The Invisible Fraction within Melanin Capable of Absorbing UV Light and with Fluorescent Properties: Is It Lacking Consideration?
Abstract
:1. Introduction
2. Results
2.1. Observations during Synthesis of Materials
2.1.1. DHI
2.1.2. Serotonin
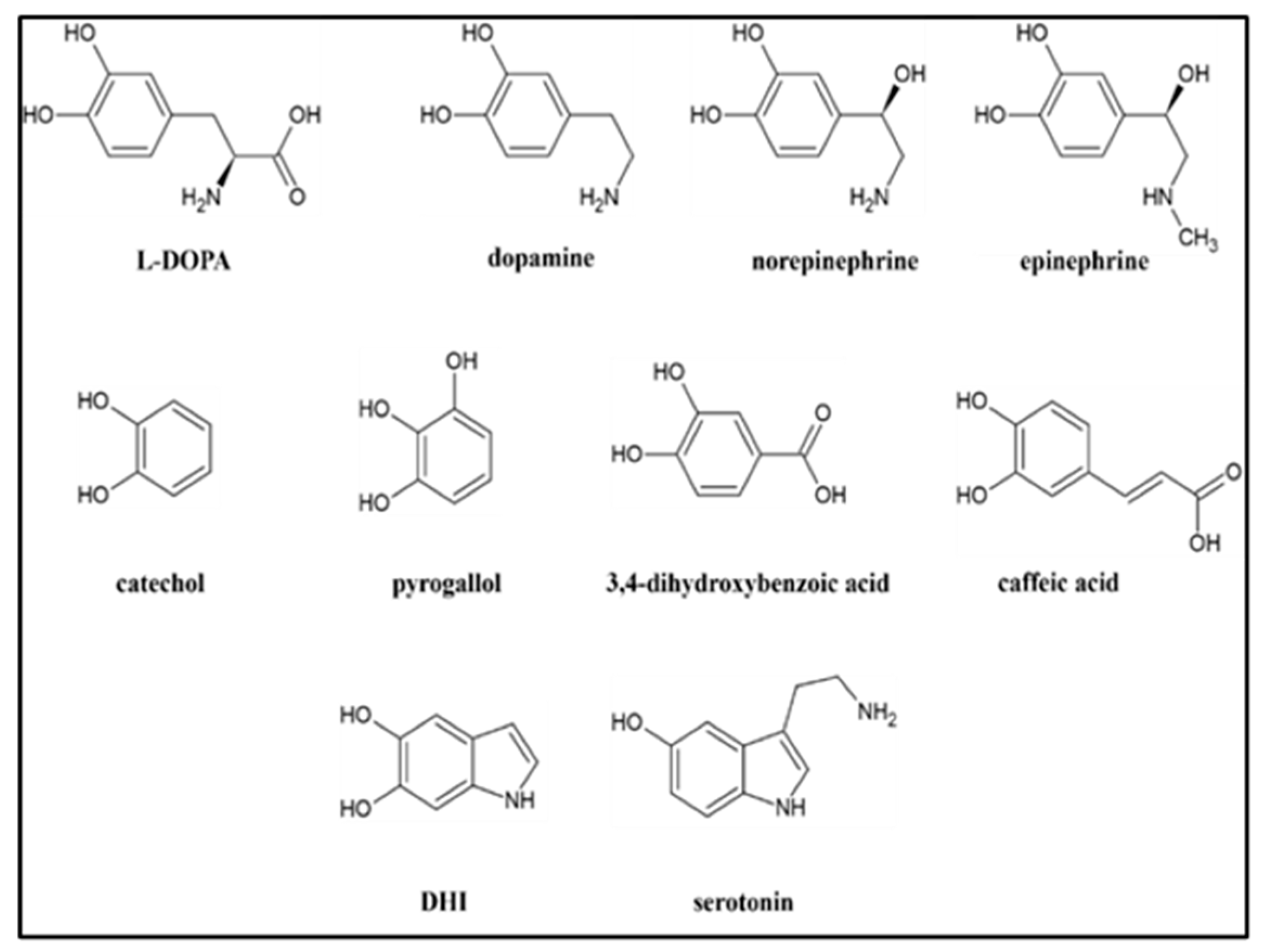
2.1.3. Other Precursors
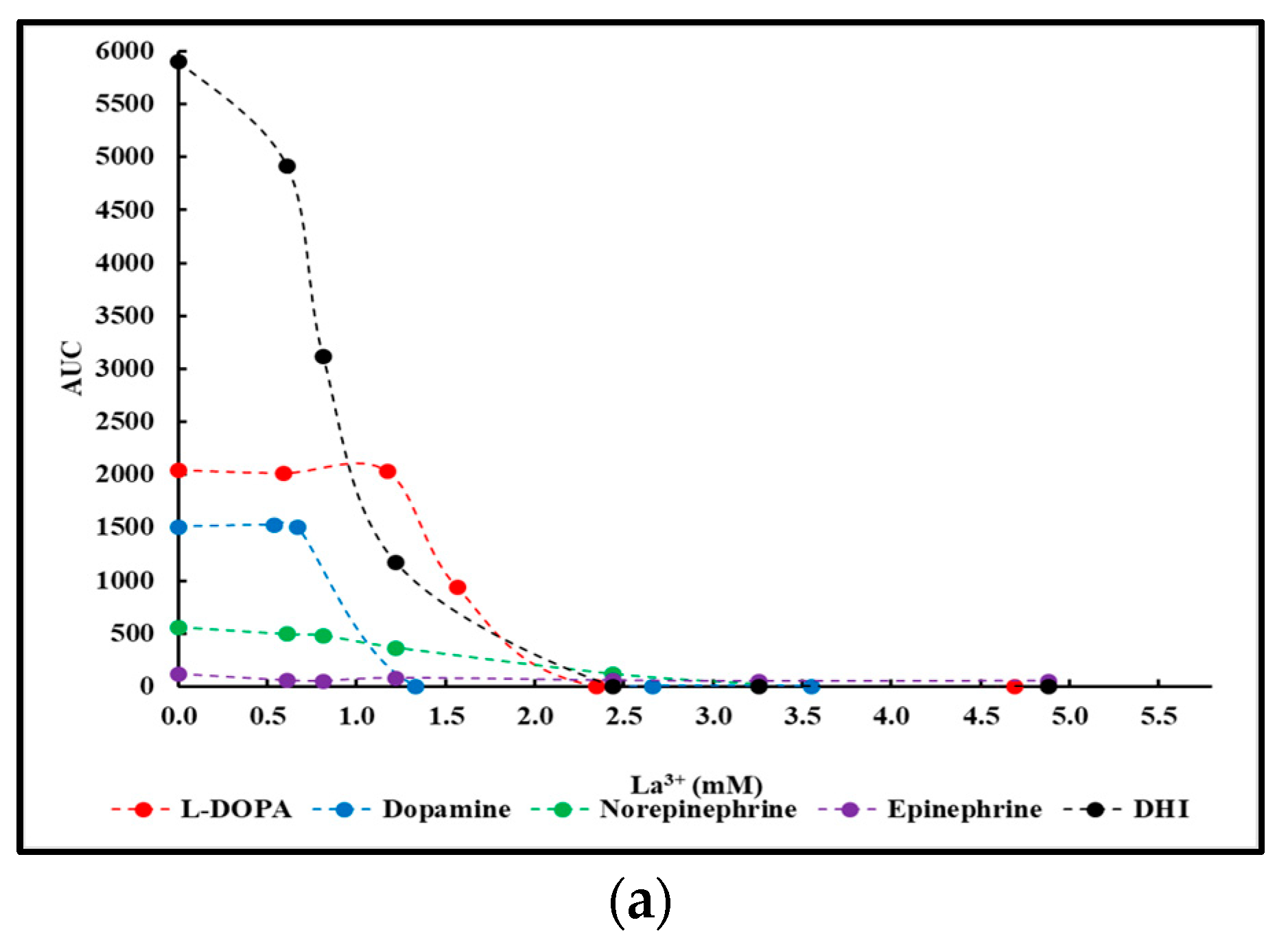
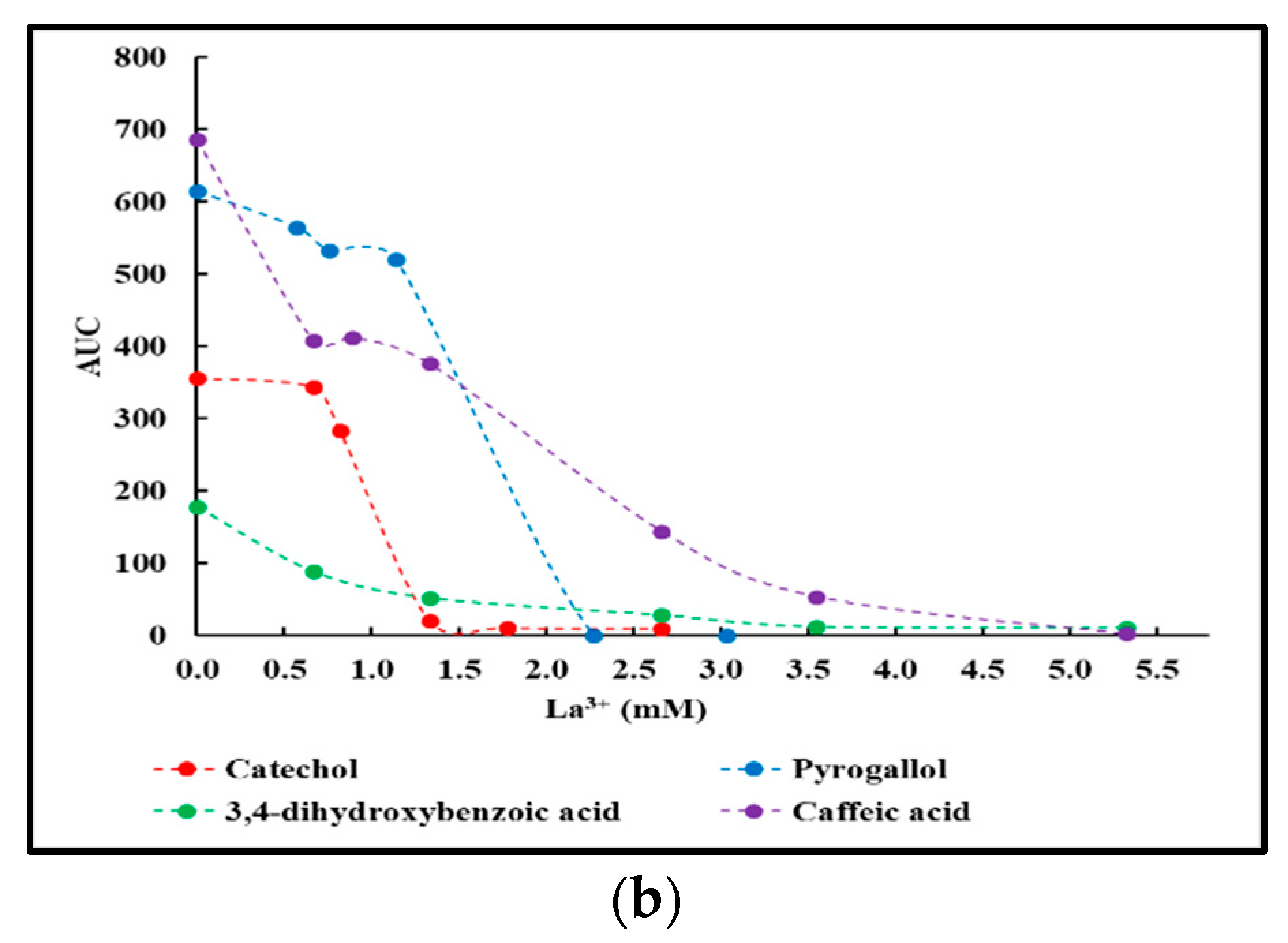
2.2. La3+ Precipitation Tests of Crude Reaction Mixtures
2.3. Fractionation of MN Materials
2.4. Characterization of Fractions
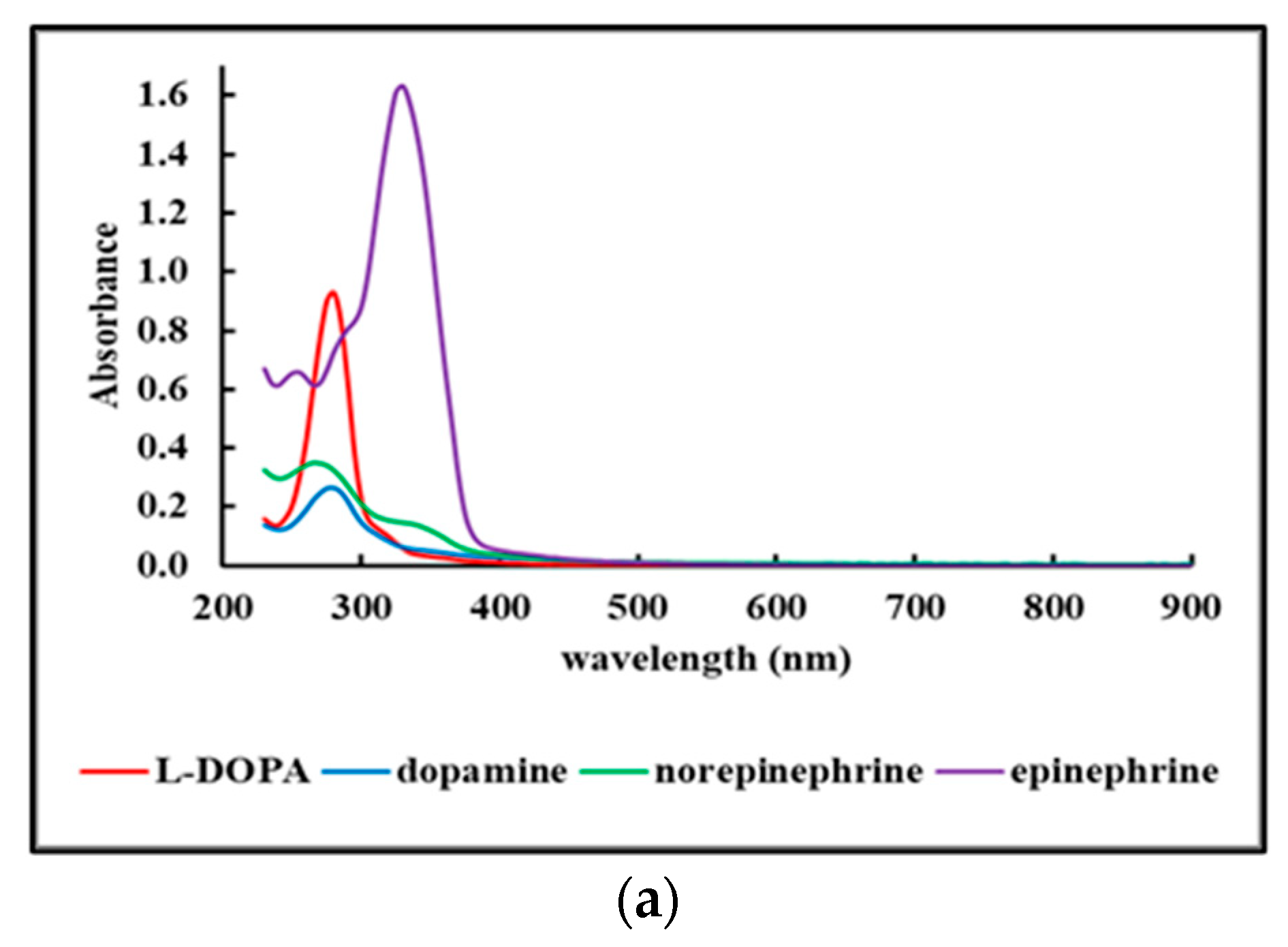
2.4.1. UV-Vis Spectra of Fdisp Fractions
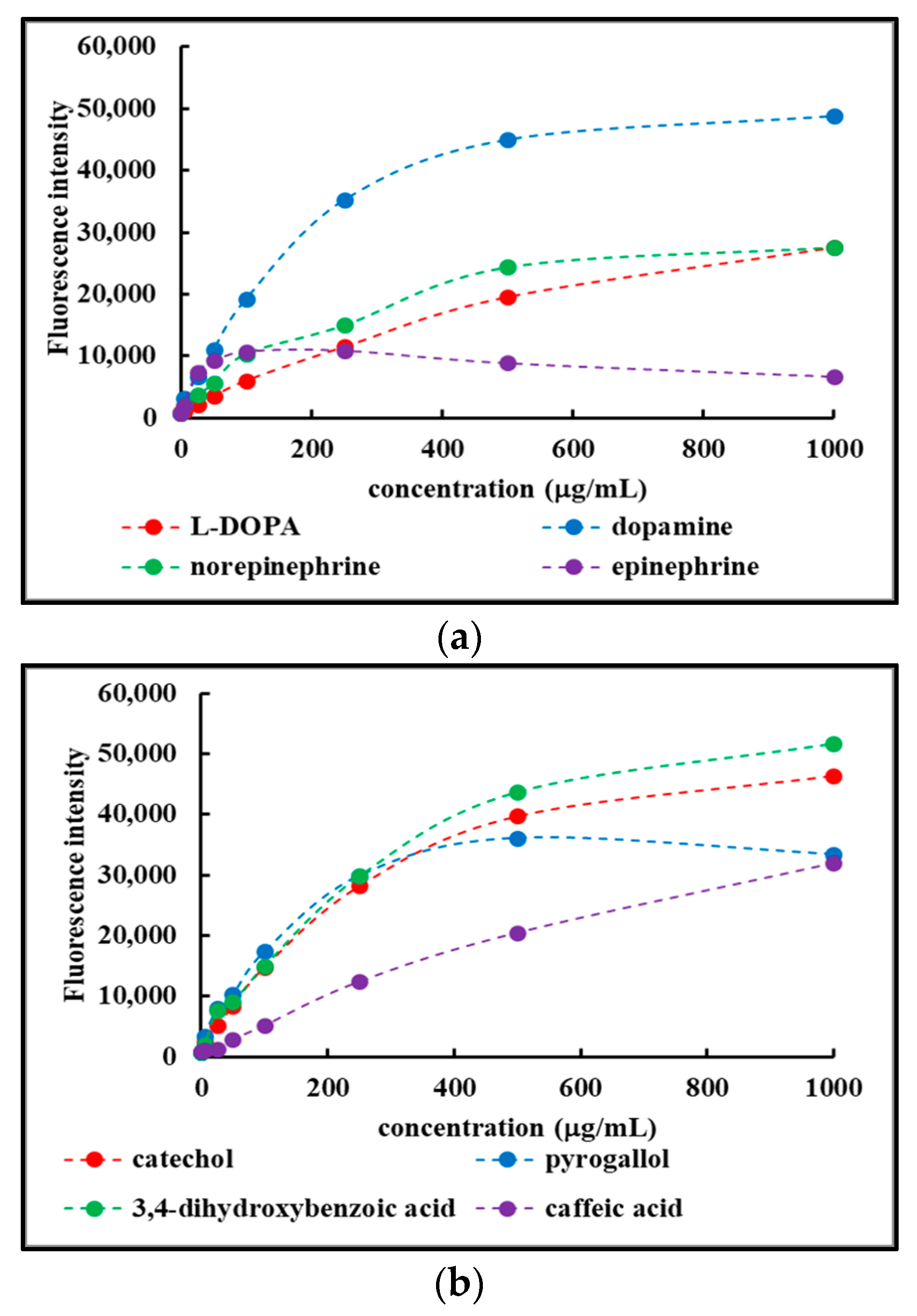
2.4.2. Concentration-Dependent Fluorescence of Fdisp Fractions
2.4.3. FT-IR Spectroscopy
3. Discussion
4. Materials and Methods
4.1. Materials and Solutions
4.2. Synthesis of MN Materials
4.3. Fractionation of Dialyzed MN Reaction Mixtures
4.4. Washing of Materials with HCl
4.5. UV-Vis Spectroscopy
4.6. Fluorescence Spectroscopy
4.7. High-Performance Liquid Chromatography (HPLC)
4.8. FT-IR Spectroscopy
4.9. Dialysis and Freeze Drying
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, D.; Gong, S.; Yang, J.; Li, W.; Ge, Y.; Wei, Y. Melanin deposition ruled out as cause of color changes in the red-eared sliders (Trachemys scripta elegans). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2018, 217, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Maranduca, M.A.; Branisteanu, D.; Serban, D.N.; Branisteanu, D.C.; Stoleriu, G.; Manolache, N.; Serban, I.L. Synthesis and physiological implications of melanic pigments. Oncol. Lett. 2019, 17, 4183–4187. