossRef]
- Jönsson, K.I.; Rabbow, E.; Schill, R.O.; Harms-Ringdahl, M.; Rettberg, P. Tardigrades survive exposure to space in low Earth orbit. Curr. Biol. 2008, 18, R729–R731. [Google Scholar] [CrossRef]
- Horikawa, D.D.; Iwata, K.I.; Kawai, K.; Koseki, S.; Okuda, T.; Yamamoto, K. High hydrostatic pressure tolerance of four different anhydrobiotic animal species. Zool. Sci. 2009, 26, 238–242. [Google Scholar] [CrossRef]
- Ono, F.; Mori, Y.; Sougawa, M.; Takarabe, K.; Hada, Y.; Nishihira, N.; Motose, H.; Saigusa, M.; Matsushima, Y.; Yamazaki, D.; et al. Effect of very high pressure on life of plants and animals. J. Phys. Conf. Ser. 2012, 377, 012053. [Google Scholar] [CrossRef]
- Traspas, A.; Burchell, M.J. Tardigrade survival limits in high-speed impacts—Implications for panspermia and collection of samples from plumes emitted by ice worlds. Astrobiology 2021, 21, 845–852. [Google Scholar] [CrossRef]
- Ono, F.; Saigusa, M.; Uozumi, T.; Matsushima, Y.; Ikeda, H.; Saini, N.L.; Yamashita, M. Effect of high hydrostatic pressure on to life of the tiny animal tardigrade. J. Phys. Chem. Solids 2008, 69, 2297–2300. [Google Scholar] [CrossRef]
- Altiero, T.; Guidetti, R.; Caselli, V.; Cesari, M.; Rebecchi, L. Ultraviolet radiation tolerance in hydrated and desiccated eutardigrades. J. Zool. Syst. Evol. Res. 2011, 49, 104–110. [Google Scholar] [CrossRef]
- Horikawa, D.D.; Cumbers, J.; Sakakibara, I.; Rogoff, D.; Leuko, S.; Harnoto, R.; Arakawa, K.; Katayama, T.; Kunieda, T.; Toyoda, A.; et al. Analysis of DNA repair and protection in the Tardigrade Ramazzottius varieornatus and Hypsibius dujardini after exposure to UVC radiation. PLoS ONE 2013, 8, e64793. [Google Scholar] [CrossRef]
- Jönsson, K.I. Radiation tolerance in tardigrades: Current knowledge and potential applications in medicine. Cancers 2019, 11, 1333. [Google Scholar] [CrossRef]
- Jönsson, K.I.; Hygum, T.L.; Andersen, K.N.; Clausen, L.K.; Møbjerg, N. Tolerance to gamma radiation in the marine heterotardigrade, Echiniscoides sigismundi. PLoS ONE 2016, 11, e0168884. [Google Scholar] [CrossRef]
- Beltrán-Pardo, E.; Jönsson, K.I.; Harms-Ringdahl, M.; Haghdoost, S.; Wojcik, A. Tolerance to gamma radiation in the tardigrade Hypsibius dujardini from embryo to adult correlate inversely with cellular proliferation. PLoS ONE 2015, 10, e0133658. [Google Scholar]
- May, R. Action différentielle des rayons x et ultraviolets sur le tardigrade Macrobiotus areolatus, a l’état actif et desséché. Bull. Biol. Fr. Belg. 1964, 98, 349–367. [Google Scholar]
- Horikawa, D.D.; Sakashita, T.; Katagiri, C.; Watanabe, M.; Kikawada, T.; Nakahara, Y.; Hamada, N.; Wada, S.; Funayama, T.; Higashi, S.; et al. Radiation tolerance in the tardigrade Milnesium tardigradum. Int. J. Radiat. Biol. 2006, 82, 843–848. [Google Scholar] [CrossRef]
- Jönsson, K.I.; Harms-Ringdahl, M.; Torudd, J. Radiation tolerance in the eutardigrade Richtersius coronifer. Int. J. Radiat. Biol. 2005, 81, 649–656. [Google Scholar] [CrossRef]
- Jönsson, K.I.; Wojcik, A. Tolerance to X-rays and heavy ions (Fe, He) in the tardigrade Richtersius coronifer and the bdelloid rotifer Mniobia russeola. Astrobiology 2017, 17, 163–167. [Google Scholar] [CrossRef]
- Jönsson, I.; Beltran-Pardo, E.; Haghdoost, S.; Wojcik, A.; Bermúdez-Cruz, R.M.; Bernal Villegas, J.E.; Harms-Ringdahl, M. Tolerance to gamma-irradiation in eggs of the tardigrade Richtersius coronifer depends on stage of development. J. Limnol. 2013, 72, 73–79. [Google Scholar] [CrossRef]
