Thermoneutrality Inhibits Thermogenic Markers and Exacerbates Nonalcoholic Fatty Liver Disease in Mice
Abstract
1. Introduction
2. Results
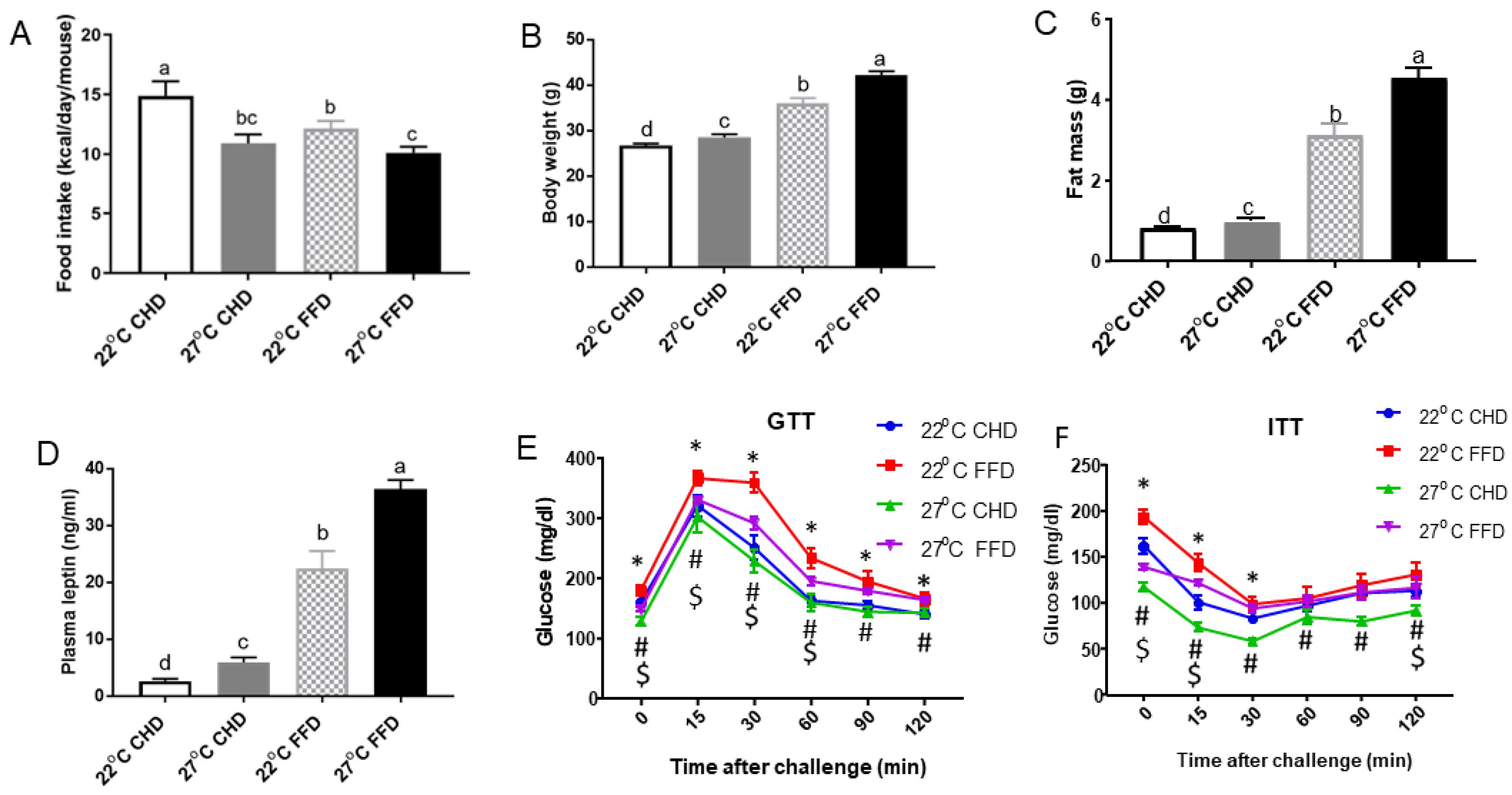
2.1. Thermoneutrality Inhibited Food Intake but Increased Body Weight Gain in Mice
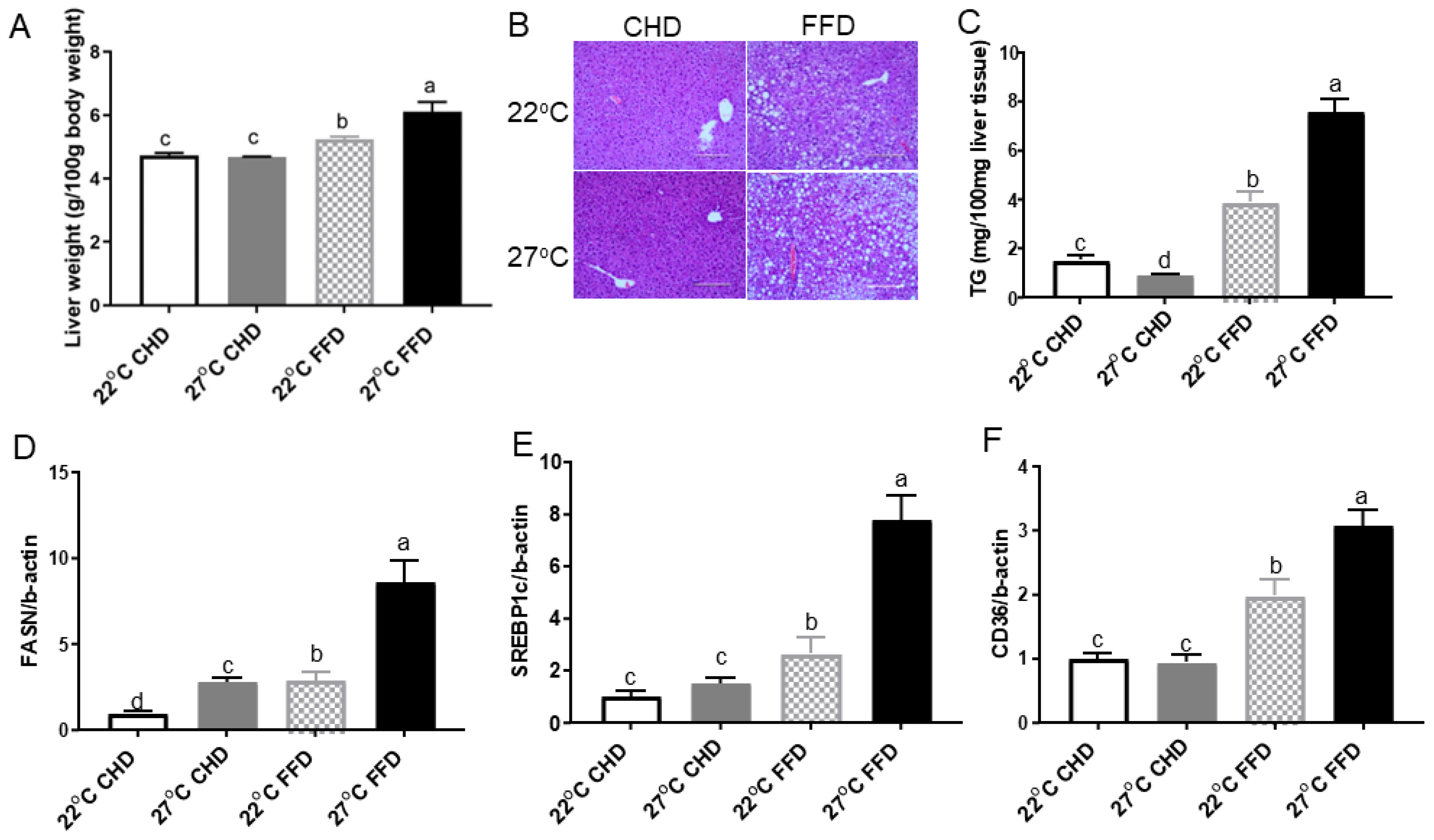
2.2. Thermoneutrality Exacerbated Development NAFLD in Mice Fed FFD
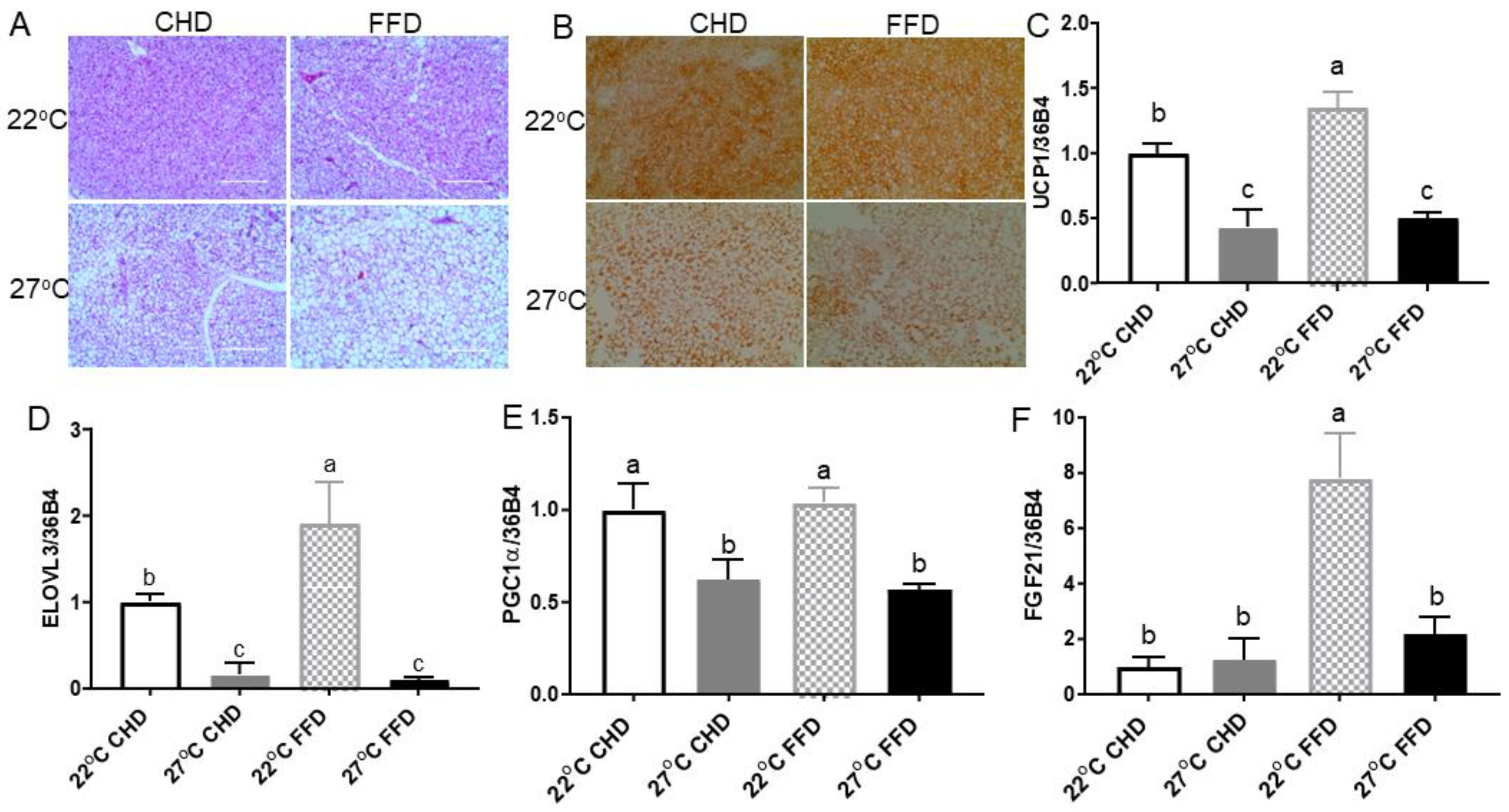
2.3. Thermoneutrality Suppressed the Thermogenic Program in BAT
2.4. Thermoneutrality Increased Adiposity and Suppressed Thermogenic Markers in Inguinal WAT (iWAT)
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Quantitative Real-Time PCR
4.3. Glucose and Insulin Tolerance Tests
4.4. Histology
4.5. Liver TG
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hardy, T.; Oakley, F.; Anstee, Q.M.; Day, C.P. Nonalcoholic Fatty Liver Disease: Pathogenesis and Disease Spectrum. Annu. Rev. Pathol. 2016, 11, 451–496. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Albhaisi, S.; Issa, D.; Alkhouri, N. Non-alcoholic fatty liver disease: A pandemic disease with multisystem burden. Hepatobiliary Surg. Nutr. 2018, 7, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Muzurović, E.; Peng, C.C.; Belanger, M.J.; Sanoudou, D.; Mikhailidis, D.P.; Mantzoros, C.S. Nonalcoholic Fatty Liver Disease and Cardiovascular Disease: A Review of Shared Cardiometabolic Risk Factors. Hypertension 2022, 79, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Kingwell, K. NASH field celebrates ‘hurrah moment’ with a first FDA drug approval for the liver disease. Nat. Rev. Drug Discov. 2024, 23, 235–237. [Google Scholar] [CrossRef]
