Radiation-Induced miRNAs Changes and cf mtDNA Level in Trauma Surgeons: Epigenetic and Molecular Biomarkers of X-ray Exposure
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Experiment Participants
2.2. Individual Annual Effective Doses for Trauma Surgeons
2.3. The Level of miRNA Expression in the Blood Plasma of Individuals Exposed to X-rays
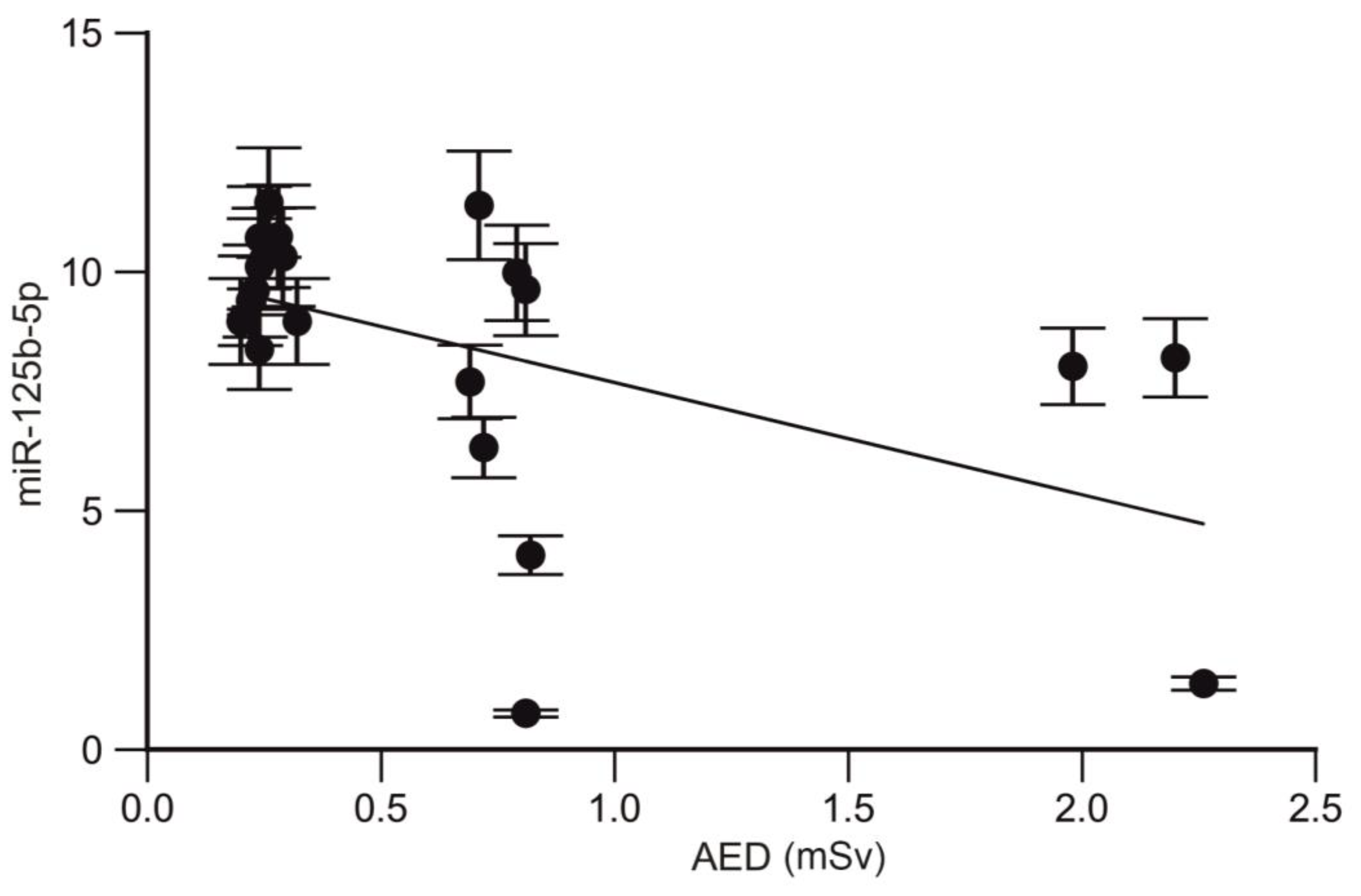
2.4. Analysis of the Correlation between the miRNA Profile and Annual Effective Dose of Trauma Surgeons
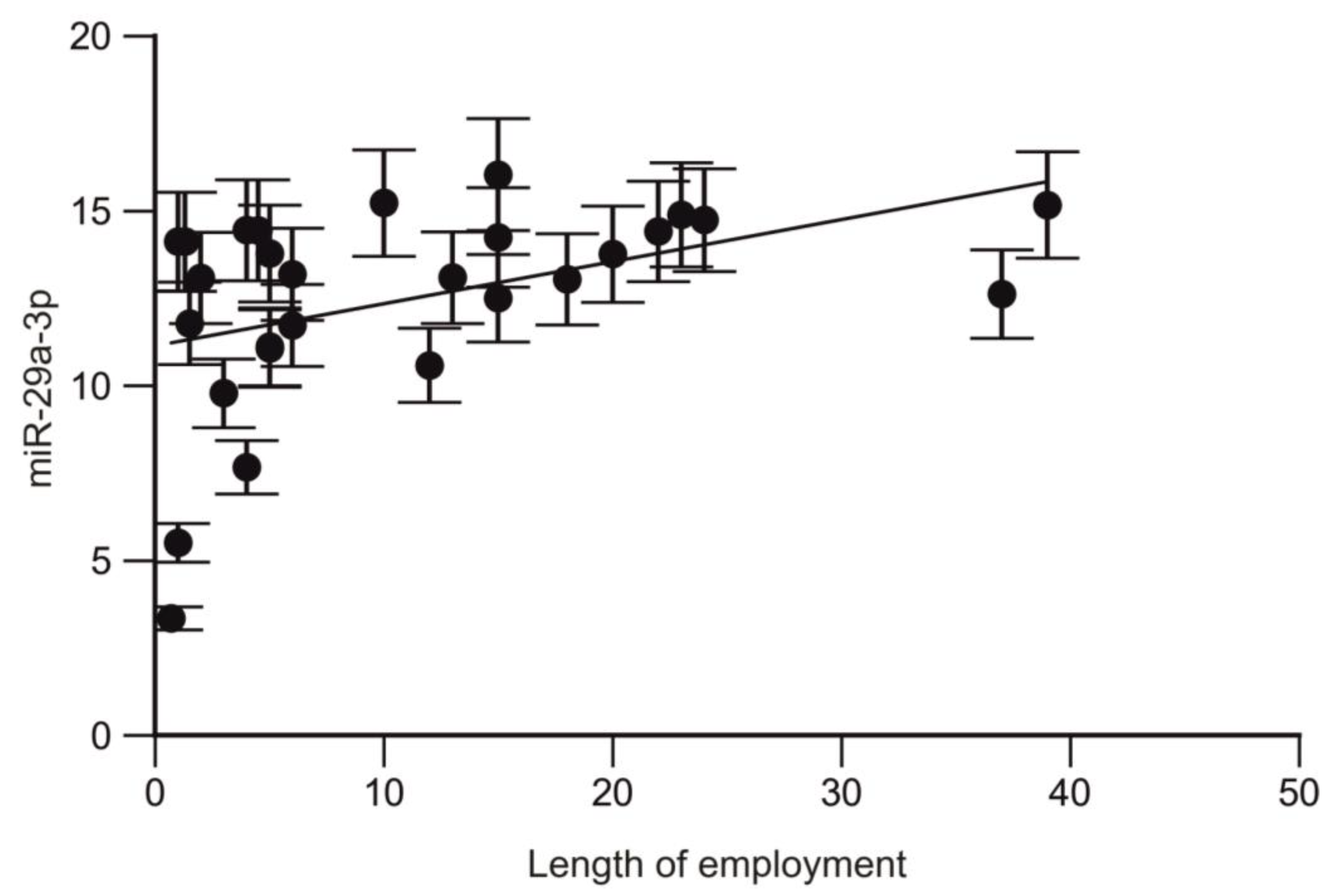
2.5. Analysis of the Correlation between the miRNA Profile and Length of Employment of Trauma Surgeons
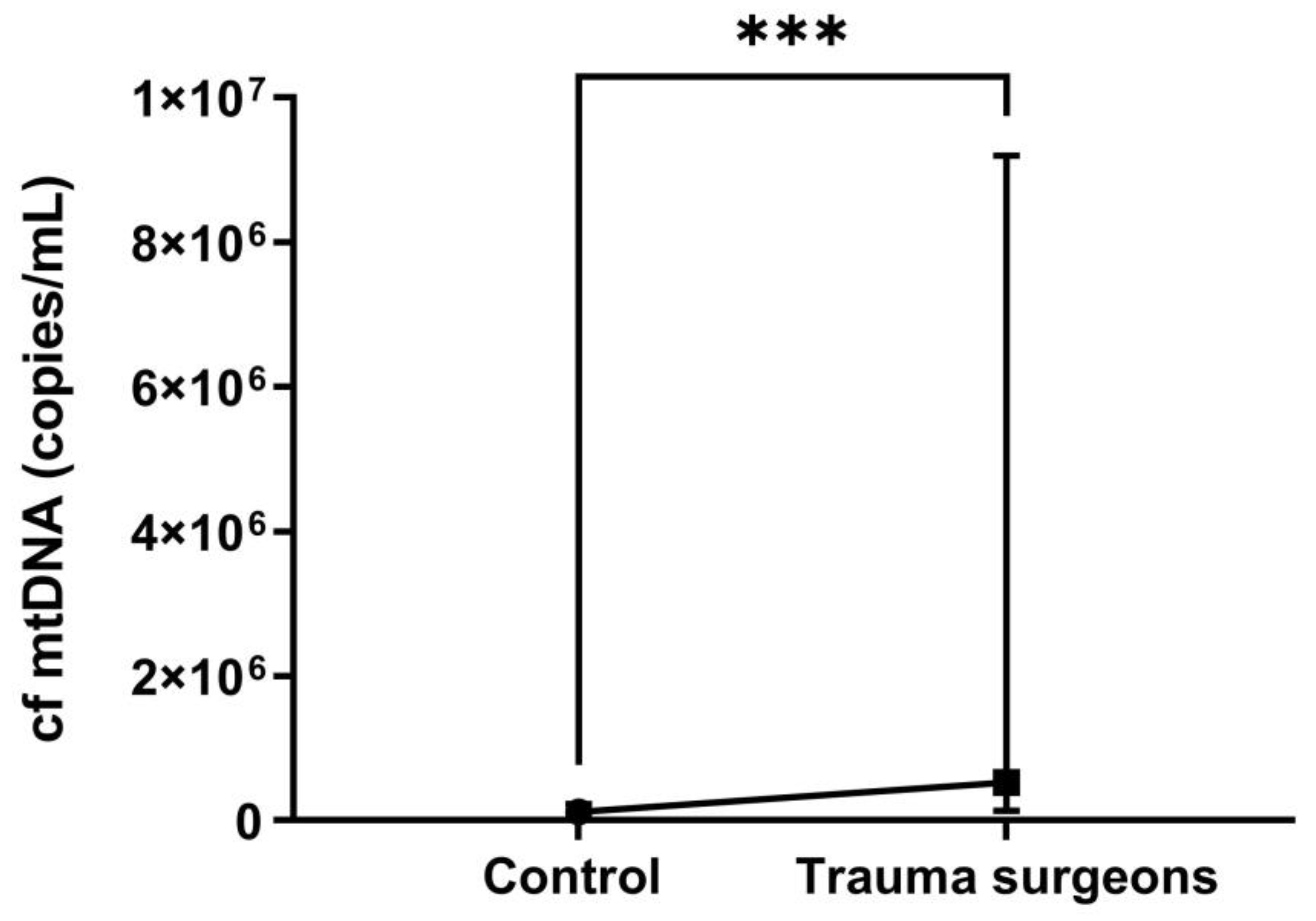
2.6. The cf mt Copy Numbers in the Blood Plasma of Individuals Exposed to X-ray
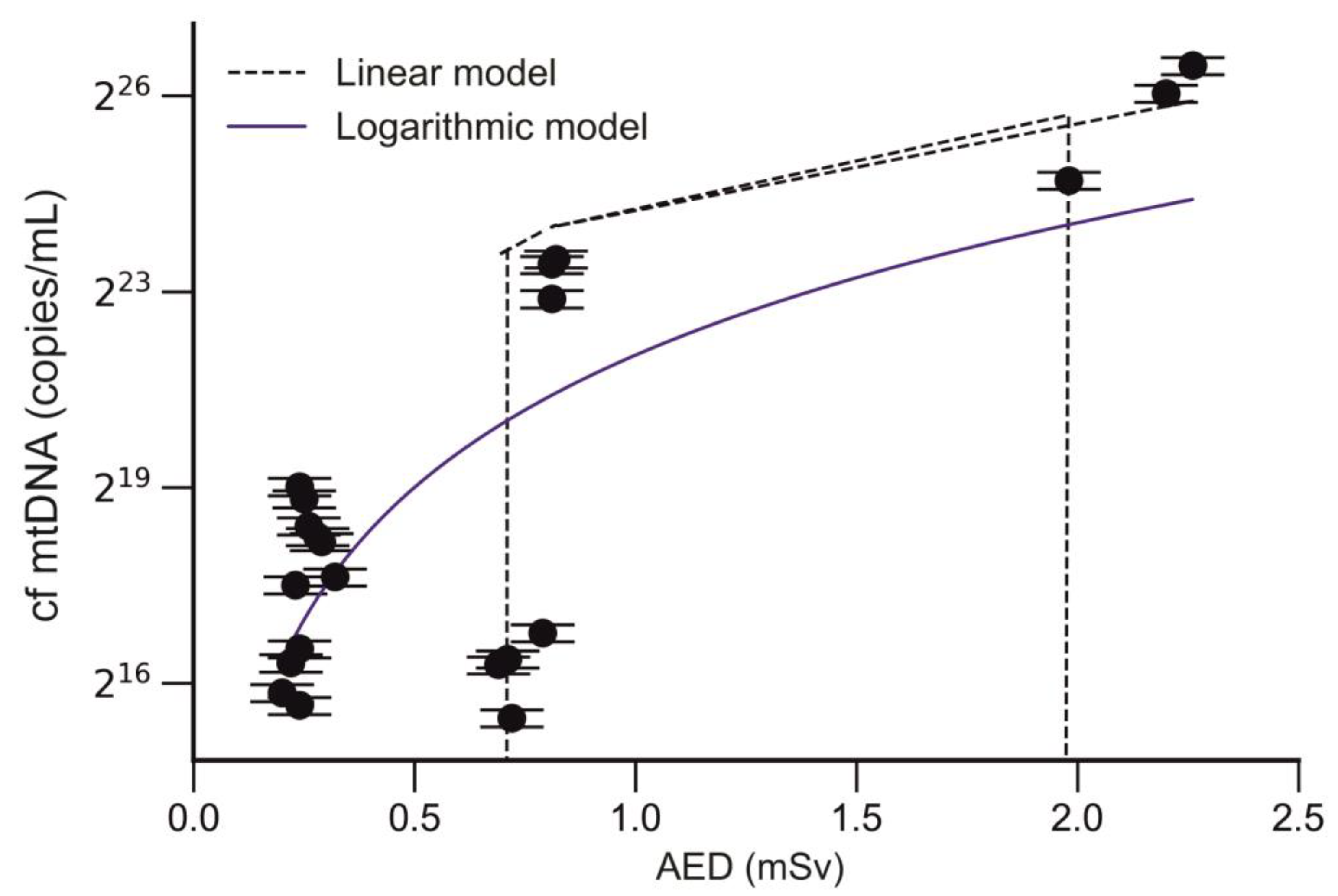
2.7. Analysis of the Correlation between the Level of cf mtDNA and Annual Effective Dose of Trauma Surgeons
2.8. Analysis of the Impact of Age, Gender and Smoking on Changes in miRNAs Profile and cf mtDNA Level
2.9. Prognostic Potential of miRNAs and cf mtDNA in Individuals Exposed to X-rays
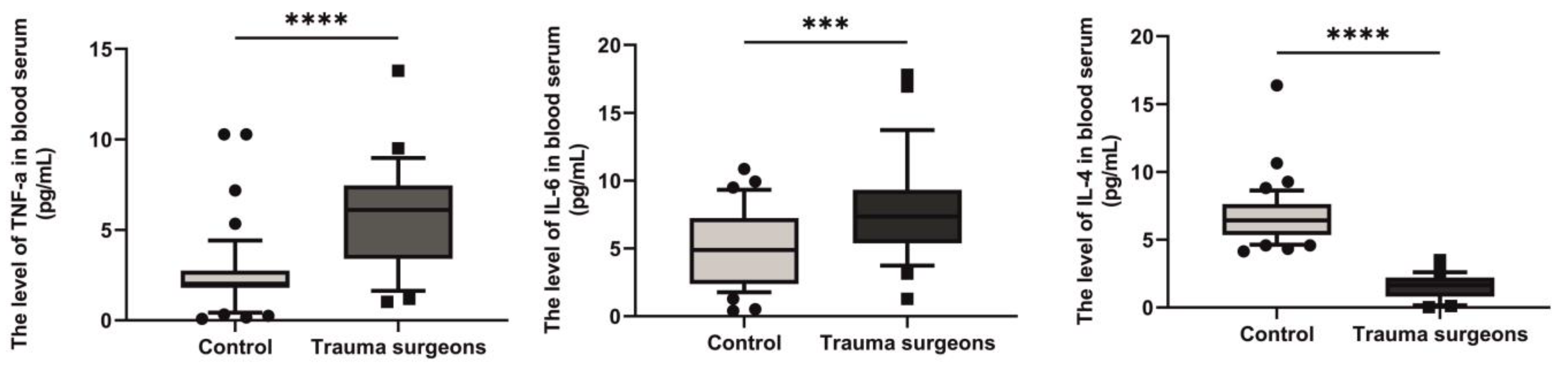
2.10. Levels of TNF-α, IL-6 and IL-4 in the Blood Serum of Individuals Exposed to X-rays
2.11. Analysis of the Correlation between the Spectrum of Pro- and Anti-Inflammatory Cytokines and Length of Employment of Trauma Surgeons
2.12. Search for a Correlation between the Profile of miRNAs and Pro- and Anti-Inflammatory Cytokines
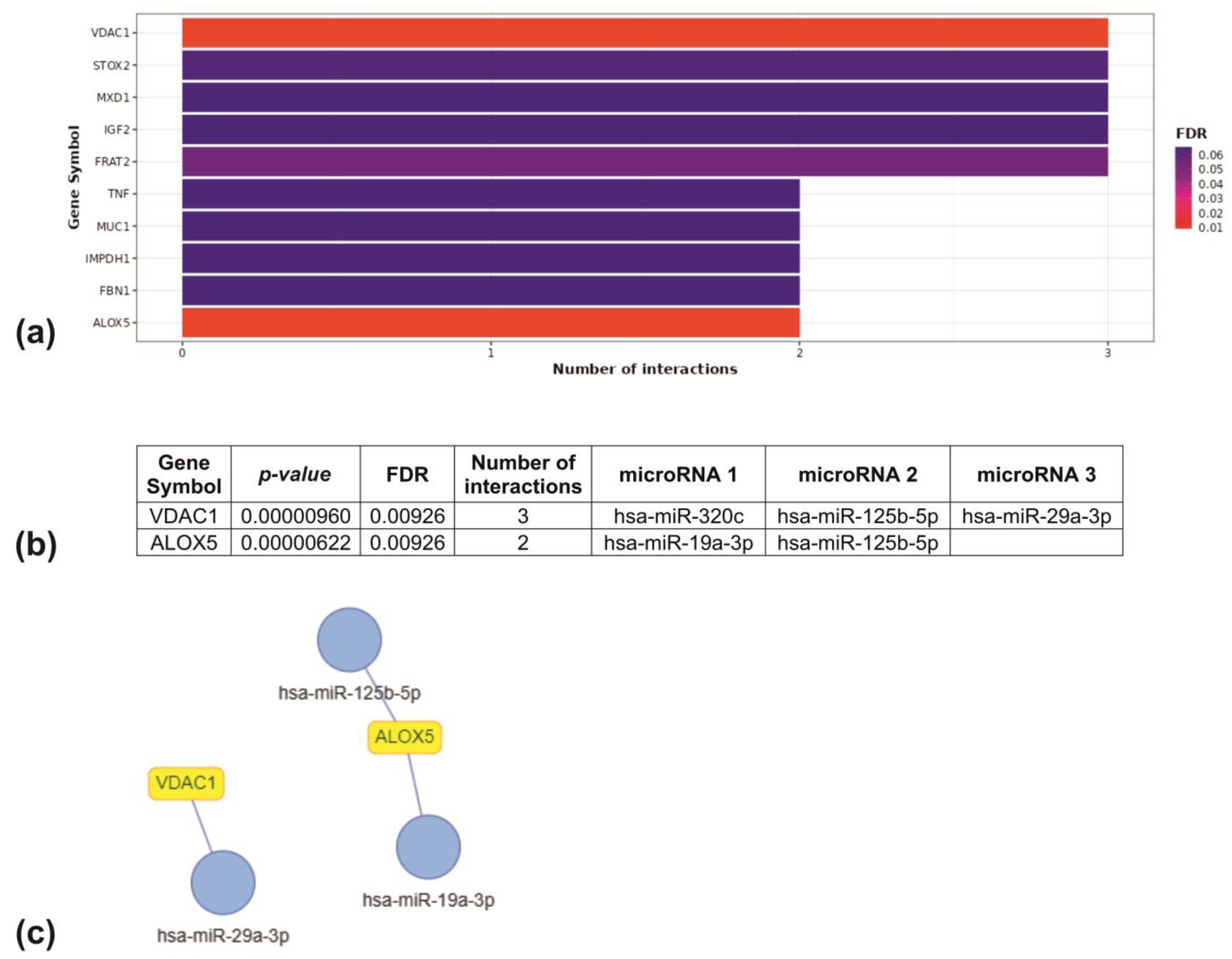
2.13. Target Enrichment Analysis Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Calculation of Individual Annual Effective Doses for Trauma Surgeons