- Nilsson, E.J.; Jönsson, K.I.; Pallon, J. Tolerance to proton irradiation in the eutardigrade Richtersius coronifer—A nuclear microprobe study. Int. J. Radiat. Biol. 2010, 6, 420–427. [Google Scholar] [CrossRef]
- Hirayama, R.; Ito, A.; Tomita, M.; Tsukada, T.; Yatagai, F.; Noguchi, M.; Matsumoto, Y.; Kase, Y.; Ando, K.; Okayasu, R.; et al. Contributions of direct and indirect actions in cell killing by high-LET radiations. Radiat. Res. 2009, 171, 212–218. [Google Scholar] [CrossRef]
- Yoshida, Y.; Satoh, T.; Ota, C.; Tanaka, S.; Horikawa, D.D.; Tomita, M.; Kato, K.; Arakawa, K. Time-series transcriptomic screening of factors contributing to the cross-tolerance to UV radiation and anhydrobiosis in tardigrades. BMC Genom. 2022, 23, 405. [Google Scholar] [CrossRef]
- Beltrán-Pardo, E.; Jönsson, K.I.; Wojcik, A.; Haghdoost, S.; Harms-Ringdahl, M.; Bermúdez-Cruz, R.M.; Bernal Villegas, J.E. Effects of ionizing radiation on embryos of the tardigrade Milnesium cf. tardigradum at different stages of development. PLoS ONE 2013, 8, e72098. [Google Scholar]
- Jönsson, K.I.; Levine, E.B.; Wojcik, A.; Haghdoost, S.; Harms-Ringdahl, M. Environmental adaptations: Radiation tolerance. In Water Bears: The Biology of Tardigrades; Schill, R.O., Ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 311–330. [Google Scholar]
- Rebecchi, L.; Altiero, T.; Guidetti, R.; Cesari, M.; Bertolani, R.; Negroni, M.; Rizzo, A.M. Tardigrade resistance to space effects: First results of experiments on the LIFE-TARSE mission on FOTON-M3 (September 2007). Astrobiology 2009, 9, 581–591. [Google Scholar] [CrossRef]
- Rebecchi, L.; Altiero, T.; Cesari, M.; Bertolani, R.; Rizzo, A.M.; Corsetto, P.A.; Guidetti, R. Resistance of the anhydrobiotic eutardigrade Paramacrobiotus richtersi to space flight (LIFE–TARSE mission on FOTON-M3). J. Zoolog. Syst. Evol. Res. 2011, 49, 98–103. [Google Scholar] [CrossRef]
- Jönsson, K.I.; Schill, R.O. Induction of Hsp70 by desiccation, ionising radiation and heat-shock in the eutardigrade Richtersius coronifer. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, 456–460. [Google Scholar] [CrossRef]
- Persson, D.; Halberg, K.A.; Jørgensen, A.; Ricci, C.; Møbjerg, N.; Kristensen, R.M. Extreme stress tolerance in tardigrades: Surviving space conditions in low earth orbit. J. Zool. Syst. Evol. Res. 2011, 49, 90–97. [Google Scholar] [CrossRef]
- Vukich, M.; Ganga, P.L.; Cavalieri, D.; Rivero, D.; Pollastri, S.; Mugnai, S.; Mancuso, S.; Pastorelli, S.; Lambreva, M.; Antonacci, A.; et al. BIOKIS: A model payload for multisciplinary experiments in microgravity. Microgravity Sci. Technol. 2012, 24, 397–409. [Google Scholar] [CrossRef]
- Johnson, A.P.; Pratt, L.M.; Vishnivetskaya, T.; Pfiffner, S.; Bryan, R.A.; Dadachova, E.; White, L.; Radtke, K.; Chan, E.; Tronnick, S.; et al. Extended survival of several microorganisms and relevant amino acid and biomarkers under simulated Martian surface conditions as a function of burial depth. Icarus 2011, 211, 1162–1178. [Google Scholar] [CrossRef]
- França, M.B.; Panek, A.D.; Eleutherio, E.C.A. Oxidative stress and its effects during dehydration. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2007, 146, 621–631. [Google Scholar] [CrossRef]
- Crowe, J.H. Trehalose as a “chemical chaperone”: Fact and fantasy. Adv. Exp. Med. Biol. 2007, 594, 143–158. [Google Scholar]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M. The role of vitrification in anhydrobiosis. Annu. Rev. Physiol. 1998, 60, 73–103. [Google Scholar] [CrossRef]