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.D.; Peles, D.N. The red and the black. Acc. Chem. Res. 2010, 43, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Melanins: Skin Pigments and Much More—Types, Structural Models, Biological Functions, and Formation Routes. New J. Sci. 2014, 2014, 498276. [Google Scholar] [CrossRef]
- Wakamatsu, K.; Ito, S. Recent Advances in Characterization of Melanin Pigments in Biological Samples. Int. J. Mol. Sci. 2023, 24, 8305. [Google Scholar] [CrossRef] [PubMed]
- Mostert, A.B. Melanin, the What, the Why and the How: An Introductory Review for Materials Scientists Interested in Flexible and Versatile Polymers. Polymers 2021, 13, 1670. [Google Scholar] [CrossRef] [PubMed]
- Pralea, I.E.; Moldovan, R.C.; Petrache, A.M.; Ilies, M.; Heghes, S.C.; Ielciu, I.; Nicoara, R.; Moldovan, M.; Ene, M.; Radu, M.; et al. From Extraction to Advanced Analytical Methods: The Challenges of Melanin Analysis. Int. J. Mol. Sci. 2019, 20, 3943. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Yang, H.; Liu, S.; Yu, H.; Li, D.; Li, P.; Xing, R. Melanin: Insights into structure, analysis, and biological activities for future development. J. Mater. Chem. B 2023, 11, 7528–7543. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K. Quantitative analysis of eumelanin and pheomelanin in humans, mice, and other animals: A comparative review. Pigment. Cell Res. 2003, 16, 523–531. [Google Scholar] [CrossRef]
- Ito, S.; Wakamatsu, K. Chemistry of Mixed Melanogenesis—Pivotal Roles of Dopaquinone. Photochem. Photobiol. 2008, 84, 582–592. [Google Scholar] [CrossRef]
