Vaccine Platform Comparison: Protective Efficacy against Lethal Marburg Virus Challenge in the Hamster Model
Abstract
1. Introduction
2. Results
2.1. Single-Dose Vaccination with LION-MARV or VSV-MARV Uniformly Protects Hamsters from Lethal MARV Infection
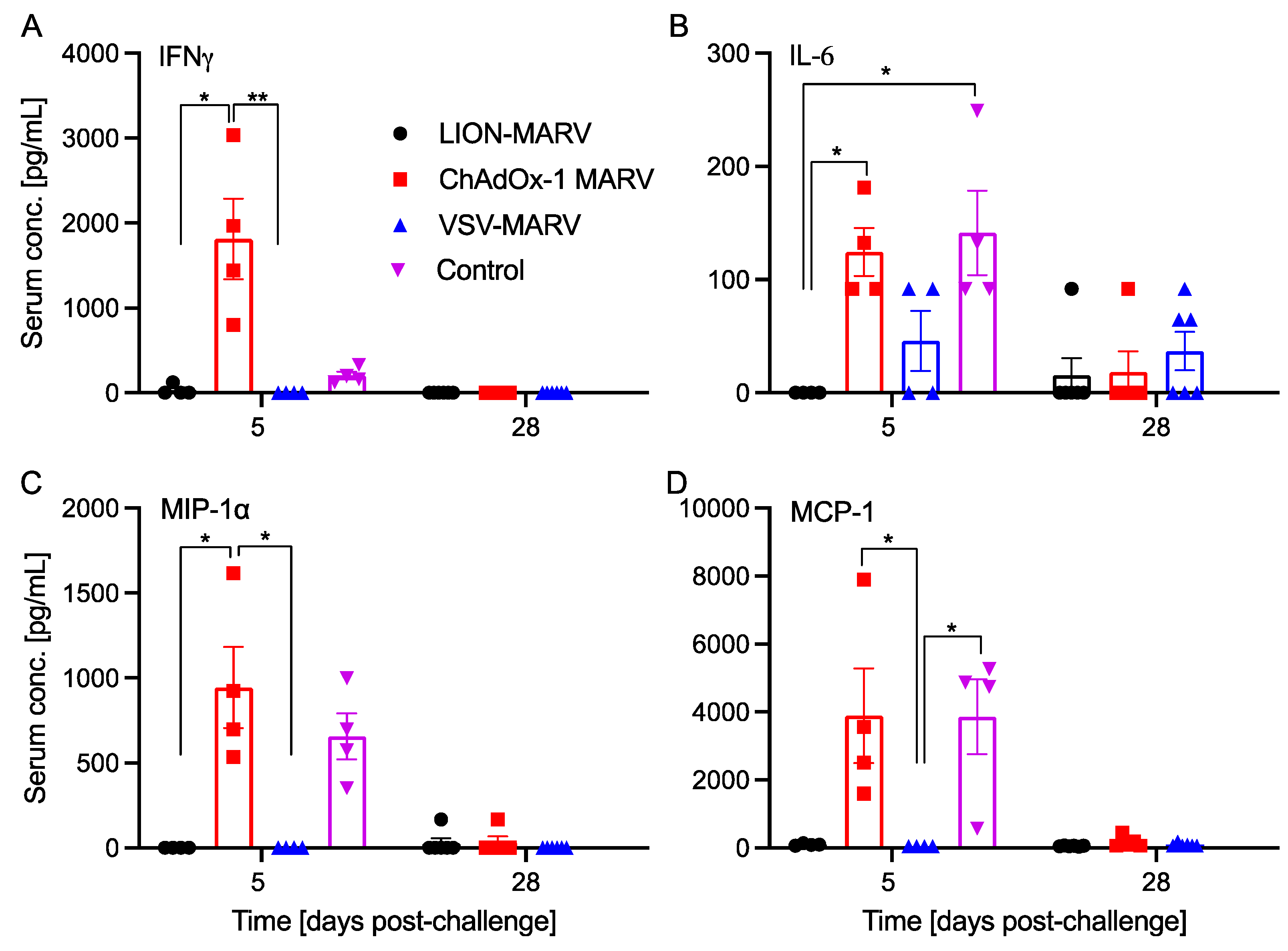
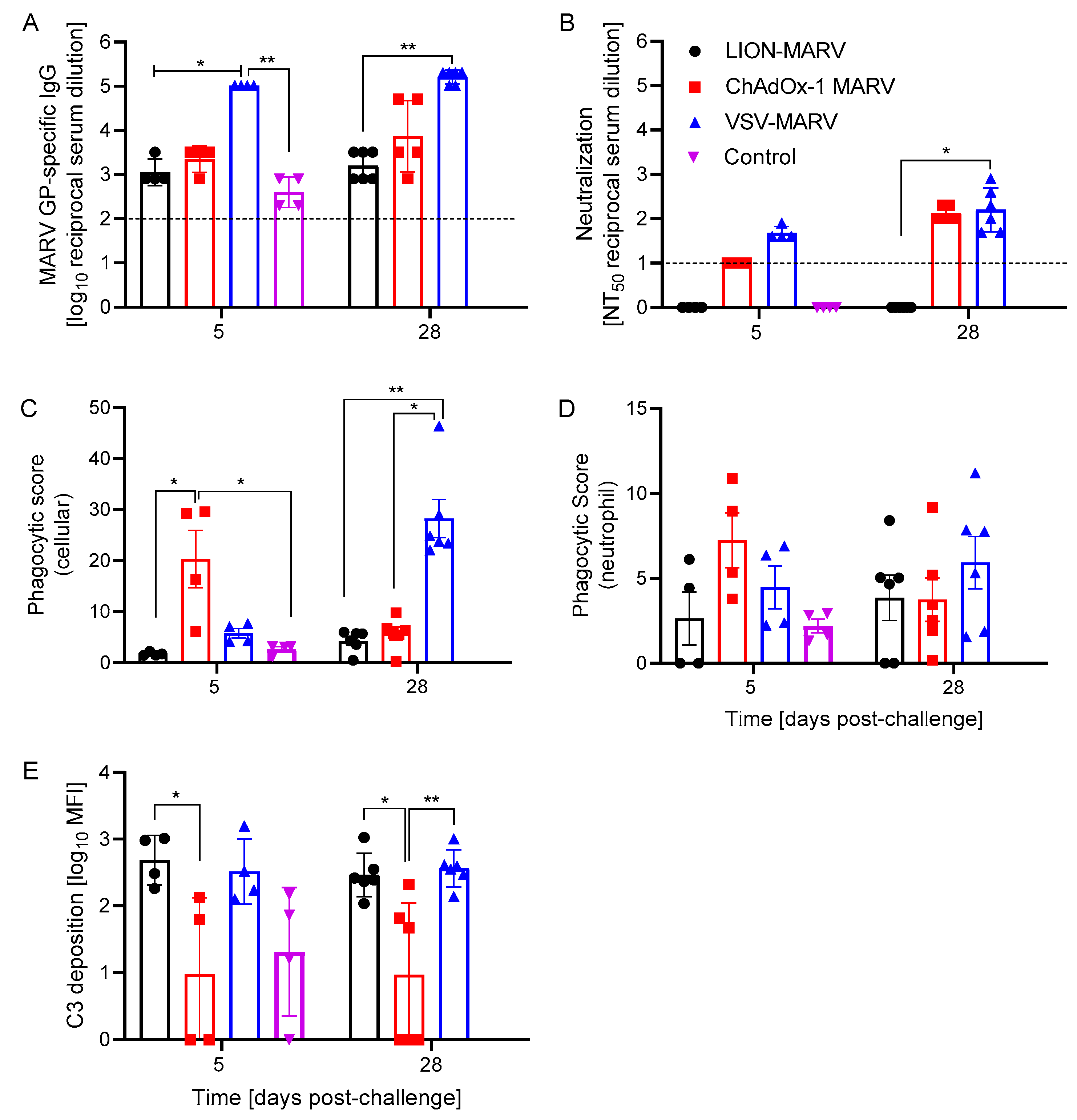
2.2. Antigen-Specific Humoral Immune Responses Post-Challenge and Functionalities Depend on the Vaccine Platform
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Cells and Viruses
4.3. Vaccines
4.4. Study Design
4.5. Viral Load Quantification
4.6. Cytokine Analysis
4.7. Assessment of Humoral Immune Response
4.8. Quantification of Antibody Effector Functions
4.9. Antibody-Dependent Complement Deposition (ADCD)
4.10. Antibody-Dependent Neutrophil Phagocytosis (ADNP)
4.11. Antibody-Dependent Cellular Phagocytosis (ADCP)
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldmann, H.; Sanchez, A.; Geisbert, T.W. Fields Virology; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Ristanovic, E.S.; Kokoskov, N.S.; Crozier, I.; Kuhn, J.H.; Gligic, A.S. A Forgotten Episode of Marburg Virus Disease: Belgrade, Yugoslavia, 1967. Microbiol. Mol. Biol. Rev. 2020, 84, e00095-19. [Google Scholar] [CrossRef] [PubMed]
- Slenczka, W. Filovirus Research: How It Began. Curr. Top. Microbiol. Immunol. 2017, 411, 3–21. [Google Scholar] [PubMed]
- Towner, J.S.; Khristova, M.L.; Sealy, T.K.; Vincent, M.J.; Erickson, B.R.; Bawiec, D.A.; Hartman, A.L.; Comer, J.A.; Zaki, S.R.; Stroher, U.; et al. Marburgvirus Genomics and Association with a Large Hemorrhagic Fever Outbreak in Angola. J. Virol. 2006, 80, 6497–6516. [Google Scholar] [CrossRef] [PubMed]
- Koundouno, F.R.; Kafetzopoulou, L.E.; Faye, M.; Renevey, A.; Soropogui, B.; Ifono, K.; Nelson, E.V.; Kamano, A.A.; Tolno, C.; Annibaldis, G.; et al. Detection of Marburg Virus Disease in Guinea. N. Engl. J. Med. 2022, 386, 2528–2530. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z. Ghana Declares Its First Outbreak of Marburg Virus Disease after Two Deaths. BMJ 2022, 378, o1797. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Deb, N. Marburg Virus Outbreak in Equatorial Guinea-What Are the Implications for India? New Microbes New Infect. 2023, 53, 101120. [Google Scholar] [CrossRef] [PubMed]
- Cuomo-Dannenburg, G.; McCain, K.; McCabe, R.; Unwin, H.J.T.; Doohan, P.; Nash, R.K.; Hicks, J.T.; Charniga, K.; Geismar, C.; Lambert, B.; et al. Marburg virus disease outbreaks, mathematical models, and disease parameters: A systematic review. Lancet Infect. Dis. 2024, 24, e307–e317. [Google Scholar] [CrossRef]