4.3. Preparation of Blood Samples
4.4. miRNA Extraction
4.5. PCR Analysis of miRNA Expression
4.6. DNA Extraction
4.7. PCR Analysis
4.8. Detection of Cytokines in Serum
4.9. Statistical Analysis
4.10. Target Enrichment Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puckett, Y.; Al-Naser, Y.A.; Nappe, T.M. Ionizing Radiation; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Hamada, N.; Azizova, T.V.; Little, M.P. An Update on Effects of Ionizing Radiation Exposure on the Eye. Br. J. Radiol. 2020, 93, 20190829. [Google Scholar] [CrossRef] [PubMed]
- Rühm, W.; Laurier, D.; Wakeford, R. Cancer Risk Following Low Doses of Ionising Radiation—Current Epidemiological Evidence and Implications for Radiological Protection. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2022, 873, 503436. [Google Scholar] [CrossRef] [PubMed]
- Hauptmann, M.; Daniels, R.D.; Cardis, E.; Cullings, H.M.; Kendall, G.; Laurier, D.; Linet, M.S.; Little, M.P.; Lubin, J.H.; Preston, D.L.; et al. Epidemiological Studies of Low-Dose Ionizing Radiation and Cancer: Summary Bias Assessment and Meta-Analysis. JNCI Monogr. 2020, 2020, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; McAlister, J.; VanderKolk, W.E.; Goldsmith, A.; Mattice, C.; Davis, A.T. Radiation Exposure in Trauma Patients. J. Trauma Inj. Infect. Crit. Care 2006, 61, 607–610. [Google Scholar] [CrossRef] [PubMed]
- National Council on Radiation Protection and Measurements (NCRP). Limitation of Exposure to Ionizing Radiation: NCRP Report No. 116; National Council on Radiation Protection and Measurements: Bethesda, MD, USA, 1993. [Google Scholar]
