IFN-β Overexpressing Adipose-Derived Mesenchymal Stem Cells Mitigate Alcohol-Induced Liver Damage and Gut Permeability
Abstract
:1. Introduction
2. Results
2.1. Overexpressing Human ASCs with IFN-β Induces HGF Secretion
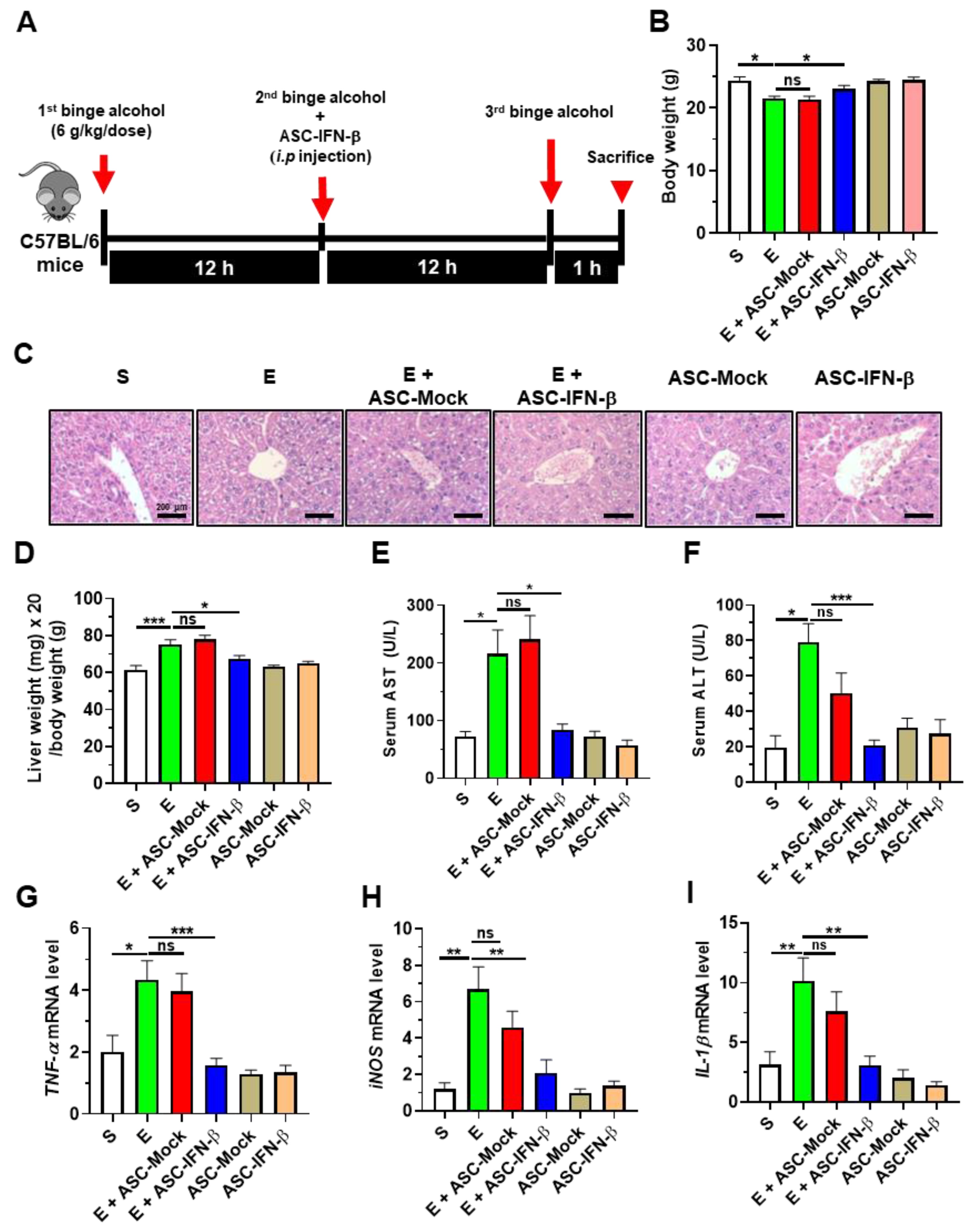
2.2. Treatment of ASCs Expressing IFN-β Decreases Binge Alcohol-Induced Liver Damage in Mice
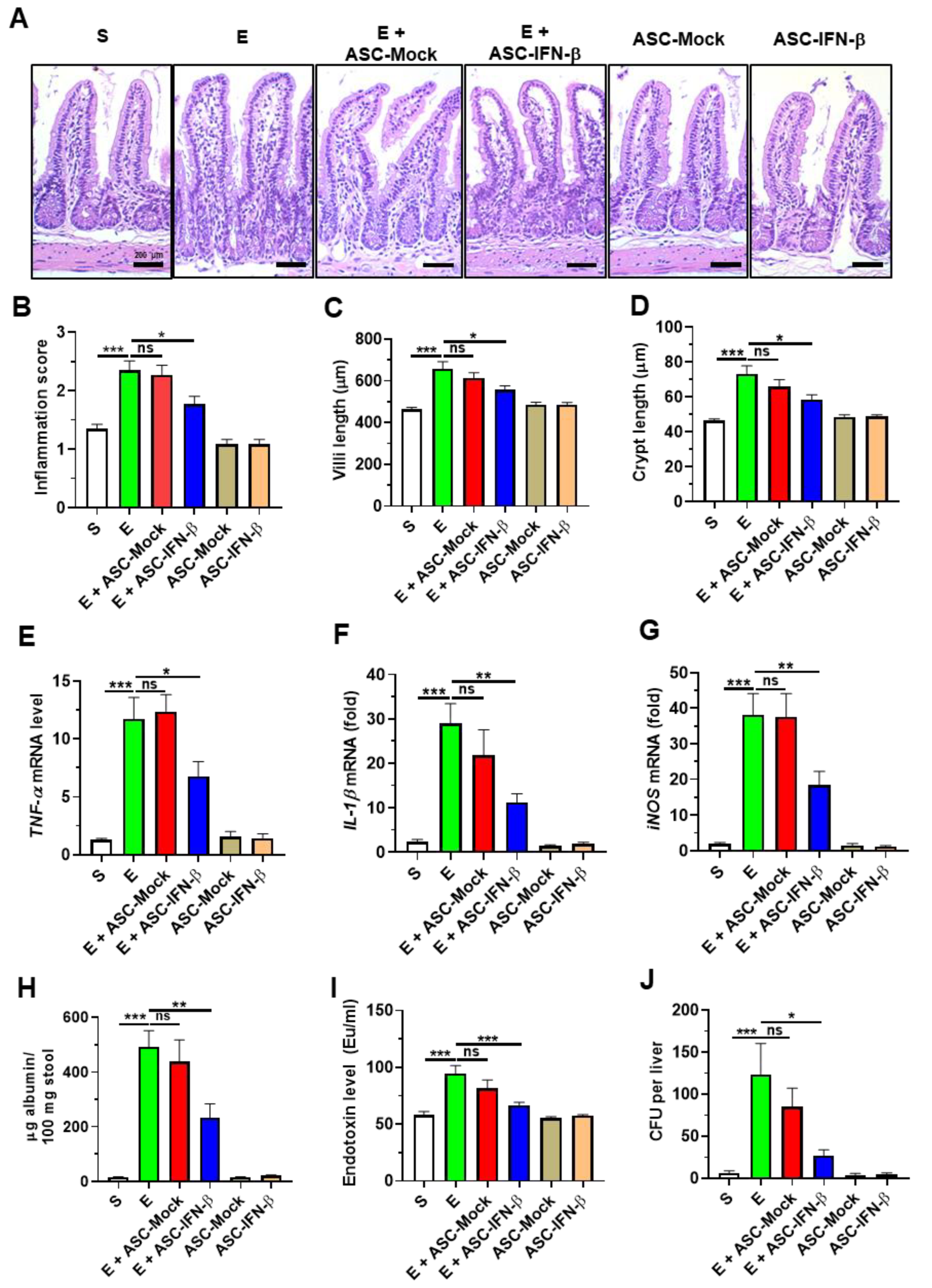
2.3. Treatment of ASCs Expressing IFN-β Reduces Binge Alcohol-Induced Gut Injury and Leakiness
2.4. HGF Reduces Ethanol-Induced Cell Death and Barrier Disruption in Intestinal Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. ASC Culture and Characterization
4.2. Transfection of Non-Viral Vector to Carry Plasmid Containing the IFN-β Gene in the ASCs
4.3. Evaluation of the Caspase Activity and Barrier Function in Caco-2 Cells
4.4. ELISA
4.5. Animal Treatments
4.6. Histology
4.7. Endotoxin Measurement
4.8. Albumin ELISA
4.9. Serum Alanine and Aspartate Transaminase Measurements
4.10. Quantitative Reverse-Transcription Polymerase Chain Reaction
4.11. Liver Enterobacteria Assay
4.12. Statistical Analysis
5. Conclusions
6. Limitations of Current Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, A.M.; Yang, J.Y.; Barritt, A.S., IV; Bataller, R.; Peery, A.F. Rising Mortality from Alcohol-Associated Liver Disease in the United States in the 21st Century. Am. J. Gastroenterol. 2020, 115, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Paula, H.; Asrani, S.K.; Boetticher, N.C.; Pedersen, R.; Shah, V.H.; Kim, W.R. Alcoholic liver disease-related mortality in the United States: 1980–2003. Am. J. Gastroenterol. 2010, 105, 1782–1787. [Google Scholar] [CrossRef] [PubMed]
- Keshavarzian, A.; Holmes, E.W.; Patel, M.; Iber, F.; Fields, J.Z.; Pethkar, S. Leaky gut in alcoholic cirrhosis: A possible mechanism for alcohol-induced liver damage. Am. J. Gastroenterol. 1999, 94, 200–207. [Google Scholar] [CrossRef]
- Liangpunsakul, S.; Toh, E.; Ross, R.A.; Heathers, L.E.; Chandler, K.; Oshodi, A.; McGee, B.; Modlik, E.; Linton, T.; Mangiacarne, D.; et al. Quantity of alcohol drinking positively correlates with serum levels of endotoxin and markers of monocyte activation. Sci. Rep. 2017, 7, 4462. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Yu, L.R.; Abdelmegeed, M.A.; Yoo, S.H.; Song, B.J. Apoptosis of enterocytes and nitration of junctional complex proteins promote alcohol-induced gut leakiness and liver injury. J. Hepatol. 