- Hengherr, S.; Heyer, A.G.; Köhler, H.R.; Schill, R.O. Trehalose and anhydrobiosis in tardigrades-evidence for divergence in responses to dehydration. FEBS J. 2008, 275, 281–288. [Google Scholar] [CrossRef]
- Jönsson, K.I.; Persson, O. Trehalose in three species of desiccation tolerant tardigrades. Open Zool. J. 2010, 3, 1–5. [Google Scholar] [CrossRef]
- Erkut, C.; Penkov, S.; Khesbak, H.; Vorkel, D.; Verbavatz, J.M.; Fahmy, K.; Kurzchalia, T.V. Trehalose renders the dauer larva of Caenorhabditis elegans resistant to extreme desiccation. Curr. Biol. 2011, 21, 1331–1336. [Google Scholar] [CrossRef]
- Tapia, H.; Koshland, D.E. Trehalose is a versatile and long-lived chaperone for desiccation tolerance. Curr. Biol. 2014, 24, 2758–2766. [Google Scholar] [CrossRef]
- Cesari, M.; Altiero, T.; Rebecchi, L. Identification of the trehalose-6-phosphate synthase (tps) gene in desiccation tolerant and intolerant tardigrades. Ital. J. Zool. 2012, 79, 530–540. [Google Scholar] [CrossRef]
- Boothby, T.C.; Tapia, H.; Brozena, A.H.; Piszkiewicz, S.; Smith, A.E.; Giovannini, I.; Rebecchi, L.; Pielak, G.J.; Koshland, D.; Goldstein, B. Tardigrades use intrinsically disordered proteins to survive desiccation. Mol. Cell 2017, 65, 975–984. [Google Scholar] [CrossRef]
- Reuner, A.; Hengherr, S.; Mali, B.; Förster, F.; Arndt, D.; Reinhardt, R.; Dandekar, T.; Frohme, M.; Brümmer, F.; Schill, R.O. Stress response in tardigrades: Differential gene expression of molecular chaperones. Cell Stress Chaperones 2010, 15, 423–430. [Google Scholar] [CrossRef]
- Tanaka, S.; Tanaka, J.; Miwa, Y.; Horikawa, D.D.; Katayama, T.; Arakawa, K.; Toyoda, A.; Kubo, T.; Kunieda, T. Novel mitochondria-targeted heat-soluble proteins identified in the anhydrobiotic Tardigrade improve osmotic tolerance of human cells. PLoS ONE 2015, 10, e0118272. [Google Scholar] [CrossRef]
- Al-Ansari, M.; Fitzsimons, T.; Wei, W.; Goldberg, M.W.; Kunieda, T.; Quinlan, R.A. The major inducible small heat shock protein HSP20-3 in the tardigrade Ramazzottius varieornatus forms filament-like structures and is an active chaperone. Cell Stress Chaperones 2024, 29, 51–65. [Google Scholar] [CrossRef]
- Hibshman, J.D.; Carra, S.; Goldstein, B. Tardigrade small heat shock proteins can limit desiccation-induced protein aggregation. Commun. Biol. 2023, 6, 121. [Google Scholar] [CrossRef]
- Wise, M.J.; Tunnacliffe, A. POPP Quest: What Do LEA Proteins Do? Trends Plant Sci. 2004, 9, 13–17. [Google Scholar] [CrossRef]
- Goyal, K.; Walton, L.J.; Tunnacliffe, A. LEA proteins prevent protein aggregation due to water stress. Biochem. J. 2005, 388, 151–157. [Google Scholar] [CrossRef]
- Liu, Y.; Chakrabortee, S.; Li, R.; Zheng, Y.; Tunnacliffe, A. Both plant and animal LEA proteins act as kinetic stabilisers of polyglutamine-dependent protein aggregation. FEBS Lett. 2011, 585, 630–634. [Google Scholar] [CrossRef]
- Hatanaka, R.; Hagiwara-Komoda, Y.; Furuki, T.; Kanamori, Y.; Fujita, M.; Cornette, R.; Sakurai, M.; Okuda, T.; Kikawada, T. An abundant LEA protein in the anhydrobiotic midge, PvLEA4, acts as a molecular shield by limiting growth of aggregating protein particles. Insect Biochem. Mol. Biol. 2013, 43, 1055–1067. [Google Scholar] [CrossRef]
