Application and Development of Cell Membrane Functionalized Biomimetic Nanoparticles in the Treatment of Acute Ischemic Stroke
Abstract
1. Introduction
2. Pathophysiology of Ischemic Stroke
2.1. Excitotoxicity
2.2. Oxidative Stress
2.3. Inflammatory Response
3. Progress of Research on Biomimetic Nanoparticles
3.1. Methods of Acquiring Cell Membranes
3.2. Membrane Preparation of Biomimetic Nanoparticles for Cell Membrane Functionalization
3.2.1. Co-Extrusion
3.2.2. Ultrasonication
3.2.3. Microfluidic Electroporation
3.3. Bionic Cell Membrane Nanoparticles for Targeted Therapy in Acute Ischemic Stroke
3.3.1. Biomimetic Nanoparticles for Red Blood Cell Membrane Functionalization
3.3.2. Biomimetic Nanoparticles for Neutrophil Membrane Functionalization
3.3.3. Biomimetic Nanoparticles for Platelet Membrane Functionalization
3.3.4. Biomimetic Nanoparticles for Exosome Membrane Functionalization
3.3.5. Macrophage Membrane-Functionalized Biomimetic Nanoparticles
3.3.6. Biomimetic Nanoparticles for Neural Stem Cell Membrane Functionalization
3.3.7. Biomimetic Nanoparticles Functionalized with Engineered Cell Membranes
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abridgement | Generic Term |
| ADEXs | astrocyte-derived exosomes |
| AIS | acute ischemic stroke |
| ANPs | amylose starch nanoparticles |
| ATP | adenosine triphosphate |
| BA-LP | baicalein liposomes |
| BBB | Blood–brain barrier |
| BMECs | brain microvascular endothelial cells |
| CIRI | cerebral ischemia and reperfusion injury |
| CMC@NPs | cell membrane-coated nanoparticles |
| Edv | edaravone |
| FTY720 | fingolimod hydrochloride |
| GB | ginkgolide B |
| GBD | global burden of disease |
| H+ | hydrogen ion |
| HBA | 4-hydroxybenzaldehyde |
| HT | hemorrhagic transformation |
| ICG | indocyanine green |
| IL-1 | interleukin-1 |
| IL-1β | interleukin-1β |
| IL-6 | interleukin-6 |
| IL-10 | interleukin-10 |
| I/R | ischemia/reperfusion |
| IS | ischemic stroke |
| MCAO | middle cerebral artery occlusion |
| MM-BA-LP | modified baicalein liposomes |
| MMPs | matrix metalloproteinases |
| MNs | magnetic nanoparticles |
| MRI | magnetic resonance imaging |
| MSC | mesenchymal stem cell |
| NMs | neutrophil membranes |
| NO | nitric oxide |
| NPs | nanoparticles |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| NSCs | neural stem cells |
| OC | oxalyl chloride |
| OGD/R | oxygen–glucose deprivation/reoxygenation |
| PAK | p21-activated protein kinase |
| PDAs | polydopamine nanospheres |
| PLTs | platelets |
| PNSs | panax notoginseng total saponins |
| PS | phosphatidylcholine |
| PTT | photothermal therapy |
| Rac | Rac2-Tyr172 |
| RAPA | rapamycin |
| RBC | red blood cell |
| Rg3 | ginsenoside Rg3 |
| ROS | reactive oxygen species |
| RvD1 | resolvin D1 |
| SHp | stroke-homing peptide |
| tPA | tissue plasminogen activator |
| TGF-β | transforming growth factor-beta |
| TNF-α | tumor necrosis factor-alpha |
| VEGF | vascular endothelial growth factor |
| VAV2 | vav guanine nucleotide exchange factor 2 |
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, Regional, and National Burden of Stroke and Its Risk Factors, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- Almalki, W.H.; Alghamdi, S.; Alzahrani, A.; Zhang, W. Emerging Paradigms in Treating Cerebral Infarction with Nanotheranostics: Opportunities and Clinical Challenges. Drug Discov. Today 2021, 26, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Barthels, D.; Das, H. Current Advances in Ischemic Stroke Research and Therapies. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165260. [Google Scholar] [CrossRef] [PubMed]
