Correlations of Imaging and Therapy in Breast Cancer Based on Molecular Patterns: An Important Issue in the Diagnosis of Breast Cancer
Abstract
1. Introduction
2. Diagnostic Methods
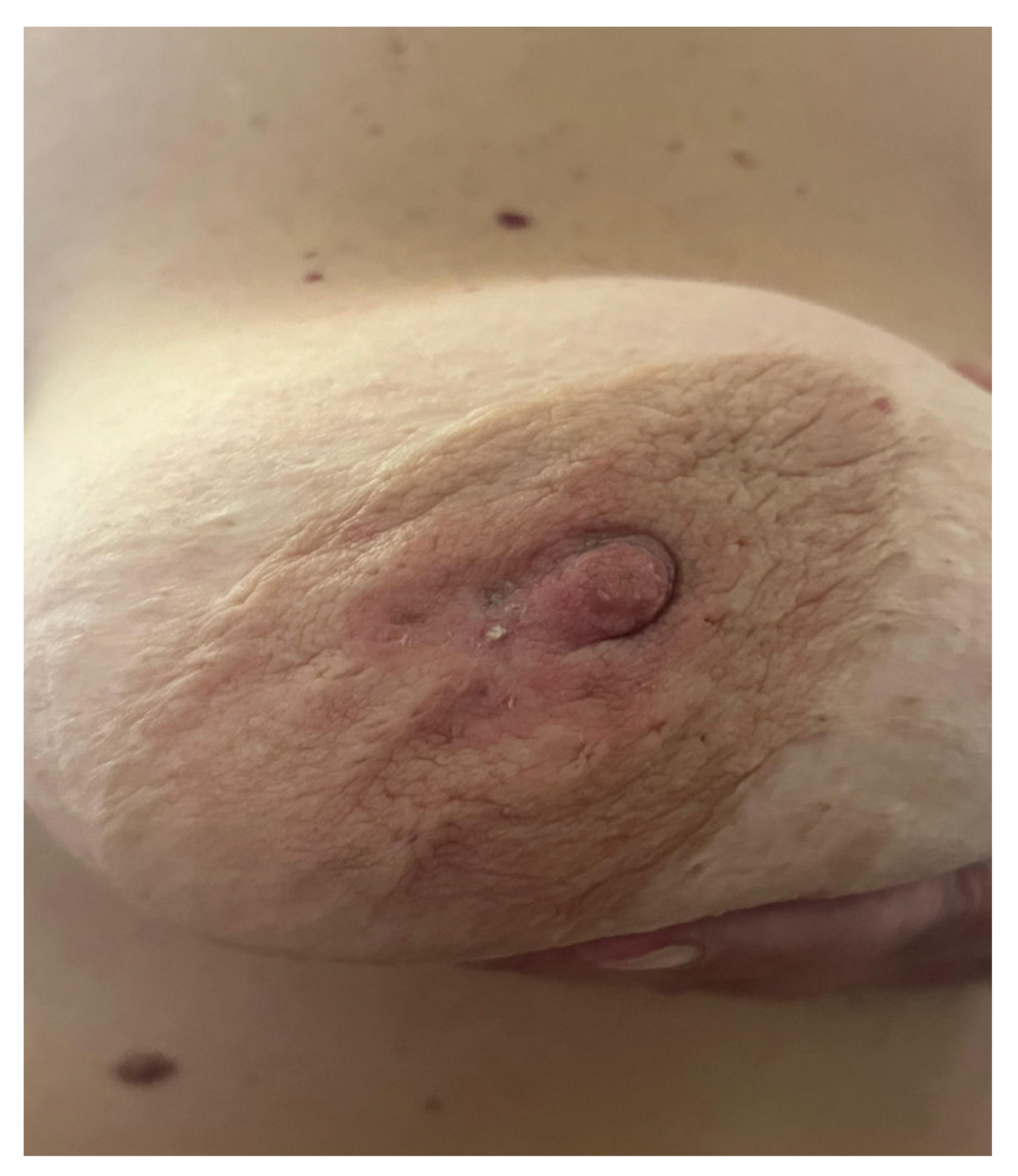
2.1. Clinical Breast Examination (CBE)
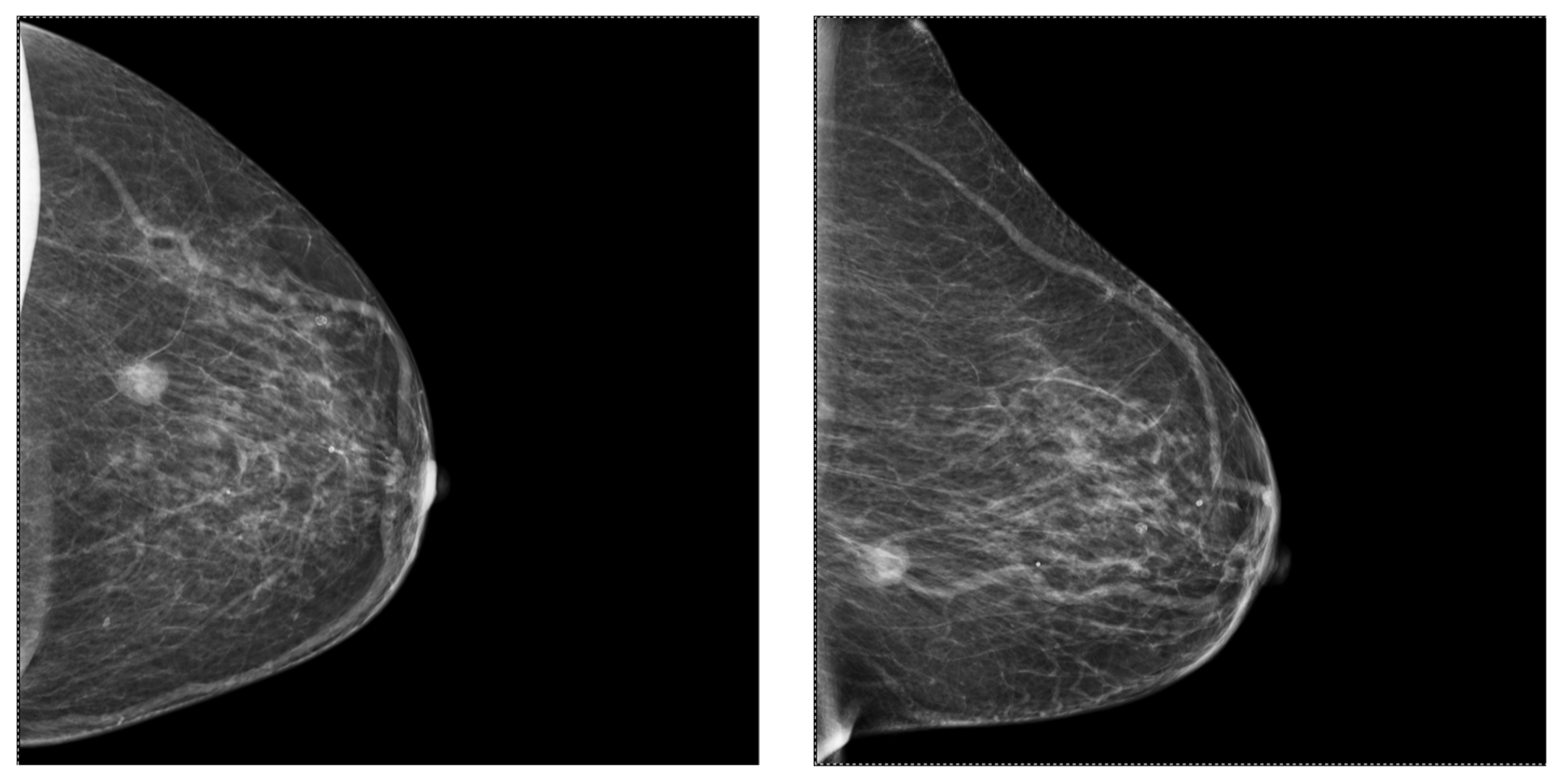
2.2. Mammography (MG)
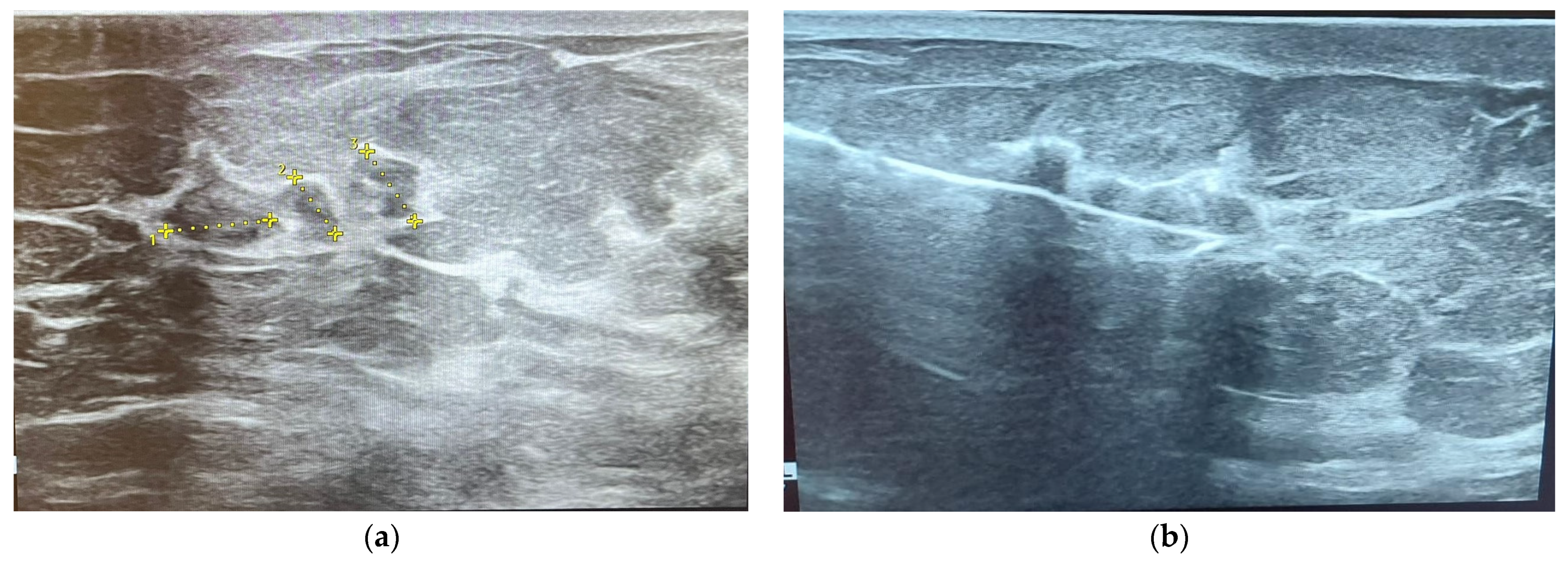
2.3. Ultrasonography (US)
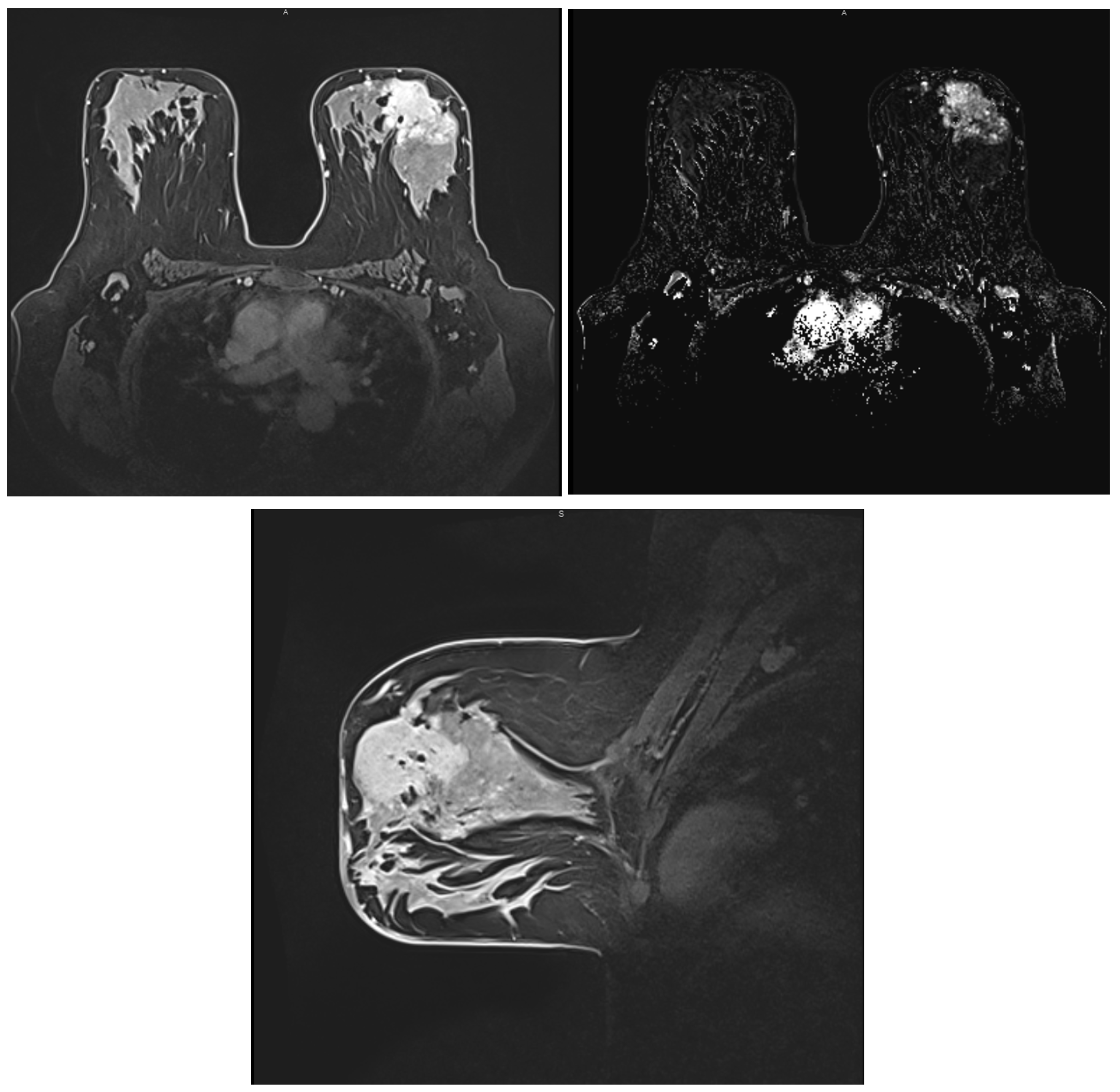
2.4. Magnetic Resonance Imaging (MRI)
2.5. Guided Biopsy
3. Discussion
3.1. BC Screening, Including Tumor Biology Manifestations in Laboratory Results
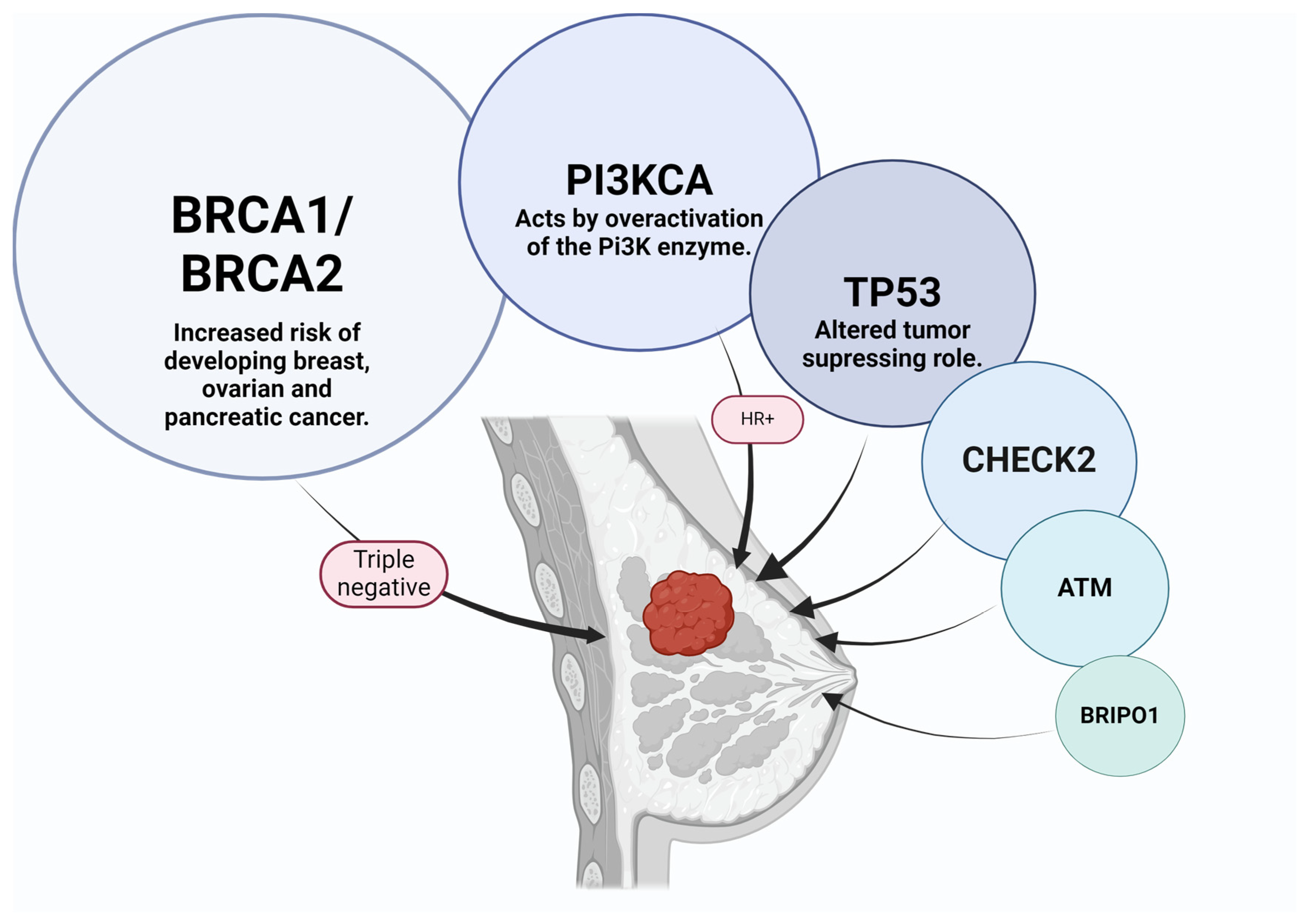
3.2. Correlations between Histological BC Types and Subtypes and Their Genomic Profile
3.3. Correlations between BC Histological Types and Subtypes and Imaging Features
3.4. Molecular Classification of BC
3.5. Correlations between Molecular Subtypes of BC and Their Mammographic Features
3.6. Correlations between Molecular Subtypes of BC and Their Ultrasonographic Features
3.7. Correlations between Molecular Subtypes of BC and Their Features on MRI
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast cancer—Epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—An updated review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sharma, R. Global, regional, national burden of breast cancer in 185 countries: Evidence from GLOBOCAN 2018. Breast Cancer Res. Treat. 2021, 187, 557–567. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Zadrożna Nowak, A.; Romanowicz, H. Breast Cancer—Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef] [PubMed]
- Duggan, C.; Dvaladze, A.; Rositch, A.F.; Ginsburg, O.; Yip, C.H.; Horton, S.; Camacho Rodriguez, R.; Eniu, A.; Mutebi, M.; Bourque, J.M.; et al. The Breast Health Global Initiative 2018 Global Summit on Improving Breast Healthcare Through Resource-Stratified Phased Implementation: Methods and Overview. Cancer 2020, 126, 2339–2352. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- VanderWalde, A.; Hurria, A. Early breast cancer in the older woman. Clin. Geriatr. Med. 2012, 28, 73–91. [Google Scholar] [CrossRef][Green Version]
- Yap, Y.S. Outcomes in breast cancer—Does ethnicity matter? ESMO Open 2023, 8, 101564. [Google Scholar] [CrossRef]
- Yoshida, R. Hereditary breast and ovarian cancer (HBOC): Review of its molecular characteristics, screening, treatment, and prognosis. Breast Cancer 2021, 28, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Tan, W.; Song, Q.; Pei, H.; Li, J. BRCA1 and Breast Cancer: Molecular Mechanisms and Therapeutic Strategies. Front. Cell Dev. Biol. 2022, 10, 813457. [Google Scholar] [CrossRef]