- Harrison, S.A.; Taub, R.; Neff, G.W.; Lucas, K.J.; Labriola, D.; Moussa, S.E.; Alkhouri, N.; Bashir, M.R. Resmetirom for nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 2023, 29, 2919–2928. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Loh, R.K.C.; Kingwell, B.A.; Carey, A.L. Human brown adipose tissue as a target for obesity management; beyond cold-induced thermogenesis. Obes. Rev. 2017, 18, 1227–1242. [Google Scholar] [CrossRef]
- Giralt, M.; Villarroya, F. White, brown, beige/brite: Different adipose cells for different functions? Endocrinology 2013, 154, 2992–3000. [Google Scholar] [CrossRef]
- Cereijo, R.; Giralt, M.; Villarroya, F. Thermogenic brown and beige/brite adipogenesis in humans. Ann. Med. 2015, 47, 169–177. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P.; Virtanen, K.A.; Richard, D.; Haman, F.; Turcotte, E.E. Brown Adipose Tissue Energy Metabolism in Humans. Front. Endocrinol. 2018, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Chondronikola, M.; Volpi, E.; Borsheim, E.; Porter, C.; Annamalai, P.; Enerback, S.; Lidell, M.E.; Saraf, M.K.; Labbe, S.M.; Hurren, N.M.; et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014, 63, 4089–4099. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Smith, S.; Linderman, J.; Courville, A.B.; Brychta, R.J.; Dieckmann, W.; Werner, C.D.; Chen, K.Y.; Celi, F.S. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 2014, 63, 3686–3698. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, M.J.; Hoeks, J.; Brans, B.; van der Lans, A.A.; Schaart, G.; van den Driessche, J.J.; Jorgensen, J.A.; Boekschoten, M.V.; Hesselink, M.K.; Havekes, B.; et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med. 2015, 21, 863–865. [Google Scholar] [CrossRef]
- Lee, P.; Bova, R.; Schofield, L.; Bryant, W.; Dieckmann, W.; Slattery, A.; Govendir, M.A.; Emmett, L.; Greenfield, J.R. Brown Adipose Tissue Exhibits a Glucose-Responsive Thermogenic Biorhythm in Humans. Cell Metab. 