Recent Progress of Induced Spermatogenesis In Vitro
Abstract
:1. Introduction
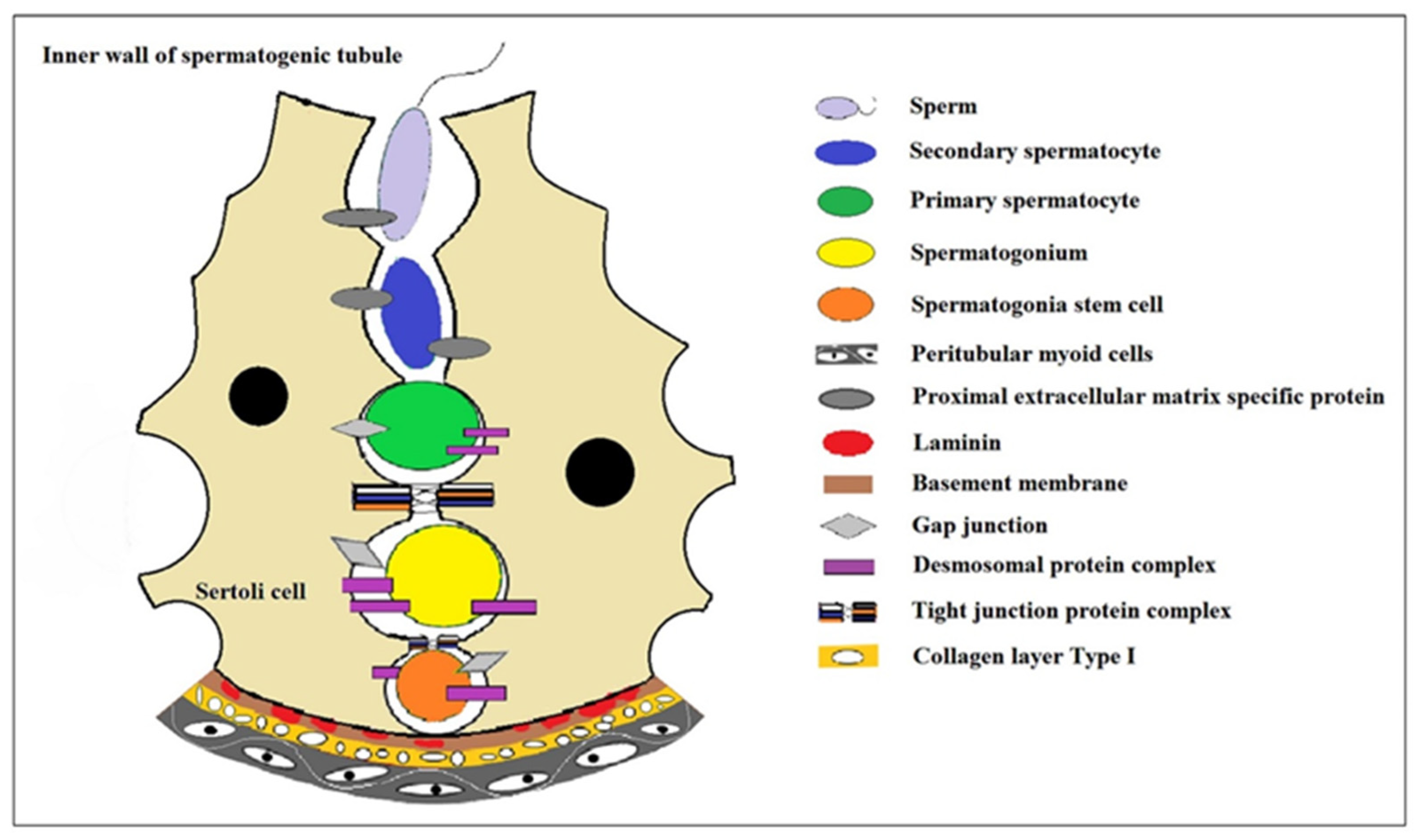
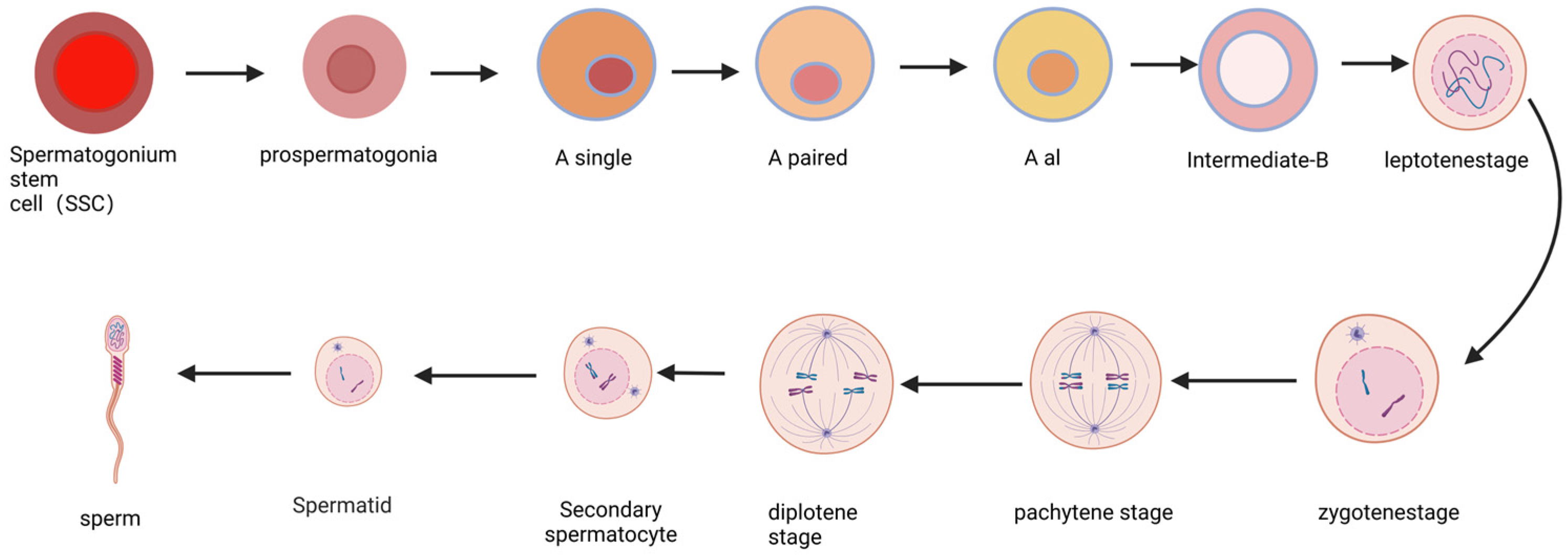
2. The Progress of Spermatogenesis
3. The Induced Spermatogenesis In Vitro
3.1. Scaffold-Based Induced Spermatogenesis In Vitro
3.1.1. Cell-Scaffold-Based Induced Spermatogenesis
3.1.2. Biomaterial-Based Scaffold Induced Spermatogenesis
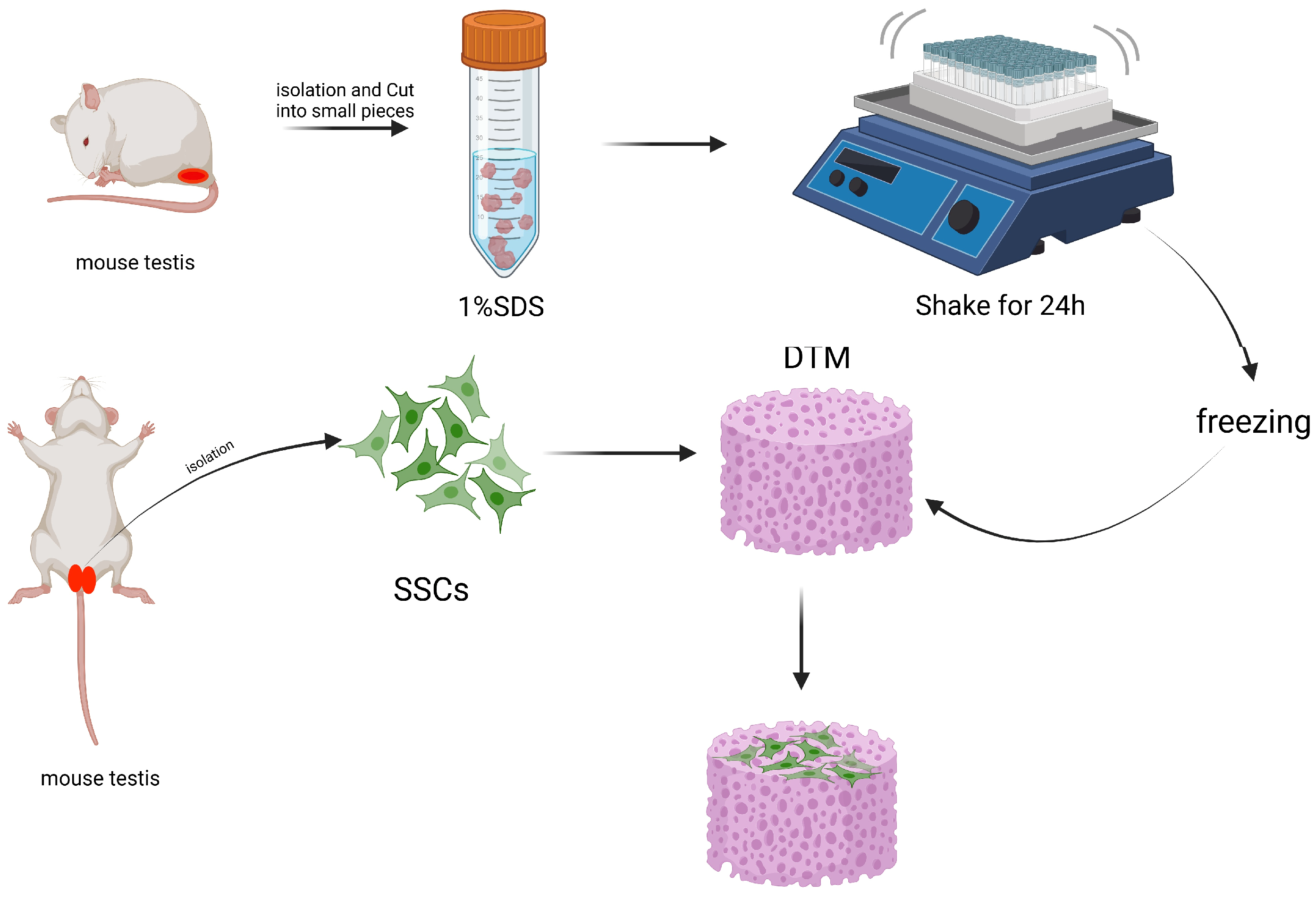
3.1.3. Non-Biomaterial-Based Scaffold Induced Spermatogenesis
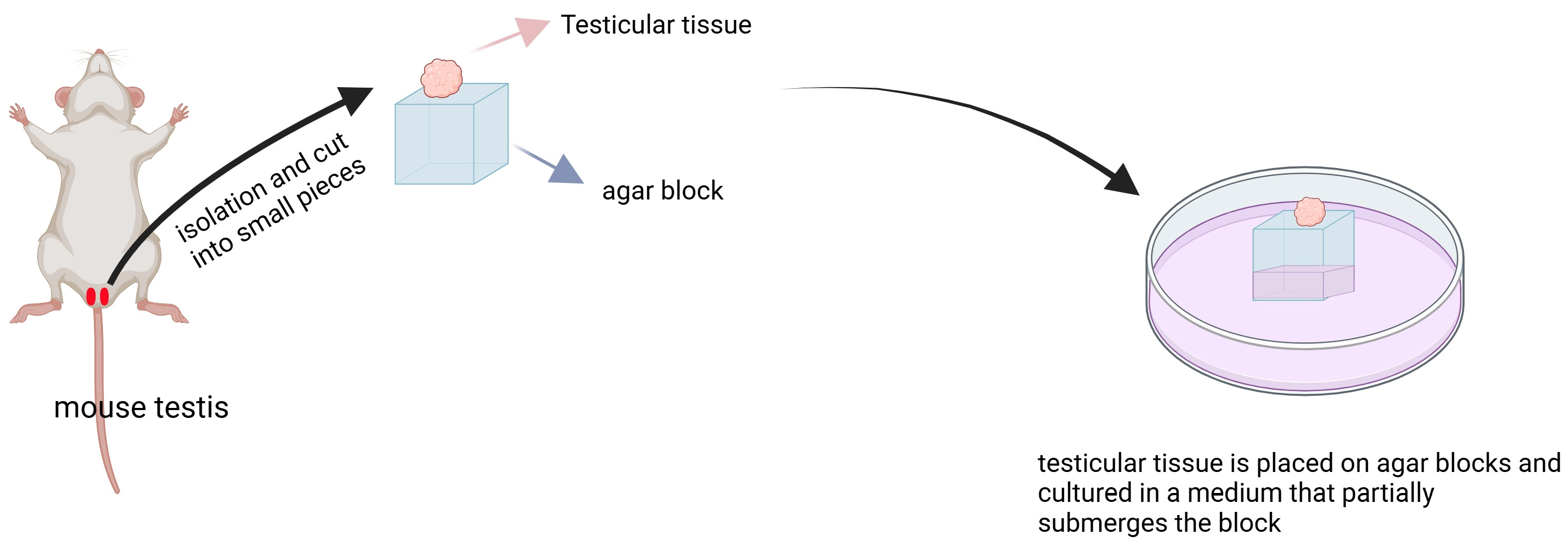
3.1.4. Organ Culture Method Induced Spermatogenesis
3.2. Non-Scaffold-Based Induced Spermatogenesis In Vitro
3.2.1. RA-Induced Spermatogenesis
3.2.2. Hormones or Growth Factors Induced Spermatogenesis
| Source of SSCs | Culture Substrate of SSCs | Markers of SSCs | Whether to Produce Offspring of SSCs | Reference of SSCs |
|---|---|---|---|---|
| Sheep | medium | The expression of post-meiosis marker genes Dnmt3a and Bcl-2 was upregulated | Sperm-like cell | [73] |
| Mice | medium | Id4 and Plzf levels were elevated, but C-kit levels were not significantly different compared with controls | unresearched | [74] |
| Mice | medium | The expression of GFP was enhanced | Early-round sperm cells and late-round sperm cells were seen | [67] |
| Pig | STO-containing medium | UCHL1, CDH1, and OCT4 positive cells showed obvious haploid peaks by flow cytometry | unresearched | [68] |
4. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thumfart, K.M.; Mansuy, I.M. What are Sertoli cells? Historical, methodological, and functional aspects. Andrology 2023, 11, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Teng, Z.; Wang, Z.; Zhu, P.; Wang, Z.; Liu, F.; Liu, X. Human umbilical cord mesenchymal stem cells (hUC-MSCs) alleviate paclitaxel-induced spermatogenesis defects and maintain male fertility. Biol. Res. 2023, 56, 47. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.T.; Eisenberg, M.L. Male infertility as a window to health. Fertil. Steril. 2018, 110, 810–814. [Google Scholar] [CrossRef] [PubMed]
- Agrimson, K.S.; Oatley, M.J.; Mitchell, D.; Oatley, J.M.; Griswold, M.D.; Hogarth, C.A. Retinoic acid deficiency leads to an increase in spermatogonial stem number in the neonatal mouse testis, but excess retinoic acid results in no change. Dev. Biol. 2017, 432, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kang, K.; Liu, S.; Ma, Y.; Yu, M.; Zhao, X. Recent Progress of In Vitro 3D Culture of Male Germ Stem Cells. J. Funct. Biomater. 2023, 14, 543. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Cheng, C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.R.; Kaproth, M.T.; Parks, J.E. In vitro production of haploid germ cells from fresh or frozen-thawed testicular cells of neonatal bulls. Biol. Reprod. 2001, 65, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Gye, M.C.; Choi, K.W.; Hong, J.Y.; Lee, Y.B.; Park, D.W.; Lee, S.J.; Min, C.K. In vitro differentiation of germ cells from nonobstructive azoospermic patients using three-dimensional culture in a collagen gel matrix. Fertil. Steril. 2007, 87, 824–833. [Google Scholar] [CrossRef]
- Oatley, J.M.; Brinster, R.L. Regulation of spermatogonial stem cell self-renewal in mammals. Annu. Rev. Cell Dev. Biol. 2008, 24, 263–286. [Google Scholar] [CrossRef]
- Staub, C.; Johnson, L. Review: Spermatogenesis in the bull. Animal 2018, 12, s27–s35. [Google Scholar] [CrossRef] [PubMed]
- Reda, A.; Hou, M.; Winton, T.R.; Chapin, R.E.; Soder, O.; Stukenborg, J.B. In vitro differentiation of rat spermatogonia into round spermatids in tissue culture. Mol. Hum. Reprod. 2016, 22, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Blanchard, T.L.; Varner, D.D.; Scrutchfield, W.L. Factors affecting spermatogenesis in the stallion. Theriogenology 1997, 48, 1199–1216. [Google Scholar] [CrossRef]