- Meisinger, Q.C.; Stahl, C.M.; Andre, M.P.; Kinney, T.B.; Newton, I.G. Radiation Protection for the Fluoroscopy Operator and Staff. Am. J. Roentgenol. 2016, 207, 745–754. [Google Scholar] [CrossRef]
- Hurley, R.J.; McCabe, F.J.; Turley, L.; Maguire, D.; Lucey, J.; Hurson, C.J. Whole-Body Radiation Exposure in Trauma and Orthopaedic Surgery. Bone Jt. Open 2022, 3, 907–912. [Google Scholar] [CrossRef]
- Schaue, D.; Kachikwu, E.L.; McBride, W.H. Cytokines in Radiobiological Responses: A Review. Radiat. Res. 2012, 178, 505–523. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Rincon, M. Interleukin-6: From an Inflammatory Marker to a Target for Inflammatory Diseases. Trends Immunol. 2012, 33, 571–577. [Google Scholar] [CrossRef]
- Lenski, M.; Tonn, J.-C.; Siller, S. Interleukin-6 as Inflammatory Marker of Surgical Site Infection Following Spinal Surgery. Acta Neurochir. 2021, 163, 1583–1592. [Google Scholar] [CrossRef]
- Naik, S.P.; Mahesh, P.A.; Jarayaj, B.S.; Madhunapantula, S.V.; Jahromi, S.R.; Yadav, M.K. Evaluation of Inflammatory Markers Interleukin-6 (IL-6) and Matrix Metalloproteinase-9 (MMP-9) in Asthma. J. Asthma 2017, 54, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Netea, M.G.; van Riel, P.L.C.M.; van der Meer, J.W.M.; Stalenhoef, A.F.H. The Role of TNF-α in Chronic Inflammatory Conditions, Intermediary Metabolism, and Cardiovascular Risk. J. Lipid Res. 2007, 48, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Wang, Z. MicroRNAs as Biomarkers for Ionizing Radiation Injury. Front. Cell Dev. Biol. 2022, 10, 861451. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.; McTiernan, C.D.; Thome, C.; Khaper, N.; Lees, S.J.; Boreham, D.R.; Tai, T.C.; Tharmalingam, S. Identification of Radiation-Induced MiRNA Biomarkers Using the CGL1 Cell Model System. Bioengineering 2022, 9, 214. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Xie, D.; Gao, S.; Bai, C.-J.; Zhu, M.-X.; Guan, H.; Zhou, P.-K. A Biomarker Panel of Radiation-Upregulated MiRNA as Signature for Ionizing Radiation Exposure. Life 2020, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Borghini, A.; Vecoli, C.; Mercuri, A.; Carpeggiani, C.; Piccaluga, E.; Guagliumi, G.; Picano, E.; Andreassi, M.G. Low-Dose Exposure to Ionizing Radiation Deregulates the Brain-Specific MicroRNA-134 in Interventional Cardiologists. Circulation 2017, 136, 2516–2518. [Google Scholar] [CrossRef]
- Abend, M.; Azizova, T.; Müller, K.; Dörr, H.; Senf, S.; Kreppel, H.; Rusinova, G.; Glazkova, I.; Vyazovskaya, N.; Unger, K.; et al. Independent Validation of Candidate Genes Identified after a Whole Genome Screening on Mayak Workers Exposed to Prolonged Occupational Radiation. Radiat. Res. 2014, 182, 299. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological Roles of Mitochondrial Reactive Oxygen Species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pachouri, U.C.; Khaidem, D.C.; Kundu, A.; Chopra, C.; Singh, P. Mitochondrial DNA Damage and Diseases. F1000Research 2015, 4, 176. [Google Scholar] [CrossRef]
- Kim, M.M.; Clinger, J.D.; Masayesva, B.G.; Ha, P.K.; Zahurak, M.L.; Westra, W.H.; Califano, J.A. Mitochondrial DNA Quantity Increases with Histopathologic Grade in Premalignant and Malignant Head and Neck Lesions. Clin. Cancer Res. 2004, 10, 8512–8515. [Google Scholar] [CrossRef]
