Copper(II) and Zinc(II) Complexes with Bacterial Prodigiosin Are Targeting Site III of Bovine Serum Albumin and Acting as DNA Minor Groove Binders
Abstract
1. Introduction
2. Results and Discussion
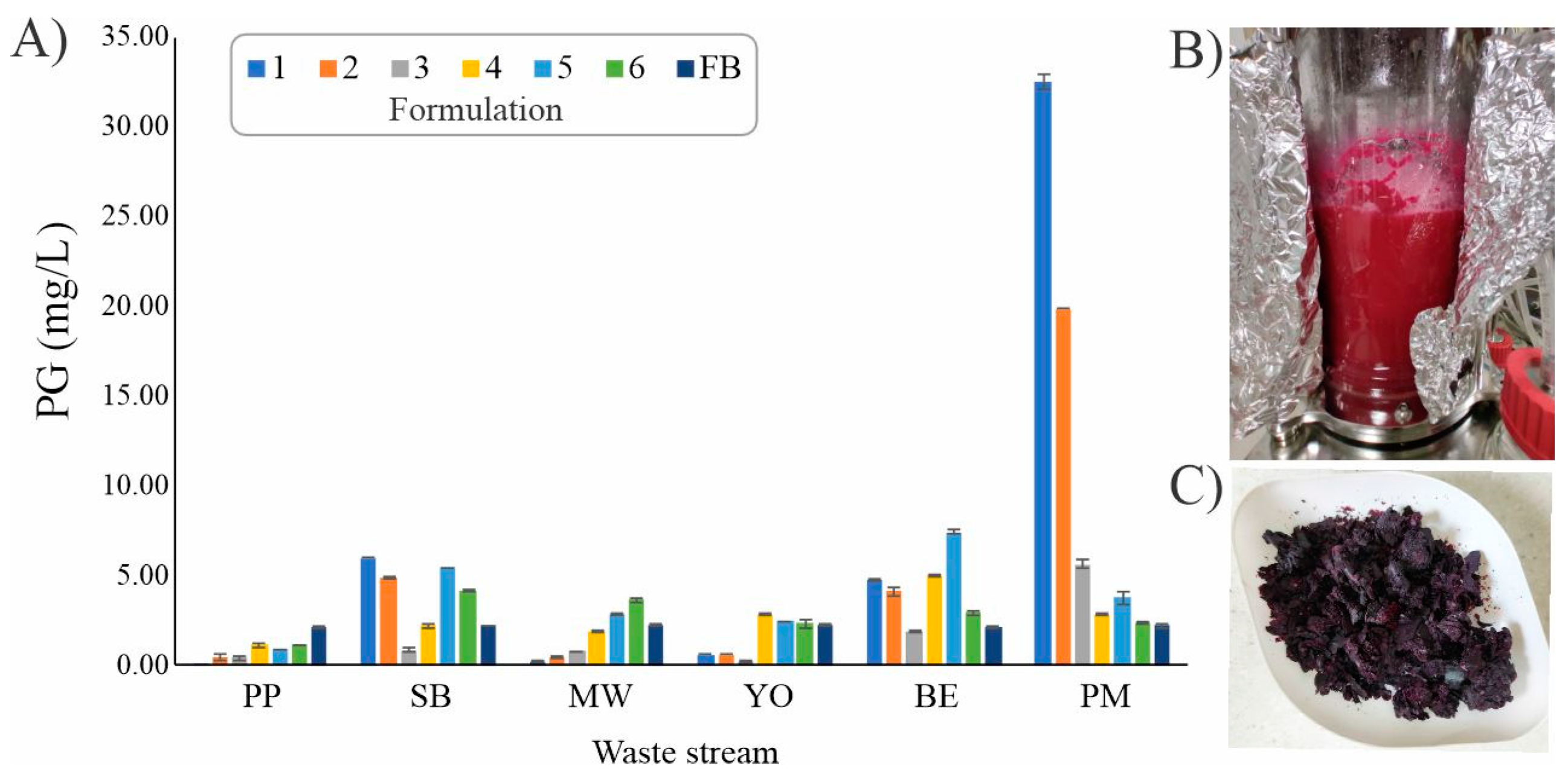
2.1. PG Production Optimization Using Waste Streams
2.2. PG Production and Purification in the Bioreactor
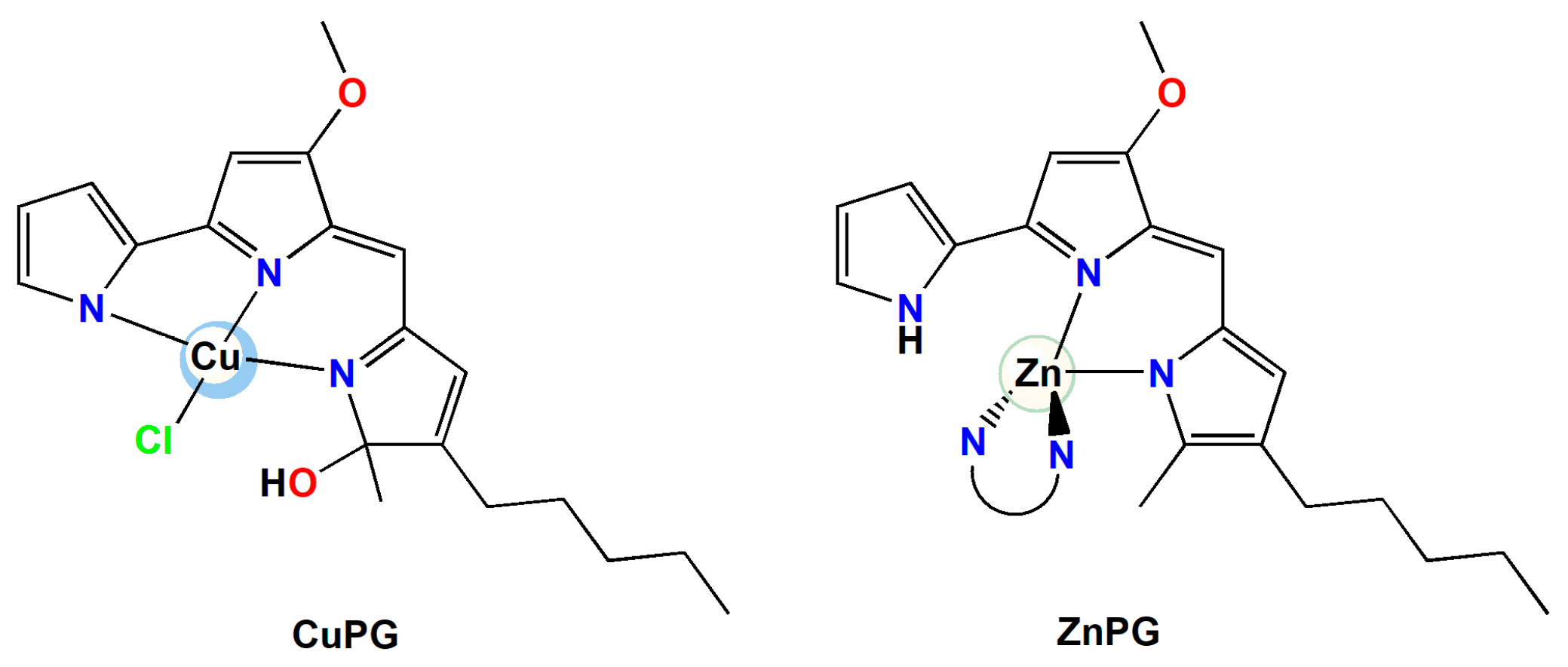
2.3. Copper(II) and Zinc(II) Complexes with PG
2.4. Antimicrobial Activity—Disc Diffusion Assay
2.5. Antiproliferative Activity
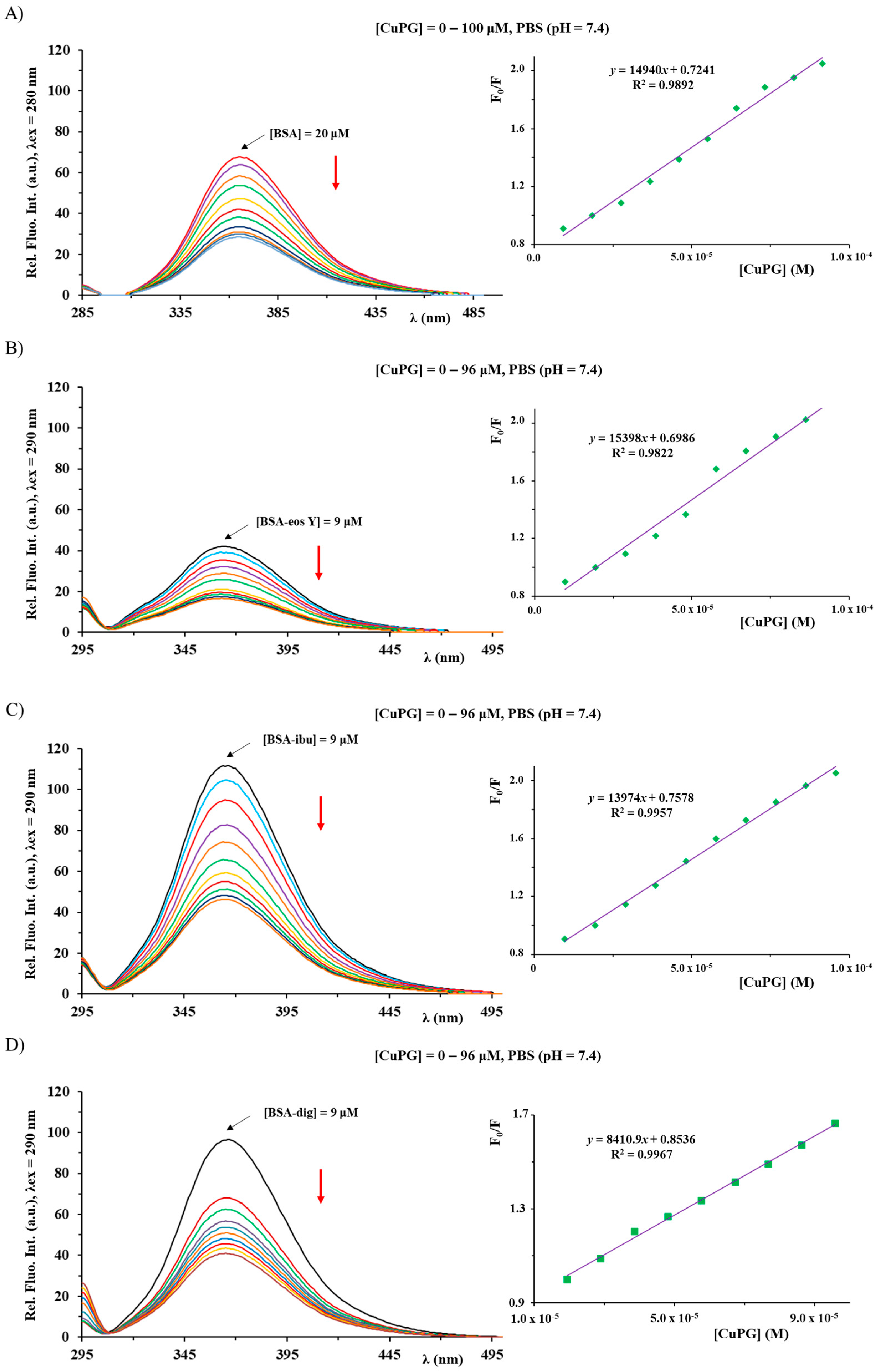
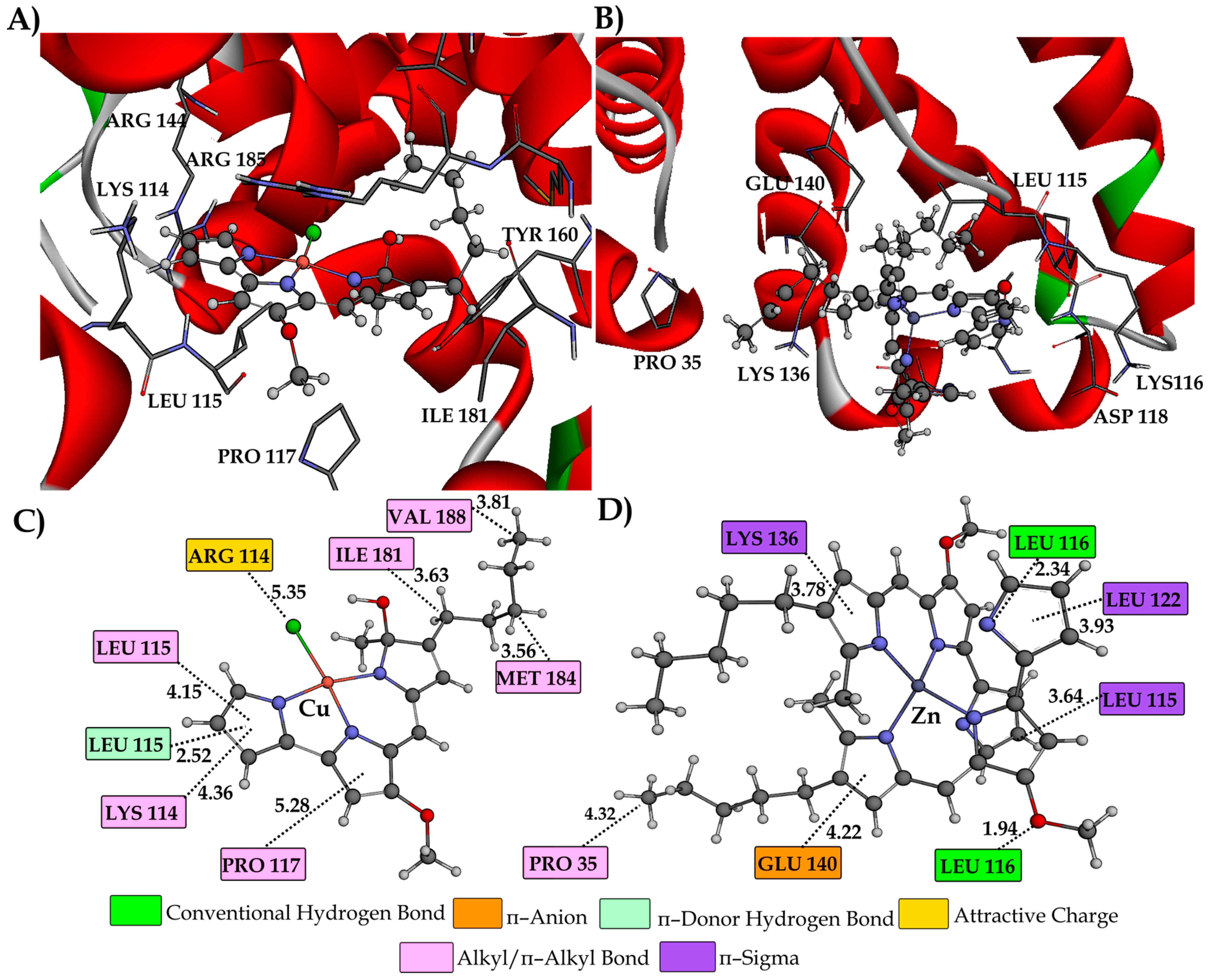
2.6. BSA Binding Study
2.7. ct-DNA Binding Study
3. Materials and Methods
3.1. PG Production Optimization Using Waste Streams
3.2. PG Production and Purification in the Bioreactor
3.3. Synhesis of Copper(II) and Zinc(II) Complexes with PG
3.4. Antimicrobial Activity—Disc Diffusion Assay
3.5. Antiproliferative Activity
3.6. BSA Binding Study
3.7. ct-DNA Binding Study
3.8. Molecular Docking Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; van Otterdijk, R.; Meybeck, A. Global Food Losses and Food Waste: Extent, Causes and Prevention; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Said, Z.; Sharma, P.; Thi Bich Nhuong, Q.; Bora, B.J.; Lichtfouse, E.; Khalid, H.M.; Luque, R.; Nguyen, X.P.; Hoang, A.T. Intelligent approaches for sustainable management and valorisation of food waste. Bioresour. Technol. 2023, 377, 128952. [Google Scholar] [CrossRef]
- Vyas, S.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Patel, A. Insights into hydrophobic waste valorization for the production of value-added oleochemicals. Microb. Biotechnol. 2023, 16, 177. [Google Scholar] [CrossRef]
- Leong, H.Y.; Chang, C.K.; Khoo, K.S.; Chew, K.W.; Chia, S.R.; Lim, J.W.; Chang, J.-S.; Show, P.L. Waste biorefinery towards a sustainable circular bioeconomy: A solution to global issues. Biotechnol. Biofuels 2021, 14, 87. [Google Scholar] [CrossRef]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial pigments and their applications. Process Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Darshan, N.; Manonmani, H.K. Prodigiosin and its potential applications. J. Food Sci. Technol. 2015, 52, 5393–5407. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, C.; Shobana, C.; Selvankumar, T.; Selvam, K. Prodigiosin production from Serratia marcescens strain CSK and their antioxidant, antibacterial, cytotoxic effect and in silico study of caspase-3 apoptotic protein. Biotechnol. Appl. Biochem. 2022, 69, 1984–1997. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Patil, C.D.; Koli, S.H.; Hallsworth, J.E.; Patil, S.V. Antimicrobial activity of prodigiosin is attributable to plasma-membrane damage. Nat. Prod. Res. 2017, 31, 572–577. [Google Scholar] [CrossRef]
- Han, R.; Xiang, R.; Li, J.; Wang, F.; Wang, C. High-level production of microbial prodigiosin: A review. J. Basic Microbiol. 2021, 61, 506–523. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Wang, S.-L.; Doan, M.D.; Nguyen, T.H.; Tran, T.H.T.; Tran, T.N.; Doan, C.T.; Ngo, V.A.; Ho, N.D.; Do, V.C. Utilization of by-product of groundnut oil processing for production of prodigiosin by microbial fermentation and its novel potent anti-nematodes effect. Agronomy 2021, 12, 41. [Google Scholar] [CrossRef]