2018, 69, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.T.L.; Reuveny, S.; Oh, S.K. Human mesenchymal stem cell therapy for cartilage repair: Review on isolation, expansion, and constructs. Stem Cell Res. 2020, 44, 101738. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Lennon, D.P.; Eaton, V.; Maier, K.; Caplan, A.I.; Miller, S.D.; Miller, R.H. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia 2009, 57, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.A.; Wang, S.Y.; Yeh, C.C.; Fu, T.W.; Fu, Y.Y.; Ko, T.L.; Chiu, M.M.; Chen, T.H.; Tsai, P.J.; Fu, Y.S. Reversal of bleomycin-induced rat pulmonary fibrosis by a xenograft of human umbilical mesenchymal stem cells from Wharton’s jelly. Theranostics 2019, 9, 6646–6664. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Joel, M.D.M.; Yuan, J.; Wang, J.; Cai, X.; Ocansey, D.K.W.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; et al. Human umbilical cord mesenchymal stem cells alleviate inflammatory bowel disease by inhibiting ERK phosphorylation in neutrophils. Inflammopharmacology 2020, 28, 603–616. [Google Scholar] [CrossRef]
- Heidari, R.; Gholamian Dehkordi, N.; Mohseni, R.; Safaei, M. Engineering mesenchymal stem cells: A novel therapeutic approach in breast cancer. J. Drug Target. 2020, 28, 732–741. [Google Scholar] [CrossRef]
- Ocansey, D.K.W.; Pei, B.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Improved therapeutics of modified mesenchymal stem cells: An update. J. Transl. Med. 2020, 18, 42. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Li, H.; Han, M.; Ruan, M.; Liu, Z.; Zhang, F.; Zhang, C.; Choi, Y.; Liu, B. Mesenchymal Stem Cells with Enhanced Bcl-2 Expression Promote Liver Recovery in a Rat Model of Hepatic Cirrhosis. Cell Physiol. Biochem. 2016, 40, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Su, D.N.; Wu, S.P.; Xu, S.Z. Mesenchymal stem cell-based Smad7 gene therapy for experimental liver cirrhosis. Stem Cell Res. Ther. 2020, 11, 395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, R.; Rong, W.; Han, M.; Cui, C.; Feng, Z.; Sun, X.; Jin, S. Therapeutic effect of hepatocyte growth factor-overexpressing bone marrow-derived mesenchymal stem cells on CCl4-induced hepatocirrhosis. Cell Death Dis. 2018, 9, 1186. [Google Scholar] [CrossRef] [PubMed]
- Jolly, D. Viral vector systems for gene therapy. Cancer Gene. Ther. 1994, 1, 51–64. [Google Scholar] [PubMed]