- Cobec, I.; Sas, I.; Moatar, A.; Moleriu, L.; Rempen, A. Ovarian cancer health politics in Romania and Germany: A comparative study. Exp. Ther. Med. 2021, 22, 1217. [Google Scholar] [CrossRef] [PubMed]
- Cobec, I.M.; Popescu, R.; Moatar, A.E.; Verdes, D. Ovarian Cancer under the Magnifying Glass. August Rom. J. Mil. Med. 2021, 124, 291–296. [Google Scholar] [CrossRef]
- Chen, H.; Wu, J.; Zhang, Z.; Tang, Y.; Li, X.; Liu, S.; Cao, S.; Li, X. Association between BRCA status and triple-negative breast cancer: A meta-analysis. Front. Pharmacol. 2018, 9, 909. [Google Scholar] [CrossRef]
- Breast Cancer Association Consortium. Breast Cancer Risk Genes—Association Analysis in More than 113,000 Women. N. Engl. J. Med. 2021, 384, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Shahbandi, A.; Nguyen, H.D.; Jackson, J.G. TP53 Mutations and Outcomes in Breast Cancer: Reading beyond the Headlines. Trends Cancer 2020, 6, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Hynes, S.O.; Kerin, M.J.; Miller, N.; Lowery, A.J. Ki-67 as a prognostic biomarker in invasive breast cancer. Cancers 2021, 13, 4455. [Google Scholar] [CrossRef]
- Fusco, N.; Malapelle, U.; Fassan, M.; Marchiò, C.; Buglioni, S.; Zupo, S.; Criscitiello, C.; Vigneri, P.; Dei Tos, A.P.; Maiorano, E.; et al. PIK3CA Mutations as a Molecular Target for Hormone Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Front. Oncol. 2021, 11, 644737. [Google Scholar] [CrossRef]
- Bhushan, A.; Gonsalves, A.; Menon, J.U. Current state of breast cancer diagnosis, treatment, and theranostics. Pharmaceutics 2021, 13, 723. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chen, Z.; Tan, M.; Elingarami, S.; Liu, Y.; Li, T.; Deng, Y.; He, N.; Li, S.; Fu, J.; et al. A review on methods for diagnosis of breast cancer cells and tissues. Cell Prolif. 2020, 53, e12822. [Google Scholar] [CrossRef]
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.N.; Saha, S. Breast cancer: Presentation, investigation and management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef]
- Turan, M.; Sozen, F.; Eminsoy, M.G.; Sencelikel, T.; Kut, A.; Yildirim, S.; Oksuz, E. Practical Utility of Diagnostic Clinical Breast Examination in the Diagnosis of Breast Cancer. Cureus 2021, 13, e17662. [Google Scholar] [CrossRef]
- Ngan, T.T.; Nguyen, N.T.Q.; Van Minh, H.; Donnelly, M.; O’Neill, C. Effectiveness of clinical breast examination as a ‘stand-alone’ screening modality: An overview of systematic reviews. BMC Cancer 2020, 20, 1070. [Google Scholar] [CrossRef] [PubMed]