- Ito, S.; Miyake, S.; Maruyama, S.; Suzuki, I.; Commo, S.; Nakanishi, Y.; Wakamatsu, K. Acid hydrolysis reveals a low but constant level of pheomelanin in human black to brown hair. Pigment Cell Melanoma Res. 2018, 31, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Wakamatsu, K.; Sarna, T. Photodegradation of Eumelanin and Pheomelanin and Its Pathophysiological Implications. Photochem. Photobiol. 2018, 94, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Ni, Q.Z.; Sierra, B.N.; La Clair, J.J.; Burkart, M.D. Chemoenzymatic elaboration of the Raper–Mason pathway unravels the structural diversity within eumelanin pigments. Chem. Sci. 2020, 11, 7836–7841. [Google Scholar] [CrossRef] [PubMed]
- Nasti, T.H.; Timares, L. MC1R, eumelanin and pheomelanin: Their role in determining the susceptibility to skin cancer. Photochem. Photobiol. 2015, 91, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Smith, K. Redhead pigment boosts skin-cancer risk. Nature 2012. [Google Scholar] [CrossRef]
- Cabaco, L.C.; Tomas, A.; Pojo, M.; Barral, D.C. The Dark Side of Melanin Secretion in Cutaneous Melanoma Aggressiveness. Front. Oncol. 2022, 12, 887366. [Google Scholar] [CrossRef]
- Saud, A.; Sagineedu, S.R.; Ng, H.S.; Stanslas, J.; Lim, J.C.W. Melanoma metastasis: What role does melanin play? (Review). Oncol. Rep. 2022, 48, 217. [Google Scholar] [CrossRef] [PubMed]
- Slominski, R.M.; Sarna, T.; Plonka, P.M.; Raman, C.; Brozyna, A.A.; Slominski, A.T. Melanoma, Melanin, and Melanogenesis: The Yin and Yang Relationship. Front. Oncol. 2022, 12, 842496. [Google Scholar] [CrossRef]