- Elkenawy, N.M.; Yassin, A.S.; Elhifnawy, H.N.; Amin, M.A. Optimization of prodigiosin production by Serratia marcescens using crude glycerol and enhancing production using gamma radiation. Biotechnol. Rep. 2017, 14, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Llagostera, E.; Soto-Cerrato, V.; Joshi, R.; Montaner, B.; Gimenez-Bonafé, P.; Pérez-Tomás, R. High cytotoxic sensitivity of the human small cell lung doxorubicin-resistant carcinoma (GLC4/ADR) cell line to prodigiosin through apoptosis activation. Anticancer Drugs 2005, 16, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, B.; Zhou, L.; Yu, S.; Su, Z.; Song, J.; Sun, Q.; Sha, O.; Wang, X.; Jiang, W.; et al. Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13150–13155. [Google Scholar] [CrossRef]
- Soto-Cerrato, V.; Llagostera, E.; Montaner, B.; Scheffer, G.L.; Perez-Tomas, R. Mitochondria-mediated apoptosis operating irrespective of multidrug resistance in breast cancer cells by the anticancer agent prodigiosin. Biochem. Pharmacol. 2004, 68, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Dalili, D.; Fouladdel, S.; Rastkari, N.; Samadi, N.; Ahmadkhaniha, R.; Ardavan, A.; Azizi, E. Prodigiosin, the red pigment of Serratia marcescens, shows cytotoxic effects and apoptosis induction in HT-29 and T47D cancer cell lines. Nat. Prod. Res. 2012, 26, 2078–2083. [Google Scholar] [PubMed]
- Yenkejeh, R.A.; Sam, M.R.; Esmaeillou, M. Targeting survivin with prodigiosin isolated from cell wall of Serratia marcescens induces apoptosis in hepatocellular carcinoma cells. Hum. Exp. Toxicol. 2017, 36, 402–411. [Google Scholar] [CrossRef]
- Chiu, W.-J.; Lin, S.-R.; Chen, Y.-H.; Tsai, M.-J.; Leong, M.K.; Weng, C.-F. Prodigiosin-emerged PI3K/Beclin-1-independent pathway elicits autophagic cell death in doxorubicin-sensitive and-resistant lung cancer. J. Clin. Med. 2018, 7, 321. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tomás, R.; Montaner, B.; Llagostera, E.; Soto-Cerrato, V. The prodigiosins, proapoptotic drugs with anticancer properties. Biochem. Pharmacol. 2003, 66, 1447–1452. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Betts, H.; Keller, S.; Cariou, K.; Gasser, G. Recent developments of metal-based compounds against fungal pathogens. Chem. Soc. Rev. 2021, 50, 10346–10402. [Google Scholar] [CrossRef]
- Chellan, P.; Sadler, P.J. Enhancing the activity of drugs by conjugation to organometallic fragments. Chemistry 2020, 26, 8676–8688. [Google Scholar] [CrossRef]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef]
- Lovering, F. Escape from Flatland 2: Complexity and promiscuity. MedChemComm 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Hung, A.W.; Ramek, A.; Wang, Y.; Kaya, T.; Wilson, J.A.; Clemons, P.A.; Young, D.W. Route to three-dimensional fragments using diversity-oriented synthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 6799–6804. [Google Scholar] [CrossRef] [PubMed]
- Sauer, W.H.; Schwarz, M.K. Molecular shape diversity of combinatorial libraries: A prerequisite for broad bioactivity. J. Chem. Inf. Comput. Sci. 2003, 43, 987–1003. [Google Scholar] [CrossRef] [PubMed]
- Galloway, W.R.; Isidro-Llobet, A.; Spring, D.R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 2010, 1, 80. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.N.; Prosser, K.E.; Stokes, R.W.; Cordes, A.; Metzler-Nolte, N.; Cohen, S.M. Expanding medicinal chemistry into 3D space: Metallofragments as 3D scaffolds for fragment-based drug discovery. Chem. Sci. 2019, 11, 1216–1225. [Google Scholar] [CrossRef]