2016, 23, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Iwen, K.A.; Backhaus, J.; Cassens, M.; Waltl, M.; Hedesan, O.C.; Merkel, M.; Heeren, J.; Sina, C.; Rademacher, L.; Windjager, A.; et al. Cold-Induced Brown Adipose Tissue Activity Alters Plasma Fatty Acids and Improves Glucose Metabolism in Men. J. Clin. Endocrinol. Metab. 2017, 102, 4226–4234. [Google Scholar] [CrossRef] [PubMed]
- Bartelt, A.; Bruns, O.T.; Reimer, R.; Hohenberg, H.; Ittrich, H.; Peldschus, K.; Kaul, M.G.; Tromsdorf, U.I.; Weller, H.; Waurisch, C.; et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 2011, 17, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Berbee, J.F.; Boon, M.R.; Khedoe, P.P.; Bartelt, A.; Schlein, C.; Worthmann, A.; Kooijman, S.; Hoeke, G.; Mol, I.M.; John, C.; et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun. 2015, 6, 6356. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Z.; Zhu, X.; Meng, M.; Li, L.; Shen, Y.; Chi, Q.; Wang, D.; Zhang, Z.; Li, C.; et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 2013, 23, 851–854. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; You, Y.; Meng, M.; Zheng, Z.; Dong, M.; Lin, J.; Zhao, Q.; Zhang, C.; Yuan, X.; et al. Brown Adipose Tissue Transplantation Reverses Obesity in Ob/Ob Mice. Endocrinology 2015, 156, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Levy, J.D.; Zhang, Y.; Frontini, A.; Kolodin, D.P.; Svensson, K.J.; Lo, J.C.; Zeng, X.; Ye, L.; Khandekar, M.J.; et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 2014, 156, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, Y.; Ones, T.; Purnak, T.; Ozguven, S.; Kurt, R.; Atug, O.; Turoglu, H.T.; Imeryuz, N. Association between the presence of brown adipose tissue and non-alcoholic fatty liver disease in adult humans. Aliment. Pharmacol. Ther. 2011, 34, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Ozguven, S.; Ones, T.; Yilmaz, Y.; Turoglu, H.T.; Imeryuz, N. The role of active brown adipose tissue in human metabolism. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Merlin, J.; Evans, B.A.; Dehvari, N.; Sato, M.; Bengtsson, T.; Hutchinson, D.S. Could burning fat start with a brite spark? Pharmacological and nutritional ways to promote thermogenesis. Mol. Nutr. Food Res. 2016, 60, 18–42. [Google Scholar] [CrossRef] [PubMed]
- Cannon, B.; Nedergaard, J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 2011, 214, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R.; Keijer, J. Not so hot: Optimal housing temperatures for mice to mimic the thermal environment of humans. Mol. Metab. 2012, 2, 5–9. [Google Scholar] [CrossRef]
- Sanchez-Gurmaches, J.; Hung, C.M.; Guertin, D.A. Emerging Complexities in Adipocyte Origins and Identity. Trends Cell Biol. 2016, 26, 313–326. [Google Scholar] [CrossRef]
- Cui, X.; Nguyen, N.L.; Zarebidaki, E.; Cao, Q.; Li, F.; Zha, L.; Bartness, T.; Shi, H.; Xue, B. Thermoneutrality decreases thermogenic program and promotes adiposity in high-fat diet-fed mice. Physiol. Rep. 2016, 4, e12799. [Google Scholar] [CrossRef]
- Uchida, K.; Shiuchi, T.; Inada, H.; Minokoshi, Y.; Tominaga, M.; Uchida, K.; Shiuchi, T.; Inada, H.; Minokoshi, Y.; Tominaga, M. Metabolic adaptation of mice in a cool environment. Pflug. Arch. 2010, 459, 765–774. [Google Scholar] [CrossRef]
- Giles, D.; Moreno-Fernandez, M.; Stankiewicz, T.; Graspeuntner, S.; Cappelletti, M.; Wu, D.; Mukherjee, R.; Chan, C.; Lawson, M.; Klarquist, J.; et al. Thermoneutral housing exacerbates nonalcoholic fatty liver disease in mice and allows for sex-independent disease modeling. Nat. Med. 2017, 23, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Small, L.; Gong, H.; Yassmin, C.; Cooney, G.J.; Brandon, A.E. Thermoneutral housing does not influence fat mass or glucose homeostasis in C57BL/6 mice. J. Endocrinol. 2018, 239, 313–324. [Google Scholar] [CrossRef]