- Ju, K.-Y.; Fischer, M.C.; Warren, W.S. Understanding the Role of Aggregation in the Broad Absorption Bands of Eumelanin. ACS Nano 2018, 12, 12050–12061. [Google Scholar] [CrossRef] [PubMed]
- Seraglia, R.; Traldi, P.; Elli, G.; Bertazzo, A.; Costa, C.; Allegri, G. Laser desorption ionization mass spectrometry in the study of natural and synthetic melanins. I—Tyrosine melanins. Biol. Mass Spectrom. 1993, 22, 687–697. [Google Scholar] [CrossRef]
- Bertazzo, A.; Costa, C.; Allegri, G.; Seraglia, R.; Traldi, P. Biosynthesis of melanin from dopamine. An investigation of early oligomerization products. Rapid Commun. Mass Spectrom. 1995, 9, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Kroesche, C.; Peter, M.G. Detection of melanochromes by MALDI-TOF mass spectrometry. Tetrahedron 1996, 52, 3947–3952. [Google Scholar] [CrossRef]
- Napolitano, A.; Pezzella, A.; Prota, G.; Seraglia, R.; Traldi, P. A Reassessment of the Structure of 5,6-Dihydroxyindole-2-carboxylic Acid Melanins by Matrix-assisted Laser Desorption/Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 1996, 10, 204–208. [Google Scholar] [CrossRef]
- Reale, S.; Crucianelli, M.; Pezzella, A.; d’Ischia, M.; De Angelis, F. Exploring the frontiers of synthetic eumelanin polymers by high-resolution matrix-assisted laser/desorption ionization mass spectrometry. J. Mass Spectrom. 2012, 47, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Pezzella, A.; Prota, G.; Seraglia, R.; Traldi, P. Structural Analysis of Synthetic Melanins from 5,6-Dihydroxyindole by Matrix-assisted Laser Desorption/Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 1996, 10, 468–472. [Google Scholar] [CrossRef]