- Mehedi, M.; Groseth, A.; Feldmann, H.; Ebihara, H. Clinical Aspects of Marburg Hemorrhagic Fever. Future Virol. 2011, 6, 1091–1106. [Google Scholar] [CrossRef]
- World Health Organization. Prioritizing Diseases for Research and Development in Emergency Contexts; Prioritizing diseases for research and development in emergency contexts (who.int); World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Hamer, M.J.; Houser, K.V.; Hofstetter, A.R.; Ortega-Villa, A.M.; Lee, C.; Preston, A.; Augustine, B.; Andrews, C.; Yamshchikov, G.V.; Hickman, S.; et al. Safety, Tolerability, and Immunogenicity of the Chimpanzee Adenovirus Type 3-Vectored Marburg Virus (Cad3-Marburg) Vaccine in Healthy Adults in the USA: A First-in-Human, Phase 1, Open-Label, Dose-Escalation Trial. Lancet 2023, 401, 294–302. [Google Scholar] [CrossRef]
- Kibuuka, H.; Berkowitz, N.M.; Millard, M.; Enama, M.E.; Tindikahwa, A.; Sekiziyivu, A.B.; Costner, P.; Sitar, S.; Glover, D.; Hu, Z.; et al. Safety and Immunogenicity of Ebola Virus and Marburg Virus Glycoprotein DNA Vaccines Assessed Separately and Concomitantly in Healthy Ugandan Adults: A Phase 1b, Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Lancet 2015, 385, 1545–1554. [Google Scholar] [CrossRef]
- Sarwar, U.N.; Costner, P.; Enama, M.E.; Berkowitz, N.; Hu, Z.; Hendel, C.S.; Sitar, S.; Plummer, S.; Mulangu, S.; Bailer, R.T.; et al. Safety and Immunogenicity of DNA Vaccines Encoding Ebolavirus and Marburgvirus Wild-Type Glycoproteins in a Phase I Clinical Trial. J. Infect. Dis. 2015, 211, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Marzi, A.; Jankeel, A.; Menicucci, A.R.; Callison, J.; O’donnell, K.L.; Feldmann, F.; Pinski, A.N.; Hanley, P.W.; Messaoudi, I. Single Dose of a VSV-Based Vaccine Rapidly Protects Macaques From Marburg Virus Disease. Front. Immunol. 2021, 12, 774026. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.L.; Feldmann, F.; Kaza, B.; Clancy, C.S.; Hanley, P.W.; Fletcher, P.; Marzi, A. Rapid protection of nonhuman primates against Marburg virus disease using a single low-dose VSV-based vaccine. EBioMedicine 2023, 89, 104463. [Google Scholar] [CrossRef] [PubMed]
- Abelson, D.; Barajas, J.; Stuart, L.; Kim, D.; Marimuthu, A.; Hu, C.; Yamamoto, B.; Ailor, E.; Whaley, K.J.; Vu, H.; et al. Long-term Prophylaxis Against Aerosolized Marburg Virus in Nonhuman Primates With an Afucosylated Monoclonal Antibody. J. Infect. Dis. 2023, 228 (Suppl. S7), S701–S711. [Google Scholar] [CrossRef] [PubMed]