- Walter, K. What Is Acute Ischemic Stroke? JAMA 2022, 327, 885. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Chu, R.; Wang, Y. Neuroprotective Treatments for Ischemic Stroke: Opportunities for Nanotechnology. Adv. Funct. Mater. 2022, 32, 2209405. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, Q.; Ma, D.; Wang, Z.; Li, L.; Han, M.; Yin, X.; Ji, X.; Wang, S.; Xin, T. Biomimetic Nanoparticles in Ischemic Stroke Therapy. Discov. Nano 2023, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, T.; Jiang, C. Recent Advances in Nanomedicines for the Treatment of Ischemic Stroke. Acta Pharm. Sin. B 2021, 11, 1767–1788. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, H.; Peng, Y.; Sun, H.; Chen, M.; Fei, A.; Pan, S. Mechanical Thrombectomy by Solitaire Stent for Treating Acute Ischemic Stroke: A Prospective Cohort Study. Int. J. Surg. 2016, 28, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Guidera, S.A.; Aggarwal, S.; Walton, J.D.; Boland, D.; Jackel, R.; Gould, J.D.; Kearins, B.; McGarvey, J.; Qi, Y.; Furlong, B. Mechanical Thrombectomy for Acute Ischemic Stroke in the Cardiac Catheterization Laboratory. JACC Cardiovasc. Interv. 2020, 13, 884–891. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Y.; Li, R.; Chen, Z.; Fan, X. Advanced Drug Delivery System against Ischemic Stroke. J. Control. Release 2022, 344, 173–201. [Google Scholar] [CrossRef]
- Nong, J.; Glassman, P.M.; Reyes-Esteves, S.; Descamps, H.C.; Kiseleva, R.Y.; Papp, T.E.; Alameh, M.-G.; Tam, Y.K.; Mui, B.L.; Omo-Lamai, S.; et al. Targeting Lipid Nanoparticles to the Blood Brain Barrier to Ameliorate Acute Ischemic Stroke. bioRxiv 2023. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, Á. Neuroprotectants in the Era of Reperfusion Therapy. J. Stroke 2018, 20, 197–207. [Google Scholar] [CrossRef]
- Liu, P.; Jiang, C. Brain-targeting Drug Delivery Systems. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1818. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, M.; Shimoni, O.; Banks, W.A.; Bush, A.I.; Gamble, J.R.; Shi, B. Development of Novel Therapeutics Targeting the Blood–Brain Barrier: From Barrier to Carrier. Adv. Sci. 2021, 8, 2101090. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zou, J.; Chen, Z.; He, W.; Wu, W. Current Research Trends of Nanomedicines. Acta Pharm. Sin. B 2023, 13, 4391–4416. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, A.; Wu, J.; Wan, Y.; You, M.; Gu, X.; Guo, H.; Tan, S.; He, Q.; Hu, B. Nanomedicine: An Emerging Novel Therapeutic Strategy for Hemorrhagic Stroke. Int. J. Nanomed. 2022, 17, 1927–1950. [Google Scholar] [CrossRef]
- He, W.; Zhang, Z.; Sha, X. Nanoparticles-Mediated Emerging Approaches for Effective Treatment of Ischemic Stroke. Biomaterials 2021, 277, 121111. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Liu, Y.; Li, S.; Lv, L.; Lu, H.; Xin, H. Advances of Nano Drug Delivery System for the Theranostics of Ischemic Stroke. J. Nanobiotechnol. 2022, 20, 248. [Google Scholar] [CrossRef]