- Bodewes, F.T.H.; van Asselt, A.A.; Dorrius, M.D.; Greuter, M.J.W.; de Bock, G.H. Mammographic breast density and the risk of breast cancer: A systematic review and meta-analysis. Breast 2022, 66, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kolb, T.M.; Lichy, J.; Newhouse, J.H. Comparison of the performance of screening mammography, physical examination, and breast ultrasound, and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology 2002, 225, 165–175. [Google Scholar] [CrossRef]
- Pisano, E.D.; Gatsonis, C.; Hendrick, E.; Yaffe, M.; Baum, J.K.; Acharyya, S.; Conant, E.F.; Fajardo, L.L.; Bassett, L.; D‘Orsi, C.; et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N. Engl. J. Med. 2005, 353, 1773–1783. [Google Scholar] [CrossRef]
- Chen, H.L.; Zhou, J.Q.; Chen, Q.; Deng, Y.C. Comparison of the sensitivity of mammography, ultrasound, magnetic resonance imaging and combinations of these imaging modalities for the detection of small (≤2 cm) breast cancer. Medicine 2021, 100, E26531. [Google Scholar] [CrossRef]
- Catalano, O.; Fusco, R.; De Muzio, F.; Simonetti, I.; Palumbo, P.; Bruno, F.; Borgheresi, A.; Agostini, A.; Gabelloni, M.; Varelli, C.; et al. Recent Advances in Ultrasound Breast Imaging: From Industry to Clinical Practice. Diagnostics 2023, 13, 980. [Google Scholar] [CrossRef]
- Yang, L.; Wang, S.; Zhang, L.; Sheng, C.; Song, F.; Wang, P.; Huang, Y. Performance of ultrasonography screening for breast cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 499. [Google Scholar] [CrossRef]
- Pan, Q.H.; Zhang, Z.P.; Yan, L.Y.; Jia, N.R.; Ren, X.Y.; Wu, B.K.; Hao, Y.B.; Li, Z.F. Association between ultrasound BI-RADS signs and molecular typing of invasive breast cancer. Front. Oncol. 2023, 13, 1110796. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, Z.; Zhang, L.; Wang, D.; Tian, J. Application of Contrast-Enhanced Ultrasound in the Differential Diagnosis of Different Molecular Subtypes of Breast Cancer. Ultrason. Imaging 2020, 42, 261–270. [Google Scholar] [CrossRef]
- Rashmi, S.; Kamala, S.; Murthy, S.S.; Kotha, S.; Rao, Y.S.; Chaudhary, K.V. Predicting the molecular subtype of breast cancer based on mammography and ultrasound findings. Indian. J. Radiol. Imaging 2018, 28, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Houser, M.; Barreto, D.; Mehta, A.; Brem, R.F. Current and future directions of breast mri. J. Clin. Med. 2021, 10, 5668. [Google Scholar] [CrossRef]