- Bertazzo, A.; Costa, C.V.; Allegri, G.; Favretto, D.; Traldi, P. Application of matrix-assisted laser desorption/ionization mass spectrometry to the detection of melanins formed from Dopa and dopamine. J. Mass Spectrom. 1999, 34, 922–929. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Wang, Y.; Chan, H.W.; Wang, L.; Chan, W. Mass Spectrometric and Spectrophotometric Analyses Reveal an Alternative Structure and a New Formation Mechanism for Melanin. Anal. Chem. 2015, 87, 7958–7963. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Micillo, R.; Panzella, L.; Crescenzi, O.; Oscurato, S.L.; Maddalena, P.; Napolitano, A.; Ball, V.; d’Ischia, M. Structural Basis of Polydopamine Film Formation: Probing 5,6-Dihydroxyindole-Based Eumelanin Type Units and the Porphyrin Issue. ACS Appl. Mater. Interfaces 2018, 10, 7670–7680. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Hsueh, N.; Chai, C.L.L. Direct Evidence for the Critical Role of 5,6-Dihydroxyindole in Polydopamine Deposition and Aggregation. Langmuir 2019, 35, 5191–5201. [Google Scholar] [CrossRef]
- Lyu, Q.; Hsueh, N.; Chai, C.L.L. Unravelling the polydopamine mystery: Is the end in sight? Polym. Chem. 2019, 10, 5771–5777. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Galeb, H.A.; Eichhorn, J.; Harley, S.; Robson, A.J.; Martocq, L.; Nicholson, S.J.; Ashton, M.D.; Abdelmohsen, H.A.M.; Pelit, E.; Baldock, S.J.; et al. Phenolic Polymers as Model Melanins. Macromol. Chem. Phys. 2023, 224, 2300025. [Google Scholar] [CrossRef]