- Tian, X.; Fan, T.; Zhao, W.; Abbas, G.; Han, B.; Zhang, K.; Li, N.; Liu, N.; Liang, W.; Huang, H.; et al. Recent Advances in the Development of Nanomedicines for the Treatment of Ischemic Stroke. Bioact. Mater. 2021, 6, 2854–2869. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Chen, J.; Liu, Y.; Cheng, X.; Yang, F.; Gu, N. Platelet Membrane Biomimetic Magnetic Nanocarriers for Targeted Delivery and in Situ Generation of Nitric Oxide in Early Ischemic Stroke. ACS Nano 2020, 14, 2024–2035. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Chen, J.; Liu, T.; Gu, Z.; Zhang, J.; Gu, X.; Teng, G.; Yang, F.; Gu, N. Platelet Bio-Nanobubbles as Microvascular Recanalization Nanoformulation for Acute Ischemic Stroke Lesion Theranostics. Theranostics 2018, 8, 4870–4883. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Han, Y.; Lu, P.; Ding, Y.; Wang, Q.; Li, Y.; Wei, J.; Huang, Q.; Wang, R.; Zhao, Y. Annexin V-Modified Platelet-Biomimetic Nanomedicine for Targeted Therapy of Acute Ischemic Stroke. Adv. Healthc. Mater. 2022, 11, 2200416. [Google Scholar] [CrossRef] [PubMed]
- Brenner, J.S.; Mitragotri, S.; Muzykantov, V.R. Red Blood Cell Hitchhiking: A Novel Approach for Vascular Delivery of Nanocarriers. Annu. Rev. Biomed. Eng. 2021, 23, 225–248. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Sha, M.; Ye, Y.; Wang, C. Cell Membrane-Derived Nanovehicles for Targeted Therapy of Ischemic Stroke: From Construction to Application. Pharmaceutics 2023, 16, 6. [Google Scholar] [CrossRef]
- Campos, F.; Pérez-Mato, M.; Agulla, J.; Blanco, M.; Barral, D.; Almeida, Á.; Brea, D.; Waeber, C.; Castillo, J.; Ramos-Cabrer, P. Glutamate Excitoxicity Is the Key Molecular Mechanism Which Is Influenced by Body Temperature during the Acute Phase of Brain Stroke. PLoS ONE 2012, 7, e44191. [Google Scholar] [CrossRef]
- Yang, X.; Yu, H.; Li, J.; Li, N.; Li, C.; Xu, D.; Zhang, H.; Fang, T.; Wang, S.; Yan, P.; et al. Excitotoxic Storms of Ischemic Stroke: A Non-Neuronal Perspective. Mol. Neurobiol. 2024, 1–20. [Google Scholar] [CrossRef]
- Li, W.; Yang, S. Targeting Oxidative Stress for the Treatment of Ischemic Stroke: Upstream and Downstream Therapeutic Strategies. Brain Circ. 2016, 2, 153–163. [Google Scholar] [PubMed]
- Yu, H.; Cai, Y.; Zhong, A.; Zhang, Y.; Zhang, J.; Xu, S. The “Dialogue” Between Central and Peripheral Immunity After Ischemic Stroke: Focus on Spleen. Front. Immunol. 2021, 12, 792522. [Google Scholar] [CrossRef]
- Yang, K.; Bao, T.; Zeng, J.; Wang, S.; Yuan, X.; Xiang, W.; Xu, H.; Zeng, L.; Ge, J. Research Progress on Pyroptosis-Mediated Immune-Inflammatory Response in Ischemic Stroke and the Role of Natural Plant Components as Regulator of Pyroptosis: A Review. Biomed. Pharmacother. 2023, 157, 113999. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Gao, H.; Zhang, X.; Sun, L.; Huang, Y.; Zhang, J.; Ding, B. Cell Membrane-Coated Biomimetic Nanoparticles in Cancer Treatment. Pharmaceutics 2024, 16, 531. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, J.; Zhang, C.; Olah, A.; Baer, E. Novel Micro-/Nano- Porous Cellular Membranes by Forced Assembly Co-Extrusion Technology. Eur. Polym. J. 2016, 83, 99–113. [Google Scholar] [CrossRef]