- Thonnes, M.; Vogt, M.; Steinborn, K.; Hausken, K.N.; Levavi-Sivan, B.; Froschauer, A.; Pfennig, F. An ex vivo Approach to Study Hormonal Control of Spermatogenesis in the Teleost Oreochromis niloticus. Front. Endocrinol. 2020, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Gholami, K.; Pourmand, G.; Koruji, M.; Sadighigilani, M.; Navid, S.; Izadyar, F.; Abbasi, M. Efficiency of colony formation and differentiation of human spermatogenic cells in two different culture systems. Reprod. Biol. 2018, 18, 397–403. [Google Scholar] [CrossRef]
- Sun, Y.Z.; Liu, S.T.; Li, X.M.; Zou, K. Progress in in vitro culture and gene editing of porcine spermatogonial stem cells. Zool. Res. 2019, 40, 343–348. [Google Scholar] [CrossRef]
- Piravar, Z.; Jeddi-Tehrani, M.; Sadeghi, M.R.; Mohazzab, A.; Eidi, A.; Akhondi, M.M. In vitro Culture of Human Testicular Stem Cells on Feeder-Free Condition. J. Reprod. Infertil. 2013, 14, 17–22. [Google Scholar]
- Xi, H.M.; Ren, Y.J.; Ren, F.; Li, Y.; Feng, T.Y.; Wang, Z.; Du, Y.Q.; Zhang, L.K.; Hu, J.H. Recent advances in isolation, identification, and culture of mammalian spermatogonial stem cells. Asian J. Androl. 2022, 24, 5–14. [Google Scholar]
- Yu, K.; Zhang, Y.; Zhang, B.L.; Wu, H.Y.; Jiang, W.Q.; Wang, S.T.; Han, D.P.; Liu, Y.X.; Lian, Z.X.; Deng, S.L. In-vitro differentiation of early pig spermatogenic cells to haploid germ cells. Mol. Hum. Reprod. 2019, 25, 507–518. [Google Scholar] [CrossRef]
- Nakagawa, T.; Nabeshima, Y.; Yoshida, S. Functional identification of the actual and potential stem cell compartments in mouse spermatogenesis. Dev. Cell 2007, 12, 195–206. [Google Scholar] [CrossRef]
- Hara, K.; Nakagawa, T.; Enomoto, H.; Suzuki, M.; Yamamoto, M.; Simons, B.D.; Yoshida, S. Mouse spermatogenic stem cells continually interconvert between equipotent singly isolated and syncytial states. Cell Stem Cell 2014, 14, 658–672. [Google Scholar] [CrossRef] [PubMed]
- Veisi, M.; Mansouri, K.; Assadollahi, V.; Jalili, C.; Pirnia, A.; Salahshoor, M.R.; Hoseinkhani, Z.; Gholami, M.R. Evaluation of co-cultured spermatogonial stem cells encapsulated in alginate hydrogel with Sertoli cells and their transplantation into azoospermic mice. Zygote 2022, 30, 344–351. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, X.; Zheng, Y.; Zhu, J.; Qin, Y.; Lv, Y.; Zeng, W. Long-Term Propagation of Porcine Undifferentiated Spermatogonia. Stem Cells Dev. 2017, 26, 1121–1131. [Google Scholar] [CrossRef]
- Khajavi, N.; Akbari, M.; Abdolsamadi, H.R.; Abolhassani, F.; Dehpour, A.R.; Koruji, M.; Habibi, R.M. Role of Somatic Testicular Cells during Mouse Spermatogenesis in Three-Dimensional Collagen Gel Culture System. Cell J. 2014, 16, 79–90. [Google Scholar]
- Kanatsu-Shinohara, M.; Miki, H.; Inoue, K.; Ogonuki, N.; Toyokuni, S.; Ogura, A.; Shinohara, T. Long-term culture of mouse male germline stem cells under serum-or feeder-free conditions. Biol. Reprod. 2005, 72, 985–991. [Google Scholar] [CrossRef]
- Tiptanavattana, N.; Thongkittidilok, C.; Techakumphu, M.; Tharasanit, T. Characterization and in vitro culture of putative spermatogonial stem cells derived from feline testicular tissue. J. Reprod. Dev. 2013, 59, 189–195. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Wang, Q.Z.; Liao, S.Y.; Zhang, Y.; Liu, Y.X.; Han, C.S. In vitro propagation of spermatogonial stem cells from KM mice. Front. Biosci. 2006, 11, 2614–2622. [Google Scholar] [CrossRef]
- Oatley, M.J.; Kaucher, A.V.; Yang, Q.E.; Waqas, M.S.; Oatley, J.M. Conditions for Long-Term Culture of Cattle Undifferentiated Spermatogonia. Biol. Reprod. 2016, 95, 14. [Google Scholar] [CrossRef] [PubMed]