- Vercruysse, K.P.; Govan, V.; Winford, J. “Invisible” ligands stabilize colloidal melanin-particles = the case of L-DOPA. ChemRxiv 2024. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Giordani, S.; Menichetti, A.; Mordini, D.; Zattoni, A.; Roda, B.; Ferrazzano, L.; Reschiglian, P.; Marassi, V.; Montalti, M. The dual nature of biomimetic melanin. Nanoscale 2024, 16, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Ishino, K.; Nishitani, S.; Man, Y.; Saito, A.; Sakata, T. Surface Characteristics and Formation of Polyserotonin Thin Films for Bioelectrical and Biocompatible Interfaces. Langmuir 2022, 38, 8633–8642. [Google Scholar] [CrossRef]
- Vercruysse, K.P. The “Unconventional” Effect of Cysteine on the In Vitro Synthesis of Melanin. ACS Omega 2024, 9, 22794–22800. [Google Scholar] [CrossRef]
- Vercruysse, K.P.; Govan, V. Melanogenesis: A Search for Pheomelanin and Also, What Is Lurking Behind Those Dark Colors? ChemRxiv 2019. [Google Scholar] [CrossRef]
- Vercruysse, K.P.; Govan, V. The yellow and the black of synthetic melanins. ChemRxiv 2021. [Google Scholar] [CrossRef]
- Smith, B.C. The C=O bond, Part III: Carboxylic Acids. Spectroscopy 2018, 33, 14–20. [Google Scholar]
- Smith, B.C. The Carbonyl Group, Part V: Carboxylates—Coming Clean. Spectroscopy 2018, 33, 20–23. [Google Scholar]
- Smith, B.C. Organic Nitrogen Compounds V: Amine Salts. Spectroscopy 2019, 34, 30–37. [Google Scholar]