- Levêque, J.-M.; Duclaux, L.; Rouzaud, J.-N.; Reinert, L.; Komatsu, N.; Desforges, A.; Afreen, S.; Sivakumar, M.; Kimura, T. Ultrasonic Treatment of Glassy Carbon for Nanoparticle Preparation. Ultrason. Sonochem. 2017, 35, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Yokose, R.; Makuta, T. Fabrication of Hollow Metal Nanoparticles by Ultrasonically Generated Microbubbles. Mater. Lett. 2018, 214, 20–22. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, X.; Wang, Q.; Ren, L.; Tong, J.; Zhou, J. Influence of Ultrasonic Treatment on Formation of Amylose Nanoparticles Prepared by Nanoprecipitation. Carbohydr. Polym. 2017, 157, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.; Cai, B.; Bu, L.-L.; Liao, Q.-Q.; Guo, S.-S.; Zhao, X.-Z.; Dong, W.-F.; Liu, W. Microfluidic Electroporation-Facilitated Synthesis of Erythrocyte Membrane-Coated Magnetic Nanoparticles for Enhanced Imaging-Guided Cancer Therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhang, K.; Qiu, J.; Wang, N.; Qu, K.; Cui, Y.; Huang, J.; Luo, L.; Zhong, Y.; Tian, T.; et al. Uptake of Oxidative Stress-Mediated Extracellular Vesicles by Vascular Endothelial Cells under Low Magnitude Shear Stress. Bioact. Mater. 2022, 9, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; He, Y. Migration of a Red Blood Cell in a Permeable Microvessel. Med. Nov. Technol. Devices 2019, 3, 100023. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Xu, J.; Liu, G.; Di, C.; Zhao, X.; Li, X.; Li, Y.; Pang, N.; Yang, C.; et al. Engineered Nanoplatelets for Targeted Delivery of Plasminogen Activators to Reverse Thrombus in Multiple Mouse Thrombosis Models. Adv. Mater. 2020, 32, 1905145. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Yin, H.; Cao, X.; Hu, Q.; Lv, W.; Xu, Q.; Gu, Z.; Xin, H. Sequentially Site-Specific Delivery of Thrombolytics and Neuroprotectant for Enhanced Treatment of Ischemic Stroke. ACS Nano 2019, 13, 8577–8588. [Google Scholar] [CrossRef]
- Vankayala, R.; Corber, S.R.; Mac, J.T.; Rao, M.P.; Shafie, M.; Anvari, B. Erythrocyte-Derived Nanoparticles as a Theranostic Agent for Near-Infrared Fluorescence Imaging and Thrombolysis of Blood Clots. Macromol. Biosci. 2018, 18, 1700379. [Google Scholar] [CrossRef]
- Lv, W.; Xu, J.; Wang, X.; Li, X.; Xu, Q.; Xin, H. Bioengineered Boronic Ester Modified Dextran Polymer Nanoparticles as Reactive Oxygen Species Responsive Nanocarrier for Ischemic Stroke Treatment. ACS Nano 2018, 12, 5417–5426. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, X.; Xu, L.; Tu, F.; Rui, X.; Zhang, L.; Yan, Z.; Liu, Y.; Hu, R. Neutrophil Membrane-Coated Nanoparticles Exhibit Increased Antimicrobial Activities in an Anti-Microbial Resistant K. Pneumonia Infection Model. Nanomed. Nanotechnol. Biol. Med. 2023, 48, 102640. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Billich, A.; Baumruker, T.; Heining, P.; Schmouder, R.; Francis, G.; Aradhye, S.; Burtin, P. Fingolimod (FTY720): Discovery and Development of an Oral Drug to Treat Multiple Sclerosis. Nat. Rev. Drug Discov. 2010, 9, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, Y.; Bai, S.; Xiang, W.; Zhou, X.; Han, L.; Zhu, D.; Guan, Y. Imaging of Microglia in Post-Stroke Inflammation. Nucl. Med. Biol. 2023, 118–119, 108336. [Google Scholar] [CrossRef] [PubMed]
- Camm, J.; Hla, T.; Bakshi, R.; Brinkmann, V. Cardiac and Vascular Effects of Fingolimod: Mechanistic Basis and Clinical Implications. Am. Heart J. 2014, 168, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, Q.; Niu, J.; Guo, E.; Zhao, C.; Zhang, J.; Liu, X.; Wang, L.; Rao, L.; Chen, X.; et al. Neutrophil Membrane-Camouflaged Polyprodrug Nanomedicine for Inflammation Suppression in Ischemic Stroke Therapy. Adv. Mater. 2024, 36, 2311803. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Tang, L.; Zhang, Y.; Ma, X.; Yin, Y.; Kuang, L.; Fan, Q.; Wang, B.; Hu, X.; Yin, T.; et al. A Homing Peptide Modified Neutrophil Membrane Biomimetic Nanoparticles in Response to ROS/Inflammatory Microenvironment for Precise Targeting Treatment of Ischemic Stroke. Adv. Funct. Mater. 2024, 34, 2309167. [Google Scholar] [CrossRef]