- Marzi, A.; Menicucci, A.R.; Engelmann, F.; Callison, J.; Horne, E.J.; Feldmann, F.; Jankeel, A.; Feldmann, H.; Messaoudi, I. Protection Against Marburg Virus Using a Recombinant VSV-Vaccine Depends on T and B Cell Activation. Front. Immunol. 2018, 9, 3071. [Google Scholar] [CrossRef] [PubMed]
- Bosaeed, M.; Balkhy, H.H.; Almaziad, S.; Aljami, H.A.; Alhatmi, H.; Alanazi, H.; Alahmadi, M.; Jawhary, A.; Alenazi, M.W.; Almasoud, A.; et al. Safety and Immunogenicity of Chadox1 Mers Vaccine Candidate in Healthy Middle Eastern Adults (Mers002): An Open-Label, Non-Randomised, Dose-Escalation, Phase 1b Trial. Lancet Microbe 2022, 3, e11–e20. [Google Scholar] [CrossRef] [PubMed]
- Ewer, K.J.; Barrett, J.R.; Belij-Rammerstorfer, S.; Sharpe, H.; Makinson, R.; Morter, R.; Flaxman, A.; Wright, D.; Bellamy, D.; Bittaye, M.; et al. T Cell and Antibody Responses Induced by a Single Dose of Chadox1 Ncov-19 (Azd1222) Vaccine in a Phase 1/2 Clinical Trial. Nat. Med. 2021, 27, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Bloom, K.; Berg, F.v.D.; Arbuthnot, P. Self-amplifying RNA vaccines for infectious diseases. Gene Ther. 2021, 28, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.B.; Lambert, L.; Kinnear, E.; Busse, D.; Erbar, S.; Reuter, K.C.; Wicke, L.; Perkovic, M.; Beissert, T.; Haas, H.; et al. Self-Amplifying RNA Vaccines Give Equivalent Protection against Influenza to mRNA Vaccines but at Much Lower Doses. Mol. Ther. 2018, 26, 446–455. [Google Scholar] [CrossRef]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An Mrna Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Pollock, K.M.; Cheeseman, H.M.; Szubert, A.J.; Libri, V.; Boffito, M.; Owen, D.; Bern, H.; O’Hara, J.; McFarlane, L.R.; Lemm, N.M.; et al. Safety and Immunogenicity of a Self-Amplifying Rna Vaccine against COVID-19: Covac1, a Phase I, Dose-Ranging Trial. EClinicalMedicine 2022, 44, 101262. [Google Scholar] [CrossRef]
- Low, J.G.; de Alwis, R.; Chen, S.; Kalimuddin, S.; Leong, Y.S.; Mah, T.K.L.; Yuen, N.; Tan, H.C.; Zhang, S.L.; Sim, J.X.Y.; et al. A phase I/II randomized, double-blinded, placebo-controlled trial of a self-amplifying COVID-19 mRNA vaccine. NPJ Vaccines 2022, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Leal, J.M.; Simpson, A.; Warner, N.L.; Berube, B.J.; Archer, J.F.; Park, S.; Kurtz, R.; Hinkley, T.; Nicholes, K.; et al. A localizing nanocarrier formulation enables multi-target immune responses to multivalent replicating RNA with limited systemic inflammation. Mol. Ther. 2023, 31, 2360–2375. [Google Scholar] [CrossRef]