- d’Ischia, M.; Wakamatsu, K.; Napolitano, A.; Briganti, S.; Garcia-Borron, J.C.; Kovacs, D.; Meredith, P.; Pezzella, A.; Picardo, M.; Sarna, T.; et al. Melanins and melanogenesis: Methods, standards, protocols. Pigment Cell Melanoma Res. 2013, 26, 616–633. [Google Scholar] [CrossRef] [PubMed]
- Ogemdi, I. Properties and Uses of Colloids: A Review. Colloid Surf. Sci. 2019, 4, 24–28. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.K.; Kleszczyński, K.; Semak, I.; Janjetovic, Z.; Sweatman, T.; Skobowiat, C.; Steketee, J.D.; Lin, Z.; Postlethwaite, A.; et al. Characterization of serotonin and N-acetylserotonin systems in the human epidermis and skin cells. J. Pineal Res. 2020, 68, e12626. [Google Scholar] [CrossRef] [PubMed]
- Derizhanova, I.S. Melanosis of the large intestine. Arkhiv Patol. 1975, 37, 54–59. [Google Scholar]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Mavridi-Printezi, A.; Menichetti, A.; Guernelli, M.; Montalti, M. The Photophysics and Photochemistry of Melanin- Like Nanomaterials Depend on Morphology and Structure. Chemistry 2021, 27, 16309–16319. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.; Vanna, R.; Bellei, C.; Zucca, F.A.; Wakamatsu, K.; Monzani, E.; Ito, S.; Casella, L.; Zecca, L. Neuromelanins of human brain have soluble and insoluble components with dolichols attached to the melanic structure. PLoS ONE 2012, 7, e48490. [Google Scholar] [CrossRef]
- Leupold, D.; Szyc, L.; Stankovic, G.; Strobel, S.; Volker, H.U.; Fleck, U.; Muller, T.; Scholz, M.; Riederer, P.; Monoranu, C.M. Melanin and Neuromelanin Fluorescence Studies Focusing on Parkinson’s Disease and Its Inherent Risk for Melanoma. Cells 2019, 8, 592. [Google Scholar] [CrossRef]