- Nayak, S.; Herzog, R.W. Progress and prospects: Immune responses to viral vectors. Gene. Ther. 2010, 17, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Jung, P.Y.; Ryu, H.; Rhee, K.J.; Hwang, S.; Lee, C.G.; Gwon, S.Y.; Kim, J.; Kim, J.; Yoo, B.S.; Baik, S.K.; et al. Adipose tissue-derived mesenchymal stem cells cultured at high density express IFN-beta and TRAIL and suppress the growth of H460 human lung cancer cells. Cancer Lett. 2019, 440–441, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Byun, C.S.; Hwang, S.; Woo, S.H.; Kim, M.Y.; Lee, J.S.; Lee, J.I.; Kong, J.H.; Bae, K.S.; Park, I.H.; Kim, S.H.; et al. Adipose Tissue-Derived Mesenchymal Stem Cells Suppress Growth of Huh7 Hepatocellular Carcinoma Cells via Interferon (IFN)-beta-Mediated JAK/STAT1 Pathway in vitro. Int. J. Med. Sci. 2020, 17, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.; Oh, J.E.; Rhee, K.J.; Baik, S.K.; Kim, J.; Kang, S.J.; Sohn, J.H.; Choi, E.; Shin, H.C.; Kim, Y.M.; et al. Adipose tissue-derived mesenchymal stem cells cultured at high density express IFN-beta and suppress the growth of MCF-7 human breast cancer cells. Cancer Lett. 2014, 352, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Guarda, G.; Braun, M.; Staehli, F.; Tardivel, A.; Mattmann, C.; Forster, I.; Farlik, M.; Decker, T.; Du Pasquier, R.A.; Romero, P.; et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 2011, 34, 213–223. [Google Scholar] [CrossRef]
- Yoo, C.H.; Yeom, J.H.; Heo, J.J.; Song, E.K.; Lee, S.I.; Han, M.K. Interferon beta protects against lethal endotoxic and septic shock through SIRT1 upregulation. Sci. Rep. 2014, 4, 4220. [Google Scholar] [CrossRef] [PubMed]
- Kawaratani, H.; Tsujimoto, T.; Douhara, A.; Takaya, H.; Moriya, K.; Namisaki, T.; Noguchi, R.; Yoshiji, H.; Fujimoto, M.; Fukui, H. The effect of inflammatory cytokines in alcoholic liver disease. Mediat. Inflamm. 2013, 2013, 495156. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Ye, D.; Dokladny, K.; Ma, T.Y. Mechanism of IL-1beta-induced increase in intestinal epithelial tight junction permeability. J. Immunol. 2008, 180, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Guo, S.; Ye, D.; Ma, T.Y. TNF-alpha modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am. J. Pathol. 2013, 183, 1871–1884. [Google Scholar] [CrossRef] [PubMed]