- Chakrabortee, S.; Tripathi, R.; Watson, M.; Schierle, G.S.; Kurniawan, D.P.; Kaminski, C.F.; Wise, M.J.; Tunnacliffe, A. Intrinsically disordered proteins as molecular shields. Mol. Biosyst. 2012, 8, 210–219. [Google Scholar] [CrossRef]
- Hesgrove, C.; Boothby, T.C. The biology of tardigrade disordered proteins in extreme stress tolerance. Cell Commun. Signal. 2020, 18, 1–15. [Google Scholar] [CrossRef]
- Arakawa, K.; Numata, K. Reconsidering the “glass transition” hypothesis of intrinsically unstructured CAHS proteins in desiccation tolerance of tardigrades. Mol. Cell 2021, 81, 409–410. [Google Scholar] [CrossRef]
- Nguyen, K.; Kc, S.; Gonzalez, T.; Tapia, H.; Boothby, T.C. Trehalose and tardigrade CAHS proteins work synergistically to promote desiccation tolerance. Commun. Biol. 2022, 5, 1046. [Google Scholar] [CrossRef]
- Lim, S.; Reilly, C.B.; Barghouti, Z.; Marelli, B.; Way, J.C.; Silver, P.A. Tardigrade secretory proteins protect biological structures from desiccation. Commun. Biol. 2024, 7, 633. [Google Scholar] [CrossRef]
- Hygum, T.L.; Clausen, L.K.; Halberg, K.A.; Jørgensen, A.; Møbjerg, N. Tun formation is not a prerequisite for desiccation tolerance in the marine tidal tardigrade Echiniscoides sigismundi. Zool. J. Linn. Soc. 2016, 178, 907–911. [Google Scholar] [CrossRef]
- Sørensen-Hygum, T.L.; Stuart, R.M.; Jørgensen, A.; Møbjerg, N. Modelling extreme desiccation tolerance in a marine tardigrade. Sci. Rep. 2018, 8, 11495. [Google Scholar] [CrossRef]
- Anoud, M.; Delagoutte, E.; Helleu, Q.; Brion, A.; Duvernois-Berthet, E.; As, M.; Marques, X.; Lamribet, K.; Senamaud, C.; Jourdren, L.; et al. Comparative transcriptomics reveal a novel tardigrade specific DNA binding protein induced in response to ionizing radiation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Clark-Hachtel, C.M.; Hibshman, J.D.; De Buysscher, T.; Stair, E.R.; Hicks, L.M.; Goldstein, B. The tardigrade Hypsibius exemplaris dramatically upregulates DNA repair pathway genes in response to ionizing radiation. Curr. Biol. 2024, 34, 1819–1830.e6. [Google Scholar] [CrossRef]
- Hashimoto, T.; Horikawa, D.D.; Saito, Y.; Kuwahara, H.; Kozuka-Hata, H.; Shin-I, T.; Minakuchi, Y.; Ohishi, K.; Motoyama, A.; Aizu, T.; et al. Extremotolerant tardigrade genome and improved radiotolerance of human cultured cells by tardigrade-unique protein. Nat. Commun. 2016, 7, 12808. [Google Scholar] [CrossRef] [PubMed]
- Kasianchuk, N.; Rzymski, P.; Kaczmarek, Ł. The biomedical potential of tardigrade proteins: A review. Biomed. Pharmacother. 2023, 158, 114063. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Riolo, G.; Marzocchi, C.; Brunetti, J.; Pini, A.; Cantara, S. The tardigrade damage suppressor protein modulates transcription factor and DNA repair genes in human cells treated with hydroxyl radicals and UV-C. Biology 2021, 10, 970. [Google Scholar] [CrossRef] [PubMed]
- Kirke, J.; Jin, X.L.; Zhang, X.H. Expression of a tardigrade dsup gene enhances genome protection in plants. Mol. Biotechnol. 2020, 62, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Murai, Y.; Yagi-Utsumi, M.; Fujiwara, M.; Tanaka, S.; Tomita, M.; Kato, K.; Arakawa, K. Multiomics study of a heterotardigrade, Echinisicus testudo, suggests the possibility of convergent evolution of abundant heat-soluble proteins in Tardigrada. BMC Genom. 2021, 22, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chavez, C.; Cruz-Becerra, G.; Fei, J.; Kassavetis, G.A.; Kadonaga, J.T. The tardigrade damage suppressor protein binds to nucleosomes and protects DNA from hydroxyl radicals. eLife 2019, 8, e47682. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Kubo, T.; Kunieda, T. Suggested Involvement of PP1/PP2A Activity and de novo gene expression in anhydrobiotic survival in a tardigrade, Hypsibius dujardini, by chemical genetic approach. PLoS ONE 2015, 10, e0144803. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Mori, M.; Tomita, M.; Arakawa, K. AMPK activity is required for the induction of anhydrobiosis in a tardigrade Hypsibius exemplaris, and its potential up-regulator is PP2A. Genes. Cells 2019, 24, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Mori, M.; Tomita, M.; Arakawa, K. Pre-treatment with D942, a furancarboxylic acid derivative, increases desiccation tolerance in an anhydrobiotic tardigrade Hypsibius exemplaris. FEBS Open Bio 2020, 10, 1774–1781. [Google Scholar] [CrossRef]
- Halberg, K.A.; Jørgensen, A.; Møbjerg, N. Desiccation tolerance in the tardigrade Richtersius coronifer relies on muscle mediated structural reorganization. PLoS ONE 2013, 8, e85091. [Google Scholar] [CrossRef]
- Boothby, T.C.; Tenlen, J.R.; Smith, F.W.; Wang, J.R.; Patanella, K.A.; Nishimura, E.O.; Tintori, S.C.; Li, Q.; Jones, C.D.; Yandell, M.; et al. Evidence for extensive horizontal gene transfer from the draft genome of a tardigrade. Proc. Natl. Acad. Sci. USA 2015, 112, 15976–15981. [Google Scholar] [CrossRef]
- Koutsovoulos, G.; Kumar, S.; Laetsch, D.R.; Stevens, L.; Daub, J.; Conlon, C.; Maroon, H.; Thomas, F.; Aboobaker, A.A.; Blaxter, M. No evidence for extensive horizontal gene transfer in the genome of the tardigrade Hypsibius dujardini. Proc. Natl. Acad. Sci. USA 2016, 113, 5053–5058. [Google Scholar] [CrossRef]
- Pereira Ede, J.; Panek, A.D.; Eleutherio, E.C. Protection against oxidation during dehydration of yeast. Cell Stress Chaperones 2003, 8, 120–124. [Google Scholar] [CrossRef]
- Potts, M. Desiccation tolerance of prokaryotes. Microbiol. Rev. 1994, 58, 755–805. [Google Scholar] [CrossRef]
- Hussain, H.A.; Hussain, S.; Khaliq, A.; Ashraf, U.; Anjum, S.A.; Men, S.; Wang, L. Chilling and drought stresses in crop plants: Implications, cross talk, and potential management opportunities. Front. Plant Sci. 2018, 9, 393. [Google Scholar] [CrossRef]
- Cruz de Carvalho, R.; Catalá, M.; Marques da Silva, J.; Branquinho, C.; Barreno, E. The impact of dehydration rate on the production and cellular location of reactive oxygen species in an aquatic moss. Ann. Bot. 2012, 110, 1007–1016. [Google Scholar] [CrossRef]
- Joanisse, D.R.; Storey, K.B. Oxidative stress and antioxidants in stress and recovery of cold-hardy insects. Insect Biochem. Mol. Biol. 1998, 28, 23–30. [Google Scholar] [CrossRef]
- Kranner, I.; Birtić, S. A modulating role for antioxidants in desiccation tolerance. Integr. Comp. Biol. 2005, 45, 734–740. [Google Scholar] [CrossRef]
- Sun, W.Q.; Leopold, A.C.; Crowe, L.M.; Crowe, J.H. Stability of dry liposomes in sugar glasses. Biophys. J. 1996, 70, 1769–1776. [Google Scholar] [CrossRef]
- Rebecchi, L. Dry and survive: The role of antioxidant defences in anhydrobiotic organisms. J. Limnol. 2013, 72, 62–72. [Google Scholar] [CrossRef]
- Savic, A.G.; Guidetti, R.; Turi, A.; Pavicevic, A.; Giovannini, I.; Rebecchi, L.; Mojovic, M. Superoxide anion radical production in the tardigrade Paramacrobiotus richtersi, the first Electron Paramagnetic Resonance spin-trapping study. Physiol. Biochem. Zool. 2015, 88, 451–454. [Google Scholar] [CrossRef]