- Zhankina, R.; Baghban, N.; Askarov, M.; Saipiyeva, D.; Ibragimov, A.; Kadirova, B.; Khoradmehr, A.; Nabipour, I.; Shirazi, R.; Zhanbyrbekuly, U.; et al. Mesenchymal stromal/stem cells and their exosomes for restoration of spermatogenesis in non-obstructive azoospermia: A systemic review. Stem Cell Res. Ther. 2021, 12, 229. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, Z.; Shu, M.; Zhang, L.; Xia, W.; Tang, L.; Li, J.; Huang, B.; Li, H. Human placental mesenchymal stem cells ameliorate chemotherapy-induced damage in the testis by reducing apoptosis/oxidative stress and promoting autophagy. Stem Cell Res. Ther. 2021, 12, 199. [Google Scholar] [CrossRef]
- Onen, S.; Kose, S.; Yersal, N.; Korkusuz, P. Mesenchymal stem cells promote spermatogonial stem/progenitor cell pool and spermatogenesis in neonatal mice in vitro. Sci. Rep. 2022, 12, 11494. [Google Scholar] [CrossRef] [PubMed]
- Onen, S.; Atik, A.C.; Gizer, M.; Kose, S.; Yaman, O.; Kulah, H.; Korkusuz, P. A pumpless monolayer microfluidic device based on mesenchymal stem cell-conditioned medium promotes neonatal mouse in vitro spermatogenesis. Stem Cell Res. Ther. 2023, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Baert, Y.; Stukenborg, J.B.; Landreh, M.; De Kock, J.; Jornvall, H.; Soder, O.; Goossens, E. Derivation and characterization of a cytocompatible scaffold from human testis. Hum. Reprod. 2015, 30, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Noghani, A.E.; Asadpour, R.; Saberivand, A.; Mazaheri, Z.; Hamidian, G. Effect of NMDA receptor agonist and antagonist on spermatogonial stem cells proliferation in 2- and 3- dimensional culture systems. Mol. Biol. Rep. 2022, 49, 2197–2207. [Google Scholar] [CrossRef]
- Noghani, A.E.; Asadpour, R.; Saberivand, A.; Mazaheri, Z.; Rodriguez-Wallberg, K.A.; Hamidian, G. Differentiation of neonate mouse spermatogonia on two-dimensional and three-dimensional culture systems supplemented with d-Serine and Dizocilpine (MK-801). Theriogenology 2022, 191, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Ashouri, M.S.; Ashouri, M.S.; Banitalebi, D.M.; Pourmand, G.; Gholami, K.; Talebi, A.; Esfandyari, S.; Jabari, A.; Samadian, A.; Abbasi, M. Isolation, identification and differentiation of human spermatogonial cells on three-dimensional decellularized sheep testis. Acta Histochem. 2020, 122, 151623. [Google Scholar] [CrossRef] [PubMed]
- Bashiri, Z.; Gholipourmalekabadi, M.; Falak, R.; Amiri, I.; Asgari, H.; Chauhan, N.; Koruji, M. In vitro production of mouse morphological sperm in artificial testis bioengineered by 3D printing of extracellular matrix. Int. J. Biol. Macromol. 2022, 217, 824–841. [Google Scholar] [CrossRef]
- Ashouri, M.S.; Banitalebi, D.M.; Koruji, M.; Pourmand, G.; Farzaneh, P.; Ashouri, M.S.; Jabari, A.; Samadian, A.; Khadivi, F.; Abbasi, M. In Vitro Spermatogenesis by Three-dimensional Culture of Spermatogonial Stem Cells on Decellularized Testicular Matrix. Galen. Med. J. 2019, 8, e1565. [Google Scholar] [CrossRef] [PubMed]
- Majidi, G.N.; Movahedin, M.; Mazaheri, Z. Three-Dimensional Culture of Mouse Spermatogonial Stem Cells Using a Decellularised Testicular Scaffold. Cell J. 2020, 21, 410–418. [Google Scholar]
- Rahbar, M.; Asadpour, R.; Azami, M.; Mazaheri, Z.; Hamali, H. Improving the process of spermatogenesis in azoospermic mice using spermatogonial stem cells co-cultured with epididymosomes in three-dimensional culture system. Life Sci. 2022, 310, 121057. [Google Scholar] [CrossRef]