- Vigo, T.; La Rocca, C.; Faicchia, D.; Procaccini, C.; Ruggieri, M.; Salvetti, M.; Centonze, D.; Matarese, G.; Uccelli, A.; Network, M. IFNbeta enhances mesenchymal stromal (Stem) cells immunomodulatory function through STAT1-3 activation and mTOR-associated promotion of glucose metabolism. Cell Death Dis. 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Molnarfi, N.; Benkhoucha, M.; Bjarnadottir, K.; Juillard, C.; Lalive, P.H. Interferon-beta induces hepatocyte growth factor in monocytes of multiple sclerosis patients. PLoS ONE 2012, 7, e49882. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, T.; Kakishita, E.; Hamano, T.; Kataoka, Y.; Seto, Y.; Iwata, N.; Kaneda, Y.; Matsumoto, K.; Nakamura, T.; Ueki, T.; et al. Hepatocyte growth factor ameliorates acute graft-versus-host disease and promotes hematopoietic function. J. Clin. Investig. 2001, 107, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Arthur, L.G.; Kuenzler, K.A.; Schwartz, M.Z. Hepatocyte growth factor ameliorates inflammatory bowel disease in a rat model. J. Gastrointest Surg. 2003, 7, 1062–1068, discussion 1068. [Google Scholar] [CrossRef] [PubMed]
- Mou, S.; Wang, Q.; Shi, B.; Gu, L.; Ni, Z. Hepatocyte growth factor ameliorates progression of interstitial injuries in tubular epithelial cells. Scand. J. Urol. Nephrol. 2010, 44, 121–128. [Google Scholar] [CrossRef]
- Abadie, A.; Wietzerbin, J. Involvement of TNF-related apoptosis-inducing ligand (TRAIL) induction in interferon gamma-mediated apoptosis in Ewing tumor cells. Ann. N. Y. Acad. Sci. 2003, 1010, 117–120. [Google Scholar] [CrossRef]
- Semnani, R.T.; Venugopal, P.G.; Mahapatra, L.; Skinner, J.A.; Meylan, F.; Chien, D.; Dorward, D.W.; Chaussabel, D.; Siegel, R.M.; Nutman, T.B. Induction of TRAIL- and TNF-alpha-dependent apoptosis in human monocyte-derived dendritic cells by microfilariae of Brugia malayi. J. Immunol. 2008, 181, 7081–7089. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, S.; Bandil, K.; Proksch, P.; Murugiyan, K.; Bharadwaj, M. Effects of A.marina-Derived Isoquercitrin on TNF-Related Apoptosis-Inducing Ligand Receptor (TRAIL-R) Expression and Apoptosis Induction in Cervical Cancer Cells. Appl. Biochem. Biotechnol. 2017, 182, 697–707. [Google Scholar] [CrossRef]
- Ling, X.; Marini, F.; Konopleva, M.; Schober, W.; Shi, Y.; Burks, J.; Clise-Dwyer, K.; Wang, R.Y.; Zhang, W.; Yuan, X.; et al. Mesenchymal Stem Cells Overexpressing IFN-beta Inhibit Breast Cancer Growth and Metastases through Stat3 Signaling in a Syngeneic Tumor Model. Cancer Microenviron. 2010, 3, 83–95. [Google Scholar] [CrossRef]
- Xu, G.; Guo, Y.; Seng, Z.; Cui, G.; Qu, J. Bone marrow-derived mesenchymal stem cells co-expressing interleukin-18 and interferon-beta exhibit potent antitumor effect against intracranial glioma in rats. Oncol. Rep. 2015, 34, 1915–1922. [Google Scholar] [CrossRef]