- Erasmus, J.H.; Khandhar, A.P.; O’Connor, M.A.; Walls, A.C.; Hemann, E.A.; Murapa, P.; Archer, J.; Leventhal, S.; Fuller, J.T.; Lewis, T.B.; et al. An Alphavirus-Derived Replicon Rna Vaccine Induces SARS-CoV-2 Neutralizing Antibody and T Cell Responses in Mice and Nonhuman Primates. Sci. Transl. Med. 2020, 12, eabc9396. [Google Scholar] [CrossRef] [PubMed]
- Hawman, D.W.; Meade-White, K.; Archer, J.; Leventhal, S.S.; Wilson, D.; Shaia, C.; Randall, S.; Khandhar, A.P.; Krieger, K.; Hsiang, T.Y.; et al. SARS-CoV-2 Variant-Specific Replicating Rna Vaccines Protect from Disease Following Challenge with Heterologous Variants of Concern. eLife 2022, 11, e75537. [Google Scholar] [CrossRef]
- Hawman, D.W.; Meade-White, K.; Clancy, C.; Archer, J.; Hinkley, T.; Leventhal, S.S.; Rao, D.; Stamper, A.; Lewis, M.; Rosenke, R.; et al. Replicating Rna Platform Enables Rapid Response to the SARS-CoV-2 Omicron Variant and Elicits Enhanced Protection in Naive Hamsters Compared to Ancestral Vaccine. EBioMedicine 2022, 83, 104196. [Google Scholar] [CrossRef]
- Leventhal, S.S.; Meade-White, K.; Rao, D.; Haddock, E.; Leung, J.; Scott, D.; Archer, J.; Randall, S.; Erasmus, J.H.; Feldmann, H.; et al. Replicating RNA vaccination elicits an unexpected immune response that efficiently protects mice against lethal Crimean-Congo hemorrhagic fever virus challenge. EBioMedicine 2022, 82, 104188. [Google Scholar] [CrossRef]
- Pennock, N.D.; White, J.T.; Cross, E.W.; Cheney, E.E.; Tamburini, B.A.; Kedl, R.M. T cell responses: Naïve to memory and everything in between. Adv. Physiol. Educ. 2013, 37, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Whitmire, J.K.; Eam, B.; Whitton, J.L. Tentative T Cells: Memory Cells Are Quick to Respond, but Slow to Divide. PLoS Pathog. 2008, 4, e1000041. [Google Scholar] [CrossRef]
- Marzi, A.; Banadyga, L.; Haddock, E.; Thomas, T.; Shen, K.; Horne, E.J.; Scott, D.P.; Feldmann, H.; Ebihara, H. A hamster model for Marburg virus infection accurately recapitulates Marburg hemorrhagic fever. Sci. Rep. 2016, 6, 39214. [Google Scholar] [CrossRef]