- Rahmani, F.; Movahedin, M.; Mazaheri, Z.; Soleimani, M. Transplantation of mouse iPSCs into testis of azoospermic mouse model: In vivo and in vitro study. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Khadivi, F.; Koruji, M.; Akbari, M.; Jabari, A.; Talebi, A.; Ashouri, M.S.; Panahi, B.A.; Feizollahi, N.; Nikmahzar, A.; Pourahmadi, M.; et al. Application of platelet-rich plasma (PRP) improves self-renewal of human spermatogonial stem cells in two-dimensional and three-dimensional culture systems. Acta Histochem. 2020, 122, 151627. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Park, M.H.; Kim, M.S.; Park, Y.R.; Yun, J.I.; Cheong, H.T.; Kim, M.; Choi, J.H.; Lee, E.; Lee, S.T. Porcine spermatogonial stem cells self-renew effectively in a three dimensional culture microenvironment. Cell Biol. Int. 2017, 41, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- AbuMadighem, A.; Solomon, R.; Stepanovsky, A.; Kapelushnik, J.; Shi, Q.; Meese, E.; Lunenfeld, E.; Huleihel, M. Development of Spermatogenesis In Vitro in Three-Dimensional Culture from Spermatogonial Cells of Busulfan-Treated Immature Mice. Int. J. Mol. Sci. 2018, 19, 3804. [Google Scholar] [CrossRef] [PubMed]
- Huleihel, M.; Nourashrafeddin, S.; Plant, T.M. Application of three-dimensional culture systems to study mammalian spermatogenesis, with an emphasis on the rhesus monkey (Macaca mulatta). Asian J. Androl. 2015, 17, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Abofoul-Azab, M.; Lunenfeld, E.; Levitas, E.; Zeadna, A.; Younis, J.S.; Bar-Ami, S.; Huleihel, M. Identification of Premeiotic, Meiotic, and Postmeiotic Cells in Testicular Biopsies Without Sperm from Sertoli Cell-Only Syndrome Patients. Int. J. Mol. Sci. 2019, 20, 470. [Google Scholar] [CrossRef] [PubMed]
- Hemadi, M.; Assadollahi, V.; Saki, G.; Pirnia, A.; Alasvand, M.; Zendehdel, A.; Gholami, M. Use of alginate hydrogel to improve long-term 3D culture of spermatogonial stem cells: Stemness gene expression and structural features. Zygote 2022, 30, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Eslahi, N.; Hadjighassem, M.R.; Joghataei, M.T.; Mirzapour, T.; Bakhtiyari, M.; Shakeri, M.; Pirhajati, V.; Shirinbayan, P.; Koruji, M. The effects of poly L-lactic acid nanofiber scaffold on mouse spermatogonial stem cell culture. Int. J. Nanomed. 2013, 8, 4563–4576. [Google Scholar]
- Ziloochi, K.M.; Bagher, Z.; Asgari, H.R.; Najafi, M.; Koruji, M.; Mehraein, F. Differentiation of neonate mouse spermatogonial stem cells on three-dimensional agar/polyvinyl alcohol nanofiber scaffold. Syst. Biol. Reprod. Med. 2020, 66, 202–215. [Google Scholar] [CrossRef]
- Bashiri, Z.; Zahiri, M.; Allahyari, H.; Esmaeilzade, B. Proliferation of human spermatogonial stem cells on optimized PCL/Gelatin nanofibrous scaffolds. Andrologia 2022, 54, e14380. [Google Scholar] [CrossRef]
- Jabari, A.; Gholami, K.; Khadivi, F.; Koruji, M.; Amidi, F.; Gilani, M.; Mahabadi, V.P.; Nikmahzar, A.; Salem, M.; Movassagh, S.A.; et al. In vitro complete differentiation of human spermatogonial stem cells to morphologic spermatozoa using a hybrid hydrogel of agarose and laminin. Int. J. Biol. Macromol. 2023, 235, 123801. [Google Scholar] [CrossRef] [PubMed]
- Jabari, A.; Sadighi, G.M.; Koruji, M.; Gholami, K.; Mohsenzadeh, M.; Rastegar, T.; Khadivi, F.; Ghanami, G.N.; Nikmahzar, A.; Mojaverrostami, S.; et al. Three-dimensional co-culture of human spermatogonial stem cells with Sertoli cells in soft agar culture system supplemented by growth factors and Laminin. Acta Histochem. 2020, 122, 151572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wan, W.; Li, B.; Zhang, X.; Zhang, M.; Wu, Z.; Yang, H. Isolation and in vitro expansion of porcine spermatogonial stem cells. Reprod. Domest. Anim. 2022, 57, 210–220. [Google Scholar] [CrossRef] [PubMed]
- He, B.R.; Lu, F.; Zhang, L.; Hao, D.J.; Yang, H. An alternative long-term culture system for highly-pure mouse spermatogonial stem cells. J. Cell Physiol. 2015, 230, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Feng, Q.; Ma, X.; Deng, Y.; Zhang, K.; Ooi, H.S.; Yang, B.; Zhang, Z.Y.; Feng, B.; Bian, L. Dynamic gelatin-based hydrogels promote the proliferation and self-renewal of embryonic stem cells in long-term 3D culture. Biomaterials 2022, 289, 121802. [Google Scholar] [CrossRef]