- Gasser, G.; Metzler-Nolte, N. The potential of organometallic complexes in medicinal chemistry. Curr. Opin. Chem. Biol. 2012, 16, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Gianferrara, T.; Bratsos, I.; Alessio, E. A categorization of metal anticancer compounds based on their mode of action. Dalton Trans. 2009, 7588–7598. [Google Scholar] [CrossRef] [PubMed]
- Frei, A.; Zuegg, J.; Elliott, A.G.; Baker, M.; Braese, S.; Brown, C.; Chen, F.; Dowson, C.G.; Dujardin, G.; Jung, N.; et al. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef] [PubMed]
- Cortat, Y.; Zobi, F. Resurgence and repurposing of antifungal azoles by transition metal coordination for drug discovery. Pharmaceutics 2023, 15, 2398. [Google Scholar] [CrossRef]
- Navarro-Peñaloza, R.; Landeros-Rivera, B.; López-Sandoval, H.; Castro-Ramírez, R.; Barba-Behrens, N. New insights on transition metal coordination compounds with biological active azole and nitroimidazole derivatives. Coord. Chem. Rev. 2023, 494, 215360. [Google Scholar] [CrossRef]
- Stanković, M.; Kljun, J.; Stevanović, N.L.; Lazic, J.; Skaro Bogojevic, S.; Vojnovic, S.; Zlatar, M.; Nikodinovic-Runic, J.; Turel, I.; Djuran, M.I.; et al. Silver(I) complexes containing antifungal azoles: Significant improvement of the anti-Candida potential of the azole drug after its coordination to the silver(I) ion. Dalton Trans. 2024, 53, 2218–2230. [Google Scholar] [CrossRef]
- Stanković, M.; Skaro Bogojevic, S.; Kljun, J.; Milanović, Ž.; Stevanović, N.L.; Lazic, J.; Vojnovic, S.; Turel, I.; Djuran, M.I.; Glišić, B. Silver(I) complexes with voriconazole as promising anti-Candida agents. J. Inorg. Biochem. 2024, 256, 112572. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, N.L.; Kljun, J.; Aleksic, I.; Bogojevic, S.S.; Milivojevic, D.; Veselinovic, A.; Turel, I.; Djuran, M.I.; Nikodinovic-Runic, J.; Glišić, B. Clinically used antifungal azoles as ligands for gold(III) complexes: The influence of the Au(III) ion on the antimicrobial activity of the complex. Dalton Trans. 2022, 51, 5322–5334. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, N.L.; Aleksic, I.; Kljun, J.; Skaro Bogojevic, S.; Veselinovic, A.; Nikodinovic-Runic, J.; Turel, I.; Djuran, M.I.; Glišić, B. Copper(II) and zinc(II) complexes with the clinically used fluconazole: Comparison of antifungal activity and therapeutic potential. Pharmaceuticals 2020, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Zang, C.-Z.; Yeh, C.-W.; Chang, W.-F.; Lin, C.-C.; Kan, S.-C.; Shieh, C.-J.; Liu, Y.-C. Identification and enhanced production of prodigiosin isoform pigment from Serratia marcescens N10612. J. Taiwan Inst. Chem. Eng. 2014, 45, 1133–1139. [Google Scholar] [CrossRef]
- Chen, W.-C.; Yu, W.-J.; Chang, C.-C.; Chang, J.-S.; Huang, S.-H.; Chang, C.-H.; Chen, S.-Y.; Chien, C.-C.; Yao, C.-L.; Chen, W.-M. Enhancing production of prodigiosin from Serratia marcescens C3 by statistical experimental design and porous carrier addition strategy. Biochem. Eng. J. 2013, 78, 93–100. [Google Scholar] [CrossRef]
- Pantelic, L.; Skaro Bogojevic, S.; Vojnovic, S.; Oliveira, R.; Lazic, J.; Ilic-Tomic, T.; Milivojevic, D.; Nikodinovic-Runic, J. Upcycling of food waste streams to valuable biopigments pyocyanin and 1-hydroxyphenazine. Enzyme Microb. Technol. 2023, 171, 110322. [Google Scholar] [CrossRef]