- Cho, Y.E.; Kim, D.K.; Seo, W.; Gao, B.; Yoo, S.H.; Song, B.J. Fructose Promotes Leaky Gut, Endotoxemia, and Liver Fibrosis Through Ethanol-Inducible Cytochrome P450-2E1-Mediated Oxidative and Nitrative Stress. Hepatology 2021, 73, 2180–2195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Wang, X. Recent advances in natural products from plants for treatment of liver diseases. Eur. J. Med. Chem. 2013, 63, 570–577. [Google Scholar] [CrossRef]
- Tarantino, G.; Finelli, C. Systematic review on intervention with prebiotics/probiotics in patients with obesity-related nonalcoholic fatty liver disease. Future Microbiol. 2015, 10, 889–902. [Google Scholar] [CrossRef]
- Milone, M.C.; O’Doherty, U. Clinical use of lentiviral vectors. Leukemia 2018, 32, 1529–1541. [Google Scholar] [CrossRef]
- Lu, Z.; Chang, W.; Meng, S.; Xu, X.; Xie, J.; Guo, F.; Yang, Y.; Qiu, H.; Liu, L. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res. Ther. 2019, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Park, B.W.; Jung, S.H.; Das, S.; Lee, S.M.; Park, J.H.; Kim, H.; Hwang, J.W.; Lee, S.; Kim, H.J.; Kim, H.Y.; et al. In vivo priming of human mesenchymal stem cells with hepatocyte growth factor-engineered mesenchymal stem cells promotes therapeutic potential for cardiac repair. Sci. Adv. 2020, 6, eaay6994. [Google Scholar] [CrossRef]
- Boicean, A.; Birlutiu, V.; Ichim, C.; Brusnic, O.; Onisor, D.M. Fecal Microbiota Transplantation in Liver Cirrhosis. Biomedicines 2023, 11, 2930. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.; Eom, Y.W.; Kang, S.H.; Baik, S.K.; Kim, M.Y. IFN-β Overexpressing Adipose-Derived Mesenchymal Stem Cells Mitigate Alcohol-Induced Liver Damage and Gut Permeability. Int. J. Mol. Sci. 2024, 25, 8509. https://doi.org/10.3390/ijms25158509
Hwang S, Eom YW, Kang SH, Baik SK, Kim MY. IFN-β Overexpressing Adipose-Derived Mesenchymal Stem Cells Mitigate Alcohol-Induced Liver Damage and Gut Permeability. International Journal of Molecular Sciences. 2024; 25(15):8509. https://doi.org/10.3390/ijms25158509
Chicago/Turabian StyleHwang, Soonjae, Young Woo Eom, Seong Hee Kang, Soon Koo Baik, and Moon Young Kim. 2024. "IFN-β Overexpressing Adipose-Derived Mesenchymal Stem Cells Mitigate Alcohol-Induced Liver Damage and Gut Permeability" International Journal of Molecular Sciences 25, no. 15: 8509. https://doi.org/10.3390/ijms25158509