- Poels, J.; Abou-Ghannam, G.; Decamps, A.; Leyman, M.; Rieux, A.; Wyns, C. Transplantation of testicular tissue in alginate hydrogel loaded with VEGF nanoparticles improves spermatogonial recovery. J. Control Release 2016, 234, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Gholami, K.; Vermeulen, M.; Del, V.F.; de Michele, F.; Giudice, M.G.; Wyns, C. The air-liquid interface culture of the mechanically isolated seminiferous tubules embedded in agarose or alginate improves in vitro spermatogenesis at the expense of attenuating their integrity. In Vitro Cell Dev. Biol. Anim. 2020, 56, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Gholami, K.; Pourmand, G.; Koruji, M.; Ashouri, S.; Abbasi, M. Organ culture of seminiferous tubules using a modified soft agar culture system. Stem Cell Res. Ther. 2018, 9, 249. [Google Scholar] [CrossRef] [PubMed]
- Kojima, K.; Sato, T.; Naruse, Y.; Ogawa, T. Spermatogenesis in Explanted Fetal Mouse Testis Tissues. Biol. Reprod. 2016, 95, 63. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, K.; Kojima, K.; Komeya, M.; Yao, M.; Ogawa, T. In Vitro Spermatogenesis in Explanted Adult Mouse Testis Tissues. PLoS ONE 2015, 10, e130171. [Google Scholar] [CrossRef]
- Matsumura, T.; Sato, T.; Abe, T.; Sanjo, H.; Katagiri, K.; Kimura, H.; Fujii, T.; Tanaka, H.; Hirabayashi, M.; Ogawa, T. Rat in vitro spermatogenesis promoted by chemical supplementations and oxygen-tension control. Sci. Rep. 2021, 11, 3458. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Katagiri, K.; Kubota, Y.; Ogawa, T. In vitro sperm production from mouse spermatogonial stem cell lines using an organ culture method. Nat. Protoc. 2013, 8, 2098–2104. [Google Scholar] [CrossRef] [PubMed]
- Busada, J.T.; Geyer, C.B. The Role of Retinoic Acid (RA) in Spermatogonial Differentiation. Biol. Reprod. 2016, 94, 10. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Teletin, M.; Vernet, N.; Ghyselinck, N.B. Role of retinoic acid receptor (RAR) signaling in post-natal male germ cell differentiation. Biochim. Biophys. Acta 2015, 1849, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Hogarth, C.A.; Arnold, S.; Kent, T.; Mitchell, D.; Isoherranen, N.; Griswold, M.D. Processive pulses of retinoic acid propel asynchronous and continuous murine sperm production. Biol. Reprod. 2015, 92, 37. [Google Scholar] [CrossRef] [PubMed]
- Paik, J.; Haenisch, M.; Muller, C.H.; Goldstein, A.S.; Arnold, S.; Isoherranen, N.; Brabb, T.; Treuting, P.M.; Amory, J.K. Inhibition of retinoic acid biosynthesis by the bisdichloroacetyldiamine WIN 18,446 markedly suppresses spermatogenesis and alters retinoid metabolism in mice. J. Biol. Chem. 2014, 289, 15104–15117. [Google Scholar] [CrossRef] [PubMed]
- Sanjo, H.; Komeya, M.; Sato, T.; Abe, T.; Katagiri, K.; Yamanaka, H.; Ino, Y.; Arakawa, N.; Hirano, H.; Yao, T.; et al. In vitro mouse spermatogenesis with an organ culture method in chemically defined medium. PLoS ONE 2018, 13, e192884. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.M.; Yang, H.; Luo, F.H.; Li, M.X.; Zhang, S.; Yang, X.G.; Lu, Y.Q.; Lu, S.S.; Wu, Y.J.; Lu, K.H. Isolation, proliferation, and induction of Bama mini-pig spermatogonial stem cells in vitro. Genet. Mol. Res. 2016, 15, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Thurston, L.; Abbara, A.; Dhillo, W.S. Investigation and management of subfertility. J. Clin. Pathol. 2019, 72, 579–587. [Google Scholar] [CrossRef]