- Liu, W.; Yang, J.; Tian, Y.; Zhou, X.; Wang, S.; Zhu, J.; Sun, D.; Liu, C. An in situ extractive fermentation strategy for enhancing prodigiosin production from Serratia marcescens BWL1001 and its application to inhibiting the growth of Microcystis aeruginosa. Biochem. Eng. J. 2021, 166, 107836. [Google Scholar] [CrossRef]
- Tao, J.; Wang, X.; Shen, Y.; Wei, D. Strategy for the improvement of prodigiosin production by a Serratia marcescens mutant through fed-batch fermentation. World J. Microbiol. Biotechnol. 2005, 21, 969–972. [Google Scholar] [CrossRef]
- Wei, Y.-H.; Chen, W.-C. Enhanced production of prodigiosin-like pigment from Serratia marcescens SMΔR by medium improvement and oil-supplementation strategies. J. Biosci. Bioeng. 2005, 99, 616–622. [Google Scholar] [CrossRef]
- Lazic, J.; Skaro Bogojevic, S.; Vojnovic, S.; Aleksic, I.; Milivojevic, D.; Kretzschmar, M.; Gulder, T.; Petkovic, M.; Nikodinovic-Runic, J. Synthesis, anticancer potential and comprehensive toxicity studies of novel brominated derivatives of bacterial biopigment prodigiosin from Serratia marcescens ATCC 27117. Molecules 2022, 27, 3729. [Google Scholar] [CrossRef] [PubMed]
- Kurbanoglu, E.B.; Ozdal, M.; Ozdal, O.G.; Algur, O.F. Enhanced production of prodigiosin by Serratia marcescens MO-1 using ram horn peptone. Braz. J. Microbiol. 2015, 46, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Salas-Villalobos, U.A.; Torres-Acosta, M.A.; Aguilar, O. Techno-economic analysis as a valuable tool for the selection of high-potential extractive fermentation approaches for large-scale prodigiosin production. Chem. Eng. J. 2024, 481, 148279. [Google Scholar] [CrossRef]
- Arivizhivendhan, K.V.; Mahesh, M.; Boopathy, R.; Swarnalatha, S.; Regina Mary, R.; Sekaran, G. Antioxidant and antimicrobial activity of bioactive prodigiosin produces from Serratia marcescens using agricultural waste as a substrate. J. Food Sci. Technol. 2018, 55, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, A.; Padalia, U. Optimization of prodigiosin biosynthesis by Serratia marcescens using unconventional bioresources. J. Genet. Eng. Biotechnol. 2020, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Wang, S.L.; Nguyen, D.-N.; Nguyen, A.-D.; Nguyen, T.-H.; Doan, M.-D.; Ngo, V.-A.; Doan, C.-T.; Kuo, Y.-H.; Nguyen, V.-B. Bioprocessing of marine chitinous wastes for the production of bioactive prodigiosin. Molecules 2021, 26, 3138. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Shi, K.; Song, D.; Quan, L.; Wu, Z. The pigment characteristics and productivity shifting in high cell density culture of Monascus anka mycelia. BMC Biotechnol. 2015, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Tomlinson, J.T.; Melvin, M.S.; Wright, M.W.; Day, C.S.; Manderville, R.A. Zinc and copper complexes of prodigiosin: Implications for copper-mediated double-strand DNA cleavage. Org. Lett. 2003, 5, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Lapenda, J.C.; Silva, P.A.; Vicalvi, M.C.; Sena, K.X.F.R.; Nascimento, S.C. Antimicrobial activity of prodigiosin isolated from Serratia marcescens UFPEDA 398. World J. Microbiol. Biotechnol. 2015, 31, 399–406. [Google Scholar] [CrossRef]