- Türk, G.; Özdemir, M.; Çoban, M.; Koç, A. Is biopsy necessary? Role of DCE-MRI in BIRADS-3 lesions. Diagn. Interv. Radiol. 2020, 26, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, E.; Singh, R.; Mishra, S.; Hari, S. Image-Guided Breast Interventions: Biopsy and beyond. Indian. J. Radiol. Imaging 2021, 31, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Rusu-Moldovan, A.O.; Radu, M.G.; Gruia, M.I.; Pătroi, D.N.; Mîrza, C.M.; Mihu, D. The impact of imagistic evaluation of premalignant and malignant lesions of the breast confirmed in histopathological terms. Rom. J. Morphol. Embryol. = Rev. Roum. Morphol. Embryol. 2019, 60, 1275–1283, Correction in Rom. J. Morphol. Embryol. 2020, 61, 599. [Google Scholar] [CrossRef]
- Kong, Y.C.; Bhoo-Pathy, N.; O’Rorke, M.; Subramaniam, S.; Bhoo-Pathy, N.T.; See, M.H.; Jamaris, S.; Teoh, K.H.; Bustam, A.Z.; Looi, L.M.; et al. The association between methods of biopsy and survival following breast cancer: A hospital registry based cohort study. Medicine 2020, 99, e19093. [Google Scholar] [CrossRef]
- Sennerstam, R.B.; Franzén, B.S.H.; Wiksell, H.O.T.; Auer, G.U. Core-needle biopsy of breast cancer is associated with a higher rate of distant metastases 5 to 15 years after diagnosis than FNA biopsy. Cancer Cytopathol. 2017, 125, 748–756. [Google Scholar] [CrossRef]
- Varzaru, V.B.; Eftenoiu, A.-E.; Vlad, D.C.; Vlad, C.S.; Moatar, A.E.; Popescu, R.; Cobec, I.M. The Influence of Tumor-Specific Markers in Breast Cancer on Other Blood Parameters. Life 2024, 14, 458. [Google Scholar] [CrossRef] [PubMed]
- Monticciolo, D.L.; Malak, S.F.; Friedewald, S.M.; Eby, P.R.; Newell, M.S.; Moy, L.; Destounis, S.; Leung, J.W.T.; Hendrick, R.E.; Smetherman, D. Breast Cancer Screening Recommendations Inclusive of All Women at Average Risk: Update from the ACR and Society of Breast Imaging. J. Am. Coll. Radiol. 2021, 18, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Hammonds, L.S.; Madsen, D.; Dale, P. Mammography in 40-year-old women: What difference does it make? The potential impact of the U.S. Preventative Services Task Force (USPSTF) mammography guidelines. Ann. Surg. Oncol. 2011, 18, 3066–3071. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.W.; Tabár, L.; Yen, A.M.; Dean, P.B.; Smith, R.A.; Jonsson, H.; Törnberg, S.; Chen, S.L.; Chiu, S.Y.; Fann, J.C.; et al. Mammography screening reduces rates of advanced and fatal breast cancers: Results in 549,091 women. Cancer 2020, 126, 2971–2979. [Google Scholar] [CrossRef] [PubMed]