- Cicchillitti, L.; Corrado, G.; De Angeli, M.; Mancini, E.; Baiocco, E.; Patrizi, L.; Zampa, A.; Merola, R.; Martayan, A.; Conti, L.; et al. Circulating Cell-Free DNA Content as Blood Based Biomarker in Endometrial Cancer. Oncotarget 2017, 8, 115230–115243. [Google Scholar] [CrossRef] [PubMed]
- Bulgakova, O.; Kussainova, A.; Kakabayev, A.; Aripova, A.; Baikenova, G.; Izzotti, A.; Bersimbaev, R. The Level of Free-Circulating MtDNA in Patients with Radon-Induced Lung Cancer. Environ. Res. 2022, 207, 112215. [Google Scholar] [CrossRef] [PubMed]
- Kam, W.W.-Y.; Banati, R.B. Effects of Ionizing Radiation on Mitochondria. Free Radic. Biol. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Bulgakova, O.; Kausbekova, A.; Kussainova, A.; Kalibekov, N.; Serikbaiuly, D.; Bersimbaev, R. Involvement of Circulating Cell-Free Mitochondrial DNA and Proinflammatory Cytokines in Pathogenesis of Chronic Obstructive Pulmonary Disease and Lung Cancer. Asian Pac. J. Cancer Prev. 2021, 22, 1927–1933. [Google Scholar] [CrossRef] [PubMed]
- Giordano, B.D.; Baumhauer, J.F.; Morgan, T.L.; Rechtine, G.R. Patient and Surgeon Radiation Exposure: Comparison of Standard and Mini-C-Arm Fluoroscopy. J. Bone Jt. Surg. Am. Vol. 2009, 91, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Tunçer, N.; Kuyucu, E.; Sayar, Ş.; Polat, G.; Erdil, İ.; Tuncay, İ. Orthopedic Surgeons’ Knowledge Regarding Risk of Radiation Exposition: A Survey Analysis. SICOT J. 2017, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- The Order of the Minister of Health of the Republic of Kazakhstan Dated August 2, 2022 No. KR DSM-71. Registered with the Ministry of Justice of the Republic of Kazakhstan on August 3, 2022 No. 29012. Available online: https://adilet.zan.kz/rus/docs/V2200029012 (accessed on 30 July 2024).