- Doniz Kettenmann, S.; White, M.; Colard-Thomas, J.; Kraft, M.; Feßler, A.T.; Danz, K.; Wieland, G.; Wagner, S.; Schwarz, S.; Wiehe, A. Investigating alkylated prodigiosenes and their Cu(II)-dependent biological activity: Interactions with DNA, antimicrobial and photoinduced anticancer activity. ChemMedChem 2022, 17, e202100702. [Google Scholar] [CrossRef]
- Lazic, J. Structure Optimisation of Biopigment Prodigiosin from Serratia marcescens ATCC 27117 and Antimicrobial and Anticancer Properties of Novel Halogenated Derivatives; University of Nova Gorica (Slovenia): Nova Gorica, Slovenia, 2022. [Google Scholar]
- Hong, B.; Prabhu, V.V.; Zhang, S.; van den Heuvel, A.P.J.; Dicker, D.T.; Kopelovich, L.; El-Deiry, W.S. Prodigiosin rescues deficient p53 signaling and antitumor effects via upregulating p73 and disrupting its interaction with mutant p53. Cancer Res. 2014, 74, 1153–1165. [Google Scholar] [CrossRef]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin-more than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Q.; Zhang, H.-M.; Zhang, G.-C.; Tao, W.-H.; Tang, S.-H. Interaction of the flavonoid hesperidin with bovine serum albumin: A fluorescence quenching study. J. Lumin. 2007, 126, 211–218. [Google Scholar] [CrossRef]
- Međedović, M.; Mijatović, A.; Baošić, R.; Lazić, D.; Milanović, Ž.; Marković, Z.; Milovanović, J.; Arsenijević, D.; Stojanović, B.; Arsenijević, M.; et al. Synthesis, characterization, biomolecular interactions, molecular docking, and in vitro and in vivo anticancer activities of novel ruthenium(III) Schiff base complexes. J. Inorg. Biochem. 2023, 248, 112363. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Guo, C.; Sun, Y.; Liu, Z.; Xu, F.; Zhang, Y.; Wen, Z.; Li, Z. Interaction between DNA and microcystin-LR studied by spectra analysis and atomic force microscopy. Biomacromolecules 2011, 12, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-C.; Tang, J.; Zhang, X.-H.; Gao, Y.-Y.; Ma, F.; Yang, Q. Study on the interaction of prodigiosin with bovine serum albumin by spectroscopic methods. J. Spectrosc. 2012, 27, 19–26. [Google Scholar] [CrossRef][Green Version]
- Rastegari, B.; Karbalaei-Heidari, H.R.; Yousefi, R.; Zeinali, S.; Nabavizadeh, M. Interaction of prodigiosin with HSA and β-Lg: Spectroscopic and molecular docking studies. Bioorg. Med. Chem. 2016, 24, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Jahanban-Esfahlan, R.; Roufegarinejad, L.; Tabibiazar, M.; Amarowicz, R. Recent developments in the detection of bovine serum albumin. Int. J. Biol. Macromol. 2019, 138, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Manjushree, M.; Revanasiddappa, H.D. A diversified spectrometric and molecular docking technique to biophysical study of interaction between bovine serum albumin and sodium salt of risedronic acid, a bisphosphonate for skeletal disorders. Bioinorg. Chem. Appl. 2018, 2018, 6954951. [Google Scholar] [CrossRef]
- Ahmed, N.; Dobler, D.; Dean, M.; Thornalley, P.J. Peptide mapping identifies hotspot site of modification in human serum albumin by methylglyoxal involved in ligand binding and esterase activity. J. Biol. Chem. 2005, 280, 5724–5732. [Google Scholar] [CrossRef]