- Fernando, L.; Qiu, X.; Melito, P.L.; Williams, K.J.N.; Feldmann, F.; Feldmann, H.; Jones, S.M.; Alimonti, J.B. Immune Response to Marburg Virus Angola Infection in Nonhuman Primates. J. Infect. Dis. 2015, 212 (Suppl. S2), S234–S241. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Goldsmith, C.S.; Metcalfe, M.G.; Spipopoulou, C.F.; E Rollin, P. Reduced virus replication, proinflammatory cytokine production, and delayed macrophage cell death in human PBMCs infected with the newly discovered Bundibugyo ebolavirus relative to Zaire ebolavirus. Virology 2010, 402, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Wauquier, N.; Becquart, P.; Padilla, C.; Baize, S.; Leroy, E.M. Human Fatal Zaire Ebola Virus Infection Is Associated with an Aberrant Innate Immunity and with Massive Lymphocyte Apoptosis. PLoS Neglected Trop. Dis. 2010, 4, e837. [Google Scholar] [CrossRef] [PubMed]
- Gunn, B.M.; Yu, W.-H.; Karim, M.M.; Brannan, J.M.; Herbert, A.S.; Wec, A.Z.; Halfmann, P.J.; Fusco, M.L.; Schendel, S.L.; Gangavarapu, K.; et al. A Role for Fc Function in Therapeutic Monoclonal Antibody-Mediated Protection against Ebola Virus. Cell Host Microbe 2018, 24, 221–233.e5. [Google Scholar] [CrossRef] [PubMed]
- Saphire, E.O.; Schendel, S.L.; Fusco, M.L.; Gangavarapu, K.; Gunn, B.M.; Wec, A.Z.; Halfmann, P.J.; Brannan, J.M.; Herbert, A.S.; Qiu, X.; et al. Systematic Analysis of Monoclonal Antibodies against Ebola Virus GP Defines Features that Contribute to Protection. Cell 2018, 174, 938–952.e13. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B.; Mahalingam, S.; Marovich, M.A.; Ubol, S.; Mosser, D.M. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: Disease regulation by immune complexes. Lancet Infect. Dis. 2010, 10, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Sawant, J.; Patil, A.; Kurle, S. A Review: Understanding Molecular Mechanisms of Antibody-Dependent Enhancement in Viral Infections. Vaccines 2023, 11, 1240. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Foo, S.; Bruzzone, R.; Dinh, L.V.; King, N.J.C.; Mahalingam, S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol. Rev. 2015, 268, 340–364. [Google Scholar] [CrossRef] [PubMed]
- Capuano, C.; De Federicis, D.; Ciuti, D.; Turriziani, O.; Angeloni, A.; Anastasi, E.; Giannini, G.; Belardinilli, F.; Molfetta, R.; Alvaro, D.; et al. Impact of SARS-CoV-2 Vaccination on Fcgammariiia/Cd16 Dynamics in Natural Killer Cells: Relevance for Antibody-Dependent Functions. Front. Immunol. 2023, 14, 1285203. [Google Scholar] [CrossRef]
- Kaplonek, P.; Deng, Y.; Lee, J.S.-L.; Zar, H.J.; Zavadska, D.; Johnson, M.; Lauffenburger, D.A.; Goldblatt, D.; Alter, G. Hybrid Immunity Expands the Functional Humoral Footprint of Both Mrna and Vector-Based SARS-CoV-2 Vaccines. Cell Rep. Med. 2023, 4, 101048. [Google Scholar] [CrossRef]