- Senchenkova, E.Y.; Ansari, J.; Becker, F.; Vital, S.A.; Al-Yafeai, Z.; Sparkenbaugh, E.M.; Pawlinski, R.; Stokes, K.Y.; Carroll, J.L.; Dragoi, A.-M.; et al. Novel Role for the AnxA1-Fpr2/ALX Signaling Axis as a Key Regulator of Platelet Function to Promote Resolution of Inflammation. Circulation 2019, 140, 319–335. [Google Scholar] [CrossRef]
- Saccaro, L.F.; Pico, F.; Chadenat, M.-L.; Richard, O.; Launay, J.-M.; Bastenaire, B.; Jullien, P.; Lambert, J.; Feuga, V.; Macquet, M.; et al. Platelet, Plasma, Urinary Tryptophan-Serotonin-Kynurenine Axis Markers in Hyperacute Brain Ischemia Patients: A Prospective Study. Front. Neurol. 2022, 12, 782317. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, M.; Chen, X.; Zhang, R.; Le, A.; Hong, M.; Zhang, Y.; Jia, L.; Zang, W.; Jiang, C.; et al. Tryptophan Metabolism in Central Nervous System Diseases: Pathophysiology and Potential Therapeutic Strategies. Aging Dis. 2023, 14, 858–878. [Google Scholar] [CrossRef] [PubMed]
- Sansbury, B.E.; Li, X.; Wong, B.; Patsalos, A.; Giannakis, N.; Zhang, M.J.; Nagy, L.; Spite, M. Myeloid ALX/FPR2 Regulates Vascularization Following Tissue Injury. Proc. Natl. Acad. Sci. USA 2020, 117, 14354–14364. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, H.; Wang, Z.; Zhao, C.; Xu, J.; Chen, Q. Resolvin D1 Improves Post-Resuscitation Cardiac and Cerebral Outcomes in a Porcine Model of Cardiac Arrest. Shock 2020, 54, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wu, J.; Hua, Q.; Lin, Z.; Ye, L.; Zhang, W.; Wu, G.; Du, J.; Xia, J.; Chu, M.; et al. Resolvin D1 Mitigates Energy Metabolism Disorder after Ischemia–Reperfusion of the Rat Lung. J. Transl. Med. 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Shu, H.-H.; Chang, L.; Ye, F.; Xu, K.-Q.; Huang, W.-Q. Resolvin D1 Protects against Hepatic Ischemia/Reperfusion Injury in Rats. Int. Immunopharmacol. 2015, 28, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.-W.; Feng, H.-C.; Xu, C.; Jiang, D.-Y.; Zhang, K.-H.; Gao, N.; Wang, Y.; Tian, H.; Liu, C. Platelet Membrane-Encapsulated Ginkgolide B Biomimetic Nanoparticles for the Treatment of Ischemic Stroke. ACS Appl. Nano Mater. 2023, 6, 17560–17571. [Google Scholar] [CrossRef]
- Ge, Y.; Xu, W.; Zhang, L.; Liu, M. Ginkgolide B Attenuates Myocardial Infarction-Induced Depression-like Behaviors via Repressing IL-1β in Central Nervous System. Int. Immunopharmacol. 2020, 85, 106652. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, L.; Cheng, H. Ginkgolide B Protects Against Ischemic Stroke via Targeting AMPK/PINK1. Front. Pharmacol. 2022, 13, 941094. [Google Scholar] [CrossRef] [PubMed]
- Maclennan, K. The CNS Effects of Ginkgo Biloba Extracts and Ginkgolide B. Prog. Neurobiol. 2002, 67, 235–257. [Google Scholar] [CrossRef]