- Miura, T.; Yamauchi, K.; Takahashi, H.; Nagahama, Y. Human chorionic gonadotropin induces all stages of spermatogenesis in vitro in the male Japanese eel (Anguilla japonica). Dev. Biol. 1991, 146, 258–262. [Google Scholar] [CrossRef]
- Nader, M.R.; Miura, T.; Ando, N.; Miura, C.; Yamauchi, K. Recombinant human insulin-like growth factor I stimulates all stages of 11-ketotestosterone-induced spermatogenesis in the Japanese eel, Anguilla japonica, in vitro. Biol. Reprod. 1999, 61, 944–947. [Google Scholar] [CrossRef]
- Miura, T.; Ando, N.; Miura, C.; Yamauchi, K. Comparative studies between in vivo and in vitro spermatogenesis of Japanese eel (Anguilla japonica). Zoolog Sci. 2002, 19, 321–329. [Google Scholar] [CrossRef]
- Deng, S.L.; Chen, S.R.; Wang, Z.P.; Zhang, Y.; Tang, J.X.; Li, J.; Wang, X.X.; Cheng, J.M.; Jin, C.; Li, X.Y.; et al. Melatonin promotes development of haploid germ cells from early developing spermatogenic cells of Suffolk sheep under in vitro condition. J. Pineal Res. 2016, 60, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Navid, S.; Rastegar, T.; Baazm, M.; Alizadeh, R.; Talebi, A.; Gholami, K.; Khosravi-Farsani, S.; Koruji, M.; Abbasi, M. In vitro effects of melatonin on colonization of neonate mouse spermatogonial stem cells. Syst. Biol. Reprod. Med. 2017, 63, 370–381. [Google Scholar] [CrossRef]
- Yang, Q.; Li, F.; Miao, Y.; Luo, X.; Dai, S.; Liu, J.; Niu, W.; Sun, Y. CdSe/ZnS quantum dots induced spermatogenesis dysfunction via autophagy activation. J. Hazard. Mater. 2020, 398, 122327. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef]
- Peng, Y.J.; Tang, X.T.; Shu, H.S.; Dong, W.; Shao, H.; Zhou, B.O. Sertoli cells are the source of stem cell factor for spermatogenesis. Development 2023, 150, dev200706. [Google Scholar] [CrossRef] [PubMed]
- Picazo, C.M.; Castano, C.; Boveda, P.; Toledano-Diaz, A.; Velazquez, R.; Pequeno, B.; Esteso, M.C.; Gadea, J.; Villaverde-Morcillo, S.; Cerdeira, J.; et al. Cryopreservation of testicular tissue from the dog (Canis familiaris) and wild boar (Sus scrofa) by slow freezing and vitrification: Differences in cryoresistance according to cell type. Theriogenology 2022, 190, 65–72. [Google Scholar] [CrossRef]
- Yildiz, C.; Mullen, B.; Jarvi, K.; McKerlie, C.; Lo, K.C. Comparison of cryosurvival and spermatogenesis efficiency of cryopreserved neonatal mouse testicular tissue between three vitrification protocols and controlled-rate freezing. Cryobiology 2018, 84, 4–9. [Google Scholar] [CrossRef]
- Onofre, J.; Baert, Y.; Faes, K.; Goossens, E. Cryopreservation of testicular tissue or testicular cell suspensions: A pivotal step in fertility preservation. Hum. Reprod. Update 2016, 22, 744–761. [Google Scholar] [CrossRef]
- Peris-Frau, P.; Benito-Blanco, J.; Martinez-Nevado, E.; Toledano-Diaz, A.; Castano, C.; Velazquez, R.; Pequeno, B.; Martinez-Madrid, B.; Esteso, M.C.; Santiago-Moreno, J. DNA integrity and viability of testicular cells from diverse wild species after slow freezing or vitrification. Front. Vet. Sci. 2022, 9, 1114695. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Pereira, A.G.; Bezerra, L.; Jeronimo, M.S.; Pereira, A.F.; Oliveira, M.F.; Comizzoli, P.; Silva, A.R. Cryopreservation of Testicular Tissue from Adult Red-Rumped Agoutis (Dasyprocta leporina Linnaeus, 1758). Animals 2022, 12, 738. [Google Scholar] [CrossRef] [PubMed]
| Source of SSCs | Culture Substrate of SSCs | Markers of SSCs | Whether to Produce Offspring of SSCs | Reference of SSCs |
|---|---|---|---|---|
| Mice | alginate hydrogel with Sertoli cells | The expressions of integrin alpha-6, integrin beta-1, Nanog, Plzf, Thy-1, Oct4 and Bcl2 were increased, while the expressions of P53, Fas and Bax were decreased | unresearched | [22] |