- Braitmaier, M.; Kollhorst, B.; Heinig, M.; Langner, I.; Czwikla, J.; Heinze, F.; Buschmann, L.; Minnerup, H.; García-Albéniz, X.; Hense, H.W.; et al. Effectiveness of Mammography Screening on Breast Cancer Mortality—A Study Protocol for Emulation of Target Trials Using German Health Claims Data. Clin. Epidemiol. 2022, 14, 1293–1303. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M.G.; Altman, D.G.; Cameron, D.A.; Dewar, J.A.; Thompson, S.G.; Wilcox, M. The benefits and harms of breast cancer screening: An independent review. Br. J. Cancer 2013, 108, 2205–2240. [Google Scholar] [CrossRef] [PubMed]
- Clift, A.K.; Dodwell, D.; Lord, S.; Petrou, S.; Brady, S.M.; Collins, G.S.; Hippisley-Cox, J. The current status of risk-stratified breast screening. Br. J. Cancer 2022, 126, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022. JNCCN J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Nascimento RGd; Otoni, K.M. Histological and molecular classification of breast cancer: What do we know? Mastology 2020, 30, 1–8. [Google Scholar]
- Rakha, E.A.; Tse, G.M.; Quinn, C.M. An update on the pathological classification of breast cancer. Histopathology 2023, 82, 5–16. [Google Scholar] [CrossRef]
- Sinn, H.P.; Kreipe, H. A brief overview of the WHO classification of breast tumors, 4th edition, focusing on issues and updates from the 3rd edition. Breast Care 2013, 8, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, T.S.; Lee, Y.; Cho, M. Histopathological classification of breast cancer images using a multi-scale input and multi-feature network. Cancers 2020, 12, 2031. [Google Scholar] [CrossRef] [PubMed]
- Cserni, G. Histological type and typing of breast carcinomas and the WHO classification changes over time. Pathologica 2020, 112, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Thennavan, A.; Beca, F.; Xia, Y.; Recio, S.G.; Allison, K.; Collins, L.C.; Tse, G.M.; Chen, Y.Y.; Schnitt, S.J.; Hoadley, K.A.; et al. Molecular analysis of TCGA breast cancer histologic types. Cell Genom. 2021, 1, 100067. [Google Scholar] [CrossRef] [PubMed]
- Galati, F.; Rizzo, V.; Moffa, G.; Caramanico, C.; Kripa, E.; Cerbelli, B.; D‘Amati, G.; Pediconi, F. Radiologic-pathologic correlation in breast cancer: Do MRI biomarkers correlate with pathologic features and molecular subtypes? Eur. Radiol. Exp. 2022, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Surabhi, D.M.; Wilson, J.C.; Singh, M.; Green, L. Recognizing invasive breast carcinoma of no special type with medullary pattern. Radiol. Case Rep. 2023, 18, 1788–1792. [Google Scholar] [CrossRef] [PubMed]