- Karatasakis, A.; Brilakis, H.S.; Danek, B.A.; Karacsonyi, J.; Martinez-Parachini, J.R.; Nguyen-Trong, P.J.; Alame, A.J.; Roesle, M.K.; Rangan, B.V.; Rosenfield, K.; et al. Radiation-associated Lens Changes in the Cardiac Catheterization Laboratory: Results from the IC-CATARACT (CATaracts Attributed to RAdiation in the CaTh Lab) Study. Catheter. Cardiovasc. Interv. 2018, 91, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Benn, D.K.; Vig, P.S. Estimation of X-Ray Radiation Related Cancers in US Dental Offices: Is It Worth the Risk? Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2021, 132, 597–608. [Google Scholar] [CrossRef]
- Han, M.A.; Kim, J.H. Diagnostic X-Ray Exposure and Thyroid Cancer Risk: Systematic Review and Meta-Analysis. Thyroid. 2018, 28, 220–228. [Google Scholar] [CrossRef]
- Fink, C.A.; Bates, M.N. Melanoma and Ionizing Radiation: Is There a Causal Relationship? Radiat. Res. 2005, 164, 701–710. [Google Scholar] [CrossRef]
- Prasad, G.; Haas-Kogan, D.A. Radiation-Induced Gliomas. Expert. Rev. Neurother. 2009, 9, 1511–1517. [Google Scholar] [CrossRef] [PubMed]
- Metz-Flamant, C.; Samson, E.; Caër-Lorho, S.; Acker, A.; Laurier, D. Leukemia Risk Associated with Chronic External Exposure to Ionizing Radiation in a French Cohort of Nuclear Workers. Radiat. Res. 2012, 178, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, S.; Hauptmann, M.; Sigurdson, A.J.; Doody, M.M.; Freedman, D.M.; Alexander, B.H.; Linet, M.S.; Ron, E.; Mabuchi, K. Nonmelanoma Skin Cancer in Relation to Ionizing Radiation Exposure among U.S. Radiologic Technologists. Int. J. Cancer 2005, 115, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.A.; Preston-Martin, S.; Ross, R.K.; Bernstein, L. Medical Radiation, Family History of Cancer, and Benign Breast Disease in Relation to Breast Cancer Risk in Young Women, USA. Cancer Causes Control 2002, 13, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Kussainova, A.; Bulgakova, O.; Aripova, A.; Khalid, Z.; Bersimbaev, R.; Izzotti, A. The Role of Mitochondrial MiRNAs in the Development of Radon-Induced Lung Cancer. Biomedicines 2022, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Ibragimova, M.; Kussainova, A.; Aripova, A.; Bersimbaev, R.; Bulgakova, O. The Molecular Mechanisms in Senescent Cells Induced by Natural Aging and Ionizing Radiation. Cells 2024, 13, 550. [Google Scholar] [CrossRef] [PubMed]
- Templin, T.; Amundson, S.A.; Brenner, D.J.; Smilenov, L.B. Whole Mouse Blood MicroRNA as Biomarkers for Exposure to γ-Rays and 56Fe Ions. Int. J. Radiat. Biol. 2011, 87, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Jacob, N.K.; Cooley, J.V.; Yee, T.N.; Jacob, J.; Alder, H.; Wickramasinghe, P.; Maclean, K.H.; Chakravarti, A. Identification of Sensitive Serum MicroRNA Biomarkers for Radiation Biodosimetry. PLoS ONE 2013, 8, e57603. [Google Scholar] [CrossRef]
- Halimi, M.; Shahabi, A.; Moslemi, D.; Parsian, H.; Asghari, S.M.; Sariri, R.; Yeganeh, F.; Zabihi, E. Human Serum MiR-34a as an Indicator of Exposure to Ionizing Radiation. Radiat. Environ. Biophys. 2016, 55, 423–429. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zhou, L.; Hao, S.; Ding, Z.; Xiao, L.; Zhou, M. Exosomal Small RNA Sequencing Uncovers Dose-Specific MiRNA Markers for Ionizing Radiation Exposure. Dose-Response 2020, 18, 155932582092673. [Google Scholar] [CrossRef]
- Małachowska, B.; Tomasik, B.; Stawiski, K.; Kulkarni, S.; Guha, C.; Chowdhury, D.; Fendler, W. Circulating MicroRNAs as Biomarkers of Radiation Exposure: A Systematic Review and Meta-Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Peng, L.; Hua, S.; Li, X.; Ma, L.; Jie, J.; Chen, D.; Wang, Y.; Li, D. MiR-144-5p Enhances the Radiosensitivity of Non-Small-Cell Lung Cancer Cells via Targeting ATF2. Biomed. Res. Int. 2018, 2018, 5109497. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.; Malachowska, B.; Meghani, K.; Konstantinopoulos, P.A.; Guha, C.; Singh, V.K.; Chowdhury, D. Evolutionarily Conserved Serum MicroRNAs Predict Radiation-Induced Fatality in Nonhuman Primates. Sci. Transl. Med. 2017, 9, eaal2408. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.S.; Fendler, W.; Watson, J.; Hamilton, A.; Pan, Y.; Gaudiano, E.; Moskwa, P.; Bhanja, P.; Saha, S.; Guha, C.; et al. Serum MicroRNAs Are Early Indicators of Survival after Radiation-Induced Hematopoietic Injury. Sci. Transl. Med. 2015, 7, 287ra69. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-K.; Song, J.-Y.; Yun, Y.-S.; Yi, S.-Y. Effect of Gamma Radiation on Cytokine Expression and Cytokine-Receptor Mediated STAT Activation. Int. J. Radiat. Biol. 2006, 82, 686–697. [Google Scholar] [CrossRef] [PubMed]
- Barjaktarovic, Z.; Schmaltz, D.; Shyla, A.; Azimzadeh, O.; Schulz, S.; Haagen, J.; Dörr, W.; Sarioglu, H.; Schäfer, A.; Atkinson, M.J.; et al. Radiation–Induced Signaling Results in Mitochondrial Impairment in Mouse Heart at 4 Weeks after Exposure to X-Rays. PLoS ONE 2011, 6, e27811. [Google Scholar] [CrossRef] [PubMed]
- Averbeck, D.; Rodriguez-Lafrasse, C. Role of Mitochondria in Radiation Responses: Epigenetic, Metabolic, and Signaling Impacts. Int. J. Mol. Sci. 2021, 22, 11047. [Google Scholar] [CrossRef] [PubMed]