- Giovannini, I.; Boothby, T.C.; Cesari, M.; Goldstein, B.; Guidetti, R.; Rebecchi, L. Production of reactive oxygen species and involvement of bioprotectants during anhydrobiosis in the tardigrade Paramacrobiotus spatialis. Sci. Rep. 2022, 12, 1938. [Google Scholar] [CrossRef]
- Rizzo, A.M.; Negroni, M.; Altiero, T.; Montorfano, G.; Corsetto, P.; Berselli, P.; Berra, B.; Guidetti, R.; Rebecchi, L. Antioxidant defences in hydrated and desiccated states of the tardigrade Paramacrobiotus richtersi. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 156, 115–121. [Google Scholar] [CrossRef]
- Kuzmic, M.; Richaud, M.; Cuq, P.; Frelon, S.; Galas, S. Carbonylation accumulation of the Hypsibius exemplaris anydrobiote reveals age-associated marks. PLoS ONE 2018, 13, e0208617. [Google Scholar] [CrossRef]
- Neumann, S.; Reuner, A.; Brümmer, F.; Schill, R.O. DNA damage in storage cells of anhydrobiotic tardigrades. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 425–429. [Google Scholar] [CrossRef]
- Carrero, D.; Pérez-Silva, J.G.; Quesada, V.; López-Otín, C. Differential mechanisms of tolerance to extreme environmental conditions in tardigrades. Sci. Rep. 2019, 9, 14938. [Google Scholar] [CrossRef]
- Giovannini, I.; Corsetto, P.A.; Altiero, T.; Montorfano, G.; Guidetti, R.; Rizzo, A.M.; Rebecchi, L. Antioxidant response during the kinetics of anhydrobiosis in two eutardigrade species. Life 2022, 12, 817. [Google Scholar] [CrossRef]
- Goldstein, B. The emergence of the tardigrade Hypsibius exemplaris as a model system. Cold Spring Harb. Protoc. 2018, 2018, pdb-emo102301. [Google Scholar] [CrossRef]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Møller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef]
- Schokraie, E.; Hotz-Wagenblatt, A.; Warnken, U.; Mali, B.; Frohme, M.; Förster, F.; Dandekar, T.; Hengherr, S.; Schill, R.O.; Schnölzer, M. Proteomic analysis of tardigrades: Towards a better understanding of molecular mechanisms by anhydrobiotic organisms. PLoS ONE 2010, 5, e9502. [Google Scholar] [CrossRef] [PubMed]
- Schokraie, E.; Warnken, U.; Hotz-Wagenblatt, A.; Grohme, M.A.; Hengherr, S.; Förster, F.; Schill, R.O.; Frohme, M.; Dandekar, T.; Schnölzer, M. Comparative proteome analysis of Milnesium tardigradum in early embryonic state versus adults in active and anhydrobiotic state. PLoS ONE 2012, 7, e45682. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.S.; Inoue, T. Structure of a superoxide dismutase from a tardigrade: Ramazzottius varieornatus strain YOKOZUNA-1. Acta Crystallogr. F Struct. Biol. Commun. 2023, 79, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Lyall, R.; Nikoloski, Z.; Gechev, T. Comparative analysis of ROS network genes in extremophile eukaryotes. Int. J. Mol. Sci. 2020, 21, 9131. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, R.; Cesari, M.; Bertolani, R.; Altiero, T.; Rebecchi, L. High diversity in species, reproductive modes and distribution within the Paramacrobiotus richtersi complex (Eutardigrada, Macrobiotidae). Zool. Lett. 2019, 5, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Satoh, T.; Ota, C.; Tanaka, S.; Horikawa, D.D.; Tomita, M.; Kato, K.; Arakawa, K. A novel Mn-dependent peroxidase contributes to tardigrade anhydrobiosis. bioRxiv 2020. [Google Scholar] [CrossRef]