- Hameed, Z.; Zahia, S.; Garcia-Zapirain, B.; Aguirre, J.J.; Vanegas, A.M. Breast cancer histopathology image classification using an ensemble of deep learning models. Sensors 2020, 20, 4373. [Google Scholar] [CrossRef] [PubMed]
- Durhan, G.; Poker, A.; Settarzade, E.; Karakaya, J.; Kösemehmetoğlu, K.; Akpınar, M.G.; Demirkazık, F.B. Magnetic resonance imaging findings of invasive breast cancer in different histological grades and different histopathological types. Clin. Imaging 2021, 76, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Alaref, A.; Hassan, A.; Sharma Kandel, R.; Mishra, R.; Gautam, J.; Jahan, N. Magnetic Resonance Imaging Features in Different Types of Invasive Breast Cancer: A Systematic Review of the Literature. Cureus 2021, 13, e13854. [Google Scholar] [CrossRef]
- Mann, R.M. The effectiveness of MR imaging in the assessment of invasive lobular carcinoma of the breast. Magn. Reson. Imaging Clin. N. Am. 2010, 18, 259–276. [Google Scholar] [CrossRef]
- Mann, R.M.; Huisman, H.; Veltman, J.; Boetes, C.; Mus, R. Morphologic and dynamic differences between invasive ductal and invasive lobular carcinomas of the breast. Proc. Intl Soc. Mag. Reson. Med. 2008, 16, 3769. [Google Scholar]
- Dietzel, M.; Baltzer, P.A.; Vag, T.; Gröschel, T.; Gajda, M.; Camara, O.; Kaiser, W. Magnetic resonance mammography of invasive lobular versus ductal carcinoma: Systematic comparison of 811 patients reveals high diagnostic accuracy irrespective of typing. J. Comput. Assist. Tomogr. 2010, 34, 587–595. [Google Scholar] [CrossRef]
- Tsang, J.Y.S.; Tse, G.M. Molecular Classification of Breast Cancer. 2019. Available online: www.anatomicpathology.com (accessed on 31 May 2024).
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast cancer: Biology, biomarkers, and treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef] [PubMed]
- Peppercorn, J.; Perou, C.M.; Carey, L.A. Molecular subtypes in breast cancer evaluation and management: Divide and conquer. Cancer Investig. 2008, 26, 1–10. [Google Scholar] [CrossRef]
- Burguin, A.; Diorio, C.; Durocher, F. Breast cancer treatments: Updates and new challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Shaikh, S.; Rasheed, A. Predicting Molecular Subtypes of Breast Cancer with Mammography and Ultrasound Findings: Introduction of Sono-Mammometry Score. Radiol. Res. Pract. 2021, 2021, 6691958. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gong, W.; Xie, Y.; Sheng, L. Correlation between the CEM imaging characteristics and different molecular subtypes of breast cancer. Breast 2023, 72, 103595. [Google Scholar] [CrossRef]