| Mice | MEF | EpCAM, CD9, α6- and β1-integrin were strongly expressed, while c-kit was weakly expressed | SSCs cultured in vitro were transplanted into the testis of sterile mice to produce normal, fertile offspring | [25] |
| Mice | supporting cells along with collagen protein | Meiosis markers SCP3 and post-meiosis markers Crem and TTF1 | unresearched | [24] |
| Mice | MEF | The expressions of Oct4 and Sox2 were detected | unresearched | [27] |
| Mice | hPMSC | The expression of proliferating genes (PCNA and KI67) increased, and the mRNA levels of apoptotic genes such as γ-H2AX, BRCA1, and PARP1 decreased | unresearched | [30] |
| Mice | BM-MSC | The proportion of c-Kit (+) differentiated spermatocytes to whole testicular cells was significantly higher in the BM-MSC co-culture group, and the number of SCP3 (+) primary and secondary spermatocytes and Acrosin (+) round spermatocytes at days 14, 28, and 42 were higher | unresearched | [31] |
| Mice | BM-MSC | C-KIT, VASA | unresearched | [32] |
| Pig | Porcine Sertoli cell | Differentiation gene C-kit, Stra8 | unresearched | [23] |
| Cat | MEF | The cells expressed SSC marker GFRα-1 and germ cell marker DDX-4 but did not express the differentiation gene c-kit | unresearched | [26] |
| Bull | BFF | The cells expressed undifferentiated cell markers ZBTB16 and LIN28 and SSC markers GFRA1 and NANOS2 | unresearched | [28] |
| Source of SSCs | Culture Substrate of SSCs | Markers of SSCs | Whether to Produce Offspring of SSCs | Reference of SSCs |
|---|---|---|---|---|
| Mice | mice DTM | The expression of Plzf was increased | unresearched | [34] |
| Mice | DTM | The expressions of Plzf, Sycp3, and Tnp1 were significantly increased | unresearched | [35] |
| Mice | Ram DTM | The expressions of pre-meiosis markers Plzf, Gfrα1, and Id4 were increased | unresearched | [37] |
| Mice | Mice DTM | The expression of the Plzf gene did not change much, but the expression of the Sycp3 gene increased significantly | unresearched | [39] |
| Mice | DTM with epididymosomes | The expressions of Plzf, miR-10b, and TGF-β were increased, while the expression of caspase-3 was decreased. Homing occurred after transplantation | unresearched | [40] |
| Human | Sheep DTM | Compared with 2D group, SCP3, Boule, Crem and Protamine2 expressions were increased | unresearched | [36] |
| Human | Sheep DTM | The expressions of pre-meiosis genes OCT4, Plzf, SCP3, BOULE, and post-meiosis genes CREM and Protamine2 were significantly increased | unresearched | [38] |
| Human | PRP | The expressions of GFRa1 and c-KIT were significantly increased | unresearched | [42] |
| Human | collagen | The expression of RMP2 increased | unresearched | [9] |
| Source of SSCs | Culture Substrate of SSCs | Markers of SSCs | Whether to Produce Offspring of SSCs | Reference of SSCs |
|---|---|---|---|---|
| Pig | agarose | Compared with the 2D microenvironment, the transcription levels of NANOG, EPCAM, UCHL1, GFRA1, and Plzf were significantly increased when cultured in 0.2% (w/v) agarose 3D hydrogel, and the protein levels of Plzf, OCT4, SOX2, and TRA-1-81 were significantly increased. Transcription of OCT4 and THY1 is upregulated, the transcription level of spermatogonial differentiation marker C-KIT is significantly down-regulated, and the translation of NANOG and TRA-1-60 is upregulated | Undifferentiated porcine SSCs transplanted into recipient mouse testis are still differentiated into sperm. | [43] |
| Rhesus monkey | MCS | The expression of the meiotic gene VASA, SALL4, and GFR-α1, meiotic gene CREM-1, and post-meiotic gene acrosin were observed after 30 days of culture | unresearched | [45] |
| Human | SACS | The expression of INTEGRIN α6 and SCP3 were increased in the SACS group, and the number and size of spermatogonial stem cell clones were significantly increased | unresearched | [15] |