- Tian, X.; Ganeshan, K.; Hong, C.; Nguyen, K.; Qiu, Y.; Kim, J.; Tangirala, R.; Tontonoz, P.; Tonotonoz, P.; Chawla, A.; et al. Thermoneutral housing accelerates metabolic inflammation to potentiate atherosclerosis but not insulin resistance. Cell Metab. 2016, 23, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Dudele, A.; Rasmussen, G.M.; Mayntz, D.; Malte, H.; Lund, S.; Wang, T. Effects of ambient temperature on glucose tolerance and insulin sensitivity test outcomes in normal and obese C57 male mice. Physiol. Rep. 2015, 3, e12396. [Google Scholar] [CrossRef]
- Jun, J.C.; Shin, M.K.; Yao, Q.; Devera, R.; Fonti-Bevans, S.; Polotsky, V.Y. Thermoneutrality modifies the impact of hypoxia on lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E424–E435. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, C.; Ma, Y.; Liu, D. Cold Exposure Improves the Anti-diabetic Effect of T0901317 in Streptozotocin-Induced Diabetic Mice. Aaps J. 2015, 17, 700–710. [Google Scholar] [CrossRef][Green Version]
- Vallerand, A.L.; Lupien, J.; Bukowiecki, L.J. Cold exposure reverses the diabetogenic effects of high-fat feeding. Diabetes 1986, 35, 329–334. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef] [PubMed]
- Low, Z.S.; Chua, D.; Cheng, H.S.; Tee, R.; Tan, W.R.; Ball, C.; Sahib, N.B.E.; Ng, S.S.; Qu, J.; Liu, Y.; et al. The LIDPAD Mouse Model Captures the Multisystem Interactions and Extrahepatic Complications in MASLD. Adv. Sci. 2024, e2404326. [Google Scholar] [CrossRef]
- Cypess, A.M.; Lehman, S.; Williams, G.; Tal, I.; Rodman, D.; Goldfine, A.B.; Kuo, F.C.; Palmer, E.L.; Tseng, Y.H.; Doria, A.; et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 2009, 360, 1509–1517. [Google Scholar] [CrossRef]