- Ian, T.W.M.; Tan, E.Y.; Chotai, N. Role of mammogram and ultrasound imaging in predicting breast cancer subtypes in screening and symptomatic patients. World J. Clin. Oncol. 2021, 12, 808–822. [Google Scholar] [CrossRef]
- Zhu, J.Y.; He, H.L.; Jiang, X.C.; Bao, H.W.; Chen, F. Multimodal ultrasound features of breast cancers: Correlation with molecular subtypes. BMC Med. Imaging 2023, 23, 57. [Google Scholar] [CrossRef]
- Szep, M.; Pintican, R.; Boca, B.; Perja, A.; Duma, M.; Feier, D.; Fetica, B.; Eniu, D.; Dudea, S.M.; Chiorean, A. Multiparametric MRI Features of Breast Cancer Molecular Subtypes. Medicina 2022, 58, 1716. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, V.S.; Polat, Y.D.; Soyder, A.; Tanyeri, A.; Karaman, C.Z.; Taşkın, F. The Relationship Between MRI Findings and Molecular Subtypes in Women With Breast Cancer. Curr. Probl. Diagn. Radiol. 2020, 49, 417–421. [Google Scholar] [CrossRef]
- Ab Mumin, N.; Ramli Hamid, M.T.; Wong, J.H.D.; Rahmat, K.; Ng, K.H. Magnetic Resonance Imaging Phenotypes of Breast Cancer Molecular Subtypes: A Systematic Review. Acad. Radiol. 2022, 29, S89–S106. [Google Scholar] [CrossRef] [PubMed]
- Yoon, G.Y.; Choi, W.J.; Cha, J.H.; Shin, H.J.; Chae, Y.E.; Kim, H.H. The role of MRI and clinicopathologic features in predicting the invasive component of biopsy-confirmed ductal carcinoma in situ. BMC Med. Imaging 2020, 20, 95. [Google Scholar] [CrossRef]
- Maiti, S.; Nayak, S.; Hebbar, K.D.; Pendem, S. Differentiation of invasive ductal and lobular carcinoma of the breast using MRI radiomic features: A pilot study. F1000Res 2024, 13, 91. [Google Scholar] [CrossRef]
- Cho, N. Imaging features of breast cancer molecular subtypes: State of the art. J. Pathol. Transl. Med. 2021, 55, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Cho, N. Molecular subtypes and imaging phenotypes of breast cancer. Ultrasonography 2016, 35, 281–288. [Google Scholar] [CrossRef]
- Sumkin, J.H.; Berg, W.A.; Carter, G.J.; Bandos, A.I.; Chough, D.M.; Ganott, M.A.; Hakim, C.M.; Kelly, A.E.; Zuley, M.L.; Houshmand, G.; et al. Diagnostic performance of MRI, molecular breast imaging, and contrast-enhanced mammography in women with newly diagnosed breast cancer. Radiology 2019, 293, 531–540. [Google Scholar] [CrossRef]
- Akin, M.; Orguc, S.; Aras, F.; Kandiloglu, A.R. Molecular subtypes of invasive breast cancer: Correlation between PET/computed tomography and MRI findings. Nucl. Med. Commun. 2020, 41, 810–816. [Google Scholar] [CrossRef]




| HISTOPATHOLOGICAL EXAM- GOLD STANDARD | ||