- Leventhal, S.S.; Meade-White, K.; Shaia, C.; Tipih, T.; Lewis, M.; Mihalakakos, E.A.; Hinkley, T.; Khandhar, A.P.; Erasmus, J.H.; Feldmann, H.; et al. Single dose, dual antigen RNA vaccines protect against lethal Crimean-Congo haemorrhagic fever virus infection in mice. eBioMedicine 2024, 101, 105017. [Google Scholar] [CrossRef] [PubMed]
- Bowman, K.A.; Kaplonek, P.; McNamara, R.P. Understanding Fc function for rational vaccine design against pathogens. mBio 2024, 15, e0303623. [Google Scholar] [CrossRef] [PubMed]
- Kacen, A.; Javitt, A.; Kramer, M.P.; Morgenstern, D.; Tsaban, T.; Shmueli, M.D.; Teo, G.C.; Leprevost, F.d.V.; Barnea, E.; Yu, F.; et al. Post-translational modifications reshape the antigenic landscape of the MHC I immunopeptidome in tumors. Nat. Biotechnol. 2023, 41, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, F.; Vidarsson, G.; Cragg, M.S. Effect of posttranslational modifications and subclass on IgG activity: From immunity to immunotherapy. Nat. Immunol. 2023, 24, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Ojha, R.; Prajapati, V.K. Cognizance of posttranslational modifications in vaccines: A way to enhanced immunogenicity. J. Cell. Physiol. 2021, 236, 8020–8034. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.M.; Feldmann, H.; Ströher, U.; Geisbert, J.B.; Fernando, L.; Grolla, A.; Klenk, H.-D.; Sullivan, N.J.; Volchkov, V.E.; Fritz, E.A.; et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 2005, 11, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liu, G.; Cao, W.; He, S.; Leung, A.; Stroher, U.; Fairchild, M.J.; Nichols, R.; Crowell, J.; Fusco, J.; et al. A Cloned Recombinant Vesicular Stomatitis Virus-Vectored Marburg Vaccine, Phv01, Protects Guinea Pigs from Lethal Marburg Virus Disease. Vaccines 2022, 10, 1004. [Google Scholar] [CrossRef] [PubMed]
- Dicks, M.D.J.; Spencer, A.J.; Edwards, N.J.; Wadell, G.; Bojang, K.; Gilbert, S.C.; Hill, A.V.S.; Cottingham, M.G. A Novel Chimpanzee Adenovirus Vector with Low Human Seroprevalence: Improved Systems for Vector Derivation and Comparative Immunogenicity. PLoS ONE 2012, 7, e40385. [Google Scholar] [CrossRef] [PubMed]
- Cottingham, M.G.; Carroll, F.; Morris, S.J.; Turner, A.V.; Vaughan, A.M.; Kapulu, M.C.; Colloca, S.; Siani, L.; Gilbert, S.C.; Hill, A.V. Preventing spontaneous genetic rearrangements in the transgene cassettes of adenovirus vectors. Biotechnol. Bioeng. 2012, 109, 719–728. [Google Scholar] [CrossRef]