- Krasowska, D.; Malek, A.; Kurzepa, J.; Kapka-Skrzypczak, L.; Krasowska, D.; Kurzepa, J. Melanin—The Eminence Grise of Melanoma and Parkinson’s Disease Development. Cancers 2023, 15, 5541. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, K.P. Evaluating the “Darkness” of Melanin Materials. ChemRxiv 2020. [Google Scholar] [CrossRef]
- Ozeki, H.; Ito, S.; Wakamatsu, K.; Thody, A.J. Spectrophotometric characterization of eumelanin and pheomelanin in hair. Pigment Cell Res. 1996, 9, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, K.; Nagao, A.; Watanabe, M.; Nakao, K.; Ito, S. Pheomelanogenesis is promoted at a weakly acidic pH. Pigment Cell Melanoma Res. 2017, 30, 372–377. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flake, A.; Vercruysse, K. The Invisible Fraction within Melanin Capable of Absorbing UV Light and with Fluorescent Properties: Is It Lacking Consideration? Int. J. Mol. Sci. 2024, 25, 8490. https://doi.org/10.3390/ijms25158490
Flake A, Vercruysse K. The Invisible Fraction within Melanin Capable of Absorbing UV Light and with Fluorescent Properties: Is It Lacking Consideration? International Journal of Molecular Sciences. 2024; 25(15):8490. https://doi.org/10.3390/ijms25158490
Chicago/Turabian StyleFlake, Aaliyah, and Koen Vercruysse. 2024. "The Invisible Fraction within Melanin Capable of Absorbing UV Light and with Fluorescent Properties: Is It Lacking Consideration?" International Journal of Molecular Sciences 25, no. 15: 8490. https://doi.org/10.3390/ijms25158490