| Human | MCS | The expression of pre-meiosis markers VASA, c-KIT, GFRa1, CD-9, α-6-Integrin, OCT4, Plzf, meiosis markers CREM-1, LDH, BOULE, and post-meiosis markers protamine and acrosin could be detected | unresearched | [40] |
| Human | PCL/Gelatin nanofibrous scaffolds | The number of spermatogonocytes increased, the expression of the Plzf gene increased significantly, and the expression of the c-Kit gene decreased significantly | unresearched | [50] |
| Human | agarose and laminin | Plzf, SCP3, PRM2, Acrosin positive cells were observed | Sperm-like cells are seen | [51] |
| Human | SACS and laminin | The expression of Plzf, α6-Integrin, Bcl2, and c-KIT genes can be seen | unresearched | [52] |
| Mice | MCS | After 4 weeks of culture, the number of clones increased, and the expressions of CD9, VASA, CREM, BOULE, and ACROSIN increased | Sperm-like cells are seen | [44] |
| Mice | alginate hydrogel | The expression of Oct4, Sox2, Nanog, Nanos2, Bcl6b and Plzf genes was observed | unresearched | [47] |
| Mice | PLLA | The expression of spermatogonial specific genes Plzf, Oct4, GFRα-1, VASA, Itgα6, Itgβ1 and germ cell differentiation gene c-Kit was observed | unresearched | [48] |
| Mice | PVA | Premeiosis markers ID-4 and GFRα-1 were significantly decreased, while the expressions of SYCP-3 Tektin 1 and TEKT-1 were increased after meiosis | unresearched | [49] |
| Mice | alginate hydrogel with Sertoli cells | The expression levels of integrin alpha-6, integrin beta-1, Nanog, Plzf, Thy-1, Oct4 a and Bcl2 were significantly increased | unresearched | [22] |
| Mice | PLL and laminin | VASA, GPR125, UCHL1, GFR-A1 and DAZL were expressed | unresearched | [54] |
| Mice | GelCD hydrogel | Pluripotent markers such as NANOG and OCT3/4 were significantly expressed, and nestin-positive, α-fetoprotein-positive, and α-SMA-positive cells represented differentiated cells from all three blastoderms | unresearched | [55] |
| Mice | Plzf and KI67 positive cells were observed | unresearched | [56] |
| Source of SSCs | Culture Substrate of SSCs | Markers of SSCs | Whether to Produce Offspring of SSCs | Reference of SSCs |
|---|---|---|---|---|
| Mice | agarose | Plzf, SCP3, KI67 positive cells were observed | [57] | |
| Mice | agarose | The expressions of Plzf, Integrin α6, Scp3 and Mvh were increased | [58] | |
| Mice | agarose | The testicular tissue volume increased by more than 3 times, and the expression of GFP was observed | There are round or elongated sperm cells produced | [59] |
| Mice | agarose | GFP-positive cells were observed | Round sperm cell | [60] |
| Mice | agarose | Tissue section observation | Round sperm cell | [61] |
| Mice | agarose | GFP begins to express after 18–30 days of culture and can last 15–45 or even longer | Sperm cell | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Wu, J.; Zhao, X.; Yu, M.; Taniguchi, M.; Bao, H.; Kang, K. Recent Progress of Induced Spermatogenesis In Vitro. Int. J. Mol. Sci. 2024, 25, 8524. https://doi.org/10.3390/ijms25158524
Liu S, Wu J, Zhao X, Yu M, Taniguchi M, Bao H, Kang K. Recent Progress of Induced Spermatogenesis In Vitro. International Journal of Molecular Sciences. 2024; 25(15):8524. https://doi.org/10.3390/ijms25158524
Chicago/Turabian StyleLiu, Siqi, Jiang Wu, Xin Zhao, Meng Yu, Masayasu Taniguchi, Huimingda Bao, and Kai Kang. 2024. "Recent Progress of Induced Spermatogenesis In Vitro" International Journal of Molecular Sciences 25, no. 15: 8524. https://doi.org/10.3390/ijms25158524
APA StyleLiu, S., Wu, J., Zhao, X., Yu, M., Taniguchi, M., Bao, H., & Kang, K. (2024). Recent Progress of Induced Spermatogenesis In Vitro. International Journal of Molecular Sciences, 25(15), 8524. https://doi.org/10.3390/ijms25158524