- Tantimongcolwat, T.; Prachayasittikul, S.; Prachayasittikul, V. Unravelling the interaction mechanism between clioquinol and bovine serum albumin by multi-spectroscopic and molecular docking approaches. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 216, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Najaran, A.; Divsalar, A.; Saboury, A.A.; Roodbari, N.H. Probing the interaction of newly synthesized Pt(II) complex on human serum albumin using competitive binding site markers. J. Fluoresc. 2019, 29, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Mrkalić, E.; Jelić, R.; Stojanović, S.; Sovrlić, M. Interaction between olanzapine and human serum albumin and effect of metal ions, caffeine and flavonoids on the binding: A spectroscopic study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 249, 119295. [Google Scholar] [CrossRef] [PubMed]
- Turel, I.; Kljun, J. Interactions of metal ions with DNA, its constituents and derivatives, which may be relevant for anticancer research. Curr. Top. Med. Chem. 2011, 11, 2661–2687. [Google Scholar] [CrossRef] [PubMed]
- Smoleński, P.; Pettinari, C.; Marchetti, F.; Guedes da Silva, M.F.; Lupidi, G.; Badillo Patzmay, G.V.; Petrelli, D.; Vitali, L.A.; Pombeiro, A.J. Syntheses, structures, and antimicrobial activity of new remarkably light-stable and water-soluble tris(pyrazolyl)methanesulfonate silver(I) derivatives of N-methyl-1,3,5-triaza-7-phosphaadamantane salt-[mPTA]BF4. Inorg. Chem. 2015, 54, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Pjura, P.E.; Grzeskowiak, K.; Dickerson, R.E. Binding of Hoechst 33258 to the minor groove of B-DNA. J. Mol. Biol. 1987, 197, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Zhou, Y.; Huang, X.; Xiao, M.; Zhou, L.; Zhou, J.; Wang, A.; Shen, J. A multi-spectroscopic approach to investigate the interaction of prodigiosin with ct-DNA. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 123, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond: In Structural Chemistry and Biology; International Union of Crystal: Chester, UK, 2001; Volume 9. [Google Scholar]
- Kumar, S.; Pande, V.; Das, A. π-Hydrogen bonding wins over conventional hydrogen bonding interaction: A jet-cooled study of indole···furan heterodimer. J. Phys. Chem. A 2012, 116, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Ryazantseva, I.N.; Saakov, V.S.; Andreyeva, I.N.; Ogorodnikova, T.I.; Zuev, Y.F. Response of pigmented Serratia marcescens to the illumination. J. Photochem. Photobiol. B Biol. 2012, 106, 18–23. [Google Scholar] [CrossRef]
- Faraag, A.H.; El-Batal, A.I.; El-Hendawy, H.H. Characterization of prodigiosin produced by Serratia marcescens strain isolated from irrigation water in Egypt. Nat. Sci. 2017, 15, 55–68. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Kurutos, A.; Nikodinovic-Runic, J.; Veselinovic, A.; Veselinović, J.B.; Kamounah, F.S.; Ilic-Tomic, T. RNA-targeting low-molecular-weight fluorophores for nucleoli staining: Synthesis, in silico modelling and cellular imaging. New J. Chem. 2021, 45, 12818–12829. [Google Scholar] [CrossRef]
- Wolfe, A.; Shimer, G.H., Jr.; Meehan, T. Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry 1987, 26, 6392–6396. [Google Scholar] [CrossRef] [PubMed]
- Bera, R.; Sahoo, B.K.; Ghosh, K.S.; Dasgupta, S. Studies on the interaction of isoxazolcurcumin with calf thymus DNA. Int. J. Biol. Macromol. 2008, 42, 14–21. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, J.; Rurainski, A.; Lenhof, H.P.; Neumann, D. A new Lamarckian genetic algorithm for flexible ligand-receptor docking. J. Comput. Chem. 2010, 31, 1911–1918. [Google Scholar] [CrossRef]