- Pearce, L.L.; Epperly, M.W.; Greenberger, J.S.; Pitt, B.R.; Peterson, J. Identification of Respiratory Complexes I and III as Mitochondrial Sites of Damage Following Exposure to Ionizing Radiation and Nitric Oxide. Nitric Oxide 2001, 5, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Taneja, N.; Tjalkens, R.; Philbert, M.; Rehemtulla, A. Irradiation of Mitochondria Initiates Apoptosis in a Cell Free System. Oncogene 2001, 20, 167–177. [Google Scholar] [CrossRef]
- Shimura, T.; Kobayashi, J.; Komatsu, K.; Kunugita, N. Severe Mitochondrial Damage Associated with Low-Dose Radiation Sensitivity in ATM- and NBS1-Deficient Cells. Cell Cycle 2016, 15, 1099–1107. [Google Scholar] [CrossRef]
- Borghini, A.; Mercuri, A.; Turchi, S.; Chiesa, M.R.; Piccaluga, E.; Andreassi, M.G. Increased Circulating Cell-free DNA Levels and mtDNA Fragments in Interventional Cardiologists Occupationally Exposed to Low Levels of Ionizing Radiation. Environ. Mol. Mutagen. 2015, 56, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Shoshan-Barmatz, V.; Shteinfer-Kuzmine, A.; Verma, A. VDAC1 at the Intersection of Cell Metabolism, Apoptosis, and Diseases. Biomolecules 2020, 10, 1485. [Google Scholar] [CrossRef] [PubMed]
- Arbel, N.; Ben-Hail, D.; Shoshan-Barmatz, V. Mediation of the Antiapoptotic Activity of Bcl-XL Protein upon Interaction with VDAC1 Protein. J. Biol. Chem. 2012, 287, 23152–23161. [Google Scholar] [CrossRef] [PubMed]
- Skonieczna, M.; Cieslar-Pobuda, A.; Saenko, Y.; Foksinski, M.; Olinski, R.; Rzeszowska-Wolny, J.; Wiechec, E. The Impact of DIDS-Induced Inhibition of Voltage-Dependent Anion Channels (VDAC) on Cellular Response of Lymphoblastoid Cells to Ionizing Radiation. Med. Chem. 2017, 13, 477–483. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, A.; Solodka, K.; Zanini, G.; Selleri, V.; Mattioli, A.V.; Nasi, M.; Pinti, M. Molecular Mechanisms of MtDNA-Mediated Inflammation. Cells 2021, 10, 2898. [Google Scholar] [CrossRef] [PubMed]
- Rencelj, A.; Gvozdenovic, N.; Cemazar, M. MitomiRs: Their Roles in Mitochondria and Importance in Cancer Cell Metabolism. Radiol. Oncol. 2021, 55, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.-Y.; Zhou, H.-H.; Mao, X.-Y. Emerging Roles of 5-Lipoxygenase Phosphorylation in Inflammation and Cell Death. Oxid. Med. Cell Longev. 2019, 2019, 2749173. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Wang, M.; Zeng, G.; Xiong, B.; Zhu, X.; Guo, J.; Chen, D.; Zhang, S.; Luo, M.; Guo, L.; Cai, L. ALOX5 Promotes Autophagy-Dependent Ferroptosis by Activating the AMPK/MTOR Pathway in Melanoma. Biochem. Pharmacol. 2023, 212, 115554. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, Y.-K.; Zhang, C.-G.; Wu, B.-Y. MiR-19a/b-3p Promotes Inflammation during Cerebral Ischemia/Reperfusion Injury via SIRT1/FoxO3/SPHK1 Pathway. J. Neuroinflammation 2021, 18, 122. [Google Scholar] [CrossRef]
- Sahebdel, F.; Morse, L.; Battaglino, R.; Olson, J.K. MiR-19a-3p and MiR-19b-3p Promote Microglia Activation Associated with Neuroinflammation Following Spinal Cord Injury. J. Immunol. 2023, 210, 167.21. [Google Scholar] [CrossRef]
- Liu, G.; Wan, Q.; Li, J.; Hu, X.; Gu, X.; Xu, S. Silencing MiR-125b-5p Attenuates Inflammatory Response and Apoptosis Inhibition in Mycobacterium Tuberculosis-Infected Human Macrophages by Targeting DNA Damage-Regulated Autophagy Modulator 2 (DRAM2). Cell Cycle 2020, 19, 3182–3194. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Rasheed, N.; Al Abdulmonem, W.; Khan, M.I. Author Correction: MicroRNA-125b-5p Regulates IL-1β Induced Inflammatory Genes via Targeting TRAF6-Mediated MAPKs and NF-ΚB Signaling in Human Osteoarthritic Chondrocytes. Sci. Rep. 2019, 9, 14729. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Motevaseli, E.; Shirazi, A.; Geraily, G.; Rezaeyan, A.; Norouzi, F.; Rezapoor, S.; Abdollahi, H. Mechanisms of Inflammatory Responses to Radiation and Normal Tissues Toxicity: Clinical Implications. Int. J. Radiat. Biol. 2018, 94, 335–356. [Google Scholar] [CrossRef] [PubMed]
- Rho, H.-S.; Kim, S.-H.; Lee, C.-E. Mechanism of NF-ΚB Activation Induced by γ-Irradiation in B Lymphoma Cells: Role of Ras. J. Toxicol. Environ. Health A 2005, 68, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Bai, L.; Chen, W.; Xu, S. The NF-ΚB Activation Pathways, Emerging Molecular Targets for Cancer Prevention and Therapy. Expert. Opin. Ther. Targets 2010, 14, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Beetz, R.U.P.T.O.W.K.A. NF-ΚB and AP-1 Are Responsible for Inducibility of the IL-6 Promoter by Ionizing Radiation in HeLa Cells. Int. J. Radiat. Biol. 2000, 76, 1443–1453. [Google Scholar] [CrossRef] [PubMed]
- Linard, C.; Marquette, C.; Mathieu, J.; Pennequin, A.; Clarençon, D.; Mathé, D. Acute Induction of Inflammatory Cytokine Expression after γ-Irradiation in the Rat: Effect of an NF-ΚB Inhibitor. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.-X.; Jin, S.-Z.; Liu, X.-D.; Liu, Y.; Liu, S.-Z. Ionizing Radiation Stimulates Secretion of Pro-Inflammatory Cytokines: Dose–Response Relationship, Mechanisms and Implications. Radiat. Environ. Biophys. 2007, 46, 21–29. [Google Scholar] [CrossRef]