- Jang, Y.; Park, J.; Kim, P.; Park, E.-J.; Sun, H.; Baek, Y.; Jung, J.; Song, T.; Doh, J.; Kim, H. Development of Exosome Membrane Materials-Fused Microbubbles for Enhanced Stability and Efficient Drug Delivery of Ultrasound Contrast Agent. Acta Pharm. Sin. B 2023, 13, 4983–4998. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.; Liu, P.; Liu, P.; Hua, W.; Zhang, Y.; Zhang, L.; Li, Z.; Xing, P.; Zhang, Y.; et al. Exosomes Containing miR-451a Is Involved in the Protective Effect of Cerebral Ischemic Preconditioning against Cerebral Ischemia and Reperfusion Injury. CNS Neurosci. Ther. 2021, 27, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, H.; Li, L.; Han, J.; Liu, Z.; Chu, M.; Sha, X.; Zhao, J. Anti-CHAC1 Exosomes for Nose-to-Brain Delivery of miR-760-3p in Cerebral Ischemia/Reperfusion Injury Mice Inhibiting Neuron Ferroptosis. J. Nanobiotechnol. 2023, 21, 109. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, Y.; Wang, L.; Li, S.; Zhou, J.; Tan, Y.; Song, J.; Xing, H.; Yi, K.; Zhan, Q.; et al. Glioma-Derived Exosomes Hijack the Blood–Brain Barrier to Facilitate Nanocapsule Delivery via LCN2. J. Control. Release 2022, 345, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Duan, R.; Ding, W.; Gu, Q.; Liu, M.; Zhou, J.; Sun, J.; Zhu, J. Astrocyte-Derived Exosomal Nicotinamide Phosphoribosyltransferase (Nampt) Ameliorates Ischemic Stroke Injury by Targeting AMPK/mTOR Signaling to Induce Autophagy. Cell Death Dis. 2022, 13, 1057. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Xu, J.; Chen, D.; Wu, T.; Cao, Y. Macrophage–Cancer Hybrid Membrane-Camouflaged Nanoplatforms for HIF-1α Gene Silencing-Enhanced Sonodynamic Therapy of Glioblastoma. ACS Appl. Mater. Interfaces 2023, 15, 31150–31158. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, L.; Ye, P.; Xie, M. Macrophage Membrane-Modified MoS2 Quantum Dots as a Nanodrug for Combined Multi-Targeting of Alzheimer’s Disease. Adv. Healthc. Mater. 2024, 13, 2303211. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, S.; Zhang, F.; Ma, S.; Heng, B.C.; Wang, Y.; Zhu, J.; Xu, M.; He, Y.; Wei, Y.; et al. Cell Membrane Vesicles with Enriched CXCR4 Display Enhances Their Targeted Delivery as Drug Carriers to Inflammatory Sites. Adv. Sci. 2021, 8, 2101562. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, Y.; Zhang, M.; Zhang, J.; Kang, N.; Zheng, L.; Ding, Z. The Optimization Design of Macrophage Membrane Camouflaging Liposomes for Alleviating Ischemic Stroke Injury through Intranasal Delivery. Int. J. Mol. Sci. 2024, 25, 2927. [Google Scholar] [CrossRef]
- Long, Y.; Xiang, Y.; Liu, S.; Zhang, Y.; Wan, J.; Ci, Z.; Cui, M.; Shen, L.; Li, N.; Guan, Y. Macrophage Membrane Modified Baicalin Liposomes Improve Brain Targeting for Alleviating Cerebral Ischemia Reperfusion Injury. Nanomed. Nanotechnol. Biol. Med. 2022, 43, 102547. [Google Scholar] [CrossRef]
- Jia, X.; Wang, L.; Feng, X.; Liu, W.; Wang, X.; Li, F.; Liu, X.; Yu, J.; Yu, B.; Yu, X. Cell Membrane-Coated Oncolytic Adenovirus for Targeted Treatment of Glioblastoma. Nano Lett. 2023, 23, 11120–11128. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, S.; Liu, J.; Liu, F.; Du, F.; Li, M.; Chen, A.T.; Bao, Y.; Suh, H.W.; Avery, J.; et al. Targeted Drug Delivery to Stroke via Chemotactic Recruitment of Nanoparticles Coated with Membrane of Engineered Neural Stem Cells. Small 2019, 15, 1902011. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jiang, X.; Li, Y.; Dong, Y.; Zheng, J.; Li, L.; Li, Y.; Wang, J.; Lin, X.; Zhang, X.; et al. Hybrid Stem Cell-Derived Bioresponsive Vesicles for Effective Inflamed Blood-Brain Barrier Targeting Delivery. Nano Today 2023, 49, 101800. [Google Scholar] [CrossRef]