- Bujacz, A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. D Biol. Crystallogr. 2012, 68 Pt 10, 1278–1289. [Google Scholar] [CrossRef]
- Milanović, Ž.; Antonijević, M.; Avdović, E.; Simić, V.; Milošević, M.; Dolićanin, Z.; Kojić, M.; Marković, Z. In silico evaluation of pharmacokinetic parameters, delivery, distribution and anticoagulative effects of new 4,7-dihydroxycoumarin derivative. J. Biomol. Struct. Dyn. 2023. [Google Scholar] [CrossRef]
- Drew, H.R.; Wing, R.M.; Takano, T.; Broka, C.; Tanaka, S.; Itakura, K.; Dickerson, R.E. Structure of a B-DNA dodecamer: Conformation and dynamics. Proc. Natl. Acad. Sci. USA 1981, 78, 2179–2183. [Google Scholar] [CrossRef]
- Canals, A.; Purciolas, M.; Aymamí, J.; Coll, M. The anticancer agent ellipticine unwinds DNA by intercalative binding in an orientation parallel to base pairs. Acta Crystallogr. D Biol. Crystallogr. 2005, 61 Pt 7, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Milanović, Ž.B.; Antonijević, M.R.; Amić, A.D.; Avdović, E.H.; Dimić, D.S.; Milenković, D.A.; Marković, Z.S. Inhibitory activity of quercetin, its metabolite, and standard antiviral drugs towards enzymes essential for SARS-CoV-2: The role of acid-base equilibria. RSC Adv. 2021, 11, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Avdović, E.H.; Dimić, D.S.; Fronc, M.; Kožišek, J.; Klein, E.; Milanović, Ž.B.; Kesić, A.; Marković, Z.S. Structural and theoretical analysis, molecular docking/dynamics investigation of 3-(1-m-chloridoethylidene)-chromane-2,4-dione: The role of chlorine atom. J. Mol. Struct. 2021, 1231, 129962. [Google Scholar] [CrossRef]

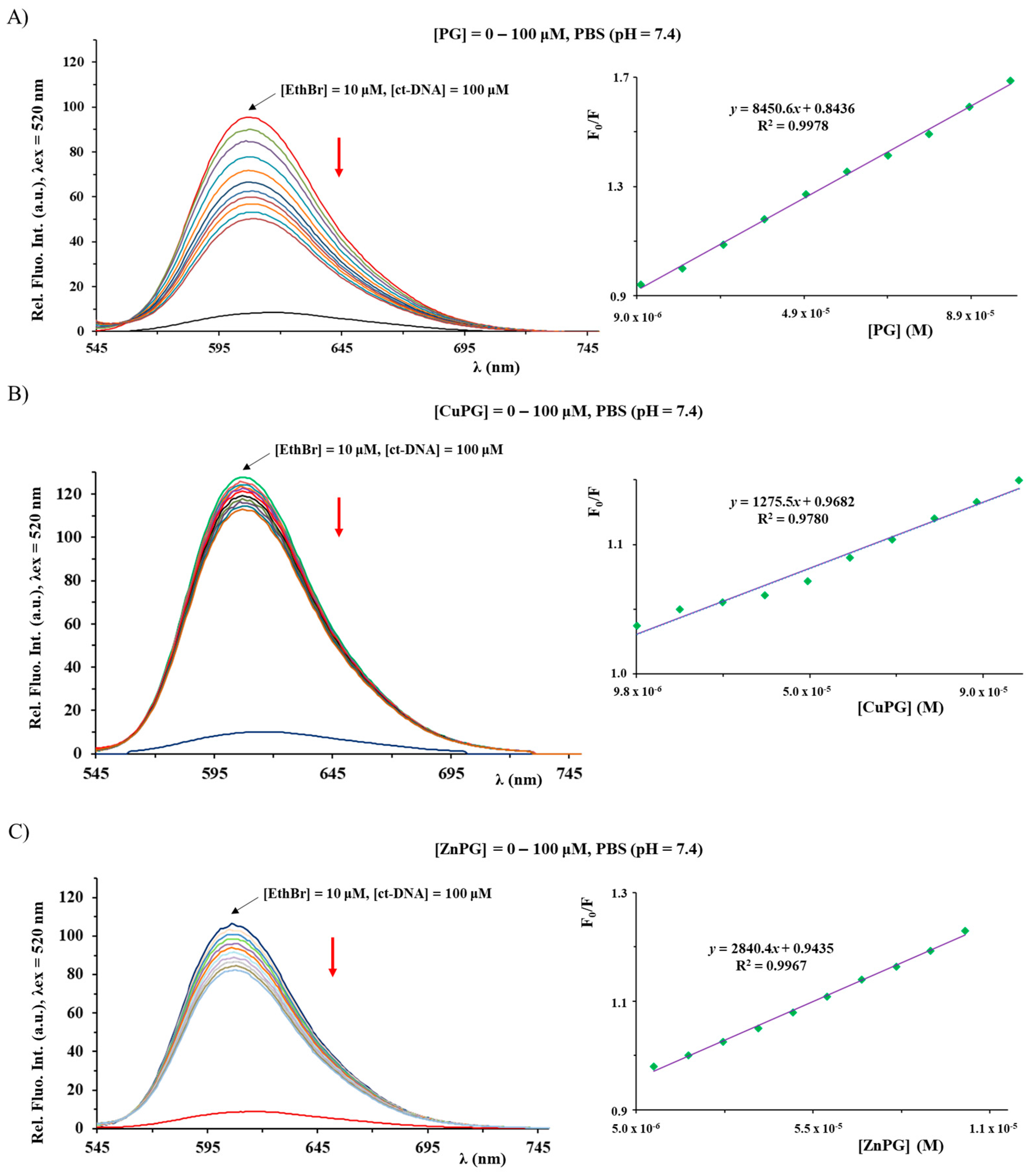

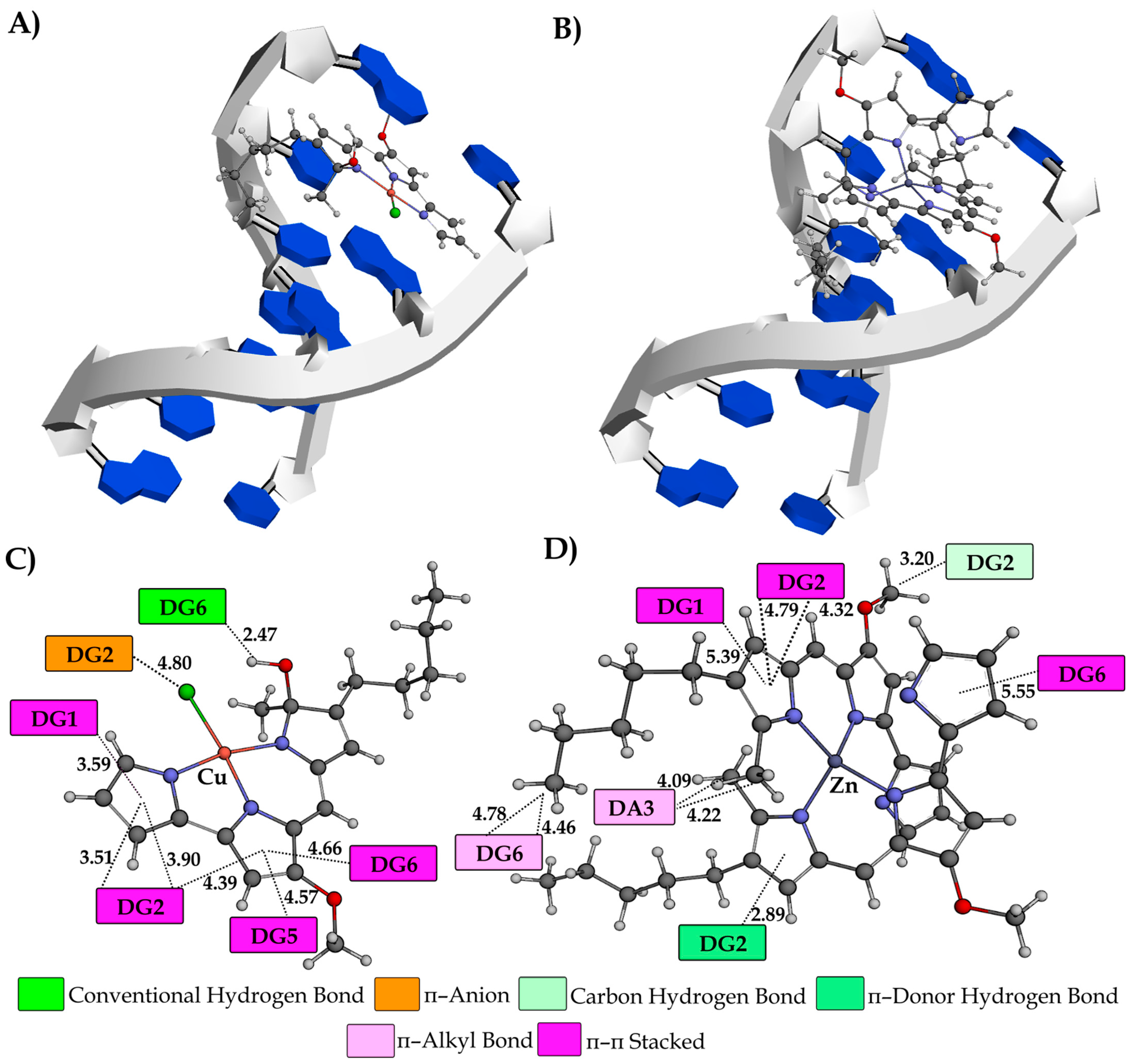
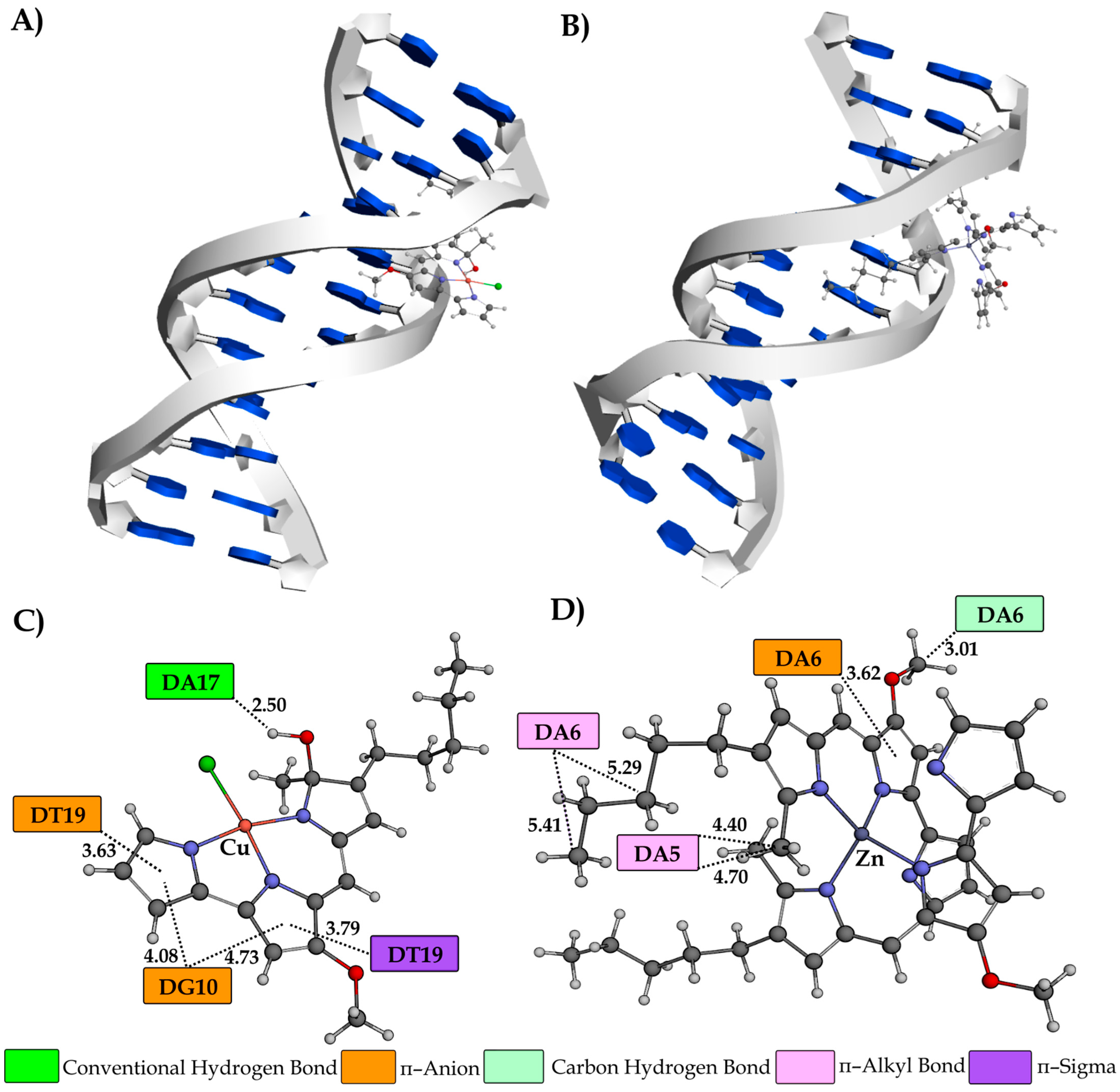
| Ksv (M−1) | Fluorescence Quenching (%) | Kq (M−1s−1) | KA (M−1) | n | |
|---|---|---|---|---|---|
| PG–BSA | (7.48 ± 0.01) × 103 | 40.8 | 7.48 × 1011 | 5.77 × 103 | 0.97 |
| PG–BSA–eos Y | (3.60 ± 0.01) × 103 | 23.9 | 3.60 × 1011 | 3.91 × 104 | 1.27 |
| PG–BSA–ibu | (1.77 ± 0.02) × 104 | 59.6 | 1.77 × 1012 | 8.33 × 104 | 1.18 |
| PG–BSA–dig | (2.35 ± 0.02) × 104 | 61.8 | 2.35 × 1012 | 3.24 × 105 | 1.31 |
| CuPG–BSA | (2.06 ± 0.03) × 104 | 57.9 | 2.06 × 1012 | 8.05 × 105 | 1.41 |
| CuPG–BSA–eos Y | (2.20 ± 0.04) × 104 | 60.6 | 2.20 × 1012 | 4.80 × 105 | 1.36 |
| CuPG–BSA–ibu | (1.84 ± 0.02) × 104 | 58.6 | 1.84 × 1012 | 3.93 × 105 | 1.34 |
| CuPG–BSA–dig | (9.85 ± 0.01) × 103 | 57.6 | 9.85 × 1011 | 1.08 × 103 | 0.72 |
| ZnPG–BSA | (3.90 ± 0.01) × 103 | 34.2 | 3.90 × 1011 | 7.14 × 103 | 1.07 |
| ZnPG–BSA–eos Y | (3.54 ± 0.01) × 103 | 32.5 | 3.54 × 1011 | 8.39 × 104 | 1.09 |
| ZnPG–BSA–ibu | (4.84 ± 0.01) × 103 | 40.2 | 4.84 × 1011 | 1.48 × 104 | 1.13 |
| ZnPG–BSA–dig | (5.69 ± 0.01) × 103 | 35.9 | 5.69 × 1011 | 6.06 × 103 | 1.00 |
| Ksv (M−1) | Fluorescence Quenching (%) | Kq (M−1s−1) | KA (M−1) | n | ||
|---|---|---|---|---|---|---|
| PG | (1.00 ± 0.01) × 104 | 47.6 | 1.00 × 1012 | 4.96 × 104 | 1.18 | |
| EthBr | CuPG | (1.32 ± 0.01) × 103 | 11.7 | 1.32 × 1011 | 4.95 × 102 | 0.91 |
| ZnPG | (3.28 ± 0.01) × 103 | 22.0 | 3.28 × 1011 | 8.86 × 103 | 1.12 | |
| PG | (8.33 ± 0.01) × 103 | 44.9 | 8.33 × 1011 | 6.84 × 103 | 0.98 | |
| Hoe | CuPG | (1.56 ± 0.01) × 103 | 7.0 | 1.56 × 1011 | 1.37 × 109 | 2.54 |