- Kiang, J.G.; Smith, J.T.; Hegge, S.R.; Ossetrova, N.I. Circulating Cytokine/Chemokine Concentrations Respond to Ionizing Radiation Doses but Not Radiation Dose Rates: Granulocyte-Colony Stimulating Factor and Interleukin-18. Radiat. Res. 2018, 189, 634–643. [Google Scholar] [CrossRef]
- Müller, K.; Meineke, V. Radiation-Induced Alterations in Cytokine Production by Skin Cells. Exp. Hematol. 2007, 35, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, S.; Panguluri, S.K.; Kumar, A. Gene Profiling Studies in Skeletal Muscle by Quantitative Real-Time Polymerase Chain Reaction Assay. In Myogenesis; Humana Press: Totowa, NJ, USA, 2012; pp. 311–324. [Google Scholar]







| Characteristic | Control n = 56 (%) | Trauma Surgeons n = 30 (%) | |
|---|---|---|---|
| Gender | Male | 25 (45) | 19 (63) |
| Female | 31 (55) | 11 (27) | |
| Age (years) | ≥40 | 15 (27) | 10 (33) |
| <40 | 41 (73) | 20 (67) | |
| Smoking | Nonsmoker | 21 (38) | 24 (80) |
| Former smoker | 2 (2) | 3 (10) | |
| Current smoker | 33 (60) | 3 (10) | |
| Work experience | 1–5 years | 13 (43) | |
| 6–10 years | 4 (16) | ||
| >10 years | 13 (43) | ||
| Number of working hours per day | 1–5 h | 11 (37) | |
| 6–8 h | 11 (37) | ||
| >8 h | 8 (26) | ||
| Sample | AED (mSv) | Sample | AED (mSv) | Sample | AED (mSv) | Sample | AED (mSv) | Sample | AED (mSv) |
|---|---|---|---|---|---|---|---|---|---|
| 1T | 2.2 | 7T | 0.2 | 13T | 0.26 | 19T | 0.72 | 25T | 0.28 |
| 2T | 2.26 | 8T | 0.22 | 14T | 0.28 | 20T | 0.69 | 26T | 0.26 |
| 3T | 0.81 | 9T | 0.23 | 15T | 0.29 | 21T | 0.79 | 27T | 0.2 |
| 4T | 0.82 | 10T | 0.24 | 16T | 0.32 | 22T | 0.29 | 28T | 0.82 |
| 5T | 0.81 | 11T | 0.24 | 17T | 0.24 | 23T | 0.29 | 29T | 0.32 |
| 6T | 1.98 | 12T | 0.25 | 18T | 0.71 | 24T | 0.32 | 30T | 0.72 |
| Parameter | Control, Copies/mL | Trauma Surgeons, Copies/mL | p Value |
|---|---|---|---|
| Median in both smokers and non-smokers | 1.21 × 105 | 5.28 × 105 | 0.0005 |
| Median nonsmokers | 1.03 × 105 | 3.18 × 105 | 0.01 |
| Median smokers | 1.45 × 105 | 1.38 × 107 | 0.003 |
| F (DFn.DFd); p Value | |||
|---|---|---|---|
| Age | Gender | Smoking | |
| miR-19a-3p | F (1.80) = 1.229 p = 0.2709 | F (1.80) = 0.5105 p = 0.4770 | F (2.80) = 0.2510 p = 0.7787 |
| miR-29a-3p | F (1.54) = 0.1488 p = 0.7012 | F (1.54) = 2.348 p = 0.1313 | F (2.54) = 0.7915 p = 0.4584 |
| miR-125b-5p | F (1.61) = 0.07672 p = 0.7827 | F (1.61) = 0.3474 p = 0.5577 | F (2.61) = 1.457 p = 0.2409 |
| miR-142-5p | F (1.81) = 0.4522 p = 0.5032 | F (1.81) = 0.9459 p = 0.3337 | F (2.81) = 3.330 p = 0.0407 |
| miR-144-5p | F (1.54) = 0.03664 p = 0.8489 | F (1.54) = 3.254 p = 0.0768 | F (2.54) = 0.5587 p = 0.5752 |
| miR-150-5p | F (1.77) = 2.176 p = 0.1442 | F (1.77) = 0.08428 p = 0.7724 | F (2.77) = 2.578 p = 0.0825 |
| miR-181a-3p | F (1.75) = 0.1840 p = 0.6692 | F (1.75) = 0.1485 p = 0.7011 | F (2.75) = 0.3031 p = 0.7394 |
| miR-320c | F (1.59) = 0.2414 p = 0.6250 | F (1.59) = 1.471 p = 0.2301 | F (2.59) = 1.304 p = 0.2790 |
| cf mtDNA | F (1.80) = 2.562 p = 0.1134 | F (1.80) = 0.2057 p = 0.6514 | - |
| Area under the ROC Curve (AUC) | miR-19a- 3p | miR-29a- 3p | miR-125b-5p | miR-142- 5p | miR-144- 5p | miR-150- 5p | miR-181a-3p | miR-320c | cf mtDNA |
|---|---|---|---|---|---|---|---|---|---|
| 95% Confidence interval | 0.615 to 0.867 | 0.674 to 0.907 | 0.604 to 0.859 | 0.852 to 0.992 | 0.650 to 0.891 | 0.599 to 0.855 | 0.717 to 0.933 | 0.737 to 0.944 | 0.700 to 0.923 |
| Significance level p | 0.0009 | <0.0001 | 0.0018 | <0.0001 | <0.0001 | 0.0019 | <0.0001 | <0.0001 | <0.0001 |
| Sensitivity | 76.19 | 80.95 | 85.71 | 85.71 | 80.95 | 80.95 | 80.95 | 85.71 | 100 |
| Specificity | 82.76 | 82.76 | 79.31 | 96.55 | 75.86 | 72.41 | 86.21 | 82.76 | 62.07 |
| Group | Primers | Nucleotide Sequence (5’−3’) | qPCR Program |
|---|---|---|---|
| Population | forward | 5’-CAGCCGCTATTAAAGGTTCG-3’ | 90 °C—10 min; (95 °C—15 s, 60 °C—60 s) × 40 |
| reverse | 5’-GGGCTCTGCCATCTTAACAA-3’ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kussainova, A.; Aripova, A.; Ibragimova, M.; Bersimbaev, R.; Bulgakova, O. Radiation-Induced miRNAs Changes and cf mtDNA Level in Trauma Surgeons: Epigenetic and Molecular Biomarkers of X-ray Exposure. Int. J. Mol. Sci. 2024, 25, 8446. https://doi.org/10.3390/ijms25158446
Kussainova A, Aripova A, Ibragimova M, Bersimbaev R, Bulgakova O. Radiation-Induced miRNAs Changes and cf mtDNA Level in Trauma Surgeons: Epigenetic and Molecular Biomarkers of X-ray Exposure. International Journal of Molecular Sciences. 2024; 25(15):8446. https://doi.org/10.3390/ijms25158446
Chicago/Turabian StyleKussainova, Assiya, Akmaral Aripova, Milana Ibragimova, Rakhmetkazhi Bersimbaev, and Olga Bulgakova. 2024. "Radiation-Induced miRNAs Changes and cf mtDNA Level in Trauma Surgeons: Epigenetic and Molecular Biomarkers of X-ray Exposure" International Journal of Molecular Sciences 25, no. 15: 8446. https://doi.org/10.3390/ijms25158446
APA StyleKussainova, A., Aripova, A., Ibragimova, M., Bersimbaev, R., & Bulgakova, O. (2024). Radiation-Induced miRNAs Changes and cf mtDNA Level in Trauma Surgeons: Epigenetic and Molecular Biomarkers of X-ray Exposure. International Journal of Molecular Sciences, 25(15), 8446. https://doi.org/10.3390/ijms25158446