- Yan, H.; Shao, D.; Lao, Y.; Li, M.; Hu, H.; Leong, K.W. Engineering Cell Membrane-Based Nanotherapeutics to Target Inflammation. Adv. Sci. 2019, 6, 1900605. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.J.C.; Kim, B.J.; Arai, Y.; Han, I.; Moon, J.J.; Paulmurugan, R.; Park, H.; Lee, S.-H. Bioengineered Stem Cell Membrane Functionalized Nanocarriers for Therapeutic Targeting of Severe Hindlimb Ischemia. Biomaterials 2018, 185, 360–370. [Google Scholar] [CrossRef]
- Yu, D.; Liu, C.; Zhang, H.; Ren, J.; Qu, X. Glycoengineering Artificial Receptors for Microglia to Phagocytose Ab Aggregates. Chem. Sci. 2021, 12, 4963–4969. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Y.; Yin, N.; Liu, C.; Zhao, Y.; Cheng, H.; Zhou, T.; Zhang, Z.; Zhang, K. Engineering CXCL12 Biomimetic Decoy-Integrated Versatile Immunosuppressive Nanoparticle for Ischemic Stroke Therapy with Management of Overactivated Brain Immune Microenvironment. Small Methods 2022, 6, 2101158. [Google Scholar] [CrossRef]
- Luo, L.; Zang, G.; Liu, B.; Qin, X.; Zhang, Y.; Chen, Y.; Zhang, H.; Wu, W.; Wang, G. Bioengineering CXCR4-Overexpressing Cell Membrane Functionalized ROS-Responsive Nanotherapeutics for Targeting Cerebral Ischemia-Reperfusion Injury. Theranostics 2021, 11, 8043–8056. [Google Scholar] [CrossRef]

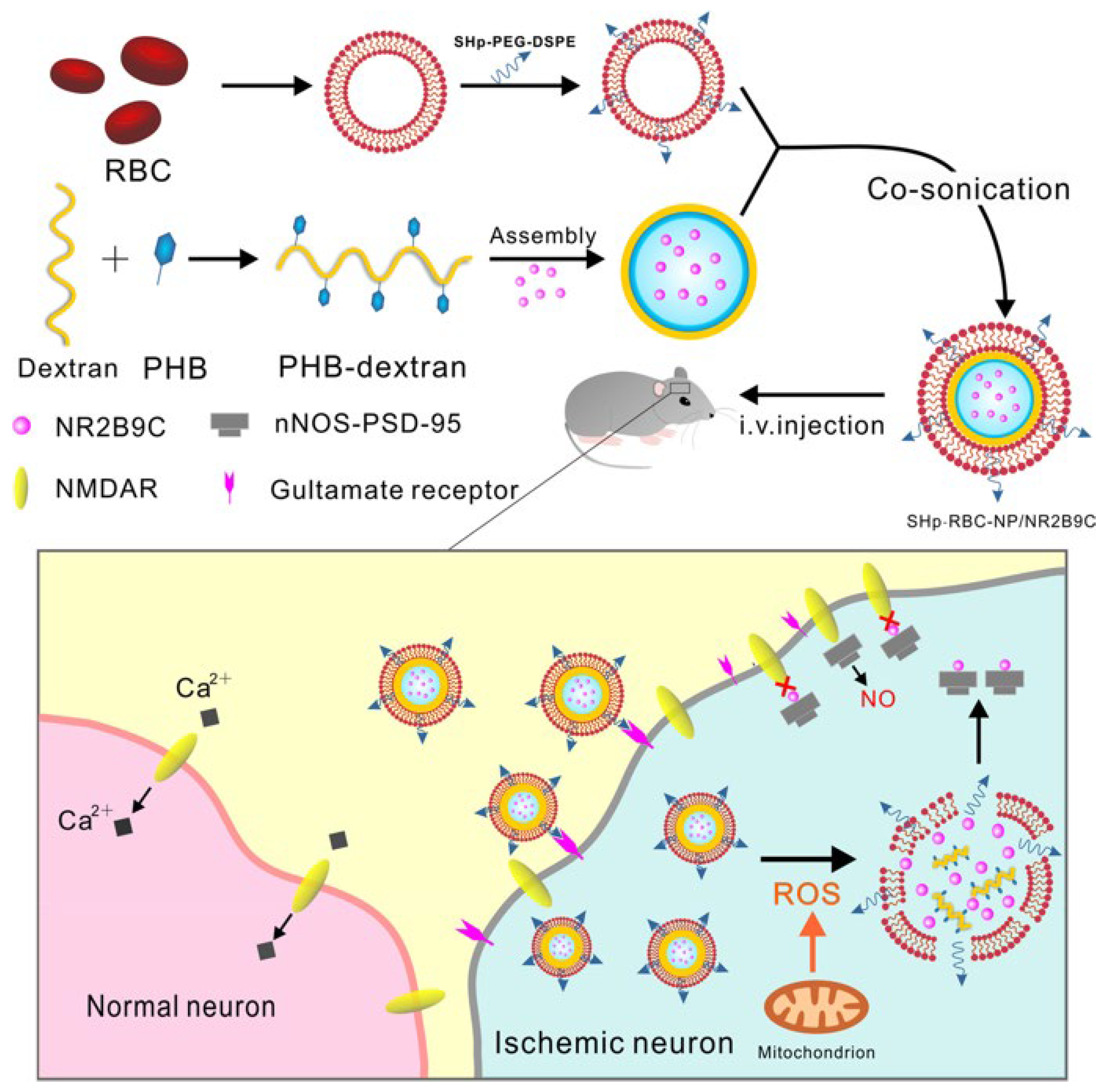

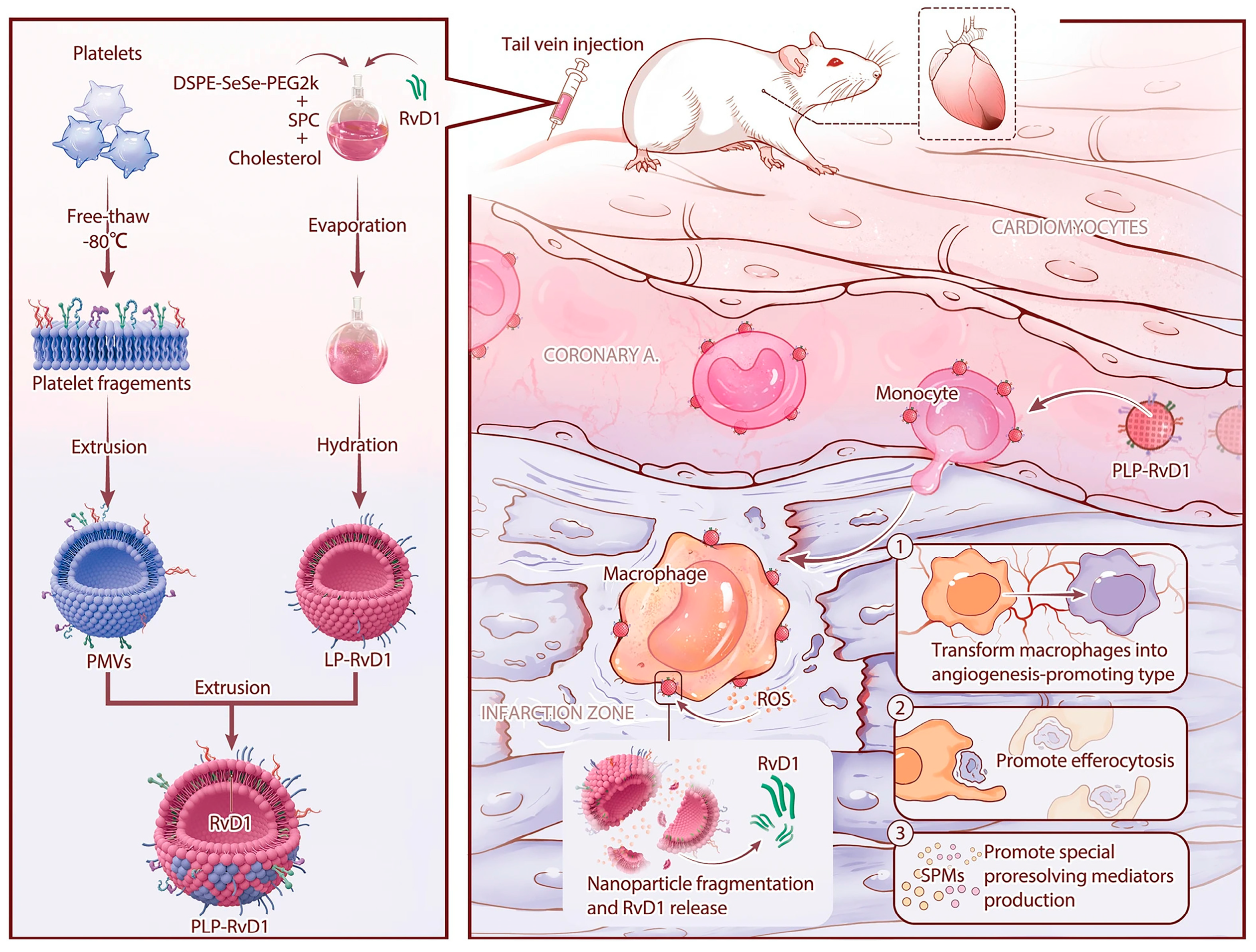
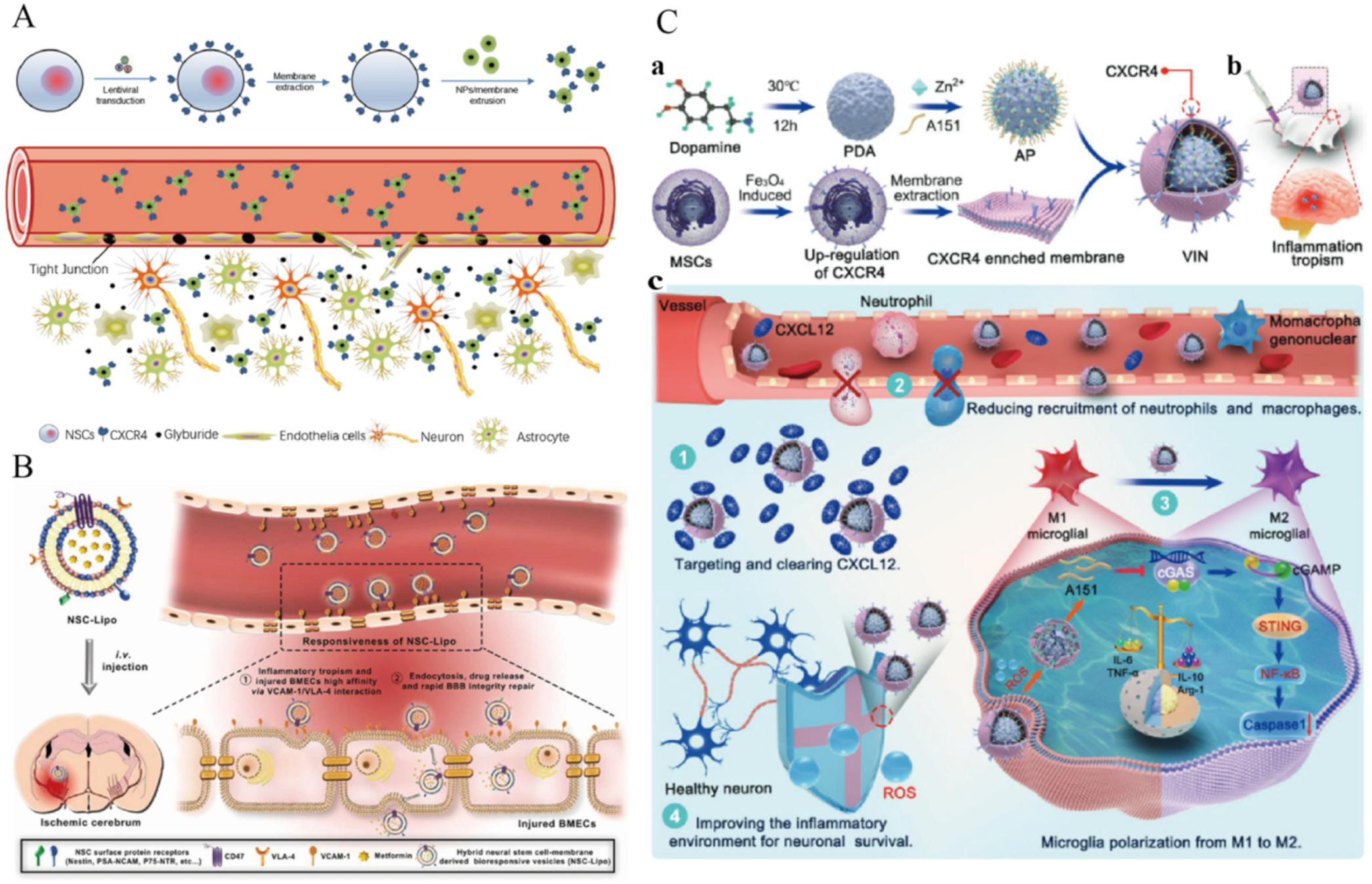
| Methods | Principle | Advantages |
|---|---|---|
| Co-extrusion | Physical shear-dependent mixing and fusion of cell membrane fragments with nanoparticles to generate biomimetic nanoparticles with specific biological functions | Ability to systematically control nanoparticle size and surface functionalization, resulting in highly consistent and bioactively stable nanocarriers for clinical studies |
| Ultrasonication | The use of high-frequency sound waves to form tiny bubbles and generate strong localized shear forces when they collapse violently helps to uniformly cover cell membrane fragments on the surface of nanoparticles | Not only is it simpler and faster, but it also effectively promotes the fusion of membrane vesicles with nanocarriers via acoustic wave technology |
| Microfluidic electroporation | Temporarily perturbing the structure of nanoparticles and cell membranes using an electric field applied to a microfluidic chip, thereby enabling the cell membrane to wrap around the nanoparticles | Efficient and precise control of cell membrane encapsulation with single-particle level processing |
| Type | Advantages | Disadvantages | Limitations of Clinical Translation |
|---|---|---|---|
| Red blood cell membrane functionalization | Prolong its circulation time in the body, slow down the clearance of the drug, and improve the bioavailability and therapeutic effect of the drug, and they can specifically recognize and bind to the target cells or tissues, and achieve precise drug delivery | Recurrence and regulation of function; complex and costly; requires special storage conditions to maintain its functionality and performance | Complex preparation and standardization; stability and long-term storage; Targeted capacity and therapeutic effects; the challenge of clinical trials |
| Neutrophil membrane functionalization | Biocompatibility and immune evasion properties, reducing recognition and clearance by the immune system | Recurrence and regulation of function; scaled production and application limitations | Complex preparation and standardization; stability and long-term storage; targeted capacity and therapeutic effects; the challenge of clinical trials |