- Förster, F.; Beisser, D.; Grohme, M.A.; Liang, C.; Mali, B.; Siegl, A.M.; Engelmann, J.C.; Shkumatov, A.V.; Schokraie, E.; Müller, T.; et al. Transcriptome analysis in tardigrade species reveals specific molecular pathways for stress adaptations. Bioinform. Biol. Insights 2012, 6, 69–96. [Google Scholar] [CrossRef] [PubMed]
- Greven, H.; Dastych, H.; Kraus, H. Notes on the integument of the glacier dwelling tardigrade Hypsibius klebelsbergi Mihelcic, 1959 (Tardigrada). Mitt. Hambur. Zool. Mus. 2005, 102, 11–20. [Google Scholar]
- Bonifacio, A.; Guidetti, R.; Altiero, T.; Sergo, V.; Rebecchi, L. Nature, source and function of pigments in tardigrades: In vivo Raman imaging of carotenoids in Echiniscus blumi. PLoS ONE 2012, 7, e50162. [Google Scholar] [CrossRef]
- Benaroudj, N.; Goldberg, A.L. Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J. Biol. Chem. 2001, 276, 24261–24267. [Google Scholar] [CrossRef]
- Oku, K.; Watanabe, H.; Kubota, M.; Fukuda, S.; Kurimoto, M.; Tsujisaka, Y.; Komori, M.; Inoue, Y.; Sakurai, M. NMR and quantum chemical study on the OH…pi and CH…O interactions between trehalose and unsaturated fatty acids: Implication for the mechanism of antioxidant function of trehalose. J. Am. Chem. Soc. 2003, 125, 12739–12748. [Google Scholar] [CrossRef] [PubMed]
- Mali, B.; Grohme, M.A.; Förster, F.; Dandekar, T.; Schnölzer, M.; Reuter, D.; Wełnicz, W.; Schill, R.O.; Frohme, M. Transcriptome survey of the anhydrobiotic tardigrade Milnesium tardigradum in comparison with Hypsibius dujardini and Richtersius coronifer. BMC Genom. 2010, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Hygum, T.L.; Fobian, D.; Kamilari, M.; Jørgensen, A.; Schiøtt, M.; Grosell, M.; Møbjerg, N. Comparative investigation of copper tolerance and identification of putative tolerance related genes in tardigrades. Front. Physiol. 2017, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Giraud-Billoud, M.; Rivera-Ingraham, G.A.; Moreira, D.C.; Burmester, T.; Castro-Vazquez, A.; Carvajalino-Fernández, J.M.; Dafre, A.; Niu, C.; Tremblay, N.; Paital, B.; et al. Twenty years of the ‘Preparation for Oxidative Stress’ (POS) theory: Ecophysiological advantages and molecular strategies. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2019, 234, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current mechanistic concepts in ischemia and reperfusion injury. Cell Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.M.; Altiero, T.; Corsetto, P.A.; Montorfano, G.; Guidetti, R.; Rebecchi, L. Space flight effects on antioxidant molecules in dry tardigrades: The TARDIKISS experiment. BioMed Res. Int. 2015, 2015, 167642. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Walker, N.J.; Tablin, F.; Crowe, J.H. Human platelets loaded with trehalose survive freeze-drying. Cryobiology 2001, 42, 79–87. [Google Scholar] [CrossRef]
- Wolkers, W.F.; Tablin, F.; Crowe, J.H. From anhydrobiosis to freeze-drying of eukaryotic cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 131, 535–543. [Google Scholar] [CrossRef]
- Ye, C.; Guo, J.; Zhou, X.Q.; Chen, D.G.; Liu, J.; Peng, X.; Jaremko, M.; Jaremko, Ł.; Guo, T.; Liu, C.G.; et al. The Dsup coordinates grain development and abiotic stress in rice. Plant Physiol. Biochem. 2023, 205, 108184. [Google Scholar] [CrossRef]
- Zarubin, M.; Azorskaya, T.; Kuldoshina, O.; Alekseev, S.; Mitrofanov, S.; Kravchenko, E. The tardigrade Dsup protein enhances radioresistance in Drosophila melanogaster and acts as an unspecific repressor of transcription. iScience 2023, 26, 106998. [Google Scholar] [CrossRef]