|---|---|---|
| SENSITIVITY | MG + US | 99.26% |
| MG | 97.73% | |
| US + ES | 88.46% | |
| SPECIFICITY | MG+ US | 44.37% |
| MG | 81.73% | |
| US + ES | 67.8% | |
| IDC (Invasive Ductal Carcinoma) | ILC (Invasive Lobular Carcinoma) |
|---|---|
| Irregular shape | Absence of smooth margins |
| Non-circumscribed appearance | Absence of rim-shaped enhancement |
| Heterogeneous/rim enhancement | Single spiculated non-homogenous |
| Intratumoral high signal | Mass/dominant lesion surrounded by many small enhancing foci—tendency to multifocality |
| Peritumoral edema on T2 WI | |
| High peak enhancement |
| MOLECULAR SUBTYPE | PROGNOSTIC |
|---|---|
| Luminal A (ER+ and/or PR+, HER−) | -Best prognosis of all subtypes -Low Ki67 expression -Good response to hormonal treatment |
| Luminal B (ER+ and/or PR+, HER+) | -Less favorable prognosis compared to Luminal A -High Ki67 expression -Good response to chemotherapy |
| HER2 enriched (ER−, PR−, HER2+) | -Poor perspective -Likely high-grade -Aggressive course -Anti-HER2 therapy improves prognosis |
| Triple Negative (ER−, PR−, HER2−) | -Worst prognosis -More aggressive -High recurrency -More common in younger women -Complex management necessary |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burciu, O.M.; Sas, I.; Popoiu, T.-A.; Merce, A.-G.; Moleriu, L.; Cobec, I.M. Correlations of Imaging and Therapy in Breast Cancer Based on Molecular Patterns: An Important Issue in the Diagnosis of Breast Cancer. Int. J. Mol. Sci. 2024, 25, 8506. https://doi.org/10.3390/ijms25158506
Burciu OM, Sas I, Popoiu T-A, Merce A-G, Moleriu L, Cobec IM. Correlations of Imaging and Therapy in Breast Cancer Based on Molecular Patterns: An Important Issue in the Diagnosis of Breast Cancer. International Journal of Molecular Sciences. 2024; 25(15):8506. https://doi.org/10.3390/ijms25158506
Chicago/Turabian StyleBurciu, Oana Maria, Ioan Sas, Tudor-Alexandru Popoiu, Adrian-Grigore Merce, Lavinia Moleriu, and Ionut Marcel Cobec. 2024. "Correlations of Imaging and Therapy in Breast Cancer Based on Molecular Patterns: An Important Issue in the Diagnosis of Breast Cancer" International Journal of Molecular Sciences 25, no. 15: 8506. https://doi.org/10.3390/ijms25158506
APA StyleBurciu, O. M., Sas, I., Popoiu, T.-A., Merce, A.-G., Moleriu, L., & Cobec, I. M. (2024). Correlations of Imaging and Therapy in Breast Cancer Based on Molecular Patterns: An Important Issue in the Diagnosis of Breast Cancer. International Journal of Molecular Sciences, 25(15), 8506. https://doi.org/10.3390/ijms25158506