| Platelet membrane functionalization | Enhanced drug targeting, improved therapeutic outcomes, and reduced side effects | Recurrence and regulation of function; scaled production and application limitations; drug loading and release efficiency | Stability and long-term storage; targeted capacity and therapeutic effects; the challenge of clinical trials |
| Exosome membrane functionalization | With their inherent immunogenicity, biocompatibility, and homing prowess, they serve as pivotal tools in immunomodulation and drug conveyance | Recurrence and regulation of function; drug loading and release efficiency; scaled production and application limitations | Complex preparation and standardization; stability and long-term storage; targeted capacity and therapeutic effects; the challenge of clinical trials |
| Macrophage membrane functionalization | Traverse the blood–brain barrier (BBB) more effectively, thereby enhancing their brain-targeting efficacy | Recurrence and regulation of function; drug loading and release efficiency; scaled production and application limitations | Stability and long-term storage; targeted capacity and therapeutic effects; the challenge of clinical trials |
| Neural stem cell membrane functionalization | Traits including self-renewal capacity, pluripotent differentiation potential, and low immunogenicity | Recurrence and regulation of function; drug loading and release efficiency; scaled production and application limitations | Complex preparation and standardization; stability and long-term storage; targeted capacity and therapeutic effects; the challenge of clinical trials |
| Engineered cell membranes functionalization | Allow for the incorporation of specific targeting molecules, facilitating precise localization of nanoparticles to target tissues or cells and thereby enhancing therapeutic efficacy | Recurrence and regulation of function; drug loading and release efficiency; scaled production and application limitations | Complex preparation and standardization; stability and long-term storage; targeted capacity and therapeutic effects; the challenge of clinical trials |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, C.; Yang, R.; Tang, J.; Li, Z.; Yi, X.; Fan, Z. Application and Development of Cell Membrane Functionalized Biomimetic Nanoparticles in the Treatment of Acute Ischemic Stroke. Int. J. Mol. Sci. 2024, 25, 8539. https://doi.org/10.3390/ijms25158539
Li Y, Wu C, Yang R, Tang J, Li Z, Yi X, Fan Z. Application and Development of Cell Membrane Functionalized Biomimetic Nanoparticles in the Treatment of Acute Ischemic Stroke. International Journal of Molecular Sciences. 2024; 25(15):8539. https://doi.org/10.3390/ijms25158539
Chicago/Turabian StyleLi, Ying, Chuang Wu, Rui Yang, Jiannan Tang, Zhanqing Li, Xue Yi, and Zhongxiong Fan. 2024. "Application and Development of Cell Membrane Functionalized Biomimetic Nanoparticles in the Treatment of Acute Ischemic Stroke" International Journal of Molecular Sciences 25, no. 15: 8539. https://doi.org/10.3390/ijms25158539
APA StyleLi, Y., Wu, C., Yang, R., Tang, J., Li, Z., Yi, X., & Fan, Z. (2024). Application and Development of Cell Membrane Functionalized Biomimetic Nanoparticles in the Treatment of Acute Ischemic Stroke. International Journal of Molecular Sciences, 25(15), 8539. https://doi.org/10.3390/ijms25158539