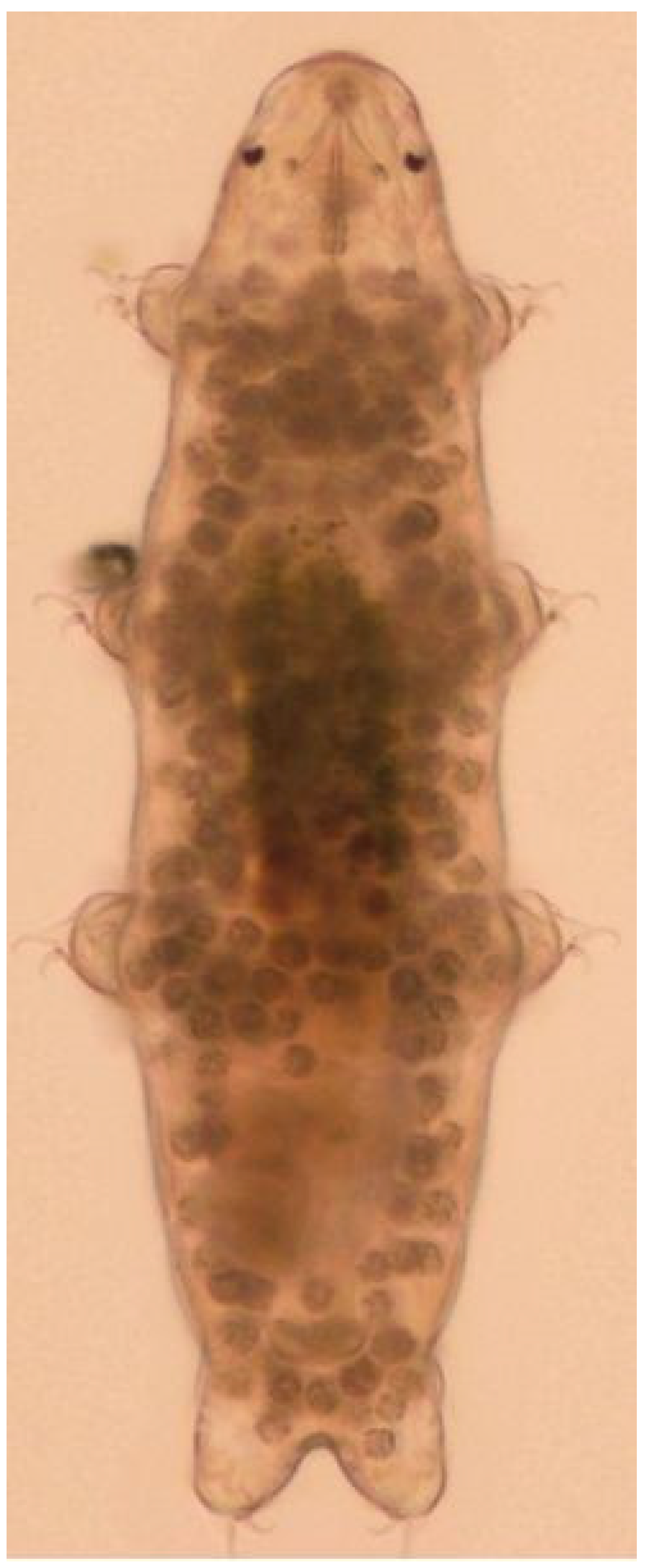

| Common Names | Water Bears; Moss Piglets |
|---|---|
| Systematic position | Phylum of Ecdysozoa |
| Number of species | About 1400 |
| Occurrence | In mosses and lichens |
| Food | Bacteria, algae, plants, and small invertebrates (species-dependent) |
| Size | 50–2100 µm |
| Number of cells | The same within species |
| Body | Barrel-shaped, four pairs of legs, and legs without joints |
| Body cover | Cuticle containing chitin |
| Reproduction | Oviparous, some species parthenogenic |
| Lifespan | 3 months–2 years |
| Resistance | Extreme resistance to various physical and chemical factors |
| Mechanisms of resistance | Mostly (but not only) cryptobiosis |
| Species | State | Ionizing Radiation | Radiation Sensitivity | Reference |
|---|---|---|---|---|
| Echiniscoides sigismundi * | H * H | γ (6.22 Gy min−1) γ (6.22 Gy min−1) | 1591 Gy (LD50/24h) 1449 Gy (LD50/7d) | [61] |
| Hypsibius dujardini | H | γ (6.04 Gy min−1) | 4180 Gy (LD50/48h) | [62] |
| Hypsibius exemplaris | H | Cs137 (1.4251 Gy min−1) | 4360 Gy (LD50) | [62] |
| Paramacrobiotus areolatus | D * | X | 5700 Gy (LD50/1d) | [63] |
| Milnesium tardigradum | H D H D | γ (5.5 to 61.7 Gy min−1) γ (5.5 to 61.7 Gy min−1) 4He (50 MeV, 16.3 keV μm−1) 4He (50 MeV, 16.3 keV μm−1) | 5000 Gy (LD50/48h) 4400 Gy (LD50/48h) 6200 Gy (LD50/48h) 5200 Gy (LD50/48h) | [61,64] [61,64] [61,64] [61,64] |
| Ramazzottius varieornatus | H D | 4He (50 MeV) 4He (50 MeV) | 4000 Gy ~100% survival 4000 Gy ~90% survival | [46] [46] |
| Richtersius coronifer | H H D D D D | γ γ γ Protons (2.55 MeV) X 4He, 56Fe | 4700 Gy (LD50/18h) 2500 Gy (LD50/30d) 3000 Gy (LD50/22h) 10,240 Gy (LD50/24h) 2000 Gy 2000 Gy | [65] [65] [65] [65] [66] [66] |
| Hypsibius exemplaris | H | Cs137 (1.4251 Gy min−1) | 4360 Gy (LD50) | [62] |
| Enzyme | Tardigrade Species | Humans | |||
|---|---|---|---|---|---|
| Echiniscoides sigismundi | Richtersius coronifer | Ramazzottius varieornatus | Hypsibius exemplaris | Homo sapiens | |
| Number of genes coding for the enzyme | |||||
| Superoxide dismutase | 8 | 14 | 17 | 15 | 3 |
| Catalase | 0 | 4 | 4 | 4 | 1 |
| Peroxiredoxins | 5 | 7 | 9 | 12 | 6 |
| Thioredoxins | 12 | 13 | 10 | 12 | 14 |
| Glutaredoxin | 5 | 4 | 3 | 3 | 4 |
| Glutathione disulfide reductase | 1 | 1 | 1 | 1 | 1 |
| Glutathione peroxidase | 2 | 1 | 1 | 1 | 8 |
| Glutathione synthetase | 1 | 2 | 2 | 3 | 1 |
| Soluble glutathione S-transferases | 35 | 30 | 31 | 34 | 22 |
| Microsomal glutathione S-transferases | 0 | 2 | 0 | 2 | 4 |
| Glucose-6-phosphate dehydrogenase | 1 | 1 | 1 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska-Bartosz, I.; Bartosz, G. Antioxidant Defense in the Toughest Animals on the Earth: Its Contribution to the Extreme Resistance of Tardigrades. Int. J. Mol. Sci. 2024, 25, 8393. https://doi.org/10.3390/ijms25158393
Sadowska-Bartosz I, Bartosz G. Antioxidant Defense in the Toughest Animals on the Earth: Its Contribution to the Extreme Resistance of Tardigrades. International Journal of Molecular Sciences. 2024; 25(15):8393. https://doi.org/10.3390/ijms25158393
Chicago/Turabian StyleSadowska-Bartosz, Izabela, and Grzegorz Bartosz. 2024. "Antioxidant Defense in the Toughest Animals on the Earth: Its Contribution to the Extreme Resistance of Tardigrades" International Journal of Molecular Sciences 25, no. 15: 8393. https://doi.org/10.3390/ijms25158393