- Wang, G.X.; Zhao, X.Y.; Meng, Z.X.; Kern, M.; Dietrich, A.; Chen, Z.; Cozacov, Z.; Zhou, D.; Okunade, A.L.; Su, X.; et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med. 2014, 20, 1436–1443. [Google Scholar] [CrossRef]
- Fisher, F.M.; Estall, J.L.; Adams, A.C.; Antonellis, P.J.; Bina, H.A.; Flier, J.S.; Kharitonenkov, A.; Spiegelman, B.M.; Maratos-Flier, E. Integrated regulation of hepatic metabolism by fibroblast growth factor 21 (FGF21) in vivo. Endocrinology 2011, 152, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Scott, S.; Abbasi, M.; Zu, Y.; Khan, M.S.H.; Yang, Y.; Wu, D.; Zhao, L.; Wang, S. Beneficial Metabolic Effects of Mirabegron In Vitro and in High-Fat Diet-Induced Obese Mice. J. Pharmacol. Exp. Ther. 2019, 369, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, L.; Khan, M.S.H.; Zu, Y.; Liu, J.; Wang, S. Thermoneutrality Inhibits Thermogenic Markers and Exacerbates Nonalcoholic Fatty Liver Disease in Mice. Int. J. Mol. Sci. 2024, 25, 8482. https://doi.org/10.3390/ijms25158482
Hao L, Khan MSH, Zu Y, Liu J, Wang S. Thermoneutrality Inhibits Thermogenic Markers and Exacerbates Nonalcoholic Fatty Liver Disease in Mice. International Journal of Molecular Sciences. 2024; 25(15):8482. https://doi.org/10.3390/ijms25158482
Chicago/Turabian StyleHao, Lei, Md Shahjalal Hossain Khan, Yujiao Zu, Jie Liu, and Shu Wang. 2024. "Thermoneutrality Inhibits Thermogenic Markers and Exacerbates Nonalcoholic Fatty Liver Disease in Mice" International Journal of Molecular Sciences 25, no. 15: 8482. https://doi.org/10.3390/ijms25158482
APA StyleHao, L., Khan, M. S. H., Zu, Y., Liu, J., & Wang, S. (2024). Thermoneutrality Inhibits Thermogenic Markers and Exacerbates Nonalcoholic Fatty Liver Disease in Mice. International Journal of Molecular Sciences, 25(15), 8482. https://doi.org/10.3390/ijms25158482