- van Doremalen, N.; Lambe, T.; Sebastian, S.; Bushmaker, T.; Fischer, R.; Feldmann, F.; Haddock, E.; Letko, M.; Avanzato, V.A.; Rissanen, I.; et al. A single-dose ChAdOx1-vectored vaccine provides complete protection against Nipah Bangladesh and Malaysia in Syrian golden hamsters. PLoS Neglected Trop. Dis. 2019, 13, e0007462. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Feldmann, F.; Shupert, W.L.; Haddock, E.; Twardoski, B.; Feldmann, H. Gamma Irradiation as an Effective Method for Inactivation of Emerging Viral Pathogens. Am. J. Trop. Med. Hyg. 2019, 100, 1275–1277. [Google Scholar] [CrossRef] [PubMed]
- Marzi, A.; Fletcher, P.; Feldmann, F.; Saturday, G.; Hanley, P.W.; Feldmann, H. Species-specific immunogenicity and protective efficacy of a vesicular stomatitis virus-based Sudan virus vaccine: A challenge study in macaques. Lancet Microbe 2023, 4, e171–e178. [Google Scholar] [CrossRef]
- Lewis, G.K.; Ackerman, M.E.; Scarlatti, G.; Moog, C.; Robert-Guroff, M.; Kent, S.J.; Overbaugh, J.; Reeves, R.K.; Ferrari, G.; Thyagarajan, B. Knowns and Unknowns of Assaying Antibody-Dependent Cell-Mediated Cytotoxicity Against HIV-1. Front. Immunol. 2019, 10, 1025. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Donnell, K.L.; Henderson, C.W.; Anhalt, H.; Fusco, J.; Erasmus, J.H.; Lambe, T.; Marzi, A. Vaccine Platform Comparison: Protective Efficacy against Lethal Marburg Virus Challenge in the Hamster Model. Int. J. Mol. Sci. 2024, 25, 8516. https://doi.org/10.3390/ijms25158516
O’Donnell KL, Henderson CW, Anhalt H, Fusco J, Erasmus JH, Lambe T, Marzi A. Vaccine Platform Comparison: Protective Efficacy against Lethal Marburg Virus Challenge in the Hamster Model. International Journal of Molecular Sciences. 2024; 25(15):8516. https://doi.org/10.3390/ijms25158516
Chicago/Turabian StyleO’Donnell, Kyle L., Corey W. Henderson, Hanna Anhalt, Joan Fusco, Jesse H. Erasmus, Teresa Lambe, and Andrea Marzi. 2024. "Vaccine Platform Comparison: Protective Efficacy against Lethal Marburg Virus Challenge in the Hamster Model" International Journal of Molecular Sciences 25, no. 15: 8516. https://doi.org/10.3390/ijms25158516
APA StyleO’Donnell, K. L., Henderson, C. W., Anhalt, H., Fusco, J., Erasmus, J. H., Lambe, T., & Marzi, A. (2024). Vaccine Platform Comparison: Protective Efficacy against Lethal Marburg Virus Challenge in the Hamster Model. International Journal of Molecular Sciences, 25(15), 8516. https://doi.org/10.3390/ijms25158516