| ZnPG | (2.48 ± 0.01) × 103 | 18.3 | 2.48 × 1011 | 1.89 × 104 | 1.22 |
| Conformations | ΔGbind | Ki (µM) | ΔGinter | ΔGvdw+hbond+desolv | ΔGelec | ΔGtotal | ΔGtor | ΔGunb |
|---|---|---|---|---|---|---|---|---|
| PG–BSA–I | −6.75 | 11.23 | −8.54 | −8.38 | −0.17 | −0.90 | 1.79 | −0.90 |
| PG–BSA–II | −8.08 | 1.19 | −9.87 | −9.73 | −0.14 | −1.00 | 1.79 | −1.00 |
| PG–BSA–III | −8.92 | 0.29 | −10.71 | −10.64 | −0.06 | −0.78 | 1.79 | −0.78 |
| CuPG–BSA–I | −3.49 | 275 | −5.14 | −4.99 | −0.15 | −0.23 | 1.65 | −0.23 |
| CuPG–BSA–II | −5.74 | 60.7 | −7.54 | −7.45 | −0.10 | −0.44 | 1.79 | −0.44 |
| CuPG–BSA–III | −7.41 | 3.69 | −10.43 | −10.29 | −0.14 | −3.28 | 3.02 | −3.28 |
| ZnPG–BSA–I | −4.12 | 961 | −7.13 | −6.91 | −0.23 | −3.04 | 3.02 | −3.04 |
| ZnPG–BSA–II | −5.84 | 52 | −9.12 | −9.11 | −0.01 | −3.45 | 3.28 | −3.45 |
| ZnPG–BSA–III | −8.71 | 0.49 | −10.36 | −10.11 | −0.25 | −0.49 | 1.65 | −0.49 |
| Conformations | ΔGbind | Ki (µM) | ΔGinter | ΔGvdw+hbond+desolv | ΔGelec | ΔGtotal | ΔGtor | ΔGunb |
|---|---|---|---|---|---|---|---|---|
| DNA (1Z3F)–PG | −7.59 | 2.73 | −9.38 | −9.23 | −0.15 | −0.94 | 1.79 | −0.94 |
| DNA (1BNA)–PG | −8.13 | 1.10 | −9.91 | −9.68 | −0.23 | −1.33 | 1.79 | −1.33 |
| DNA (1Z3F)–CuPG | −7.64 | 2.49 | −9.29 | −9.04 | −0.25 | −0.54 | 1.65 | −0.54 |
| DNA (1BNA)–CuPG | −7.82 | 1.85 | −9.47 | −9.52 | 0.05 | −0.29 | 1.65 | −0.29 |
| DNA (1Z3F)–ZnPG | −6.09 | 34.34 | −9.11 | −8.95 | −0.16 | −3.08 | 3.02 | −3.08 |
| DNA (1BNA)–ZnPG | −7.84 | 1.79 | −10.86 | −10.81 | −0.05 | −2.67 | 3.02 | −2.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantelic, L.; Skaro Bogojevic, S.; Andrejević, T.P.; Pantović, B.V.; Marković, V.R.; Ašanin, D.P.; Milanović, Ž.; Ilic-Tomic, T.; Nikodinovic-Runic, J.; Glišić, B.Đ.; et al. Copper(II) and Zinc(II) Complexes with Bacterial Prodigiosin Are Targeting Site III of Bovine Serum Albumin and Acting as DNA Minor Groove Binders. Int. J. Mol. Sci. 2024, 25, 8395. https://doi.org/10.3390/ijms25158395
Pantelic L, Skaro Bogojevic S, Andrejević TP, Pantović BV, Marković VR, Ašanin DP, Milanović Ž, Ilic-Tomic T, Nikodinovic-Runic J, Glišić BĐ, et al. Copper(II) and Zinc(II) Complexes with Bacterial Prodigiosin Are Targeting Site III of Bovine Serum Albumin and Acting as DNA Minor Groove Binders. International Journal of Molecular Sciences. 2024; 25(15):8395. https://doi.org/10.3390/ijms25158395
Chicago/Turabian StylePantelic, Lena, Sanja Skaro Bogojevic, Tina P. Andrejević, Bojana V. Pantović, Violeta R. Marković, Darko P. Ašanin, Žiko Milanović, Tatjana Ilic-Tomic, Jasmina Nikodinovic-Runic, Biljana Đ. Glišić, and et al. 2024. "Copper(II) and Zinc(II) Complexes with Bacterial Prodigiosin Are Targeting Site III of Bovine Serum Albumin and Acting as DNA Minor Groove Binders" International Journal of Molecular Sciences 25, no. 15: 8395. https://doi.org/10.3390/ijms25158395
APA StylePantelic, L., Skaro Bogojevic, S., Andrejević, T. P., Pantović, B. V., Marković, V. R., Ašanin, D. P., Milanović, Ž., Ilic-Tomic, T., Nikodinovic-Runic, J., Glišić, B. Đ., & Lazic, J. (2024). Copper(II) and Zinc(II) Complexes with Bacterial Prodigiosin Are Targeting Site III of Bovine Serum Albumin and Acting as DNA Minor Groove Binders. International Journal of Molecular Sciences, 25(15), 8395. https://doi.org/10.3390/ijms25158395











